Abstract
DNA polymerase β (polβ), a member of the X family of DNA polymerases, is the major polymerase in the base excision repair pathway. Using in vitro selection, we obtained RNA aptamers for polβ from a variable pool of 8 × 1012 individual RNA sequences containing 30 random nucleotides. A total of 60 individual clones selected after seven rounds were screened for the ability to inhibit polβ activity. All of the inhibitory aptamers analyzed have a predicted tri-lobed structure. Gel mobility shift assays demonstrate that the aptamers can displace the DNA substrate from the polβ active site. Inhibition by the aptamers is not polymerase specific; inhibitors of polβ also inhibited DNA polymerase κ, a Y-family DNA polymerase. However, the RNA aptamers did not inhibit the Klenow fragment of DNA polymerase I and only had a minor effect on RB69 DNA polymerase activity. Polβ and κ, despite sharing little sequence similarity and belonging to different DNA polymerase families, have similarly open active sites and relatively few interactions with their DNA substrates. This may allow the aptamers to bind and inhibit polymerase activity. RNA aptamers with inhibitory properties may be useful in modulating DNA polymerase actvity in cells.
INTRODUCTION
DNA polymerases (pols) replicate and maintain the integrity of cellular DNA. As a consequence, DNA pols may play key roles in both the avoidance and development of cancer (1). In addition, since DNA pols are the target of many chemotherapeutic agents, they may influence the efficacy of the treatment and possibly the development of resistance (1). DNA polymerase β (polβ), a member of the X-family of low fidelity DNA pols, is the major gap-filling polymerase in both short and long patch base excision repair (BER) pathways (2). Polβ is a distributive enzyme on long single-stranded templates (3) but acts processively on a gapped substrate (4). Tumors and tumor cell lines where polβ has been deleted or truncated have decreased polβ activity and therefore impaired BER (5,6). It has been suggested that decreased polβ activity increases the susceptibility of individuals to cancer (7). High expression of polβ has been seen in tumor cell lines and is associated with increased mutagenicity, genetic instability and tumorigenesis (8–10). Increased polβ activity also has been shown to increase tolerance to several chemotherapeutic agents including radiation, cisplatin and alkylating agents (8,11,12). The Y-family of DNA polymerases are characterized by low fidelity and are thought to be important in DNA damage tolerance pathways that involve translesion synthesis (13,14). However, increased or uncontrolled expression of error-prone DNA polymerases could lead to mutations and cancer (1). In fact, alteration of Y-family polymerase expression levels appears to be common in tumors (1). Although it is not clear whether overexpression of a Y-family polymerase is causative in the initiation or progression of tumorigenesis, high expression of certain DNA pols may provide cells with a growth advantage and/or resistance to DNA damaging agents. In view of the tremendous importance DNA pols play in human disease, the ability to modulate DNA polymerase activity in cells would provide increased understanding of their important role and could lead to the development of new therapies.
RNA aptamers are RNA oligomers that bind tightly and specifically to target molecules. Aptamers are selected in vitro via SELEX (systematic evolution of ligands by exponential enrichment) from randomized RNA libraries (15,16). RNA aptamers are currently being developed as possible therapeutic agents; e.g. RNA aptamers specific for human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) have been explored as potential RT inhibitors to inhibit HIV-1 replication (17–19). We decided to select RNA aptamers that would bind specifically to and inhibit the activity of a DNA polymerase. Owing to the natural affinity the polymerase has for nucleic acids, we expected that the aptamers would bind to the polymerase active site and be highly specific inhibitors of enzymatic activity. We report here the selection and characterization of RNA aptamers selected to inhibit the polymerase activity of polβ. Unexpectedly, the aptamers also bind to and inhibit DNA polymerase κ. These semi-selective RNA aptamers that inhibit error-prone repair polymerases could prove useful for understanding the role of repair in the initiation and progression of cancer as well as in the development of resistance to chemotherapeutic DNA damaging agents.
MATERIALS AND METHODS
Oligonucleotides, RNA pools and target RNA
Template DNA for the RNA pool and PCR primers were synthesized on a model 394 Applied Biosystems automated DNA synthesizer. The preparation of the random RNA pool has been described previously and shown to be a suitable library for automatic SELEX (20). Briefly, templates for reverse transcription were synthesized by PCR using synthetic oligonucleotides. The template was 5′-GGGAATGGATCCACATCTACGAATTC—30N—TTCACTGCAGACTTGACGAAGCTT-3′ where 30N represents 30 random nucleotide positions. PCR primers were 5′-GATAATACGACTCACTATAGGGAATGGATCCACATCTACGA-3′ and 5′-AAGCTTCGTCAAGTCTGCAGTGAA-3′, where the T7 promoter sequence is underlined. RNA pools were prepared by in vitro transcription with T7 RNA polymerase (Stratagene, La Jolla, CA, USA). Pool RNAs were refolded by heat denaturing at 73°C and rapidly cooling to room temperature in binding buffer (BB; 20 mM Tris–HCl, pH 7.7, 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2).
Protein purification
DNA pols β (21) and κ (22) were purified from overexpressed Escherichia coli as previously described.
