Abstract
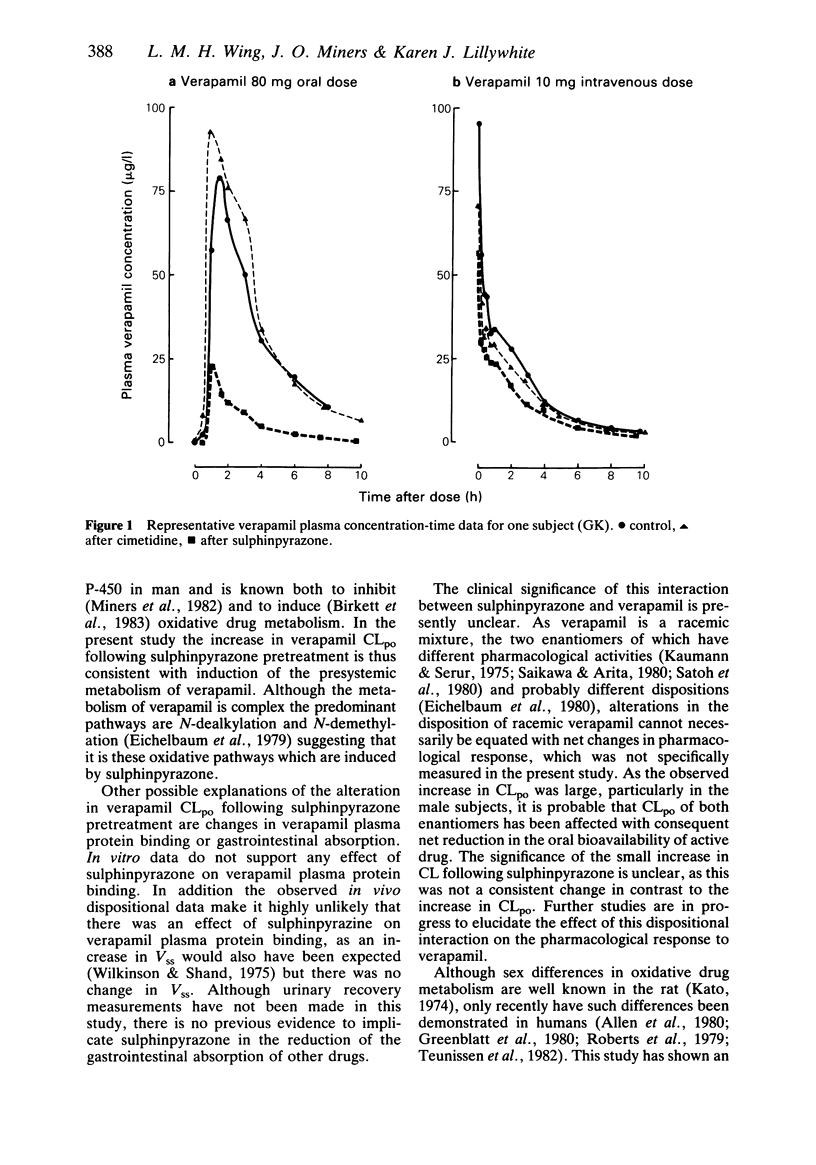
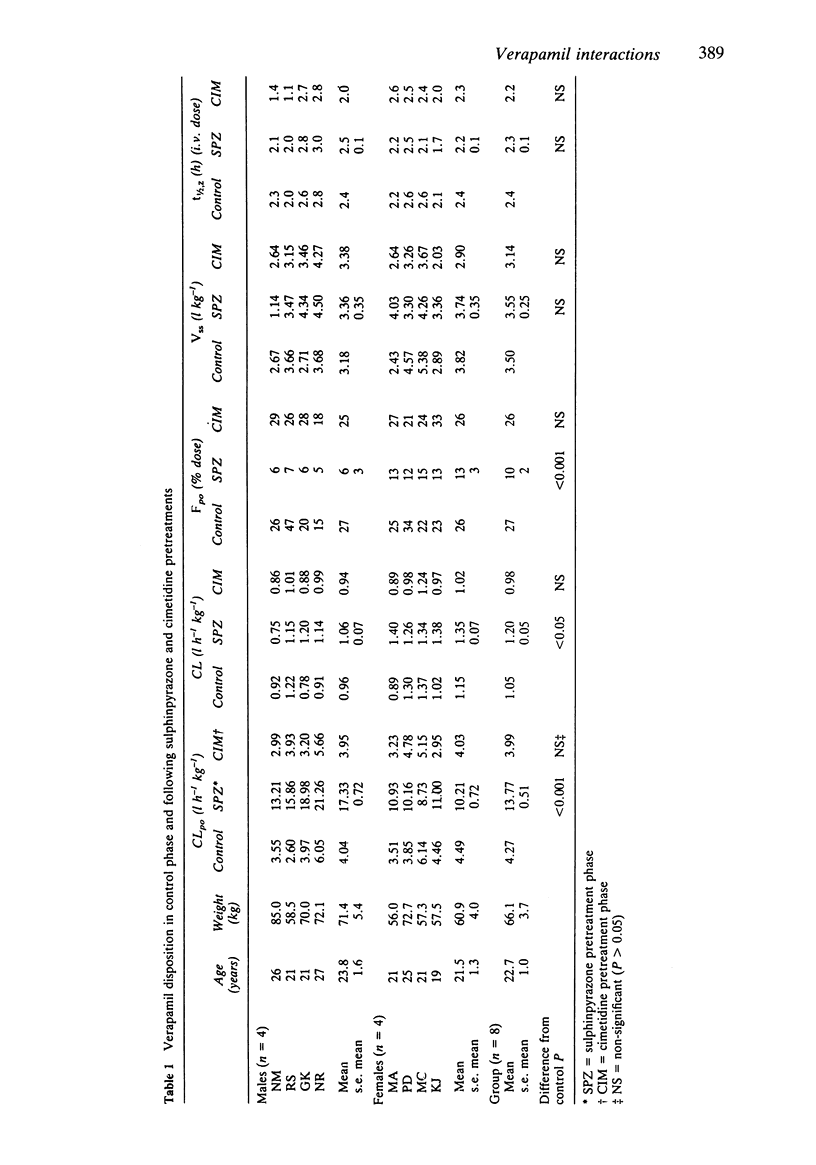
The effects of separate 7 day pretreatments with sulphinpyrazone (800 mg daily) and cimetidine (1 g daily) on the disposition of (+/-)-verapamil have been examined in eight healthy volunteers (four male, four female). Each subject received single oral (80 mg) and intravenous (0.15 mg/kg) doses of verapamil on different occasions before and after each pretreatment. Following sulphinpyrazone pretreatment, verapamil apparent oral plasma clearance (CLpo) increased from 4.27 to 13.77 l h-1 kg-1 (s.e. mean 0.51--ANOVA) (P less than 0.001); CL increased from 1.05 to 1.20 l h-1 kg-1 (s.e. mean 0.05) (P less than 0.05) and Fpo decreased from 27 to 10% administered dose (s.e. mean 2) (P less than 0.001). Vss and t1/2,z were unchanged. There was no sex difference for any dispositional parameter in the control phase, but the increase in CLpo following sulphinpyrazone pretreatment was more marked in males (4.04 to 17.33 l h-1 kg-1) than in females (4.49 to 10.21 l h-1 kg-1) (s.e. mean 0.72) (P less than 0.01). There was no significant change in any verapamil disposition parameter following cimetidine pretreatment. Verapamil unbound fraction in plasma was 0.157 (s.e. mean 0.001, n = 40). There was no alteration in verapamil plasma protein binding associated with increasing verapamil concentration (25-250 micrograms l-1) or addition of sulphinpyrazone (50-500 mg l-1) or cimetidine (0.5-5 mg l-1). The results suggest that sulphinpyrazone induces the metabolic clearance of (+/-)-verapamil with a sex difference in the response.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. D., Greenblatt D. J., Harmatz J. S., Shader R. I. Desmethyldiazepam kinetics in the elderly after oral prazepam. Clin Pharmacol Ther. 1980 Aug;28(2):196–202. doi: 10.1038/clpt.1980.150. [DOI] [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Birkett D. J., Miners J. O., Attwood J. Evidence for a dual action of sulphinpyrazone on drug metabolism in man: theophylline-sulphinpyrazone interaction. Br J Clin Pharmacol. 