Abstract
To examine the developmental course of look duration as a function of age and stimulus type, 14-to 52-week-old infants were shown static and dynamic versions of faces, Sesame Street material and achromatic patterns for 20 seconds of accumulated looking. Heart rate was recorded during looking and parsed into stimulus orienting, sustained attention, and attention termination phases of attention. Infants' peak look durations indicated that prior to 26 weeks there was a linear decrease with age for all stimuli. Older infants' look durations continued to decline for patterns but increased for Sesame Street and faces. Measures of heart rate change during sustained attention and the proportion of time spent in each phase of attention confirmed infants' greater engagement with the more complex stimuli.
Infants' Attention to Patterned Stimuli: Developmental Change from 3 to 12 Months of Age
Measures of visual attention obtained from habituation and selective looking (e.g., paired-comparison; novelty preference) procedures have provided a wealth of information about the development of infants' sensory, perceptual, and cognitive capabilities (for reviews see Haith & Benson, 1998; Kellman & Banks, 1998). More recently, these procedures have become important in their own right with growing evidence that components of infants' attention during habituation and selective looking also provide information about the functioning and early development of the human information-processing system (see Bornstein, 1998; Colombo, 1993; Fagan, 1990; Hayne, 2004; McCall, 1994; Rose, Feldman, & Jankowski, 2004). The underlying reasoning is not new and is based on the assumption that the process of habituation represents a ubiquitous and elementary form of learning during which the infant constructs an internal representation (trace, or engram) of an external stimulus. As the infant's attention to the stimulus progresses and its representation becomes complete, his or her attention to it wanes. The subsequent presentation of a novel stimulus elicits a recovery of attention (dishabituation, or a novelty preference) as the infant presumably compares its features to the internal representation of the familiar stimulus, “recognizes” at some level that it is unrepresented or weakly represented in memory, and begins the process of trace construction once more (Sokolov, 1963). These same familiarization, comparison, and recognition processes are also presumed to occur without complete habituation, as infants will show visual preference for a novel stimulus even after fairly brief exposure to a standard (see Fagan, 1990). Thus, infants' habituation and novelty preferences may be analogous to the encoding, storage and retrieval processes that have been documented in older children and adults.
Consistent with this information-processing interpretation of habituation and recovery to (or preference for) novelty, there is some evidence that older infants habituate more efficiently (e.g., in less time, at a faster rate, with shorter look durations) than do younger infants (for reviews see Bornstein, 1998; Bornstein & Sigman, 1986; Colombo, 1993). This finding has been attributed, at least in part, to an increase in speed and/or efficiency in information processing with age (e.g., Frick, Colombo, & Saxon, 1999; McCall, 1994; Rose & Feldman, 1997; Rose, Feldman, & Jankowski, 2002). Moreover, individual differences in these same look duration measures within age have also been observed. Infants who examine stimuli with short look durations compared to those who examine with long look durations appear to encode information more quickly and/or efficiently (Colombo, Freeseman, Coldren, & Frick, 1995; Colombo, Mitchell, Coldren, & Freeseman, 1991; Courage & Howe, 2001; Jankowski & Rose, 1997; Jankowski, Rose, & Feldman, 2001) and also show higher performance on certain cognitive measures in later childhood (Bornstein, Slater, Brown, Roberts, and Barrett, 1997; Colombo, Mitchell, Dodd, Coldren, & Horowitz, 1989; Fagan, 1990; Rose, Feldman & Wallace, 1992; Rose & Feldman, 1995; Sigman, Cohen & Beckwith, 1997). These robust findings have a long history, represent research from diverse perspectives, and have been established across a range of ages, tasks, and experimental manipulations. However, there are three sets of findings in the current infant attention literature that call for reconsideration and perhaps a modification of some of these important conclusions about the relationship between the development of infant looking and early information processing.
First, there is strong evidence that the primary measure of infants' visual attention (i.e., the duration of each look) does not represent a unitary attentional process and therefore may not reflect visual information processing directly. Much of this has been established by Richards and colleagues (e.g., Berg & Richards, 1997; Richards, 1985, 1989, 1997a, 2001, 2003; Richards & Casey, 1991, 1992) who proposed a model in which the phasic changes in infants' heart rates that occur as they look at a stimulus correspond to different levels of attentional engagement with the stimulus. There are four key phases in this model and these are illustrated in Figure 1.
Figure 1.
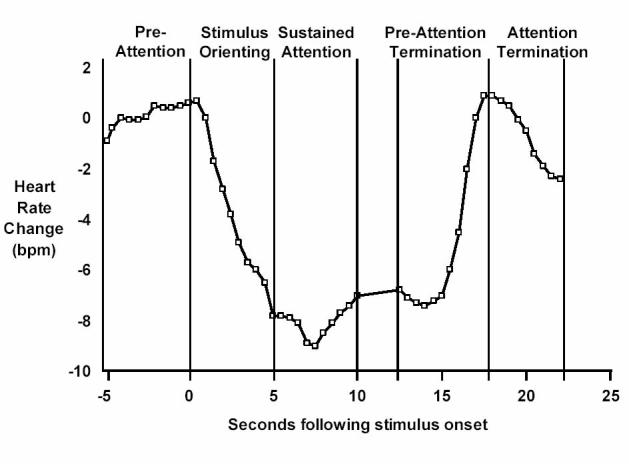
Mean heart-rate change as a function of seconds following stimulus onset during the heart-rate defined attention phases (from Richards & Casey, 1991, 1992).
First, when the stimulus is initially presented there is a brief automatic interrupt phase that reflects the detection of transient changes in environmental stimulation (see Graham, 1979). During this phase (not labeled on Figure 1) there is a very brief, reflexive, biphasic deceleration-acceleration in heart rate. The second phase of stimulus orienting reflects the beginning of attentional engagement in which the automatic interrupt system activates an orienting reflex (Sokolov, 1963) and initiates preliminary processing of the information in the stimulus. A large, rapid deceleration in heart rate from its preattention or prestimulus level occurs during this phase. The third phase of sustained attention reflects the activation of the alertness/arousal system of the brain and involves voluntary, subject-controlled cognitive processing of the stimulus information. The heart rate deceleration that was reached during stimulus orienting is maintained during this phase. Heart rate also shows decreased variability and certain other somatic changes that facilitate attentiveness may occur (e.g., inhibition of movement, decreased respiration amplitude). The fourth key phase is attention termination during which the infant continues to look at the stimulus but is no longer processing its information (i.e., is inattentive) and is briefly resistant to new stimulation. The lowered heart rate (and heart rate variability) that occurred during sustained attention begins to return to prestimulus levels during this. Figure 1 also shows the “preattention” and “preattention termination” periods that occur before the presentation of the stimulus and before the heart rate returns to its prestimulus level but after sustained attention has occurred, respectively.
There is currently an extensive database that validates this model across infancy and early childhood and shows that the phases are elicited by a wide range of stimuli and conditions (for a review see Reynolds & Richards, in press). These include brief exposures to the traditional achromatic geometric patterns used in psychophysical research with infants, more extended viewing of children's video material (Richards & Cronise, 2000; Richards & Gibson, 1997; Richards & Turner, 2001), and the visual/manual examination of novel toys (Lansink and Richards, 1997). Moreover, there is evidence that infants' information-processing activity occurs primarily during the heart rate phase of sustained attention rather than during the other phases. For example, it is during sustained attention that infants (1) encode information and demonstrate later recognition (e.g., Colombo, Richman, Shaddy, Greenhoot, & Maikranz, 2001; Frick & Richards, 2001; Richards, 1997a, 2003), (2) show attenuated localization (longer latency, lower probability) of a distracter stimulus located in the peripheral visual field and slower peak velocity of saccadic eye movements to the distracter stimulus in the presence of a central stimulus (e.g., Hicks & Richards, 1998; Hunter & Richards, 2003; Richards, 1997b; Richards & Hunter, 1997), and (3) are more resistant to distraction from competing stimuli during toy play (e.g., Richards & Lansink, 1998; Lansink, Mintz, & Richards, 2002). Collectively, these findings indicate that a sustained heart rate deceleration (relative to a prestimulus baseline rate) accompanied by visual fixation can be considered a marker for sustained attention and information processing in infants (see also, Lewis, Kagan, Campbell, & Kalafat, 1966). To date however, few studies on the development of look duration in infants have included a measure of the phasic changes in heart rate that co-occur with looking, although such information could serve to refine the interpretation of these data (but see Colombo, Shaddy, Richman, Maikranz, & Blaga, 2004; Colombo, et a., 2001; Maikranz, Colombo, Richman, & Frick, 2000).