In vitro selection
To select the aptamers that bind to polβ, seven rounds of selection were performed. The aptamers were selected from the random pool of the RNA sequences described above. The first four rounds of selection were carried out by filtration of the polβ/RNA complex through nitrocellulose filters (0.45 µm HA; Millipore, Billerica, MA, USA). The filters were washed with BB as described (23). Bound material recovery from the filters, reverse transcription, PCR amplification of synthesized cDNA and RNA synthesis for the next round of selection were performed as described (23). Binding of the initial RNA pool to polβ was close to the background. In the first round of selection, 100 pmol (200 nM) polβ was incubated with 1.2 nmols (2.4 µM; 8 × 1012 individual molecules) of variable RNA pool in 500 µl BB (Table 1). In the next three rounds of selection, the amount of the RNA was reduced to 500 pmol with the other conditions remaining the same. In the fifth and sixth rounds of selection on nitrocellulose filters, the background binding of the selected pool increased by >20%. Therefore, we went back to the pool from the fourth round of selection and performed the next three rounds of selection with polβ adsorbed to the surface of the immunological plates (Nunc Micro Well Module, Nalgene Nunc, Rochester, NY, USA) (24). In the fifth and sixth rounds, wells were coated for 1 h at room temperature with 50 pmol polβ in 200 µl of PBS. After blocking with Super Block Dry Blend (TBS) Blocking Buffer (Pierce Chemical company, Rockford, IL, USA) (24) and washing with BB, 200 µl of BB containing 150 pmol of the RNA pool from the previous round was introduced into the wells (Table 1). The wells were washed, bound RNA was recovered and cDNA synthesized as described (24). In the seventh round, the amount of polβ introduced into the well was reduced to 35 pmols (Table 1). The PCR products synthesized after seventh round of selection were cloned into the plasmid pGM-T (Promega, Madison WI, USA). Plasmid DNA was purified (Miniprep Kit, Qiagen, Valencia, CA, USA) and the sequence of the insert determined by automated sequencing.
Table 1.
Summary of the selection of polβ aptamers
| Round | Polβ (pmol) | RNA pool (pmol) | Method |
|---|---|---|---|
| 1 | 100 | 1200 | Filtration through nitrocellulose |
| 2 | 100 | 500 | |
| 3 | 100 | 500 | |
| 4 | 100 | 500 | |
| 5 | 50 | 150 | Immunological plates |
| 6 | 50 | 150 | |
| 7 | 35 | 150 |
Determination of the affinity of selected pools
After completion of rounds 4–7, each pool was assayed for its ability to bind polβ. RNA pools were radiolabeled in a reaction containing 40 mM Tris–HCl (pH 7.9), 25 mM MgCl2, 30 mM DTT, 2 mM each rNTP, 50 nM [α-32P]UTP, 40 U of RNAse inhibitor (Roche Applied Science, Indianapolis, IN, USA), 120 U of T7 RNA polymerase (Stratagene, La Jolla, CA, USA) and 45 pmol purified PCR product in a total reaction volume of 100 µl (PCR Cleanup Kit, Qiagen, Valencia, CA, USA). After 1 h of incubation at 37°C, the labeled RNA was purified by electrophoresis on an 8% denaturing polyacrylamide gel (PAG) (23). Rather than determining the binding constants of selected pools we determined the minimal concentrations of polβ at which the binding of the complex of labeled RNA with protein was detectable. After the fourth round of selection the binding was minimally detectable at 100 nM polβ. After the sixth round, binding was detectable at 30 nM polβ. After the seventh round binding was well above background at 30 nM polβ. The pool selected after the eighth round had similar binding to polβ as the pool after the seventh round, so we cloned the PCR products obtained after seventh round of selection.
Preparation of individual RNA aptamers for in vitro analysis
Individual RNA aptamers were synthesized by in vitro transcription from PCR products obtained by the direct amplification of bacterial lysates (25). In vitro transcription reactions were performed in a mixture containing: 40 mM Tris–HCl (pH 7.9), 25 mM MgCl2, 30 mM DTT, 2 mM each rNTP, 40 U of RNAse inhibitor (Roche Applied Science, Indianapolis, IN, USA), 200 U of T7 RNA polymerase (Stratagene, La Jolla, CA, USA) and 70 pmol of purified (PCR Cleanup Kit, Qiagen, Valencia, CA, USA) PCR product. The mixture was incubated overnight at 37°C and the synthesized RNA was purified by electrophoresis as described (23). After elution from denaturing PAG, the RNA was ethanol precipitated and resuspended in 20 µl of H2O. Then 2 µl of 1M NaCl was added, and the mixture was incubated for 3 min at 73°C and cooled to room temperature. RNA concentration was determined using yeast transfer RNA (tRNA) (Roche Applied Science, Indianapolis, IN, USA) as a standard (1 OD tRNA = 42 µg). The RNA was diluted in loading buffer (95% formamide, 0.1% bromphenol blue, 0.1% xylene cyanol 10 mM Na-EDTA) and electrophoresed on a 10% denaturing PAGE. The gel was stained with CYBR Green II and the amounts of RNA quantified using a Storm 840 phosphorimager (Amersham Biosciences Corp., Piscataway, NJ, USA) and ImageQuant.
Kd determination of individual RNA aptamers
Individual RNA aptamers were radiolabeled in 40 mM Tris–HCl (pH 7.9), 25 mM MgCl2, 30 mM DTT, 1 mM each rNTP, 50 nM [α-32P]UTP, 20 U of RNAse inhibitor (Roche Applied Science, Indianapolis, IN, USA), 60 U of T7 RNA polymerase (Stratagene, La Jolla, CA, USA) and 30 pmol of purified (PCR Cleanup Kit, Qiagen, Valencia, CA, USA) PCR product for 1 h at 37°C in a total reaction volume of 50 µl. The labeled RNA was used in the binding assay without purification. Radiolabeled RNAs (5 µl in each binding reaction) were incubated in 100 µl BB with increasing concentrations of protein. For binding curves, the reactions were passed through individual 0.45 µl HA nitrocellulose filters (Millipore, Billerica, MA, USA) over DE 81 filter paper (Whatman, Florham Park, NJ, USA). In this method, the nitrocellulose filter captures the protein-bound radiolabeled RNA, while the DE81 paper captures unbound radiolabeled RNA. The amount of radioactivity retained at each protein concentration was quantified using a Storm 840 phosphorimager (Amersham Biosciences Corp., Piscataway, NJ, USA) and ImageQuant.