1983 May;15(5):567–569. doi: 10.1111/j.1365-2125.1983.tb02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Birkel P., Grube E., Gütgemann U., Somogyi A. Effects of verapamil on P-R-intervals in relation to verapamil plasma levels following single I.V. and oral administration and during chronic treatment. Klin Wochenschr. 1980 Sep 15;58(18):919–925. doi: 10.1007/BF01477049. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Ende M., Remberg G., Schomerus M., Dengler H. J. The metabolism of DL-[14C]verapamil in man. Drug Metab Dispos. 1979 May-Jun;7(3):145–148. [PubMed] [Google Scholar]
- Eichelbaum M., Somogyi A., von Unruh G. E., Dengler H. J. Simultaneous determination of the intravenous and oral pharmacokinetic parameters of D,L-verapamil using stable isotope-labelled verapamil. Eur J Clin Pharmacol. 1981 Jan;19(2):133–137. doi: 10.1007/BF00568400. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Allen M. D., Harmatz J. S., Shader R. I. Diazepam disposition determinants. Clin Pharmacol Ther. 1980 Mar;27(3):301–312. doi: 10.1038/clpt.1980.40. [DOI] [PubMed] [Google Scholar]
- Harapat S. R., Kates R. E. High-performance liquid chromatographic analysis of verapamil. II. Simultaneous quantitation of verapamil and its active metabolite, norverapamil. J Chromatogr. 1980 Mar 14;181(3-4):484–489. [PubMed] [Google Scholar]
- Kato R. Sex-related differences in drug metabolism. Drug Metab Rev. 1974;3(1):1–32. doi: 10.3109/03602537408993737. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Serur J. R. Optical isomers of verapamil on canine heart. Prevention of ventricular fibrillation induced by coronary artery occlusion, impaired atrioventricular conductance and negative inotropic and chronotropic effects. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(4):347–358. doi: 10.1007/BF00501793. [DOI] [PubMed] [Google Scholar]
- McAllister R. G., Jr, Kirsten E. B. The pharmacology of verapamil. IV. Kinetic and dynamic effects after single intravenous and oral doses. Clin Pharmacol Ther. 1982 Apr;31(4):418–426. doi: 10.1038/clpt.1982.54. [DOI] [PubMed] [Google Scholar]