The second set of findings to consider indicates that although there is a large literature showing that look duration decreases linearly with age (for a review see Colombo, 1993), most of the data have been gathered from infants between 3 and 7 months of age who were tested in the habituation or selective looking paradigms (e.g., Bornstein, Pecheaux, & Lecuyer, 1988; Colombo & Mitchell, 1990; Mayes & Kessen, 1989). In a more recent meta-analysis of the extant literature that included studies with younger and older infants as well as non-human primates Colombo and colleagues (Colombo, 2001; Colombo, Harlan, and Mitchell, 1999) reported that the purported decline in look duration (e.g., the duration of the peak or longest look, the average or mean length of the looks in a trial) across age was not linear but appeared instead to follow a triphasic course (see also Ruff & Rothbart, 1996). Specifically, look duration showed an increase with age from birth to about 8 or 10 weeks before beginning to show the classic linear decline to about 6 months, followed by a plateau or even a slight increase thereafter. Notably, this meta-analysis included few studies in which look duration measures were tracked in older infants during the last quarter of their first year, a time-frame in which significant cognitive changes occur (e.g., Carver, Bauer, & Nelson, 2000; Ruff & Rothbart, 1996). An important implication of the results of this meta-analysis is that look duration may represent a number of attentional and cognitive processes that emerge during the first postnatal year. If this so, it may be especially important to use measures of heart rate change to validate infants' attentional state independently from measures of looking (see Colombo et al., 2004).
The third set of findings concerns the fact that most of the traditional habituation and selective looking research on age changes in infants' look duration has employed static, two-dimensional, usually achromatic stimulus material. However, there is some evidence from research with other paradigms (e.g., object examination, extended media viewing) that when attention is actively engaged with complex and/or interactive stimuli (e.g., toys or video clips), look duration increases across age. Interestingly, most of these studies have been done with older infants and toddlers. For example, in a series of studies Ruff and her colleagues (e.g., Ruff & Capozzoli, 2003; Ruff & Lawson, 1990; Ruff, Saltarelli, Capozzoli, & Dubiner, 1992) showed that when provided with a novel toy, infants showed a significant increase in the amount of focused attention they directed to the toy across the age range from 5 to 11 months, a trend that continued into the preschool years. Focused attention was defined as a period of concentrated looking, or intense examination of the toy as opposed to casual attention that was defined as object-directed looking but without active concentration on its features (see also Oakes and Tellinghausen, 1994). Finally, in a series of studies on infants' and toddlers' attention to video material over an extended viewing period, Richards and his colleagues (Richards & Cronise, 2000; Richards & Gibson, 1997; Richards & Turner, 2001) found evidence of “attentional inertia”, defined as a progressive increase in attentional engagement as the duration of a look toward a stimulus is sustained (see Anderson & Levin, 1976). Infants' and toddlers' from 3 months to 2 years viewed an audiovisual film clip of a Sesame Street movie and a series of computer-generated black and white patterns during two 20-minute sessions. The results showed that mean look duration increased significantly from 6 to 24 months, though only for the Sesame Street material (for a review see Richards & Anderson, 2004). The results of these studies with older infants and toddlers engaged with more complex stimulus material and toys are inconsistent with models of attention that predict a linear decrease in look duration with age but consistent with the triphasic model (Colombo, 2001) in which look duration shows a plateau or an increase after about 6 months of age.
Collectively, these three sets of research findings underscore two issues germane to the development of infants' visual attention and information processing. First, the developmental course of look duration across the first postnatal year is obscured by the confound that exists in the literature between the infant's age and the type of stimulus material presented. Studies that indicate a linear decline in look duration have been based primarily on the performance of younger (i.e., less that about 6 months) infants viewing static geometric patterns and face stimuli. Those that indicate an increase in look duration have been based on older (i.e., greater than about 6 months) infants and toddlers examining more complex (e.g., dynamic, chromatic) stimulus material. Second, the assumption that if an infant is looking at a stimulus then he or she is processing its information is oversimplified. Rather than being isomorphic, there is strong evidence that both “looking” and “attention” may independently reflect a number of underlying cognitive processes and mechanisms that also likely change with age. The goal of the present study was to address both of these issues concurrently in an experiment in which infants from ages 3 to 12 months viewed a diverse series of stimuli that included both static and dynamic versions of achromatic geometric patterns, faces, and film clips from children's video material. This age range encompassed both the early and later months of infancy in which look durations have been shown to decrease and increase, respectively. The stimulus material was selected to vary in complexity (e.g., movement, color, features) and also to represent the categories of stimuli commonly used in previous research. Finally, given the evidence that the occurrence of the heart-rate defined phase of sustained attention during looking is a sensitive and reliable index of ongoing information processing, measures of both looking and heart rate were taken as infants viewed the stimulus material.
There were three specific questions underlying this research. The first question was whether look duration would follow the triphasic course of development across the various stimulus types. We expected that the traditional pattern of linear decline that characterized the second phase of the model would be observed for all stimulus types but that look duration might be stimulus dependent – continuing to decrease for some stimuli (e.g., static pattern) and increasing for others (e.g., dynamic Sesame Street) across the third phase Note that we did not include infants between birth and 8 to 10 weeks of age whose look durations characterize the first phase of the triphasic model as identification of the heart rate defined phases of attention is not reliable until about 8 weeks of age (see Richards, 2004). The second question concerned the extent to which the heart rate measures of sustained attention would be consistent with the developmental course of the look duration measures. We expected that such convergent evidence from behavioral and physiological indices of attention could provide potentially powerful evidence on the cognitive processes underlying infants' visual (i.e., look duration) behavior. Finally, the third question concerned the relationship between looking and information processing. We expected that if infants' look durations differed as a function of stimulus type, that proportionately more of the looking period would be spent in the phase of sustained attention than in stimulus orienting or attention termination for those stimuli.
Method
Participants
A cross-sectional sample of 100 infants from five different ages groups (n = 20) were the participants. The ages of the infants in each group were 14 weeks (M = 97.80 days, SD = 8.49), 20 weeks (M = 144.60 days, SD = 4.58), 26 weeks (M = 185.80 days, SD = 5.85), 39 weeks (M = 275.95 days, SD = 5.74), or 52 weeks (M = 369.15, SD = 6.95). There were 50 males and 50 females such that gender distribution was approximately equal at each age. Infants were recruited from lists of births in the Columbia, SC metropolitan area that were purchased from INFO USA (Omaha, NE). Parents were contacted by mail and those who expressed interest in having their infants participate in research were contacted by phone and an appointment was arranged. All infants were full-term (at least 38 weeks gestation, birth weight at least 2500 grams) and healthy at birth with no known developmental anomalies. Participants were primarily White and of middle socioeconomic status. An additional 16 infants were tested but excluded from the final sample because of fussiness (n = 14) or procedural error (n = 2). All infants participated with the informed, signed consent of their parents.
Apparatus and Stimuli
Infants were seated on the parent's lap approximately 55cm from the center of a 49 cm color monitor. The monitor subtended a 44 degree visual angle and the plane of the monitor was parallel to the infant's eyes. To reduce distraction, the immediate testing area was surrounded by a frame covered with black fabric. A video camera was mounted centrally above the monitor so that an experimenter in an adjacent room could judge the infant's fixations toward or away from the monitor on-line and also for recording the session with a time code to synchronize heart rate and look duration information for later analysis. The video was digitized and saved in computer-based AVI files.
The infants were shown a series of 8 different stimuli. These stimuli were adapted (with permission) from those used in previous research (see Colombo et al, 1991; Cooper & Richards, 2003; Jankowski & Rose, 1997; Richards & Turner, 2001) as they varied widely in complexity and represented typical examples of the types of stimuli that have been used in studies of infant and toddler looking that have been reported in the literature. Although we have described these stimuli as varying in “complexity,” we have not quantified this variable precisely as has been done in the infant perception literature (e.g., Banks & Ginsberg, 1985). Rather we use the term qualitatively to describe stimuli that differ in general characteristics such as the number features, amount of color, or movement. There is a precedent for this less formal usage in the attention literature (e.g., Oakes & Tellinghuisen, 1994; Richards & Anderson, 2004; Richards & Gibson, 1997).