Inhibition of DNA polymerase activity with RNA aptamers
To detect the inhibition of the DNA polymerases, RNA aptamers were preincubated in 10 µl of 50 mM NaCl with DNA polymerase at 37°C for 10 min. Then 10 µl of the solution, containing 1–6 pmol (50–300 nM final concentration) of the oligonucleotide annealed substrate duplex [32P-5′-GGAAGAAGAAGTATGTT-3′ 5′-CCTTCTTCATTCTAACATACTTCTTCTTCC-3′ 32P-labeled and annealed as described (26)]; 50 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 10 mM DTT, 2 µg/mL BSA, 10% glycerol and 100 µM dATP, dGTP and dTTP, were added to the DNA–polymerase/aptamer mixture. After 10 min at 37°C, reactions were placed on ice and an equal volume of loading buffer (95% formamide, 0.1% bromphenol blue, 0.1% xylene cyanol and 10 mM EDTA) was added to each tube. The products of the polymerization reaction were separated on a 20% denaturing PAG and analyzed using a Storm 840 phosphorimager (Amersham Biosciences Corp., Piscataway, NJ, USA) and quantified with ImageQuant. Time course assays were performed similarly except that reaction time varied from 1 to 20 min.
Gel electrophoretic mobility shift assays
Competition between substrate DNA and aptamer binding was determined for polβ by a gel shift assay in which 500 nM (or 700 nM) polβ and 190 nM (or 250 nM) 32P-labeled 4-base gapped substrate (27) were incubated in 10 µl BB at 37°C for 15 min. Then varying amounts of purified aptamer RNA (final concentration: 0.5–1.4 µM) in 10 µl BB was added and the mixture incubated another 15 min at 37°C. Finally 4 µl of 50% glycerol with 0.05% bromphenol blue was added and the mixture electrophoresed on an 8% non-denaturing PAG at 8°C for 1.5 h at 300 V. The gel was analyzed using a Storm 840 phosphorimager (Amersham Biosciences Corp., Piscataway, NJ, USA).
RESULTS AND DISCUSSION
In vitro selection of RNA aptamers that bind polβ
For the selection of RNA aptamers that bind to and inhibit polβ, an RNA library of ∼8 × 1012 different molecules was generated with each molecule containing a 30 nt random sequence flanked by defined sequences as described in the Materials and Methods. Aptamers were obtained after four rounds of selection on nitrocellulose filters and three rounds of selection in the wells of immunological plates (Table 1). After seven rounds of selection, the amplified cDNAs were cloned.
Aptamers that inhibit polβ activity
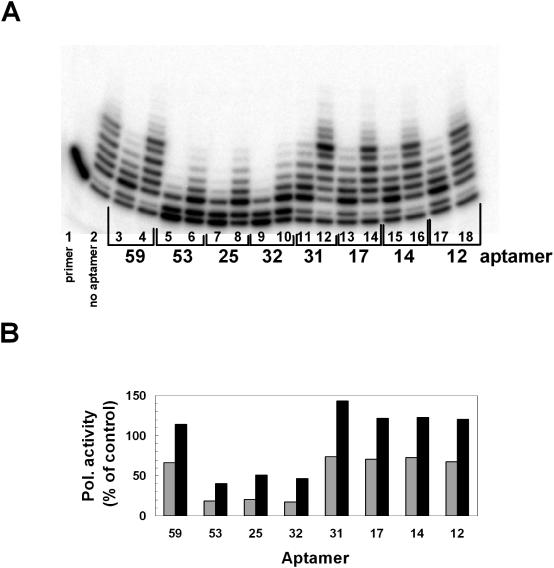
Since our selection was based on a general interaction between the aptamer and polβ, a further level of selection was necessary to distinguish which of these aptamers could inhibit polymerase activity. A total of 60 cloned aptamers were screened in primer extension DNA polymerase assays. To observe the level of inhibition, polβ was pre-incubated with two different concentrations of each individual RNA aptamer. Once equilibrated, a 32P-labeled primed oligonucleotide substrate and dNTPs were added, and the mixture incubated to permit polβ to extend the primer. A representative gel from the screening of eight individual cloned aptamers shows that at high aptamer concentration (480 nM), all eight aptamers reduced the efficiency of primer extension but only aptamers 32, 25 and 53 inhibited polymerase activity at the 10-fold lower (48 nM) concentration (Figure 1). Aptamer 45 (data not shown) also inhibited polβ at 48 nM. Of the 60 aptamers tested, seven were good inhibitors of polβ (25, 32, 33, 41, 42, 45 and 53), about 35 aptamers slightly inhibited DNA polymerase activity at high concentration similar to aptamers 12, 14, 17, 31 and 59, and the other aptamers showed virtually no ability to inhibit the polymerase activity even at high concentrations (data not shown).
Figure 1.
Inhibition of polβ polymerase activity by RNA aptamers. (A) Primer extension reactions containing 4 nM polβ and 300 nM 5′-32P-labeled primer-template were performed as described in Materials and Methods in the absence (lane 2) or presence of 48 nM (lanes 4, 6, 8, 10, 12, 14, and 16) aptamer or 480 nM (Lanes 3, 5, 7, 9, 11, 13, 15) as labeled. Reactions were then quenched and products were analyzed by denaturing PAGE. Lane 1 represents a control reaction carried out in the absence of polβ. (B) Bar graph showing quantification of the polymerase activity in each reaction calculated by summing the product of the band intensity and the number of nucleotides that had been added to the primer under the assumption of completely distributive addition. This value for lane 2, polβ activity in the absence of aptamer was set to 100% and the other lanes normalized to this value. For each aptamer, the black bars, 48 nM and gray bars, 480 nM.
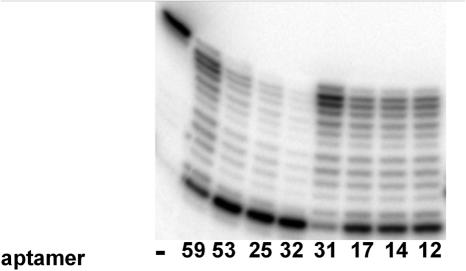
Anti-polβ aptamers also inhibit DNA polymerase κ
In order to determine the specificity of the polymerase inhibition, the individual aptamers were incubated with polκ in primer extension assays under similar conditions to that used for polβ (Figure 2) except the aptamer concentration was 240 nM. As observed with polβ, aptamers 32, 25 and 53 were the most inhibitory, 12, 14 and 17 were moderately inhibitory and 31 and 59 the least effective at inhibiting polκ activity. Aptamer 45 (data not shown) was also an effective inhibitor of polκ. All other aptamers analyzed had minimal effect on polκ activity and were not analyzed further.