- Miners J. O., Foenander T., Wanwimolruk S., Gallus A. S., Birkett D. J. The effect of sulphinpyrazone on oxidative drug metabolism in man: inhibition of tolbutamide elimination. Eur J Clin Pharmacol. 1982;22(4):321–326. doi: 10.1007/BF00548400. [DOI] [PubMed] [Google Scholar]
- Roberts R. K., Desmond P. V., Wilkinson G. R., Schenker S. Disposition of chlordiazepoxide: sex differences and effects of oral contraceptives. Clin Pharmacol Ther. 1979 Jun;25(6):826–831. doi: 10.1002/cpt1979256826. [DOI] [PubMed] [Google Scholar]
- Saikawa T., Arita M. Effects of verapamil and its optical isomers on repetitive slow responses induced by electrical depolarization in canine ventricular myocardium. Jpn Heart J. 1980 Mar;21(2):247–255. doi: 10.1536/ihj.21.247. [DOI] [PubMed] [Google Scholar]
- Satoh K., Yanagisawa T., Taira N. Coronary vasodilator and cardiac effects of optical isomers of verapamil in the dog. J Cardiovasc Pharmacol. 1980;2(3):309–318. doi: 10.1097/00005344-198005000-00008. [DOI] [PubMed] [Google Scholar]
- Somogyi A., Gugler R. Drug interactions with cimetidine. Clin Pharmacokinet. 1982 Jan-Feb;7(1):23–41. doi: 10.2165/00003088-198207010-00002. [DOI] [PubMed] [Google Scholar]
- Teunissen M. W., Srivastava A. K., Breimer D. D. Influence of sex and oral contraceptive steroids on antipyrine metabolite formation. Clin Pharmacol Ther. 1982 Aug;32(2):240–246. doi: 10.1038/clpt.1982.154. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R., Shand D. G. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975 Oct;18(4):377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]
- Wing L. M., Miners J. O., Birkett D. J., Foenander T., Lillywhite K., Wanwimolruk S. Lidocaine disposition--sex differences and effects of cimetidine. Clin Pharmacol Ther. 1984 May;35(5):695–701. doi: 10.1038/clpt.1984.97. [DOI] [PubMed] [Google Scholar]
- Wong L. T., Solomonraj G., Thomas B. H. Simple and rapid micro-determination of sulfinpyrazone (Anturan) in biological fluids by reversed-phase high-performance liquid chromatography. J Chromatogr. 1978 Mar 21;150(2):521–526. doi: 10.1016/s0021-9673(00)88214-x. [DOI] [PubMed] [Google Scholar]