These stimuli were: (1) a still frame from a video of a woman's face, (2) the same face engaged in conversation (no sound), (3) a matrix of white dots on a black background arranged in a diamond-shaped configuration, (4) the same array of white-on-black dots were activated such that selected dots appeared to flicker on and off at random, (5) a symmetrical array of eight white triangles and four white bars on a black background, (6) the same triangles and bars were overlaid by a black-and-white checkerboard that moved clockwise to each of the four quadrants of the array, (7) a single still frame from a Sesame Street video, and (8) a dynamic version of the clip from the same Sesame Street video. The series of stimuli were presented twice. Within each of the two series the stimuli were presented in a different quasi-random order with the constraint that the still face was always presented first and the dynamic Sesame Street segment was always presented last. These constraints on the random order of stimulus presentation were needed in order to examine a number of additional variables that are not reported in the current paper. We are confident that this did not undermine the integrity of the results. First, as the sequence of stimuli were presented twice (each in a different quasi-random order) the face and the dynamic Sesame Street stimuli were always followed and preceded by a different stimulus, making a novelty or a sensitization effect unlikely. Second, analysis of order effects for the remaining six stimuli and a comparison of look durations on the first and second stimulus presentations did not indicate any significant effects (see Results section).
Procedure
When the infant was seated comfortably and the heart-rate leads had been attached a 2-minute period during which baseline heart rate was measured began. During this time the parent and the experimenter engaged in the minimum amount of interaction with the infant needed to avoid restlessness and fussing. After the 2-minute baseline ended, the experimenter dimmed the lights and the presentation of the stimuli began. The order of presentation of the stimuli was programmed into the computer and each one remained on the monitor until the baby had looked at it for an accumulated 20 seconds or until a 2-minute period without a look toward the stimulus had elapsed. Periods of looking to and away from the stimuli were determined by an experimenter in the adjacent room who observed and noted by key strokes whether the baby's fixations were directed on or off the stimulus. After the 8 stimuli had been shown once there was a second 2-minute baseline HR assessment. Finally, the 8 stimuli were shown again as before but in a different quasi-random order. Thus, each infant received a maximum of 16 trials (2 exposures to each stimulus type). All 100 of the infants completed at least one series of stimulus exposures (i.e., 8 trials) and 76 infants (16, 19, 15, 13, and 13 infants at 14-, 20-, 26-, 39-, and 52-weeks, respectively) completed all 16 trials. The remaining 24 infants completed at least one series (9 infants: 1 infant at each of 14 and 20 weeks, 2 infants at each of 26 and 39 weeks, and 3 at 52 weeks) or one complete series and a portion of the second series (15 infants: 2 infant at each of 14 and 20 weeks, 3 infants at 26 weeks, 4 at 39 and 52 weeks). These infants failed to complete the two series because they became inattentive or fussy during the procedure.
Measurement and Quantification of Look Duration
The primary measure of visual attention (i.e., looking) of interest was (1) the duration of the longest or peak look toward each stimulus in the allotted 20 seconds of accumulated looking. In addition, a number of other measures were calculated for each trial as follows: (2) the mean duration of each look, (3) the number of looks, and (4) the total time to accumulate the 20 seconds. Look-duration measures were judged off-line from the videotaped recording of the procedure. Each look was judged as either looking at the stimulus or looking away from the stimulus. A time code recorded on the videotape allowed the judgments to have millisecond accuracy, although resolution was limited to a single video frame scan (half the total frame length = ∼16 msec).
To establish the interobserver reliability of the look-duration judgments two observers independently judged these look durations for 25% of the infants in each age group, although the data for the analysis came from the ratings of only one of the observers. From these data two estimates of interobserver reliability were computed: (1) the average of the Pearson correlations between the total fixation times toward (r = .98) and away from (r = .95) the stimulus reported by each observer for each infant, and (2) the average proportion of agreement between the two observers' ratings for times looking-to and looking-away from the stimulus (Cohen's kappa, κ = .89) (see Hunter & Koopman, 1990). These interobserver reliability data indicate that the reliability of the observations was high.
Measurement and Quantification of Heart Rate Variables
The electrocardiogram (ECG) was recorded with three Ag-AgCl electrodes with disposable adhesive collars that were placed on the infant's chest. The ECG was digitized online at 1000 Hz with a microcomputer running custom software. A computer algorithm identified the QRS complex in the ECG and the inter-beat interval (IBI) was defined as the time between successive R-waves (or R-R interval) in the ECG. The IBI is the reciprocal of heart rate, such that the lengthening of the IBI corresponds to heart-rate deceleration and shortening of the IBI corresponds to heart rate acceleration. Although the units of heart rate (HR) change illustrated in Figure 1 are in beats per minute, the use of these units in the analysis of cardiac cycle length is uncommon in current research. Instead, change in cycle length is more typically calculated in terms of its interbeat interval (or IBI) in millisecond units. The IBI is determined by measuring the time between cardiac cycles, typically between the peaks of successive R-waves. A number of quantitative or statistical characteristics of IBI make it preferable to the beats-per-unit of time measure for the analysis of psychophysiological data. For example, IBI differences are approximately linear across the age ranges we studied whereas HR changes in beats-per-unit-of-time are not. As HR and IBI are inversely related, we use the term “heart rate” synonymously for HR and IBI changes in the cardiac cycle length whether they are decreased HR (longer IBIs) or increased HR (shorter IBIs). Importantly, the direction of the cardiac cycle length is the underlying biological process that is changing regardless of using IBI or HR terminology. For the analyses, the IBI was assigned to 0.5-second intervals by averaging the IBIs in each interval weighted by the proportion of the interval occupied by that beat. For off-line analyses, algorithms developed by Cheung (1981) and Berntson, Quigley, Jang, & Boysen (1990) were used to identify artifacts, along with visual inspection of atypical beats in the ECG.
The IBI changes were also used to operationally define and identify the attention phases of interest in this study (i.e., stimulus orienting, sustained attention, attention termination) off-line. Specifically, the phase of stimulus orienting began with the deceleration of the infant's heart rate from a 5-second prestimulus (or preattenton) baseline level that followed the first fixation of the stimulus and ended at the point where sustained attention began. The infant was judged to be in the phase of sustained attention when the deceleration reached the point where five successive beats each had longer IBIs than the median of the five prestimulus beats of the baseline period before stimulus onset. Finally, attention termination was defined as the return of heart rate to its prestimulus level defined as five consecutive beats with IBIs shorter than the median of the five pre-stimulus beats. This heart rate acceleration had to have followed a heart rate deceleration.
For each of the trials during which 20-seconds of looking was accumulated, several measures of the infant's heart rate change were recorded. First, we calculated the average amount of IBI change that occurred during (a) stimulus orienting (b) sustained attention, and (c) attention termination. These measures were expected to provide information about the amount and direction of cardiac change that occurred as each of the stimulus types was presented. Of particular interest was the phase of sustained attention during which infants' engage in voluntary5 cognitive processing of the stimulus. According to the Richards and Casey (1992) model, the amount of heart rate deceleration (i.e., longer IBIs, greater IBI change) observed during sustained attention would be expected to vary across the different stimulus types and ages as the infants' engagement with each of the stimuli increased or decreased. The phase of stimulus orienting is brief and although the amount of IBI change might vary with stimulus type, this response was of less interest as it is involuntary or reflexive and shows little developmental change in infancy. The phase of attention termination is defined in relation to sustained attention and involves acceleration in heart rate (shorter IBIs) in the preattention termination period as heart rate returns to baseline level. Attention termination indicates that the infant is no longer engaged with the stimulus and is no longer processing its information. Second, we calculated the proportion of time that infants spent in stimulus orienting, sustained attention, and attention termination during each of the 20-seconds periods of looking at the stimulus types. Given the evidence that infants' looking time does not reflect attention directly, the proportion of time spent in the three heart-rate defined phases (and sustained attention in particular) should provide the most sensitive estimate of infants' engagement with each stimulus type and by inference, their processing of its information. This metric has been used by Colombo and colleagues (e.g., Colombo et al., 2004) and was also expected to provide convergent information about infants' differential attention to the stimulus types. Specifically, if infants' look durations and pattern of heart rate change (longer IBIs) indicated that they were more engaged with certain of the stimuli than others, this should be reflected in correspondingly greater proportion of the 20-second period spent in sustained attention for those stimuli.