Figure 2.
Inhibition of polκ polymerase activity by RNA aptamers. Primer extension reactions containing 8 nM truncated polκ and 300 nM 5′-32P-labeled primer-template were performed in the presence of 240 nM of each aptamer as indicated. Reactions were then quenched and products were analyzed by denaturing PAGE. In the left most lane (−), the reaction contained no aptamer.
Sequence of selected aptamers
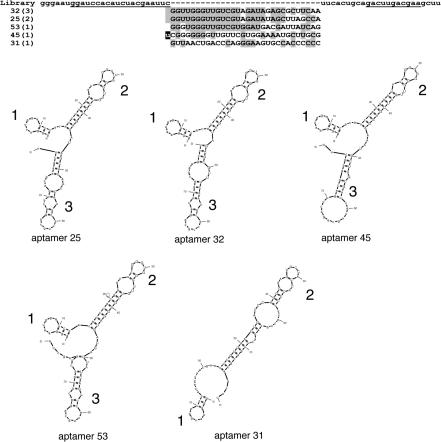
Seven strongly inhibitory aptamers and a poor inhibiting aptamer (aptamer 31) were sequenced. The eight aptamers had five different RNA sequences (Figure 3). The most stable two-dimensional structure of each RNA aptamer was predicted using MFOLD program (28,29) (Figure 3). In general, two different folded structures are predicted from the selected 80 nt long aptamer sequences. In 4 of 5 cases, the aptamers form a tri-lobed stem–loop structure with two long loops and a short loop (Aptamers 25, 32, 45, 53). Loop 1 is composed entirely of bases from the 5′ constant region of the aptamer and loops 2 and 3 are formed due to complementarities between the constant and variable regions. All the inhibitory aptamers have loops 1 and 2 which are identical, whereas loop 3 differs among the four aptamers. The other structure (Aptamer 31) is predicted to form a bi-lobed stem–loop structure. Loop 1, being formed from the constant region is also present in Aptamer 31. The structure and sequence of loop 2 is very different from the equivalent loop in the other aptamers and loop 3 is missing. Further mutation studies of these sequences will allow us to determine if the structure or the sequence are more important for binding to and inhibiting the activity of DNA polymerases.
Figure 3.
Sequences and structure of selected aptamers. (Upper) Sequences of the primers, the RNA pool and the polβ aptamers. The PCR primer annealing sites within the constant regions are underlined. For the RNA library, the randomized region (n30) is flanked by the 5′ and 3′ constant regions. The names of the representative aptamers are shown on the left. The number in parentheses indicates the number of independent clones of the identical sequence. In aptamer 45, the last C in the 5′ constant region has been mutated to a U. The sequences are shown with the most similar sequences grouped together according to Clustal (49). (Lower) The Mfold program (available at http://www.bioinfo.rpi.edu/applications/mfold) was used to derive secondary structural models for aptamers 25 (−17.6 kcal/mol, next most stable −17.0 kcal/mol), 31 (−15.3 kcal/mol, next most stable −14.5 kcal/mol), 32 (−19.3 kcal/mol, next most stable −18.3 kcal/mol), 45 (−21.2 kcal/mol, next most stable −20.6 kcal/mol) and 53 (−20.2 kcal/mol, next most stable −19.4 kcal/mol). Only the most stable predicted structure is shown.
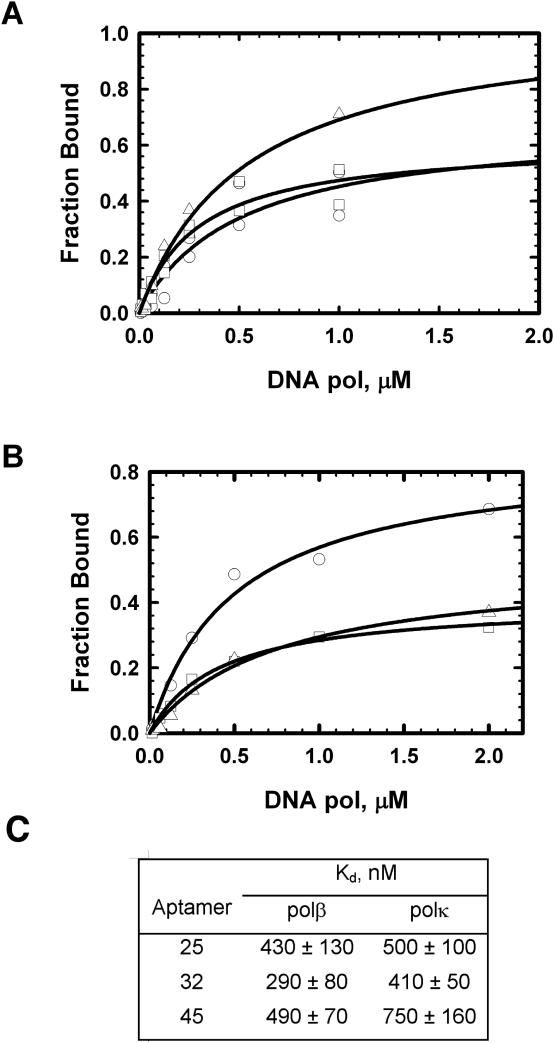
Affinity of aptamers for DNA polymerases
To evaluate binding specificities of these aptamers with polβ and polκ, a nitrocellulose binding experiment was performed using 5′-radiolabeled RNAs (Figure 4A and B) and the values of the dissociation constants (Kds) of RNAs were determined (Figure 4C). We found that the aptamers selected for polβ binding also bound polκ with similar affinities (Figure 4B and C). However, the maximum level of binding of aptamers 32 and 45 for polκ are notably lower than that of aptamer 25. Perhaps these aptamers fold into two or more slightly different conformations that interact with polκ with different affinities.
Figure 4.
