Abstract
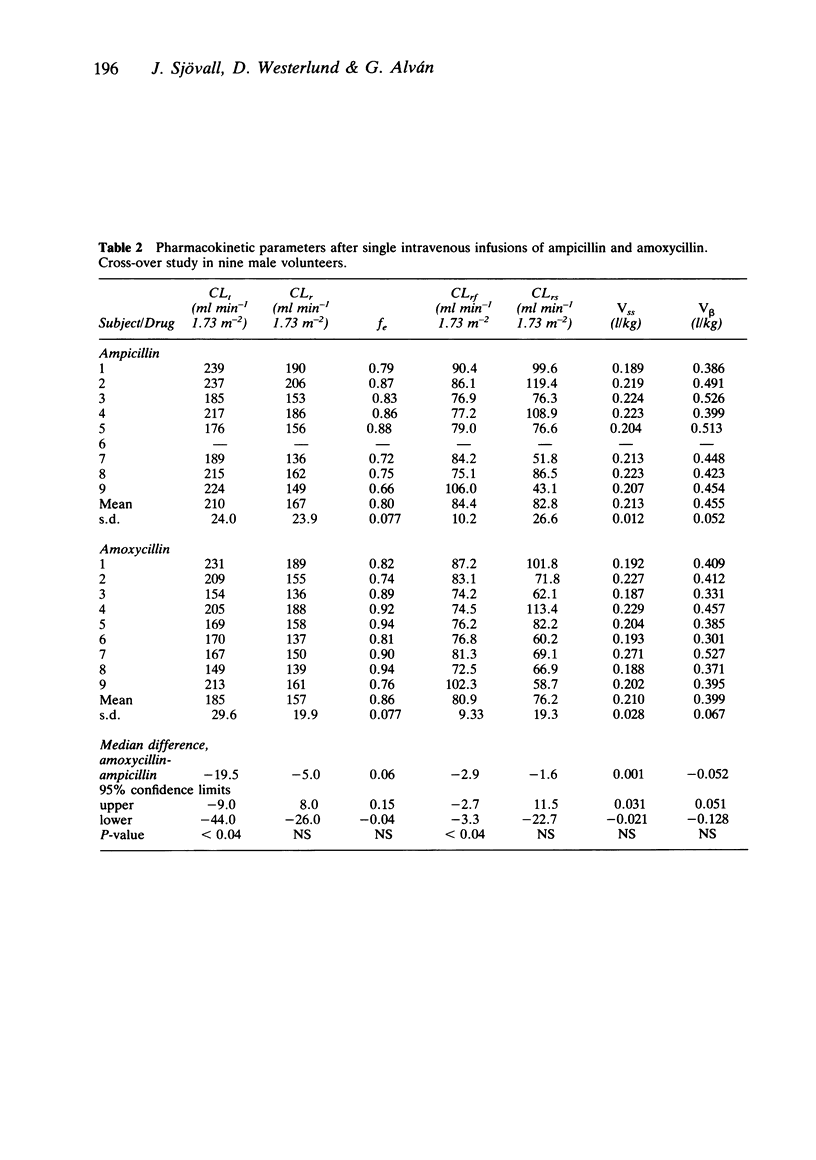
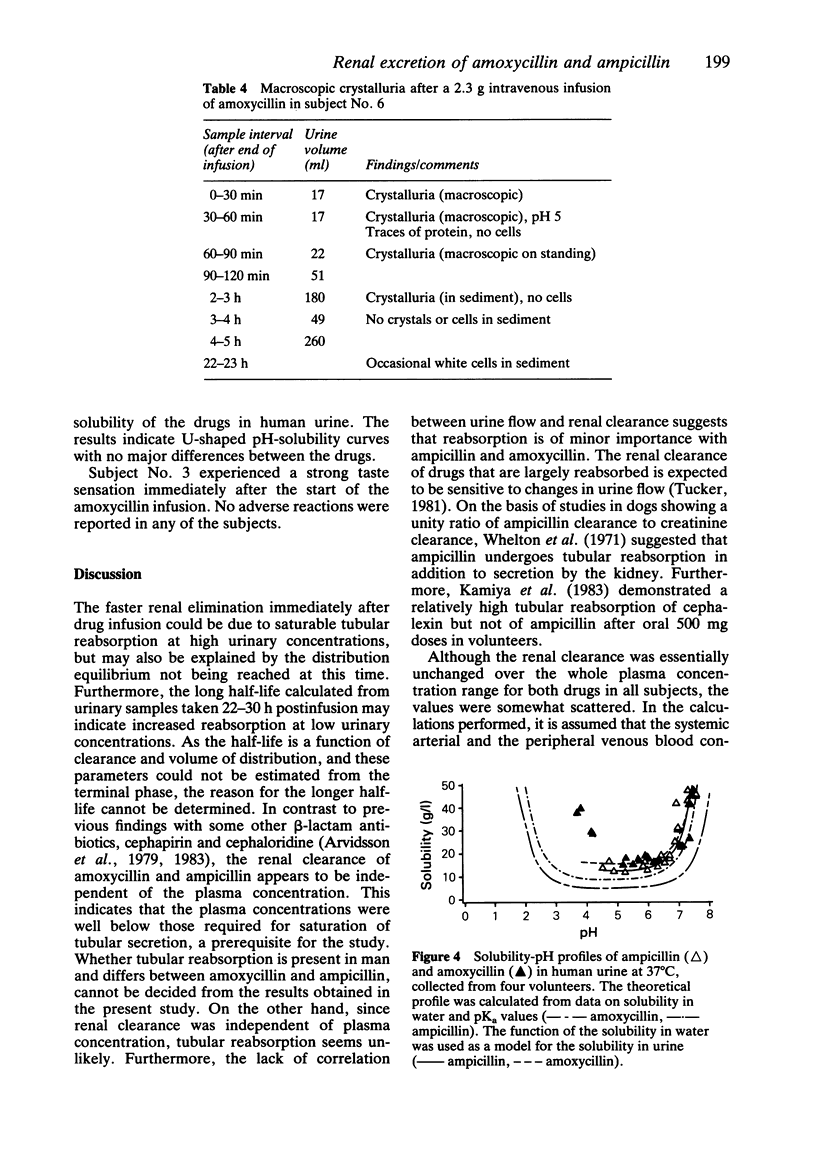
The aim of this study was to determine whether concentration-dependent renal clearance of ampicillin and amoxycillin occurs. The drugs were given as single 20 min i.v. infusions in doses ranging from 1.9 to 2.8 g to nine healthy volunteers using a cross-over design. Plasma and urinary concentrations were determined by a selective liquid chromatographic method using frequent sampling up to 10 and 30 h respectively after termination of the infusion. The renal clearance of the drugs was independent of the plasma concentration. The mean (s.d.) renal clearances of ampicillin and amoxycillin were 167 (24) and 157 (20) ml min-1 1.73 m-2 respectively. The net secretion was about 50% of the total renal clearance of both drugs. The plasma concentration and urinary excretion rate versus time curves indicated a polyexponential decline, which could be described by both a biexponential and a triexponential equation. The former proved to be more reliable, especially in the calculation of micro rate constants. There was a tendency to more sustained plasma concentrations after amoxycillin, also illustrated by a significantly lower mean (s.d.) plasma clearance of this drug, viz. 185 (30) ml min-1 1.73 m-2, as compared to ampicillin, 210 (24) ml min-1 1.73 m-2 (P less than 0.04). There were no major differences in the disposition rate constants and the distribution volumes of ampicillin and amoxycillin. The mean (s.d.) plasma half-life was 1.7 (0.3) h for both drugs. The urinary excretion rate indicated a slower terminal disposition rate however, with ampicillin and amoxycillin half-lives of 3.4 (2.0) and 3.9 (1.2) h respectively. The longer half-life in the terminal phase may be due to increased tubular reabsorption at low urinary concentrations. It was not possible to determine in this study whether the half-life was affected by changes in clearance or volume of distribution. The urinary solubility of the drugs was dependent on pH. This could explain the massive macroscopic crystalluria seen in one subject after amoxycillin. Three hours after termination of the infusion, crystals could no longer be found in the sediment. There was no clinical or laboratory evidence of renal damage.