Results
Preliminary analyses were conducted to determine the effects of gender and stimulus order on the primary dependent measures of interest (peak look duration, mean IBI change during each of the attention phases, proportion of the 20 second viewing time spent in each phase). There were no significant main effects or interactions involving these variables and they are not included as factors in the analyses reported below.
Look Duration Measures
The mean duration of each look, the duration of the longest look (peak look), the median peak look, number of looks, and the time to accumulate the 20 seconds looking time for each stimulus type for each age group are presented in Table 1.
Table1.
Mean look length, peak look length, median peak look, number of looks, and time taken to accumulate 20 seconds of looking for each stimulus and age. Standard deviations are in parentheses.
| Stimulus Type | Age (wk) | Mean look length | Peak look length | Median peak look | Number of looks | Accumulation time for 20s |
|---|---|---|---|---|---|---|
| Static face | 14 | 10.21 (5.9) | 13.00 (5.5) | 11.56 | 2.80 (2.3) | 30.90 (9.6) |
| 20 | 8.65 (5.1) | 12.13 (3.8) | 11.75 | 3.25 (2.7) | 34.49 (11.1) | |
| 26 | 4.44 (1.8) | 7.60 (2.9) | 7.97 | 5.00 (2.3) | 41.42 (13.2) | |
| 39 | 5.90 (2.5) | 10.02 (2.8) | 9.63 | 3.45 (1.4) | 34.18 (5.8) | |
| 52 |
6.14 (4.1) |
9.65 (4.1) |
8.82 |
4.00 (1.9) |
35.57 (7.9) |
|
| Dynamic face | 14 | 13.74 (5.8) | 15.44 (4.6) | 16.30 | 1.95 (1.6) | 27.53 (5.0) |
| 20 | 10.42 (5.5) | 12.54 (4.7) | 11.84 | 2.85 (2.5) | 29.48 (12.2) | |
| 26 | 6.03 (4.7) | 7.88 (4.1) | 7.27 | 4.45 (1.9) | 38.38 (11.8) | |
| 39 | 7.19 (4.1) | 10.55 (4.5) | 9.27 | 3.15 (1.4) | 31.16 (5.8) | |
| 52 |
8.91 (4.7) |
11.42 (4.4) |
11.08 |
2.85 (1.5) |
29.50 (5.9) |
|
| Static dots | 14 | 9.81 (6.0) | 12.48 (5.3) | 11.57 | 2.50 (1.4) | 27.15 (6.4) |
| 20 | 6.09 (3.9) | 9.23 (3.6) | 8.68 | 4.15 (1.9) | 35.66 (14.8) | |
| 26 | 5.28 (2.9) | 8.37 (3.2) | 8.89 | 3.95 (2.1) | 36.01 (8.5) | |
| 39 | 5.18 (3.3) | 9.19 (3.3) | 8.36 | 4.00 (2.0) | 36.40 (15.7) | |
| 52 |
5.28 (3.4) |
8.32 (3.4) |
7.69 |
3.50 (1.6) |
31.11 (7.9) |
|
| Dynamic dots | 14 | 12.32 (5.9) | 15.06 (4.4) | 15.66 | 2.30 (2.2) | 28.09 (6.6) |
| 20 | 7.56 (4.6) | 10.25 (4.3) | 9.71 | 3.60 (1.9) | 30.91 (9.2) | |
| 26 | 6.65 (3.1) | 10.64 (4.7) | 10.09 | 2.95 (1.9) | 29.53 (8.9) | |
| 39 | 8.66 (5.5) | 11.92 (5.3) | 11.62 | 2.95 (1.9) | 29.55 (7.3) | |
| 52 |
6.73 (4.6) |
10.48 (4.4) |
10.99 |
3.15 (1.5) |
34.54 (11.2) |
|
| Static angles | 14 | 7.27 (3.8) | 10.05 (4.1) | 10.52 | 3.30 (1.7) | 28.09 (6.6) |
| 20 | 4.97 (2.6) | 8.53 (3.3) | 7.15 | 4.35 (1.8) | 30.91 (9.2) | |
| 26 | 3.91 (2.6) | 7.75 (3.5) | 7.23 | 5.95 (2.1) | 29.53 (8.9) | |
| 39 | 5.12 (3.4) | 8.69 (3.8) | 8.32 | 4.55 (2.5) | 29.55 (7.3) | |
| 52 |
5.43 (2.8) |
8.55 (3.6) |
8.55 |
3.45 (2.2) |
34.54 (11.2) |
|
| Dynamic angles | 14 | 11.23 (5.8) | 13.10 (4.9) | 13.81 | 2.55 (1.8) | 33.64 (8.9) |
| 20 | 8.11 (5.5) | 10.85 (5.0) | 9.41 | 4.50 (3.2) | 38.75 (20.6) | |
| 26 | 5.54 (2.9) | 8.91 (3.5) | 9.62 | 4.25 (2.2) | 46.44 (17.2) | |
| 39 | 6.07 (3.7) | 9.19 (3.6) | 7.95 | 3.80 (1.2) | 37.89 (12.0) | |
| 52 |
7.09 (4.3) |
10.62 (4.6) |
10.89 |
3.20 (1.8) |
32.92 (15.5) |
|
| Static Sesame St | 14 | 12.75 (6.2) | 15.29 (4.3) | 15.27 | 2.00 (1.5) | 27.01 (5.8) |
| 20 | 8.43 (4.9) | 10.82 (4.3) | 9.86 | 3.55 (2.1) | 32.77 (11.0) | |
| 26 | 6.44 (3.9) | 9.30 (4.2) | 7.74 | 3.85 (2.1) | 34.59 (9.5) | |
| 39 | 8.75 (5.5) | 11.49 (4.7) | 11.34 | 3.10 (2.1) | 30.11 (6.7) | |
| 52 |
10.20 (5.9) |
13.43 (5.3) |
11.90 |
2.05 (1.2) |
27.03 (3.1) |
|
| Dynamic Sesame St | 14 | 18.32 (2.9) | 18.99 (2.2) | 20.00 | 1.05 (.22) | 25.32 (11.3) |
| 20 | 15.01 (5.1) | 16.48 (4.1) | 17.43 | 1.80 (1.51) | 28.94 (7.9) | |
| 26 | 10.09 (5.9) | 12.76 (5.3) | 13.71 | 3.35 (2.7) | 34.30 (12.9) | |
| 39 | 13.47 (6.4) | 15.49 (4.8) | 15.49 | 2.30 (2.0) | 29.84 (7.5) | |
| 52 | 15.26 (5.5) | 16.83 (4.2) | 16.83 | 1.85 (1.5) | 29.73 (9.1) |
Following Colombo and Mitchell (1990) who argued that the duration of the longest look in a trial (i.e., the peak look) is the measure that drives most of the variance in the other measures of looking (e.g., mean look, total looks) and has the most robust developmental course, the peak look was the primary behavioral measure of visual attention in the analyses reported here. However, it is important to note that peak-look length and mean-look length were positively correlated for all stimuli at all ages and ranged from r = .88 to r = .96 (all p < .001). Consistent with this, peak look length was negatively correlated with the number of looks in a trial (correlations ranged from r = −.77 to r = −0.63, all p < .001) and with the amount of time taken to accumulate the 20 seconds of looking (correlations ranged from r = −.40 to r = −.72, all p < .001). In addition, the peak-look duration measures on the first and second presentations of a stimulus type were correlated (rs ranged from .31 to .48, all ps < .01), though look durations were uniformly shorter on the second exposure compared to the first exposure for all stimuli. For accuracy and stability, the mean of the peak-look durations on the two series were used in the data analyses reported below. Where an infant missed a trial due to fussiness on the second series of stimulus presentations, the look duration from the first series presentation for that stimulus was included in the analyses.
