Abstract
Dopamine is a major neurotransmitter in the central nervous system, and its receptors are associated with a number of neuropathological disorders such as Parkinson's disease and schizophrenia. Although the precise pathophysiology of schizophrenia remains unknown, the dopaminergic hypothesis of the illness assumes that the illness results from excessive activity at dopamine synapses in the brain. Because, at present, the diagnosis of schizophrenia relies on descriptive behavioral and symptomatic information, a peripheral measurable marker may enable a simpler, more rapid, and more accurate diagnosis and monitoring. In recent years, human peripheral blood lymphocytes have been found to express several dopamine receptors (D3, D4, and D5) by using molecular biology techniques and binding assays. It has been suggested that these dopamine receptors found on lymphocytes may reflect receptors found in the brain. Here we demonstrate a correlation between the D3 dopamine receptor on lymphocytes and schizophrenia and show a significant elevation of at least 2-fold in the mRNA level of the D3, but not of the D4, dopamine receptor in schizophrenic patients. This increase is not affected by different antipsychotic drug treatments (typical or atypical). Moreover, nonmedicated patients exhibit the same pattern, indicating that this change is not a result of medical treatment. We propose the D3 receptor mRNA on blood lymphocytes as a marker for identification and followup of schizophrenia.
Schizophrenia is a neuropsychiatric disorder afflicting about one percent of the population. Although its exact pathogenesis is still not known precisely, a common belief is that excessive activity at dopaminergic synapses in the brain plays a prominent role (1). To date, a definitive diagnosis of schizophrenia requires a 6-month duration of symptomatology and relies on heterogeneous symptoms. Because there is neither an effective biological marker for identifying schizophrenia (2, 3), nor an accurate and rapid diagnosis to ensure more optimal management at an early stage in the illness (4), there remains a vital need for a convenient assay for diagnosis and followup of schizophrenia.
Most of the drugs used to treat schizophrenia act to control symptoms by neuroreceptor antagonism. Moreover, the dopaminergic basis of schizophrenia is strongly supported by the close correlation between clinical efficacy of antipsychotic medications and their potency to antagonize the binding of dopamine to its receptors (5).
Dopamine receptors are divided into two subclasses, D1 and D2. The D1 subclass contains the D1 and D5 receptor subtypes, and the D2 subclass contains the D2, D3, and D4 subtypes (6). The dopamine hypothesis of schizophrenia specifically relates to the D2 subclass. Notably, most drugs effective in treating schizophrenia exhibit D2 receptor antagonistic activity, and administration of a selective D1-like antagonist has been reported to result in the worsening of symptoms (7). The D3 receptor, one among the three in the D2 subclass, is located principally in an area of the brain that could be very relevant to schizophrenia, the nucleus accumbens (2). Studies with positron-emission tomography and postmortem brain tissue have indicated increased levels of D2-like dopamine receptors in schizophrenic patients when compared with nonschizophrenic patients (8). Thus, the level of dopamine receptor could be used as a marker for schizophrenia if it could be analyzed on an available tissue, preferably a peripheral one.
In recent years, high-affinity binding of dopaminergic ligands, as well as the presence of mRNA of several dopamine receptor subtypes (D3, D4, and D5) in human peripheral blood lymphocytes (PBLs), have been reported (9, 10). It should be noted, however, that neither D2 nor D1 dopamine receptor subtypes, which are the most abundant receptors in the brain and belong to the D2 and the D1 subclasses, respectively, have been detected in lymphocytes. Although the significance of dopamine receptors, as well as of other neurotransmitter receptors, in lymphocytes is still not clear, it has been suggested that they may reflect corresponding brain receptors. Several studies have demonstrated the increased binding of dopamine antagonists in lymphocytes of schizophrenic patients as compared with healthy individuals (11, 12). In addition, a previous study carried out in our lab has demonstrated that spiperone (a D2 antagonist) binding in peripheral blood lymphocytes is higher in neuroleptic responders as compared with treatment-resistant schizophrenic patients (13). However, the observed differences in binding studies were rather low and often not significant. The discrepancies obtained could have resulted from the crossreactivity of radioligands with different subtypes of the receptor and with other receptors (e.g., serotonergic), and from the scattered levels of binding sites. Therefore, such binding assays in lymphocytes may not be suitable as reliable assays for schizophrenia.