Binding curves for RNA aptamers with polβ and polκ. Data points were obtained by the nitrocellulose filter binding assay as described in the Materials and Methods. (A) Polβ, aptamer 25, circles; aptamer 32, squares and aptamer 45, triangles. (B) Polκ, aptamer 25, circles; aptamer 32, squares and aptamer 45, triangles. In each case, the concentration of the labeled aptamer was 5–7 nM. C. Kds derived from the filter binding assays.
RNA aptamers compete with primed oligonucleotide substrate for binding
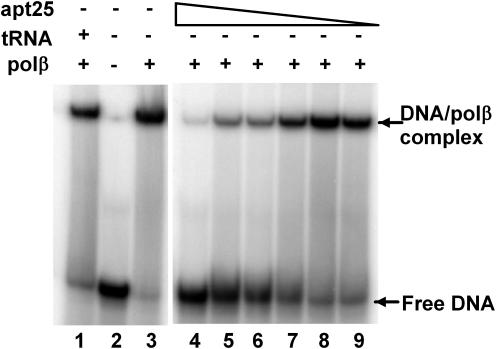
To elucidate the mode of aptamer binding, we analyzed the ability of the aptamers to displace a bound primed oligonucleotide template. Polβ was incubated with a tight binding 4-base gapped oligonucleotide substrate (27) and varying amounts of RNA aptamer. After a 15 min incubation, the mixture was electrophoresed on a non-denaturing gel. Representative results using aptamer 25 are shown in Figure 5. However, all aptamers tested (25, 32, 45 and 53) were able to displace the DNA bound in the polymerase active site at similar aptamer concentrations (data not shown).
Figure 5.
Aptamer 25 displaces bound primer-template from polβ. Gel shift competition reactions were carried out as described in Materials and Methods. Each reaction contained 250 nM 4-base gapped DNA, 700 nM polβ (except lane 2) and the indicated amount of purified aptamer 25; lanes 1–3, no aptamer; lanes 4–9, 6.0 µM, 3.0 µM, 1.5 µM, 0.75 µM, 0.38 µM and 0.19 µM. The positions of free and bound labeled primer-templates are indicated on the right.
Anti-polβ aptamers also inhibit other DNA polymerases
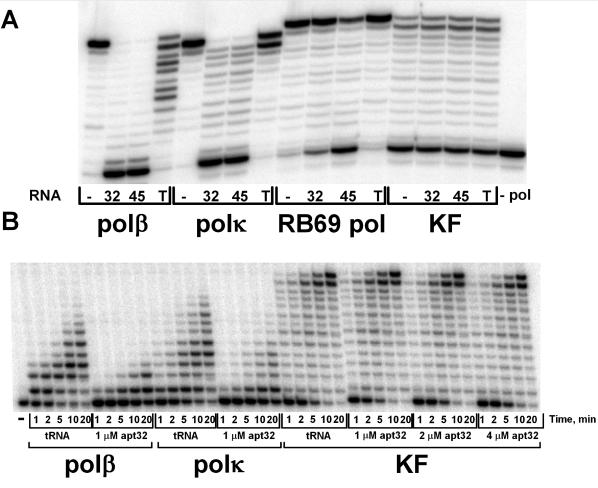
To determine if the selected aptamers inhibit DNA polymerases other than pols β and κ, we assayed RB69 DNA polymerase (RB69 pol, family B) and the Klenow fragment of DNA polymerase I (KF, family A) from E.coli in the presence of two inhibitory aptamers, 32 and 45 (Figure 6A). Yeast tRNA (up to 1 mM) only slightly reduced the extension of the primer by polβ and polκ while aptamers 32 and 45 almost completely inhibited the polymerase activity. Aptamers 32 and 45 had a minor inhibitory effect on RB69 pol, the effect being greater with aptamer 45. No inhibitory effect of the aptamers were detected for KF. We also performed a time course experiment showing dramatic inhibition of pols β and κ compared with the tRNA control (Figure 6B) by aptamer 32. In the presence of 1 µM aptamer 32 polβ has 20% remaining activity and polκ, 26% compared with the tRNA control condition. In contrast, we observed no inhibition of KF even at 4-fold higher concentrations of aptamer 32 (Figure 6B). Thus, the selected aptamers are most effective at inhibiting the lower fidelity pols from the X and Y families and have little or no effect on the higher fidelity pols from the A and B families.
Figure 6.
Inhibition of DNA pols by aptamers 32 and 45. A. In reactions containing polβ (0.9 nM), polκ (1.7 nM), RB69 pol (1.6 nM) or KF (1.1 nM) and 50 nM 5′-32P-labeled primer-template, primer extension was measured in the absence (−) or presence of 300 nM aptamer 32, aptamer 45 or 1000 nM yeast tRNA (T). (B) Time course reactions containing polβ (0.3 nM), polκ (0.5 nM) or KF (0.3 nM) and 50 nM 5′-32P-labeled primer-template, primer extension was measured in the absence (−) or presence of 1–4 µM aptamer 32 or 1000 nM yeast tRNA.
Structure of DNA polymerases may explain low specificity of inhibitory aptamers
A number of RNA aptamer structures have been determined by X-ray crystallography or NMR spectroscopy (30). These structural analyses have been useful to generalize two types of aptamers. High affinity aptamers generated against proteins that are potentially nucleic acid-binding proteins are shorter in length and bind to their protein targets through a small number of interaction sites. The major interactions between the RNA and the protein are stacking of flat moieties, hydrogen bonding and shape complementation (30). The aptamers described here appear to bind directly in the polymerase active site, displacing the bound DNA substrate (Figure 5). The Kds for the polymerase primer-template interactions are as follows: RB69 pol, 70 nM (31), KF, 5 nM (32), polβ, 49 nM (primer-template) (33) and 48 nM [1-base gapped template (34)], and the polκ homolog (Sso DNA pol Y1) has been reported to bind dsDNA with a Kd of 30 nM (35). From these approximate values one can conclude that, among these four polymerases, KF has the strongest interaction with primer-template DNA. If the mechanism by which the aptamer binds the protein and inhibits polymerase activity requires displacement of the DNA substrate, the tighter DNA binding of KF may partially explain the lack of inhibition by the aptamers. The structures of the binary (DNA–protein) complexes of these DNA polymerases have been determined (36–39) (Figure 7). The interaction of KF with primer-template DNA involves a large number of amino acid residues and the protein wraps around the bound DNA. In the polβ structure, there are fewer interactions and the DNA binding site is more open. RB69 pol and polκ are intermediate between these two extremes. Perhaps a combination of substrate binding affinity and the ‘openness’ of the active site determines whether these tri-lobed aptamers inhibit the polymerase activity.
