Abstract
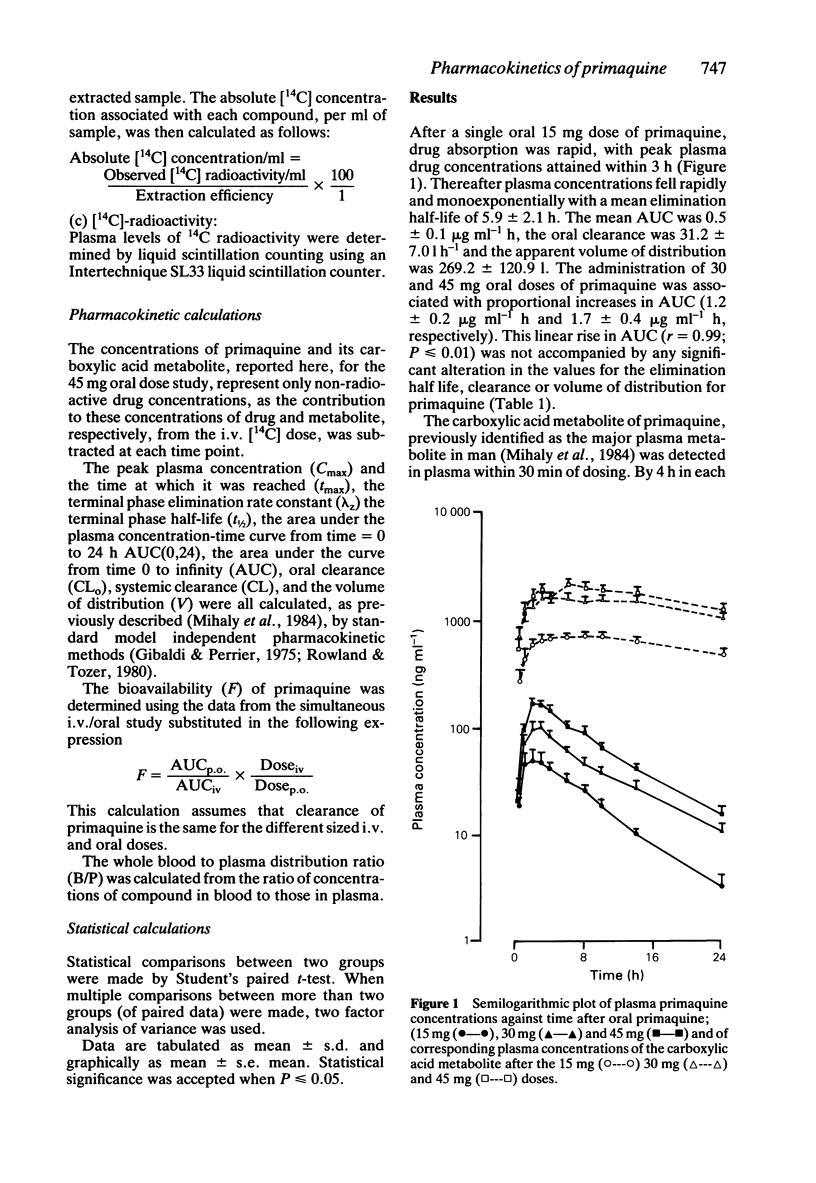
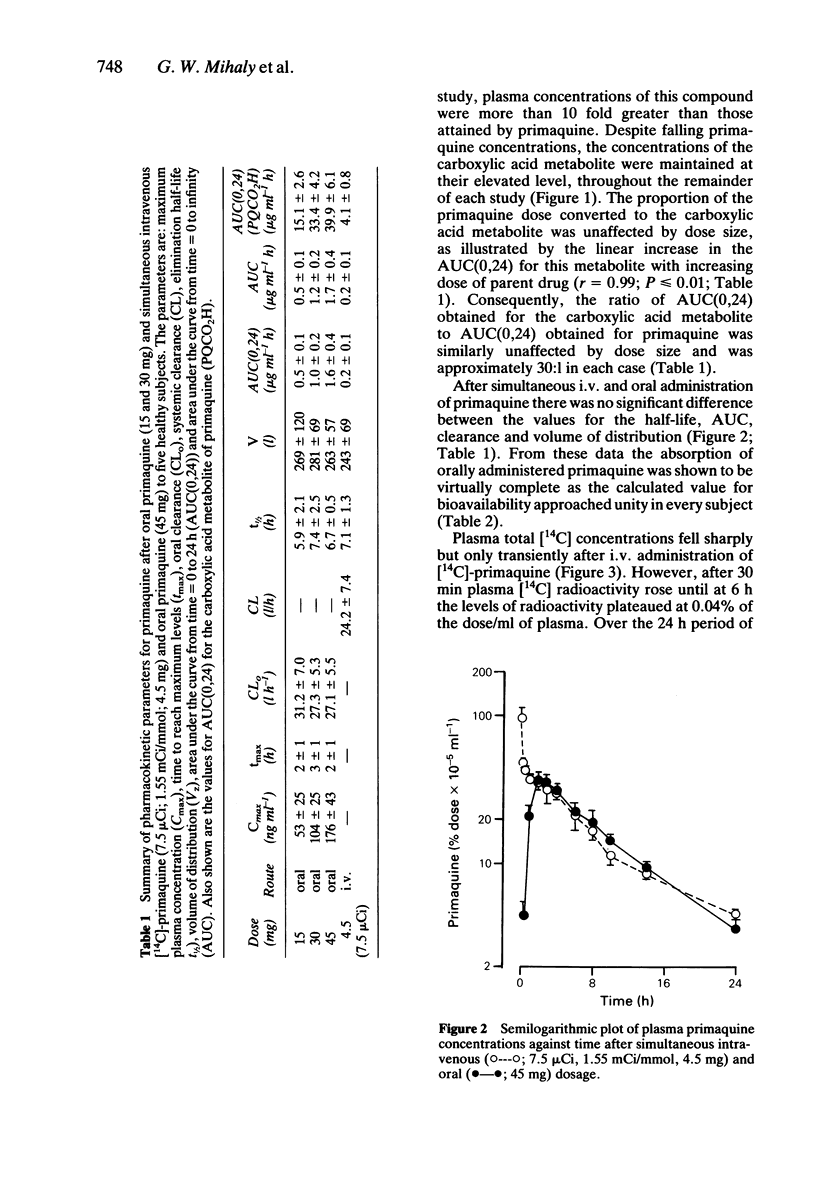
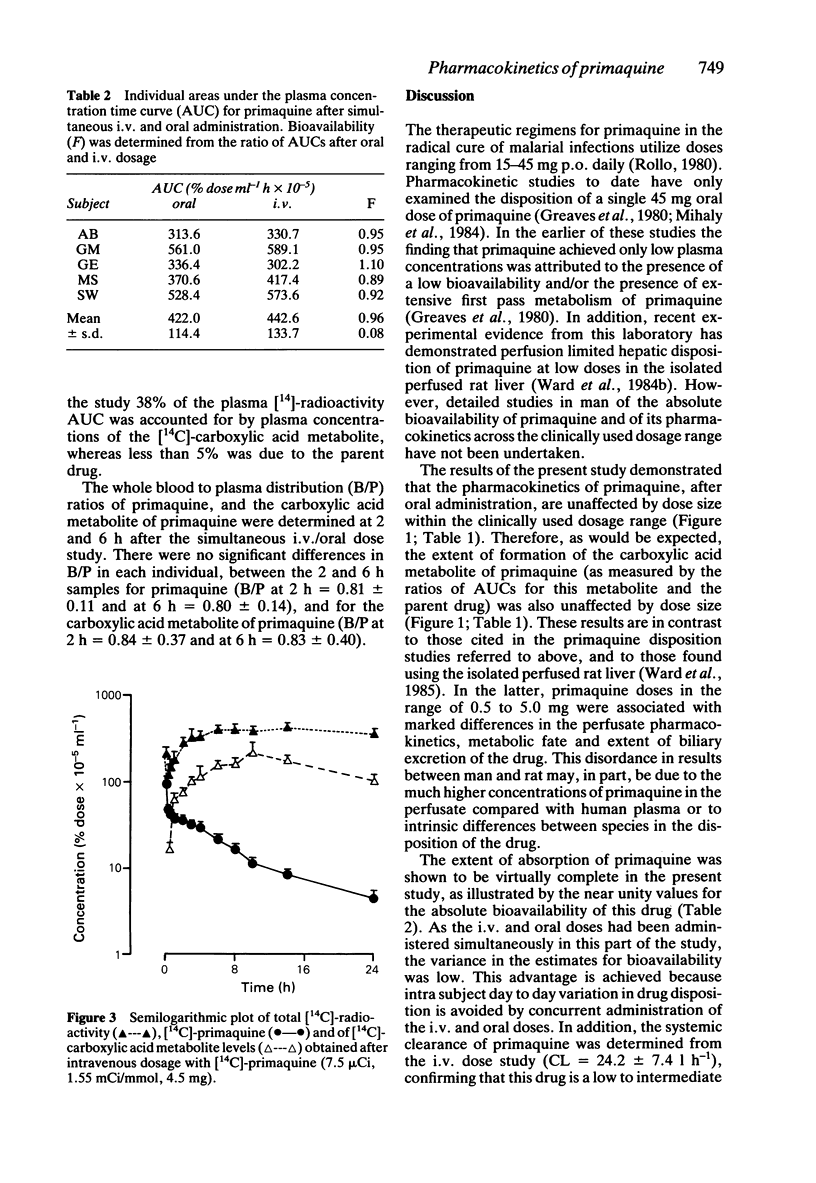
The pharmacokinetics of primaquine have been examined in five healthy volunteers who received single oral doses of 15, 30 and 45 mg of the drug, on separate occasions. Each subject received an i.v. tracer dose of [14C]-primaquine (7.5 microCi), simultaneously with the 45 mg oral dose. Absorption of primaquine was virtually complete with a mean absolute bioavailability of 0.96 +/- 0.08. Elimination half-life, oral clearance and apparent volume of distribution for both primaquine and the carboxylic acid metabolite were unaffected by either dose size, or route of administration. The relationships between area under the curve and dose size were linear for both primaquine (r = 0.99, P less than or equal to 0.01) and its carboxylic acid metabolite (r = 0.99, p less