In this work, we have measured mRNA levels of dopamine receptors in the PBLs of schizophrenic patients and healthy individuals to find out whether the receptors can serve as peripheral markers for this disorder. Because the inhibitory D2 subclass, rather than the D1 subclass, of dopamine receptors is considered to be associated with neuropsychiatric disorders, in this study we have focused on only the D3 and D4 subtypes, both belonging to the D2 subclass. In the current study, we demonstrate a correlation between the D3 dopamine receptor on lymphocytes and schizophrenia and show a significant elevation (2- to 7-fold) in the mRNA level of D3, but not of D4, in schizophrenic patients. This increase is not affected by different antipsychotic drug treatments (typical or atypical). Moreover, nonmedicated patients exhibit the same pattern, indicating that this change is not a result of medical treatment. We propose the D3 receptor mRNA as a peripheral marker for identification and followup of schizophrenia.
Materials and Methods
Patients.
Schizophrenic patients were recruited from Tyrat Hacarmel and Beer Yaacov Mental Health Centers after providing written informed consent for participation in the study. The study has been approved by the Institutional Review Boards for human studies in these two mental health centers. All patients were diagnosed formally according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; ref. 14) and were evaluated by using standard rating scales by a senior psychiatrist. Healthy individuals' ages and sexes matched the patient group as much as possible.
Lymphocyte Isolation.
Blood (40–50 ml) was drawn from the cubital vein into a heparinized plastic syringe and then transferred into a sterile 50-ml plastic tube. Blood samples were diluted with an equal volume of PBS, were placed onto Ficoll/Paque gradients, and then were centrifuged for 30 min at 400 × g. The lymphocyte layer was collected and washed twice in PBS. The resulting pellet was frozen immediately at −80°C until RNA preparation.
Reverse Transcription–PCR Analysis.
Total RNA was isolated from lymphocytes by using the guanidinum-thiocyanate method, and the amount and quality of RNA were determined by using spectrophotometry and gel electrophoresis (2% agarose; GIBCO/BRL). Two micrograms of total RNA was reverse transcribed into first-strand cDNA by using polydT-priming and 20 units of Moloney murine leukemia virus reverse transcriptase. Two microliters cDNA product (80 ng RNA) was used for PCR amplification at a final concentration of 1× PCR buffer (Perkin–Elmer) and 1 unit of Taq DNA polymerase (Perkin–Elmer), in a final volume of 25 μl. PCR was carried out in a DNA thermocycler (MiniCycler; MJ Research, Cambridge, MA) for 23 cycles (β-actin) and 38 cycles (D3 and D4) with an annealing temperature of 60°C. The amplification was found to be linear between 30 and 40 cycles for D3 and D4, and between 19 and 25 cycles for β-actin.
The PCR primers for D3, D4, and β-actin were designed to include at least one intron to eliminate amplification of genomic DNA. Their sequences were as follows:
D3 dopamine receptor—GGAGACGGAAAAGGATCCTCACTCG (nucleotides 655–680) and TCAGCAAGACAGGATCTTGAGGAAGG (nucleotides 1203–1177).
D4 dopamine receptor—CGGGATCCCACCCCAGACTCCACC (nucleotides 964–988) and CGGAATTCCGTTGCGGAACTCGGC (nucleotides 1240–1216).
β-actin—TGAAGTGTGACGTGGACATCCG (nucleotides 96–117) and GCTGTCACCTTCACCGTT CCAG (nucleotides 543–522).
The obtained PCR products corresponded to the respective dopamine receptor fragments, as confirmed by sequence and Southern blot analyses.
Quantification of PCR products was performed by using a densitometer and scion image (Frederick, MD) analysis software, and/or PCR–ELISA.
PCR–ELISA.