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson A., Alván G., Borgå O., Eklöf R. Effect of cephapirin on tubular reabsorption of amino acids, uric acid and beta 2-microglobulin in man. Br J Clin Pharmacol. 1983 Mar;15(3):339–346. doi: 10.1111/j.1365-2125.1983.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A., Borgå O., Alván G. Renal excretion of cephapirin and cephaloridine: evidence for saturable tubular reabsorption. Clin Pharmacol Ther. 1979 Jun;25(6):870–876. doi: 10.1002/cpt1979256870. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetic comparison of oral bacampicillin and parenteral ampicillin. Antimicrob Agents Chemother. 1978 Jun;13(6):971–974. doi: 10.1128/aac.13.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusch J. L., Bergeron M. G., Barza M., Weinstein L. An in vitro and pharmacological comparison of amoxicillin and ampicillin. Am J Med Sci. 1974 Jan;267(1):41–48. doi: 10.1097/00000441-197401000-00006. [DOI] [PubMed] [Google Scholar]
- Carlqvist J., Westerlund D. Determination of amoxicillin in body fluids by reversed-phase liquid chromatography coupled with a post-column derivatization procedure. J Chromatogr. 1979 Nov 11;164(3):373–381. doi: 10.1016/s0378-4347(00)81238-4. [DOI] [PubMed] [Google Scholar]
- Chiou W. L., Lam G. The significance of the arterial-venous plasma concentration difference in clearance studies. Int J Clin Pharmacol Ther Toxicol. 1982 May;20(5):197–203. [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Dalhoff A., Koeppe P. Comparative pharmacokinetic analysis of amoxycillin using open two and three-compartment models. Eur J Clin Pharmacol. 1982;22(3):273–279. doi: 10.1007/BF00545227. [DOI] [PubMed] [Google Scholar]
- Dalhoff A., Koeppe P., von Kobyletzki D. Untersuchungen zur Pharmakokinetik von Amoxicillin nach intravenöser, intramuskulärer und oraler Applikation. Arzneimittelforschung. 1981;31(7):1148–1157. [PubMed] [Google Scholar]
- Fell P. J., Stevens M. T. Pharmacokinetics--uses and abuses. Eur J Clin Pharmacol. 1975 Apr 4;8(3-4):241–248. doi: 10.1007/BF00567122. [DOI] [PubMed] [Google Scholar]