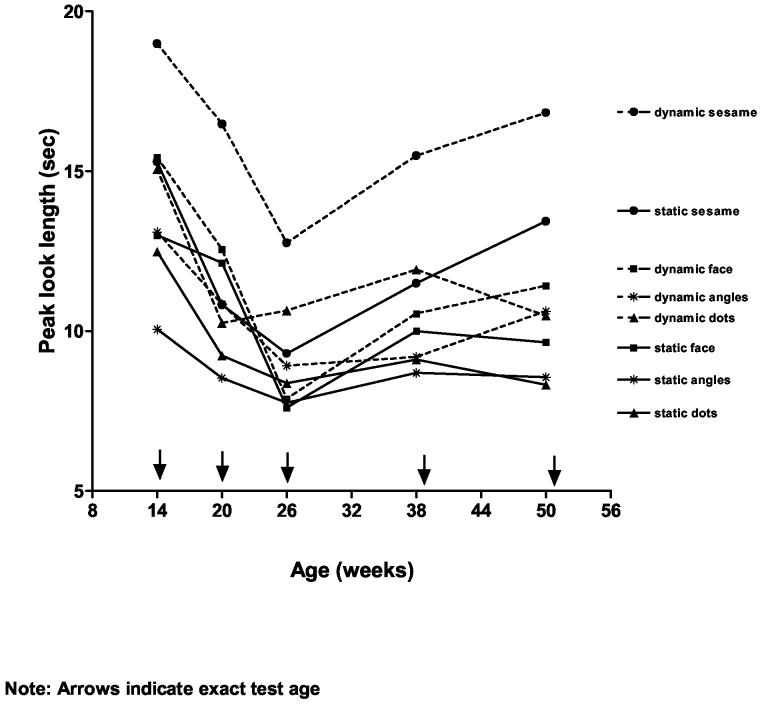
The peak-look duration data were analyzed in a 5(age: 14, 20, 26, 39, 52 wk) x 4 (stimulus type: faces, achromatic dots, achromatic angles, sesame street) x 2(movement: static, dynamic) mixed analysis of variance (ANOVA) where age was between subjects and stimulus type and movement were within-subject variables. Figure 2 illustrates infants' mean peak-look durations to the eight stimulus types as function of age.
Figure 2.
Mean peak look durations for static and dynamic versions on each stimulus type (faces, achromatic dots, achromatic angles, Sesame Street) as a function of age.
The results of the ANOVA yielded a significant main effect of movement: F(1,95) = 92.05, p < .001, η2 = .50. As can be seen in Figure 2, the dynamic version of all 4 stimulus types (dashed lines) elicited longer peak looks than did the static versions (solid lines) of the stimuli. This result was qualified by a significant Movement x Stimulus Type interaction: F(3, 285) = 8.81, p < .001, η2 = .09. Post-hoc paired t-tests (corrected for the number of comparisons: Bonferroni, p < .01) indicated that although the advantage for dynamic over static stimuli was significant for all pairs, it was most pronounced for the Sesame Street pair: t(99) = −2.54, −5.44, −4.09, and −8.61 for faces, dots, angles, and Sesame Street stimuli, respectively. The analysis also yielded significant main effects for age: F(4, 95) = 8.51, p < .001, η2 = .26 and for stimulus type: F(3, 285) = 50.99, p < .001, η2 = .35, as well as a significant Age x Stimulus Type interaction: F(12, 285) = 2.421, p = .005, η2 = .09. A series of follow-up oneway ANOVAs on the age main effect for each of the stimulus types were significant for all stimuli except the static angles: Fs(4, 95) = 5.88, 7.71, 3.96, 3.71, 2.88, 5.18, and 5.74, all ps < .01 for static and dynamic faces, static and dynamic dots, dynamic angles, and static and dynamic Sesame Street stimuli, respectively. As can be seen in Figure 2 and also as confirmed by post-hoc Bonferroni tests corrected for multiple comparisons (p < .003): (1) except for the static angles, there was a significant drop in peak-look duration for all stimulus types from 14- and 20- to 26 weeks of age, and (2) after 26 weeks, peak-look durations increased significantly to 52 weeks for certain of the stimuli (i.e., dynamic and static Sesame Street) but leveled off (i.e., static faces, dynamic faces, dynamic angles) or continued to decrease (static dots, dynamic dots) for others. Look duration to the static angles remained uniformly low across age.
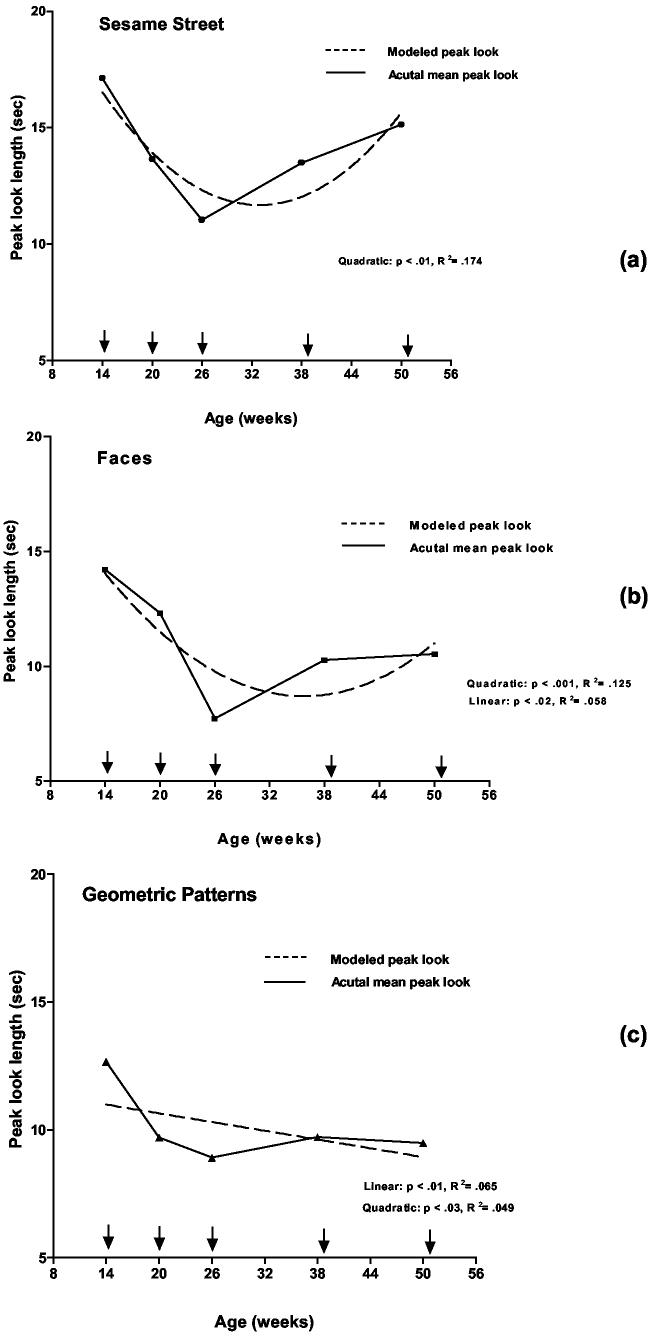
These results indicated that the developmental course of infants' look durations across the first postnatal year depended on the type of stimuli they examined. In order to make this relationship more explicit, a series of multiple regression analyses with curve estimation procedures were conducted on the look duration data. To simplify these supplementary analyses and to provide a clear picture of the developmental trajectory of look durations for each stimulus viv-a-vis the Colombo (e.g., Colombo, 2001; Colombo, et al., 1999) triphasic model, we did two things. First, we collapsed across the static and dynamic data for each stimulus type as look durations were significantly correlated (rs from .34 to .50, all ps < .001), although for each pair the dynamic stimulus elicited longer peak looks than did the static stimulus. Second, we grouped the various stimuli according to three categories that have commonly been employed in research on infants' and toddlers' visual attention – faces, achromatic geometric patterns (i.e., dots, angles), and complex chromatic material (i.e., Sesame Street).