PCR was performed as described previously except for the use of digoxigenin-labeled dNTPs. PCR products were incubated with biotinylated-specific internal primers of the tested fragments that were immobilized in streptavidin-coated microtiter plates. The biotinylated internal primers served as capture probes. The bound digoxigenin-labeled PCR products then were incubated with anti-digoxigenin-peroxidase conjugate that bound to the digoxigenin residues in the labeled PCR product. Peroxidase substrate solution was added, and the color developed was measured in a microtiter-plate reader.
Results and Discussion
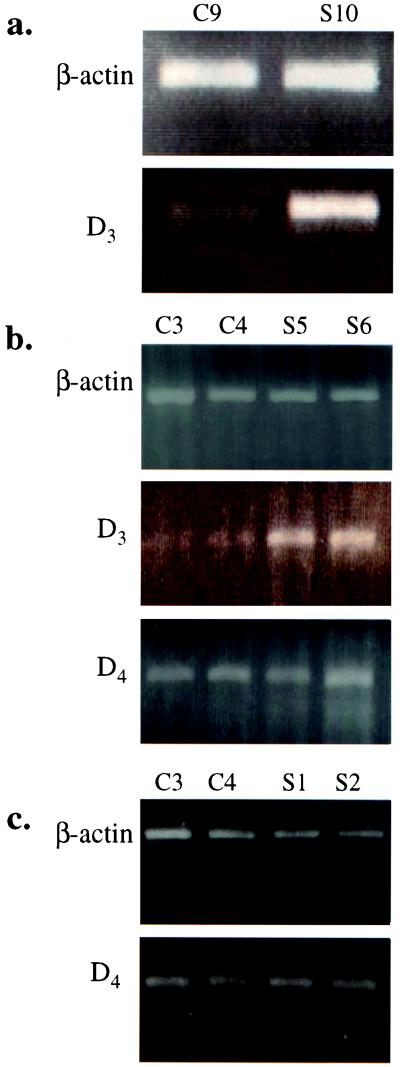
Table 1 summarizes the details (ages, sexes, and diagnoses) of schizophrenic patients and healthy controls from whom blood samples were obtained for this study. RT-PCR was performed on total RNA preparations from these blood samples with primers specific for D3 or D4 dopamine receptor and β-actin as a control. The specific PCR products were resolved on 2% agarose gels, and their sequences were verified. For each patient, a sex- and optimal-age-matched healthy control was used, and the level of specific dopamine receptor mRNAs was compared between sick and healthy patients. As depicted in Fig. 1 a and b for several representative patients, the signals for D3 receptor mRNA were significantly higher in schizophrenic patients than in healthy controls. This increase was found to apply to the D3 receptors specifically, because no significant differences in the intensities of D4 receptor bands were detected between schizophrenic patients and healthy controls (Fig. 1 b and c).
Table 1.
Characterization of patients analyzed in this study
| Number | Age | Sex | Diagnosis | Comments |
|---|---|---|---|---|
| Schizophrenic patients | ||||
| S1 | 21 | M | Schizophrenia–residual type | |
| S2 | 27 | M | Schizophrenia–paranoid type | |
| S3 | 25 | M | Schizophrenia–undifferentiated type | |
| S4 | 27 | F | Schizophrenia–unspecified type | |
| S5 | 49 | F | Schizophrenia–paranoid type | |
| S6 | 57 | F | Schizophrenia–residual type | |
| S7 | 41 | M | Schizophrenia–undifferentiated type | |
| S8 | 54 | M | Schizophrenia–paranoid type | |
| S9 | 47 | M | Schizophrenia–undifferentiated type | |
| S10 | 42 | M | Schizophrenia–undifferentiated type | |
| S11 | 42 | M | Schizophrenia–paranoid type | |
| S12 | 21 | M | Schizophrenia–undifferentiated type | Nonmedicated |
| S13 | 40 | F | Schizophrenia–paranoid type | Nonmedicated |
| S14 | 29 | M | Schizophrenia–paranoid type | Nonmedicated |
| Healthy controls | ||||
| C1 | 45 | F | — | — |
| C2 | 37 | F | — | — |
| C3 | 37 | M | — | — |
| C4 | 62 | F | — | — |
| C5 | 22 | M | — | — |
| C6 | 44 | M | — | — |
| C7 | 31 | M | — | — |
| C8 | 32 | M | — | — |
| C9 | 49 | F | — | — |
| C10 | 27 | M | — | — |
| C11 | 36 | F | — | — |
Figure 1.