Figure 7.
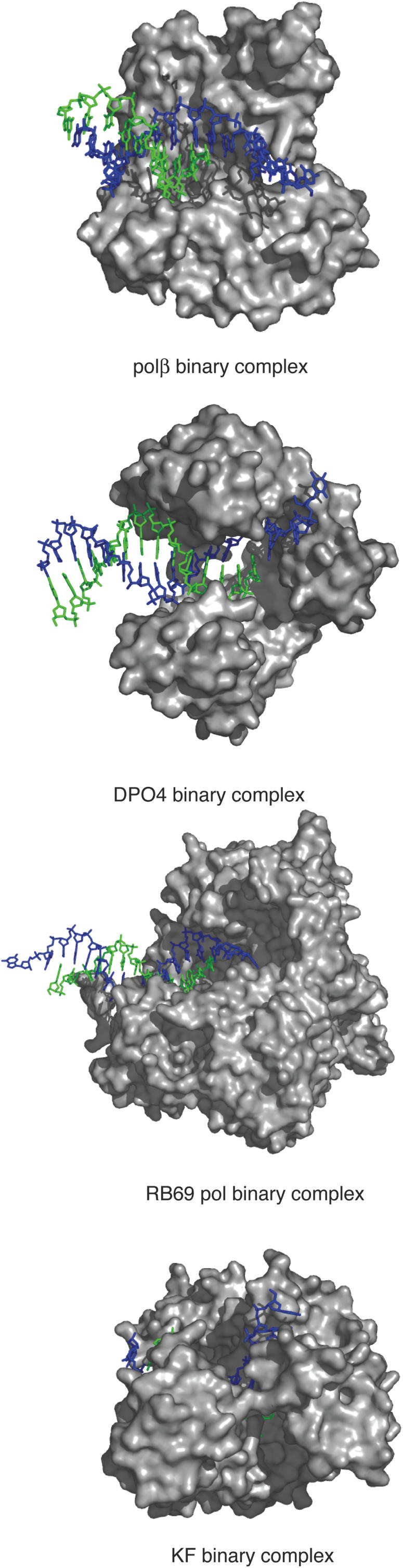
Comparison of the DNA binding modes for DNA polymerase. Polβ binary complex (Protein Data Bank accession code 1BPX), DPO4 binary complex (as a family Y model structure) (1N56), RB69 pol binary complex (1Q9X), KF (Bacillus) binary complex (1L3S). The protein residues are shown in a gray surface representation, the DNA primer strand in green and the template in dark blue. Figure was generated using PyMOL (50) (http://www.pymol.org).
Comparison with other polymerase inhibiting aptamers
RNA aptamers have been selected that bind and inhibit HIV-1 reverse transcriptase (40–44), yeast RNA polymerase II (45) and E.coli RNA polymerase (46). In general, although it has not been tested extensively, polymerase binding aptamers have been found to be specific. For instance, the HIV-1 RT aptamers did not inhibit the reverse transcriptases from Moloney murine leukemia virus or avian myeloblastosis virus (43). The yeast RNA polymerase II aptamer did not inhibit RNA polymerase I or III (45) and the E.coli RNA polymerase aptamers did not bind to Taq RNA polymerase (46). However, some of the HIV-1 RT inhibitory aptamers mimick primer-template junctions and could be extended by the target polymerase RT as well as Sequenase (T7 DNA pol) and AMV RT (47). The co-crystal structure of an RNA pseudoknot bound to HIV-1 RT shows that the position of the RNA aptamer bound to RT largely overlaps the DNA binding site (48). It is likely that the polβ aptamers we describe also occupy the DNA binding site. The low polymerase selectivity of the polβ aptamers offers an advantage over highly specific inhibitory aptamers in that they may be useful as generalized DNA repair suppression molecules. Future experiments are underway to express these aptamer in cells to explore this possibility.
Acknowledgments
The authors thank Samuel H. Wilson for the polβ overexpression plasmid and Haruo Ohmori for the bacterial expression plasmid for truncated polκ. The authors are indebted to Margaret Luk-Paszyc for helpful discussions and critical reading of the manuscript. This research was supported by grant CA17395 from the National Cancer Institute to APG. Funding to pay the Open Access publication charges for this article was provided by the National Cancer Institute (grant CA17395).
Conflict of interest statement. None declared.