The regression analyses for these data indicated that a quadratic function provided the best fit for the Sesame Street data (R2 = .17, p < .001; t = 4.53, p < .001) and for the faces data (R2 = .13, p< .001; t = 4.20, p < .001) although there was also a significant linear component for the latter (R2 = .06, p< .016; t = 2.47, p < .015). For the achromatic geometric data, a linear model provided the best fit (R2 = .07, p< .01; t = 2.66, p < .01) although there was also a significant quadratic component (R2 = .04, p< .03; t = 2.24, p< .02). These estimated curve fits are shown in Figure 3.
Figure 3.
Estimated curve fits from multiple regression analyses of the peak-look duration measures for the three grouped stimulus types: faces, achromatic geometric patterns, Sesame Street material.
Collectively, these analyses are consistent with the peak-look data in the overall analysis and confirm that there was a significant drop in the length of the peak-look to all stimulus types (except static dots) across the age range from 14 to 26 weeks. After that and toward 52 weeks of age, there was a marked increase in peak-look duration for the Sesame Street stimuli, a less prominent increase in the peak look to the faces and a leveling off or decline in peak-look duration to the achromatic geometric stimuli.
Heart Rate Measures: Interbeat Interval (IBI) Change During the Phases of Attention
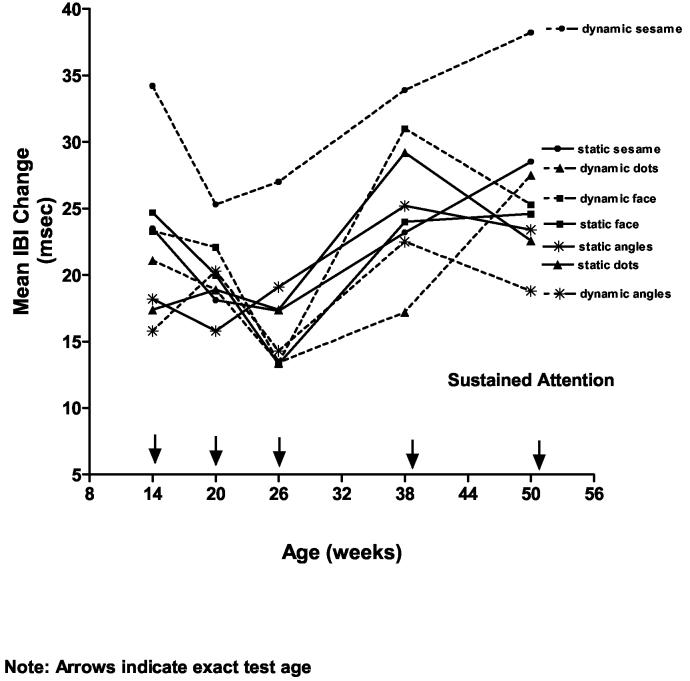
A series of ANOVAs were conducted on the average amount of change in the cardiac cycle (i.e., the length of the interbeat interval, or IBI) that occurred during stimulus orienting, sustained attention and attention termination as the infants in each age group examined each of the stimuli during the 20-second looking periods. Increasing values for IBI change indicated a heart rate deceleration and decreasing values for IBI change indicated heart rate acceleration. First, a 5(age: 14, 20, 26, 39, 52 wk) x 4 (stimulus type: faces, achromatic dots, achromatic angles, Sesame Street) x 2(movement: static, dynamic) mixed ANOVA on the average amount of IBI change that occurred during sustained attention was conducted. Figure 4 illustrates the IBI change that occurred to each stimulus types as function of age.
Figure 4.
The mean amount of heart rate (interbeat interval) change during the phase of sustained attention for each of the stimulus types as a function of age.
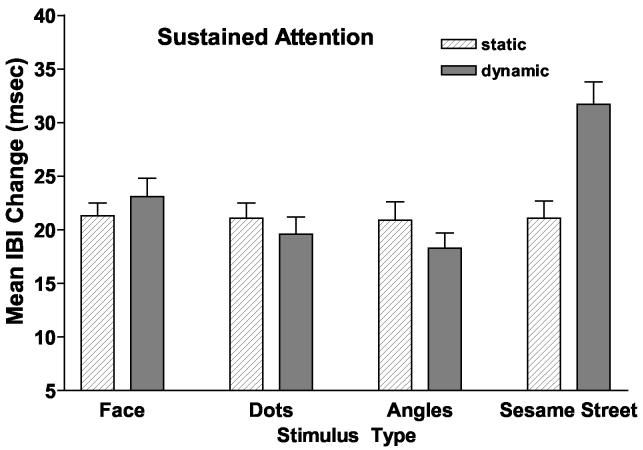
The ANOVA yielded a significant main effect for age: F(1, 95) = 4.22, p < .003, η2 = .15. As seen in Figure 4 and confirmed by post hoc pairwise Bonferroni tests (p < .005), for all stimulus types there was a slight (though not significant) decrease in the amount is IBI change from 14- to 20- to 26 weeks (M = 20.32 msec, SE = 1.86; M = 19.92 msec, SE = 1.86; and M = 16.9 msec, SE = 1.86, respectively) and a significant increase in IBI after that to 39 weeks (M = 25.8 msec, SE = 1.86) and 52 weeks (M = 25.9 msec, SE = 1.86). The analysis also yielded a significant main effect for stimulus type, F(3, 285) = 9.04, p < .001, η2 = .09 that was qualified by a significant Stimulus Type X Movement interaction: F(3, 285) = 9.28, p < .001, η2 = .09. As can be seen in Figure 5, the interaction was due primarily to the increase in IBI that occurred as the infants looked at the dynamic Sesame Street stimulus.
Figure 5.
Mean amount of heart rate (interbeat interval) change that infants showed to static and dynamic versions of the stimulus types.
A follow up ANOVAs on the amount of IBI change within each movement type confirmed this. For the static condition there was no effect of stimulus type. For the dynamic condition there was a significant effect of stimulus type: F(3, 97) = 13.93, p < .001. Post hoc Bonferroni tests (p < .005) indicated that the amount of IBI change did not differ for the face, dots, or angles but was significantly greater that these three for the Sesame Street stimulus. A parallel series of mixed ANOVAs on the average amount of IBI change during the stimulus orienting and attention termination phases of attention failed to indicate significant main effects or interactions involving age, stimulus group or movement.
Heart Rate Measures: Proportion of time spent in the Phases of Attention
An alternative way to evaluate infants' attention to the different stimulus types is to examine the proportion of the 20 seconds of looking time that they spend in each of the heart-rate defined phases of attention while looking at the different stimulus types (see Colombo et el., 2001). As noted, these analyses provide convergent evidence on the relative importance of the stimulus types in eliciting sustained attention. As this analysis was supplementary, we elected to simplify the data as was done for the curve estimation analyses reported above, by (1) collapsing across the stimulus movement variable, and (b) grouping the stimuli in to faces, achromatic geometric patterns and Sesame Street categories. To this end an Age (5: 14-, 20-, 26-, 39-, 52-weeks) x Stimulus Group (3: faces, achromatic geometric pattern, Sesame Street) x Phase (3: stimulus orienting, sustained attention, attention termination) mixed ANOVA was carried out on the proportion of the 20-second periods of accumulated looking that the infants spent in each of the heart-rate-defined phases of attention.