Ethidium bromide staining of D3, D4, and β-actin PCR products from healthy controls (C) and schizophrenics (S).
Quantification of the intensities of the specific D3 receptor bands was performed by densitometry. The results for 14 patients are summarized in Table 2. Each schizophrenic patient is compared with a sex- and optimal-age-matched healthy individual. For each of them, a ratio of the measured density value for the D3 receptor to the value for β-actin was determined. The ratio of these two values for a patient and a matched healthy control, respectively, represents the increased level (in folds) in D3-specific mRNA in a given patient. As shown in Table 2, the increased levels obtained for the 14 patients ranged from 1.59 to 7.73 (average increase 3.64 ± 2.09). This increase in D3 receptor mRNA in schizophrenic patients is significantly higher than the reported increases in binding levels and other recently suggested peripheral markers for schizophrenia (15). Furthermore, the increase in D3 receptor RNA was unaffected by different drug treatments. Although some of the patients received typical treatment and some atypical treatment (see Table 1), it can be noted that all patients exhibited a similar range of increase indicating that this was not a result of specific dopamine-receptor subtype blockade and up-regulation. Moreover, we found that this increase was not the consequence of dopamine-receptor antagonist treatment, because nonmedicated patients (S12, S13, and S14) showed a similar increase in D3 level (see Tables 1 and 2).
Table 2.
Densitometric evaluation of D3 and D4 mRNA levels in patients compared with their levels in healthy individuals
| Schizophrenic
patients
|
Controls
|
D3 fold increase, S/C | Schizophrenic
patients
|
Controls
|
D4 fold increase, S/C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | β-actin arb. units | D3 arb. units | D3/β-actin | Number | β-actin arb. units | D3 arb. units | D3/β-actin | β-actin arb. units | D4 arb. units | D4/β-actin | Number | β-actin arb. units | D4 arb. units | D4/β-actin | ||
| S1 | 67 | 51 | 0.671 | C3 | 86 | 23 | 0.267 | 2.513 | 74 | 70 | 0.945 | C3 | 67 | 70 | 1.044 | 0.905 |
| S2 | 87 | 56 | 0.643 | C3 | 86 | 23 | 0.267 | 2.408 | 71 | 72 | 1.010 | C3 | 67 | 70 | 1.044 | 0.967 |
| S3 | 82 | 60 | 0.731 | C3 | 86 | 23 | 0.267 | 2.737 | 72 | 74 | 1.027 | C3 | 67 | 70 | 1.044 | 0.983 |
| S4 | 98 | 100 | 1.020 | C2 | 95 | 61 | 0.642 | 1.588 | 67 | 71 | 1.059 | C2 | 82 | 73 | 0.890 | 1.189 |
| S5 | 55 | 143 | 2.600 | C2 | 58 | 59 | 1.017 | 2.556 | ||||||||
| S6 | 75 | 153 | 2.040 | C4 | 79 | 70 | 0.886 | 2.302 | ||||||||
| S7 | 19 | 171 | 9.000 | C10 | 85 | 163 | 1.917 | 4.694 | ||||||||
| S8 | 138 | 426 | 3.087 | C8 | 425 | 176 | 0.414 | 7.456 | ||||||||
| S9 | 89 | 60 | 0.674 | C7 | 121 | 28 | 0.231 | 2.917 | ||||||||
| S10 | 107 | 71 | 0.663 | C7 | 121 | 28 | 0.231 | 2.870 | ||||||||
| S11 | 303 | 216 | 0.714 | C3 | 271 | 29 | 0.107 | 6.644 | ||||||||
| S12 | 319 | 227 | 0.711 | C7 | 273 | 86 | 0.315 | 2.257 | ||||||||
| S13 | 237 | 354 | 1.493 | C9 | 199 | 130 | 0.653 | 2.286 | ||||||||
| S14 | 162 | 825 | 5.092 | C8 | 91 | 60 | 0.659 | 7.727 | ||||||||
arb., arbitrary.