- Jones H. M., Schrader W. A., Jr Ampicillin crystalluria. Am J Clin Pathol. 1972 Aug;58(2):220–223. doi: 10.1093/ajcp/58.2.220. [DOI] [PubMed] [Google Scholar]
- Kamiya A., Okumura K., Hori R. Quantitative investigation on renal handling of drugs in rabbits, dogs, and humans. J Pharm Sci. 1983 Apr;72(4):440–443. doi: 10.1002/jps.2600720429. [DOI] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Potter J. L., Weinberg A. G., West R. Ampicillinuria and ampicillin crystalluria. Pediatrics. 1971 Oct;48(4):636–639. [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Sjövall J. Tissue levels after administration of bacampicillin, a prodrug of ampicillin, and comparisons with other aminopenicillins: a review. J Antimicrob Chemother. 1981 Nov;8 (Suppl 100):41–58. doi: 10.1093/jac/8.suppl_c.41. [DOI] [PubMed] [Google Scholar]
- Sjövall J., Westerlund D., Alván G., Magni L., Nord C. E., Sörstad J. Rectal bioavailability of bacampicillin hydrochloride in man as determined by reversed-phase liquid chromatography. Chemotherapy. 1984;30(3):137–147. doi: 10.1159/000238260. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Nakashima E., Hamano S., Yamana T. Physicochemical properties of amphoteric beta-lactam antibiotics I: Stability, solubility, and dissolution behavior of amino penicillins as a function of pH. J Pharm Sci. 1978 Aug;67(8):1059–1066. doi: 10.1002/jps.2600670810. [DOI] [PubMed] [Google Scholar]
- Tucker G. T. Measurement of the renal clearance of drugs. Br J Clin Pharmacol. 1981 Dec;12(6):761–770. doi: 10.1111/j.1365-2125.1981.tb01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund D., Carlqvist J., Theodorsen A. Analysis of penicillins in biological material by reversed phase liquid chromatography and post-column derivatization. Acta Pharm Suec. 1979;16(3):187–214. [PubMed] [Google Scholar]
- Whelton A., Sapir D. G., Carter G. G., Kramer J., Walker W. G. Intrarenal distribution of penicillin, cephalothin, ampicillin and oxytetracycline during varied states of hydration. J Pharmacol Exp Ther. 1971 Nov;179(2):419–428. [PubMed] [Google Scholar]
- Zarowny D., Ogilvie R., Tamblyn D., MacLeod C., Ruedy J. Pharmacokinetics of amoxicillin. Clin Pharmacol Ther. 1974 Dec;16(6):1045–1051. doi: 10.1002/cpt19741661045. [DOI] [PubMed] [Google Scholar]