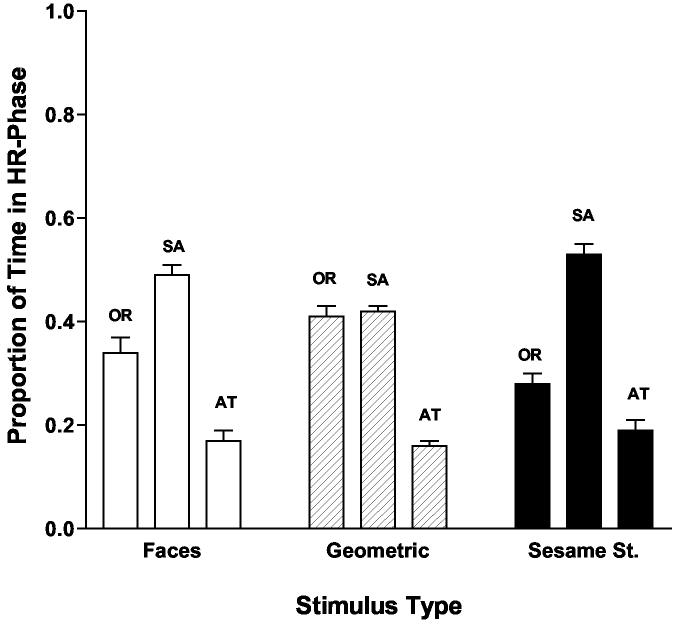
The analysis indicated a significant main effect for phase: F(2, 190) = 99.04, p < .001, η2 = .51 reflecting the fact that as expected, all infants spent significantly more time in the phase of sustained attention (M = .48, SE = .02) than in stimulus orienting (M = .35, SE = .02) or attention termination (M = .17, SE = .01), post hoc tests (Bonferroni, p < .016). This effect was qualified by a significant Phase x Stimulus Type interaction: F(3, 380) = 7.20, p < .001, η2 = .07. Follow-up ANOVAs on each of the phases with post hoc Bonferoni tests (p < .016) indicated that significantly more of the 20-second looking period was spent in sustained attention when the Sesame Street and face stimuli were viewed compared to the geometric patterns. Conversely, significantly less time was spent in stimulus orienting when faces and Sesame Street were viewed compared to the geometric patterns. There were no differences among the three stimulus groups for the phase of attention termination. The mean proportions of stimulus orienting, sustained attention, and attention termination for each stimulus type are shown in Figure 6.
Figure 6.
Proportion of time that infants spent in each of the phases of attention during presentation of the three stimulus groups (faces, achromatic geometric patterns, Sesame Street). Note: OR = stimulus orienting, SA = sustained attention, AT = attention termination
The phase main effect in the overall analysis was also qualified by a significant Phase x Age interaction: F(7, 160) = 2.59, p = .02, η2 = .10. Follow up ANOVAs with post hoc Bonferroni tests (p < .005) for each phase indicated that the interaction was largely due to the performance of the 26-week-olds who spent a smaller proportion of time in sustained attention (M = .41, SE = .02) than did the 52-week-olds (M= .52, SE = .02) and a larger proportion of time in stimulus orienting (M = .44, SE = .03) than did the 14-week-olds (M = .29, SE = .02). There were no differences in attention termination as a function of age.
In sum, the general pattern of results from the proportion of time analyses and the amount of heart rate (IBI) change analyses is consistent in one important way. That is, Sesame Street and to a lesser extent the face stimuli elicited a larger proportion of sustained attention and more IBI change during sustained attention than did the achromatic geometric patterns.
Discussion
We began this research with the intention of answering some unresolved questions about the developmental course of infants' look duration over the first year of life with particular reference to the predictions of the triphasic model proposed by Colombo and colleagues (Colombo, 2001; Colombo et al., 1999). Specifically, we sought to clarify a potential interaction between age and stimulus type in this development that had been implied but not systematically explored in the extant literature. There were several findings of note. First, for all of the stimulus types that were shown and at all ages, the dynamic versions (especially the Sesame Street clip) elicited more looking than did the static versions of the same stimuli. This finding was not unexpected as movement is a salient cue that provides an additional stimulus attribute to engage young infants beyond pattern configuration, one that they can readily exploit from an early age to extract perceptual information about the world (e.g., Arterberry & Bornstein, 2002; Haith, 1966; Kellman & Spelke, 1984; Shaddy & Colombo, 2004).
Second, and more importantly, the results indicated clearly that the developmental trajectory of look duration across the first postnatal year depended not only on age but on the type of stimulus that the infant viewed. Look duration decreased significantly from 14 to 26 weeks for all types of stimuli with the exception of the static angles. Look duration to that stimulus was uniformly low in this time frame and remained so across age. After 26 weeks, the course of look durations diverged according to stimulus type. When infants viewed the achromatic geometric patterns, their look durations continued a linear decline (or reached a plateau) across age. However, when these older infants viewed the Sesame Street material and the faces their look durations increased markedly such that their performance from 14 weeks to the end of the first year was best described by a quadratic function, a trend that was more pronounced for the Sesame Street than for the face stimuli.
These findings both complement and extend the sizeable literature in which infants' look durations have been reported to decrease linearly with age. In much of that research look duration was a dependent measure in the service of examining other cognitive processes, the test materials used were generally achromatic patterns or faces, and infant participants were in the age range between 3- and 7-months (e.g., see Bornstein, 1998; Colombo, 1993; Fagan, 1990; Rose et al., 2004), although Colombo et al. (2004) included infants as old as 9 months of age. Our data also showed this pattern under these same testing parameters. The extended look durations that we found for Sesame Street and face stimuli in our older infants are consistent with findings from research in which infants' attention to complex toys (e.g., Oakes & Tellinghuisen, 1994; Ruff & Saltarelli, 1993) and television material (see Richards & Anderson, 2004) were examined in the second year of life.
Collectively, these data fit well with Colombo's proposal that infant look duration might follow a triphasic course of development over the first year of life and that different patterns of looking might reflect the maturational state of the different neural mechanisms and processes underlying infants' attentional systems. In the first phase of this model, the early increase in look duration between birth and 2-months is believed to indicate the emergence of the alertness or arousal aspect of attention that is elicited most readily by exogenous events and is likely mediated by increased subcortical influence on higher-level structures. As we did not test infants in this age range, our data do not address this phase of attention. In the second phase, there is a decline in look duration from 3 to about 6 months that primarily reflects improvements in the ability to disengage from a stimulus. Disengagement and shifting of attention have been linked to developments in the posterior orienting network (see Posner & Raichle, 1994; Ruff & Rothbart, 1996). In addition, changes in visual function (e.g., visual acuity, binocularity), object recognition, and speed of information processing emerge in this time frame as maturation of the visual and inferior temporal cortices occur (see Ruff & Rothart, 1996). In the third phase, the plateau or increase in look duration reflects the emergence of an endogenous, apparently voluntary directing of attention as a function of the task at hand, in particular the inhibition of the tendency to shift attention away from a task that is interesting or demanding. This endogenous aspect of attention and the control of looking are mediated by developments in the frontal cortical areas (e.g., anterior cingulate, frontal eye fields, dorsolateral prefrontal cortex) that occur late in the first postnatal year. The divergent patterns of look duration as a function of stimulus that were apparent during the second half of the first year are a consistent with emergence of this selective, endogenous aspect of attention. Although describing the attentional processes of a nonverbal infant as “voluntary” or “selective” is problematic (e.g., for discussions see Colombo, 2001; Khaneman, 1973; Posner 1995; Ruff & Rothbart, 1996), if an infant's visual regard is directed, maintained, terminated or shifted as a function of the requirements of the task at hand or in this case stimulus to be processed, many would concur that attention is likely volitional.
A second issue that motivated this research was the evidence that measures of look duration do not reflect information processing directly. As Richards and colleagues have demonstrated (see Reynolds & Richards, in press), it is only in the portion of looking that occurs during the heart-rate defined phase of sustained attention that the infant is engaged in actively processing the information in the stimulus. Consequently, we sought to establish the extent of correspondence between behavioral measures of look duration and physiological measures of heart-rate change as infants viewed the varied stimulus types. Two measures were analyzed for this purpose (a) the average amount of interbeat interval (IBI) change during the 20 seconds of looking in each phase of attention and (b) the proportion of time infants spent in each phase when looking at the different stimulus types.
Concerning (a), analysis of the average amount of interbeat interval change indicated that regardless of stimulus type the older infants (39- and 52-week-olds) showed more heart-rate deceleration in sustained attention than did the younger infants (14-, 20-, and 26-week-olds). This is consistent with the developmental trend towards greater sustained attention with increasing age that has been previously reported (see Richards, 2004). The analysis also indicated that regardless of age, the dynamic Sesame Street stimulus elicited more interbeat interval change during sustained attention than did any of the other stimuli. This finding is consistent with the analysis of the peak-look duration data that also highlighted the prominence of the dynamic Sesame Street stimulus in engaging the infant's visual attention and by inference, their information processing. Although the Age x Stimulus Type interaction only approached significance (p = .10), comparison of Figures 2 and 4 suggested a parallel course of development for the behavioral (i.e., look duration) and physiological (amount of IBI change) measures of sustained attention that might be profitably explored and clarified in future research. Importantly, these findings provide convergent evidence that after about 6 months of age, infants' attention is not a monolithic phenomenon but appears to be dependent on the characteristics of the material being processed. The amount of IBI change during the phases of stimulus orienting and attention termination did not differ with age or stimulus type which provides further evidence of the central role that sustained attention plays in infants' information processing.