Another way to quantify the differences in a specific mRNA level was obtained from PCR–ELISA experiments (see Materials and Methods). Table 3 summarizes the results obtained from 6 patients. The increased mRNA levels observed are between 1.66 and 3.38 (average increase 2.30 ± 0.63). It should be noted that there is a relatively good agreement between the quantitative values obtained by densitometry or PCR–ELISA (see patients S1, S4, and S6 in Tables 2 and 3).
Table 3.
Evaluation by PCR–ELISA of D3 mRNA levels in patients compared with their levels in healthy individuals
| Schizophrenic
patients
|
Controls
|
D3 fold increase, S/C | ||||||
|---|---|---|---|---|---|---|---|---|
| Number | β-actin, OD | D3, OD | D3/β-actin | Number | β-actin, OD | D3, OD | D3/β-actin | |
| S1 | 0.556 | 0.868 | 1.561 | C8 | 0.918 | 0.552 | 0.601 | 2.597 |
| S2 | 0.808 | 2.225 | 2.753 | C9 | 0.405 | 0.330 | 0.814 | 3.382 |
| S3 | 0.224 | 0.253 | 1.129 | C8 | 0.533 | 0.272 | 0.510 | 2.214 |
| S4 | 0.629 | 0.394 | 0.626 | C8 | 0.876 | 0.316 | 0.360 | 1.738 |
| S5 | 0.340 | 0.823 | 2.420 | C2 | 0.365 | 0.533 | 1.460 | 1.657 |
| S6 | 0.339 | 0.899 | 2.652 | C3 | 0.368 | 0.444 | 1.206 | 2.199 |
OD, optical density at 405 nm.
It should be added that the use of sex- and/or age-matched controls does not appear to be critical. We have demonstrated that the differences in D3-specific mRNA levels between schizophrenic patients and healthy individuals, determined by either densitometry or PCR–ELISA, were similar when compared with additional, not necessarily matched, controls (Table 4). This observation may be valuable in designing a practical assay.
Table 4.
Evaluation of D3 mRNA levels in patients compared with their levels in healthy individuals
| Schizophrenic
patients
|
Controls
|
Ratio | ||
|---|---|---|---|---|
| Number | D3/β-actin | Number | D3/β-actin | |
| S8 | 3.087 | C8 | 0.414 | 7.456 |
| C9 | 0.498 | 6.198 | ||
| S5 | 2.600 | C2 | 1.017 | 2.556 |
| C3 | 1.145 | 2.270 | ||
| C4 | 0.886 | 2.934 | ||
| S6 | 2.040 | C2 | 1.017 | 2.005 |
| C3 | 1.145 | 1.781 | ||
| C4 | 0.886 | 2.302 | ||
| S4 | 1.020 | C2 | 0.642 | 1.588 |
| C8 | 0.656 | 1.554 | ||
As mentioned above, the elevated levels of D3 dopamine receptor in PBLs of schizophrenic patients are in agreement with other reports (7) demonstrating elevated levels of this receptor in postmortem brains of medicated and nonmedicated schizophrenic patients. Such a correlation between the status of receptors in the brain and in PBLs has also been demonstrated in Alzheimer's disease, where muscarinic receptors are reduced in both brains and lymphocytes (16). A previous study by Nagai et al. (17) demonstrates that patients with Parkinson's disease exhibit reduced levels of D3-receptor mRNA in PBLs, as compared with healthy individuals. These latter findings provide another example of a disease that is associated with an insult in the central nervous system that is reflected in PBLs. This reduction has also been detected in medicated and nonmedicated patients. The changes in mRNA levels observed in this study might reflect a systemic imbalance in D3-receptor level or in brain-receptor status. Further studies are required to determine whether the levels of D3 receptor correlate with the progression of the disease and whether they can be deployed to monitor changes in the patient's condition.