REFERENCES
- 1.Albertella M.R., Lau A., O'Connor M.J. The overexpression of specialized DNA polymerases in cancer. DNA Repair. 2005;4:583–593. doi: 10.1016/j.dnarep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Wilson S.H. Mammalian base excision repair and DNA polymerase beta. Mutat. Res. 1998;407:203–215. doi: 10.1016/s0921-8777(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 3.Abbotts J., SenGupta D.N., Zmudzka B., Widen S.G., Notario V., Wilson S.H. Expression of human DNA polymerase beta in Escherichia coli and characterization of the recombinant enzyme. Biochemistry. 1988;27:901–909. doi: 10.1021/bi00403a010. [DOI] [PubMed] [Google Scholar]
- 4.Singhal R.K., Wilson S.H. Short gap-filling synthesis by DNA polymerase beta is processive. J. Biol. Chem. 1993;268:15906–15911. [PubMed] [Google Scholar]
- 5.Husain I., Morton B.S., Beard W.A., Singhal R.K., Prasad R., Wilson S.H., Besterman J.M. Specific inhibition of DNA polymerase beta by its 14 kDa domain: role of single- and double-stranded DNA binding and 5′-phosphate recognition. Nucleic Acids Res. 1995;23:1597–1603. doi: 10.1093/nar/23.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya N., Chen H.C., Wang L., Banerjee S. Heterogeneity in expression of DNA polymerase beta and DNA repair activity in human tumor cell lines. Gene Expr. 2002;10:115–123. [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson T.E., Rogan P.K., Risinger J.I., Taylor J.A. Splice variants but not mutations of DNA polymerase beta are common in bladder cancer. Cancer Res. 2002;62:3251–3256. [PubMed] [Google Scholar]
- 8.Canitrot Y., Cazaux C., Frechet M., Bouayadi K., Lesca C., Salles B., Hoffmann J.S. Overexpression of DNA polymerase beta in cell results in a mutator phenotype and a decreased sensitivity to anticancer drugs. Proc. Natl Acad. Sci. USA. 1998;95:12586–12590. doi: 10.1073/pnas.95.21.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergoglio V., Pillaire M.J., Lacroix-Triki M., Raynaud-Messina B., Canitrot Y., Bieth A., Gares M., Wright M., Delsol G., Loeb L.A., et al. Deregulated DNA polymerase beta induces chromosome instability and tumorigenesis. Cancer Res. 2002;62:3511–3514. [PubMed] [Google Scholar]
- 10.Servant L., Cazaux C., Bieth A., Iwai S., Hanaoka F., Hoffmann J.-S. A role for DNA polymerase beta in mutagenic UV lesion bypass. J. Biol. Chem. 2002;277:50046–50053. doi: 10.1074/jbc.M207101200. [DOI] [PubMed] [Google Scholar]
- 11.Bergoglio V., Canitrot Y., Hogarth L., Minto L., Howell S.B., Cazaux C., Hoffmann J.S. Enhanced expression and activity of DNA polymerase beta in human ovarian tumor cells: impact on sensitivity towards antitumor agents. Oncogene. 2001;20:6181–6187. doi: 10.1038/sj.onc.1204743. [DOI] [PubMed] [Google Scholar]
- 12.Raaphorst G.P., Cybulski S.E., Sobol R., Ng C.E. The response of human breast tumour cell lines with altered polymerase beta levels to cisplatin and radiation. Anticancer Res. 2001;21:2079–2083. [PubMed] [Google Scholar]
- 13.Woodgate R. A plethora of lesion-replicating DNA polymerases. Genes Dev. 1999;13:2191–2195. doi: 10.1101/gad.13.17.2191. [DOI] [PubMed] [Google Scholar]
- 14.Ohmori H., Friedberg E.C., Fuchs R.P., Goodman M.F., Hanaoka F., Hinkle D., Kunkel T.A., Lawrence C.W., Livneh Z., Nohmi T., et al. The Y-family of DNA polymerases. Mol. Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 15.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 16.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 17.Held D.M., Kissel J.D., Patterson J.T., Nickens D.G., Burke D.H. HIV-1 inactivation by nucleic acid aptamers. Front Biosci. 2006;11:89–112. doi: 10.2741/1782. [DOI] [PubMed] [Google Scholar]
- 18.Nickens D.G., Patterson J.T., Burke D.H. Inhibition of HIV-1 reverse transcriptase by RNA aptamers in Escherichia coli. RNA. 2003;9:1029–1033. doi: 10.1261/rna.5550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi P.J., Fisher T.S., Prasad V.R. Anti-HIV inhibitors based on nucleic acids: emergence of aptamers as potent antivirals. Curr. Drug Targets Infect. Disord. 2003;3:383–400. doi: 10.2174/1568005033481060. [DOI] [PubMed] [Google Scholar]
- 20.Sooter L.J., Riedel T., Davidson E.A., Levy M., Cox J.C., Ellington A.D. Toward automated nucleic acid enzyme selection. Biol. Chem. 2001;382:1327–1334. doi: 10.1515/BC.2001.165. [DOI] [PubMed] [Google Scholar]
- 21.Conlon K.A., Miller H., Rosenquist T.A., Zharkov D.O., Berrios M. The murine DNA glycosylase NEIL2 (mNEIL2) and human DNA polymerase beta bind microtubules in situ and in vitro. DNA Repair. 2005;4:419–431. doi: 10.1016/j.dnarep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Ohashi E., Ogi T., Kusumoto R., Iwai S., Masutani C., Hanaoka F., Ohmori H. Error-prone bypass of certain DNA lesions by the human DNA polymerase kappa. Genes Dev. 2000;14:1589–1594. [PMC free article] [PubMed] [Google Scholar]
- 23.Conrad R.C., Giver L., Tian Y., Ellington A.D. In vitro selection of nucleic acid aptamers that bind proteins. Methods Enzymol. 1996;267:336–367. doi: 10.1016/s0076-6879(96)67022-0. [DOI] [PubMed] [Google Scholar]
- 24.Drolet D.W., Jenison R.D., Smith D.E., Pratt D., Hicke B.J. A high throughput platform for systematic evolution of ligands by exponential enrichment (SELEX) Comb. Chem. High Throughput Screen. 1999;2:271–278. [PubMed] [Google Scholar]
- 25.Gening L.V., Klincheva S.A., Gusev A.S., Surovoy A.Y., Potapov V.K. SSCP screening of individual aptamers. Biotechniques. 2001;31:828–834. doi: 10.2144/01314st10. [DOI] [PubMed] [Google Scholar]
- 26.Miller H., Grollman A.P. Kinetics of DNA polymerase I (Klenow fragment exo-) activity on damaged DNA templates: effect of proximal and distal template damage on DNA synthesis. Biochemistry. 1997;36:15336–15342. doi: 10.1021/bi971927n. [DOI] [PubMed] [Google Scholar]