than or equal to 0.01). The mean whole blood to plasma concentration ratios were determined for primaquine (0.81), and for the carboxylic acid metabolite of primaquine (0.84). Primaquine is a low clearance compound (CL = 24.2 +/- 7.4 l h-1), is extensively distributed into body tissues (V = 242.9 +/- 69.5 l) and is not subject to extensive first pass metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergqvist Y., Domeij-Nyberg B. Distribution of chloroquine and its metabolite desethyl-chloroquine in human blood cells and its implication for the quantitative determination of these compounds in serum and plasma. J Chromatogr. 1983 Jan 14;272(1):137–148. doi: 10.1016/s0378-4347(00)86110-1. [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L. J. The Manson Oration, May 1979. Man against malaria: conquest or defeat. Trans R Soc Trop Med Hyg. 1979;73(6):605–617. doi: 10.1016/0035-9203(79)90002-6. [DOI] [PubMed] [Google Scholar]
- Greaves J., Evans D. A., Gilles H. M., Fletcher K. A., Bunnag D., Harinasuta T. Plasma kinetics and urinary excretion of primaquine in man. Br J Clin Pharmacol. 1980 Oct;10(4):399–404. doi: 10.1111/j.1365-2125.1980.tb01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly G. W., Ward S. A., Edwards G., Orme M. L., Breckenridge A. M. Pharmacokinetics of primaquine in man: identification of the carboxylic acid derivative as a major plasma metabolite. Br J Clin Pharmacol. 1984 Apr;17(4):441–446. doi: 10.1111/j.1365-2125.1984.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. A., Edwards G., Orme M. L., Breckenridge A. M. Determination of primaquine in biological fluids by reversed-phase high-performance liquid chromatography. J Chromatogr. 1984 Jan 13;305(1):239–243. doi: 10.1016/s0378-4347(00)83337-x. [DOI] [PubMed] [Google Scholar]