In conclusion, our findings strongly suggest that D3-receptor mRNA levels in PBLs may function as convenient and reliable peripheral markers for schizophrenia and thus assist in the early diagnosis (which is frequently unclear, e.g., ref. 4) and possible followup of the illness. Early diagnosis and treatment of schizophrenia may have prognostic significance, because many consider that more optimal management at an early stage of the illness may alter its course (18). In this manner, our observations would be of significant clinical and practical relevance. Although our findings are certainly robust, we still do not know whether differences in the degrees of increase of specific D3-receptor mRNA reflects, in any way, the severity and prognosis of the disease. In addition to serving potentially as a peripheral marker, these changes in the D3-receptor subtype may further indicate its involvement centrally in the pathophysiology of schizophrenia and thus may potentially play a role in the development of medication suitable for management of the chronic disorder. Further studies, clearly warranted to test these observations, are now under way.
Acknowledgments
We thank Dr. Lily Raveh for encouragement, help, and fruitful discussions, and Shari Carmon for technical assistance. This research was supported in part by a research grant from the Abramson Family Foundation (to S.F.).
Abbreviation
- PBL
peripheral blood lymphocyte
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021535398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021535398
References
- 1.Carlsson A, Waters N, Carlsson M L. Eur Arch Psychiatry Clin Neurosci. 1999;249:37–43. doi: 10.1007/pl00014183. [DOI] [PubMed] [Google Scholar]
- 2.Willner P. Int Clin Psychopharmacol. 1997;12:297–308. doi: 10.1097/00004850-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hietala J, Syvalahti E. Annu Rev Med. 1996;28:557–561. doi: 10.3109/07853899608999120. [DOI] [PubMed] [Google Scholar]
- 4.Sheitman B B, Lee H, Strauss R, Lieberman J A. Schizophr Bull. 1997;23:653–661. doi: 10.1093/schbul/23.4.653. [DOI] [PubMed] [Google Scholar]
- 5.Creese I, Burt D R, Snyder S H. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 6.Levant B. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 7.Karlsson P, Smith L, Farde L, Harnryd C, Sedvall G, Wiesel F A. Psychopharmacology. 1995;121:309–316. doi: 10.1007/BF02246068. [DOI] [PubMed] [Google Scholar]
- 8.Seeman P, Niznik H B. FASEB J. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 9.Ricci A, Bronzetti E, Felici L, Tayebati S K, Amenta F. Neurosci Lett. 1997;229:130–134. doi: 10.1016/s0304-3940(97)00413-8. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi N, Nagai Y, Ueno S, Saeki Y, Yanagihara T. FEBS Lett. 1992;314:23–25. doi: 10.1016/0014-5793(92)81452-r. [DOI] [PubMed] [Google Scholar]
- 11.Bondy B, Ackenheil M, Birzle W, Elbers R, Frohler M. Biol Psychiatry. 1984;19:1377–1393. [PubMed] [Google Scholar]
- 12.Bondy B, Ackenheil M, Elbers R, Frohler M. Psychiatry Res. 1985;15:41–48. doi: 10.1016/0165-1781(85)90038-1. [DOI] [PubMed] [Google Scholar]
- 13.Grodzicki J, Pardo M, Schved G, Schlosberg A, Fuchs S, Kanety H. Biol Psychiatry. 1990;27:1327–1330. doi: 10.1016/0006-3223(90)90503-t. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: Am. Psychiatric Assoc.; 1994. [Google Scholar]
- 15.Avissar S, Nechamkin Y, Barki-Harrington L, Roitman G, Schreiber G. J Affect Disord. 1997;43:85–93. doi: 10.1016/s0165-0327(96)01400-0. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero P, Rocca P, Eva C, Benna P, Rebaudengo N, Ravizza L, Genazzani E, Bergamasco B. Brain. 1991;114:1759–1770. doi: 10.1093/brain/114.4.1759. [DOI] [PubMed] [Google Scholar]
- 17.Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T. Neurology. 1996;46:791–795. doi: 10.1212/wnl.46.3.791. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman J A. J Clin Psychiatry. 1999;60:9–12. [PubMed] [Google Scholar]