- 27.Miller H., Prasad R., Wilson S.H., Johnson F., Grollman A.P. 8-oxodGTP incorporation by DNA polymerase beta is modified by active- site residue Asn279. Biochemistry. 2000;39:1029–1033. doi: 10.1021/bi991789x. [DOI] [PubMed] [Google Scholar]
- 28.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews D.H., Sabina J., Zuker M., Turner D.H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 30.Hermann T., Patel D.J. Adaptive recognition by nucleic acid aptamers. Science. 2000;287:820–825. doi: 10.1126/science.287.5454.820. [DOI] [PubMed] [Google Scholar]
- 31.Capson T.L., Peliska J.A., Kaboord B.F., Frey M.W., Lively C., Dahlberg M., Benkovic S.J. Kinetic characterization of the polymerase and exonuclease activities of the gene 43 protein of bacteriophage T4. Biochemistry. 1992;31:10984–10994. doi: 10.1021/bi00160a007. [DOI] [PubMed] [Google Scholar]
- 32.Kuchta R.D., Mizrahi V., Benkovic P.A., Johnson K.A., Benkovic S.J. Kinetic mechanism of DNA polymerase I (Klenow) Biochemistry. 1987;26:8410–8417. doi: 10.1021/bi00399a057. [DOI] [PubMed] [Google Scholar]
- 33.Werneburg B.G., Ahn J., Zhong X., Hondal R.J., Kraynov V.S., Tsai M.D. DNA polymerase beta: pre-steady-state kinetic analysis and roles of arginine-283 in catalysis and fidelity. Biochemistry. 1996;35:7041–7050. doi: 10.1021/bi9527202. [DOI] [PubMed] [Google Scholar]
- 34.Dalal S., Kosa J.L., Sweasy J.B. The D246V mutant of DNA polymerase beta misincorporates nucleotides: evidence for a role for the flexible loop in DNA positioning within the active site. J. Biol. Chem. 2004;279:577–584. doi: 10.1074/jbc.M309607200. [DOI] [PubMed] [Google Scholar]
- 35.Gruz P., Pisani F.M., Shimizu M., Yamada M., Hayashi I., Morikawa K., Nohmi T. Synthetic activity of Sso DNA pol Y1, an archaeal DinB-like DNA polymerase, is stimulated by processivity factors PCNA and RFC. J. Biol. Chem. 2001;276:47394–47401. doi: 10.1074/jbc.M107213200. [DOI] [PubMed] [Google Scholar]
- 36.Sawaya M.R., Prasad R., Wilson S.H., Kraut J., Pelletier H. Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: evidence for an induced fit mechanism. Biochemistry. 1997;36:11205–11215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- 37.Ling H., Boudsocq F., Woodgate R., Yang W. Snapshots of replication through an abasic lesion; structural basis for base substitutions and frameshifts. Mol. Cell. 2004;13:751–762. doi: 10.1016/s1097-2765(04)00101-7. [DOI] [PubMed] [Google Scholar]
- 38.Freisinger E., Grollman A.P., Miller H., Kisker C. Lesion (in)tolerance reveals insights into DNA replication fidelity. EMBO J. 2004;23:1494–1505. doi: 10.1038/sj.emboj.7600158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson S.J., Taylor J.S., Beese L.S. Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc. Natl Acad. Sci. USA. 2003;100:3895–3900. doi: 10.1073/pnas.0630532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi P., Prasad V.R. Potent inhibition of human immunodeficiency virus Type 1 replication by template analog reverse transcriptase inhibitors derived by SELEX (Systematic Evolution of Ligands by Exponential Enrichment) J. Virol. 2002;76:6545–6557. doi: 10.1128/JVI.76.13.6545-6557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kensch O., Connolly B.A., Steinhoff H.J., McGregor A., Goody R.S., Restle T. HIV-1 reverse transcriptase-pseudoknot RNA aptamer interaction has a binding affinity in the low picomolar range coupled with high specificity. J. Biol. Chem. 2000;275:18271–18278. doi: 10.1074/jbc.M001309200. [DOI] [PubMed] [Google Scholar]
- 42.Burke D.H., Scates L., Andrews K., Gold L. Bent Pseudoknots and novel RNA inhibitors of Type 1 human immunodeficiency virus (HIV-1) reverse transcriptase. J. Mol. Biol. 1996;264:650–666. doi: 10.1006/jmbi.1996.0667. [DOI] [PubMed] [Google Scholar]
- 43.Tuerk C., MacDougal S., Gold L. RNA pseudoknots that inhibit human immunodeficiency virus type 1 reverse transcriptase. Proc. Natl Acad. Sci. USA. 1992;89:6988–6992. doi: 10.1073/pnas.89.15.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaloin L., Lehmann M.J., Sczakiel G., Restle T. Endogenous expression of a high-affinity pseudoknot RNA aptamer suppresses replication of HIV-1. Nucleic Acids Res. 2002;30:4001–4008. doi: 10.1093/nar/gkf522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas M., Chedin S., Carles C., Riva M., Famulok M., Sentenac A. Selective targeting and inhibition of yeast RNA polymerase II by RNA aptamers. J. Biol. Chem. 1997;272:27980–27986. doi: 10.1074/jbc.272.44.27980. [DOI] [PubMed] [Google Scholar]
- 46.Kulbachinskiy A., Feklistov A., Krasheninnikov I., Goldfarb A., Nikiforov V. Aptamers to Escherichia coli core RNA polymerase that sense its interaction with rifampicin, sigma-subunit and GreB. Eur. J. Biochem. 2004;271:4921–4931. doi: 10.1111/j.1432-1033.2004.04461.x. [DOI] [PubMed] [Google Scholar]
- 47.Schneider D.J., Feigon J., Hostomsky Z., Gold L. High-affinity ssDNA inhibitors of the reverse transcriptase of type 1 human immunodeficiency virus. Biochemistry. 1995;34:9599–9610. doi: 10.1021/bi00029a037. [DOI] [PubMed] [Google Scholar]
- 48.Jaeger J., Restle T., Steitz T.A. The structure of HIV-1 reverse transcriptase complexed with an RNA pseudoknot inhibitor. EMBO J. 1998;17:4535–4542. doi: 10.1093/emboj/17.15.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chenna R., Sugawara H., Koike T., Lopez R., Gibson T.J., Higgins D.G., Thompson J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLano W.L. 2002. The PyMOL Molecular Graphics System. [Google Scholar]