Concerning (b), it was not unexpected that for all stimulus types infants spent the largest proportion the 20 seconds of looking in the phase of sustained attention. When infants are presented with any stimulus that engages their attention they orient towards it and begin to encode its information (e.g., Sokolov, 1963). Further, there is ample evidence that as the infant becomes more engaged with the stimulus, looking is coincident with the heart rate defined phase of sustained attention. The subsequent return of heart rate to baseline levels indicates that processing and attention have terminated, though the infant may still be looking at the stimulus (see Reynolds and Richards, in press). Of greater significance in these analyses was that infants spent a relatively larger proportion of time in sustained attention when the stimuli presented were the Sesame Street material or faces compared to the achromatic geometric stimuli. Moreover, this pattern mirrored that of the peak look duration analyses in which infants' visual behavior also indicated a preference for Sesame Street and faces. In contrast, more time was spent in the phase of stimulus orienting when the geometric patterns were shown, indicating perhaps that infants continued to engage with these stimuli during the 20-second period, but did not need to spend as much time in processing them. Importantly, what these results confirm at a more general level is that looking and attentiveness (and information processing) are not isomorphic. Across the stimulus types only about half of the time (48%) was spent in sustained attention. The remainder of the time was spent in stimulus orienting (35%) and attention termination (17%). That these proportions also varied as a function of stimulus type indicates that the interpretation of any single period of looking should include a measure of sustained attention if developments in information processing are of experimental interest.
The proportions of time that infants spent in the phases of attention did not vary markedly with age in this study except that 26-week-old infants spent significantly less time in sustained attention than did the 52-week-olds and they spent more time in stimulus orienting than did the 14-week-olds. Although the implications of this pattern are not entirely clear, at a more general level these proportion of time analyses considered with the amount of IBI change analyses may reflect an increase in sustained attention from younger (i.e., less than 6 months) to older (i.e., greater than 6 months) infants, an increase that may occur as less time is spent in orienting to the stimulus. This interpretation of the change in the phases of attention is different from that proposed by Colombo et al. (2004) who also reported that a similar increase in sustained attention from younger to older infants that was at the expense of time spent in attention termination. This inconsistency will only be resolved in future research and may be due in part to the exclusive use of static face stimuli in the Colombo study. Importantly, the findings of the two sets of heart rate analyses reported here were in agreement in showing that regardless of age, during the phase of SA infants were more engaged with (i.e., had larger HR decelerations, spent proportionally more time in) the Sesame Street material (and to a lesser extent the faces) compared to the geometric patterns.
Collectively, the results of the peak-look duration and heart-rate (IBI change and proportion of time) analyses provide considerable clarification of the developmental course of infant look duration during the first year of life. They also provide some new directions for future research. Although the results were generally consistent with the predicted linear decrease pattern of look duration during the second period of the triphasic model, they further demonstrated that the course of look duration in the third phase was stimulus dependent. Specifically, across the second half of the first year of life, more complex and dynamic stimuli (e.g., Sesame Street) elicited more looking and greater amounts of heart rate change than did simpler stimuli (e.g., achromatic patterns), likely due to the functional emergence of the frontally controlled structures that guide the voluntary aspects of attention. At a more general level the results of these analyses underscore the importance of providing convergent evidence from independent behavioral measures (looking) and physiological measures (cardiac change) of attention when developmental changes in infants' information processing are of interest.
Contributor Information
Mary L. Courage, Memorial University, Canada
Greg D. Reynolds, Appalachian State University
John E. Richards, University of South Carolina
References
- Anderson DR, Levin SR. Young children's attention to Sesame Street. Child Development. 1979;47:806–811. [PubMed] [Google Scholar]
- Arterberry ME, Bornstein MH. Infant perceptual and conceptual categorization: the roles of static and dynamic stimulus attributes. Cognition. 2002;86:1–24. doi: 10.1016/s0010-0277(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Banks MS, Ginsberg AP. Infant visual preferences: A review and new theoretical treatment. In: Reese HW, editor. Advances in Child Development and Behavior. Vol. 19. Academic Press; Orlando, FL: 1985. [DOI] [PubMed] [Google Scholar]
- Berg WK, Richards JE. Attention across time in infant development. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 347–368. [Google Scholar]
- Berntson GG, Quigley KS, Jang JF, Boysen ST. An approach to artifact identification. Psychophysiology. 1990;27:586–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Stability of mental development from early life: Methods, measures, models, meanings, and myths. In: Simion F, Butterworth G, editors. The development of sensory, motor, and cognitive capacities in early infancy: From perception to cognition. Psychology Press; Hove, UK: 1998. pp. 301–332. [Google Scholar]
- Bornstein MH, Pecheaux MG, Lecuyer R. Visual habituation in human infants: Developmental and rearing condiitons. Psychological Research. 1988;50:130–133. doi: 10.1007/BF00309213. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Slater A, Brown E, Roberts E, Barrett J. Stability of mental development from infancy to later childhood: Three waves of research. In: Bremner G, Slater A, Butterworth G, editors. Infant development: Recent advances. Psychology Press; East Sussex, UK: 1997. pp. 191–215. [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Cheung MN. Detection and recovery from errors in cardiac inter-beat intervals. Psychophysiology. 1981;18:341–346. doi: 10.1111/j.1469-8986.1981.tb03045.x. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant cognition: Predicting later intellectual functioning. Sage; Newberry Park: 1993. [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Colombo J, Freeseman LJ, Coldren JT, Frick JE. Individual differences in infant visual fixation: Dominance of global and local stimulus properties. Cognitive Development. 1995;10:271–28. [Google Scholar]
- Colombo J, Harlan JE, Mitchell DW. Look duration in infancy: Evidence for a triphasic course?; Poster presented at the meeting of the Society for research in Child Development; Albuquerque, NM. 1999. [Google Scholar]
- Colombo J, Mitchell DW. Individual and developmental differences in infant visual attention: Fixation time and information processing. In: Colombo J, Fagen JW, editors. Individual differences in infancy: Reliability, stability, and prediction. Erlbaum; Hillsdale, NJ: 1990. pp. 193–227. [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant attention: Are short lookers faster processors or feature processors? Child Development. 1991;62:1247–1257. [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Dodd JD, Coldren JT, Horowitz FD. Longitudinal correlates of infant attention in the paired-comparison paradigm. Intelligence. 1989;13:33–42. [Google Scholar]
- Colombo J, Richman WA, Shaddy DJ, Greenhoot AF, Maikranz JM. HR-defined phases of attention, look duration, and infant performance in the paired comparison paradigm. Child Development. 2001;72:1605–1616. doi: 10.1111/1467-8624.00368. [DOI] [PubMed] [Google Scholar]
- Colombo J, Shaddy DJ, Richman WA, Maikrantz JM, Blaga O. The developmental course of habituation in infancy and preschool outcome. Infancy. 2004;5:1–38. [Google Scholar]
- Cooper RP, Richards JE. Differential heart rate activity in infants to uni- and multimodal events; Poster presented at the meeting of the Society for research in Child Development; Tampa, FL. 2003. [Google Scholar]
- Courage ML, Howe ML. Long-term retention in 3.5 month-olds: Familiarization time and individual differences. Journal of Experimental Child Psychology. 2001;79:271–293. doi: 10.1006/jecp.2000.2606. [DOI] [PubMed] [Google Scholar]
- Fagan JF. The paired-comparison paradigm and infant intelligence. In: Diamond A, editor. Annals of the New York Academy of Sciences: Vol. 68. The development and neural bases of higher cognitive functions. New York Academy of Sciences; New York: 1990. pp. 337–364. [DOI] [PubMed] [Google Scholar]
- Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Development. 1999;70:537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Frick JE, Richards JE. Individual differences in infants' recognition of briefly presented visual stimuli. Infancy. 2001;2:331–352. doi: 10.1207/S15327078IN0203_3. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing amont orienting, defense, and startle reflexes. In: Kimmel HD, van Olst HH, Orlebeke JF, editors. The orienting reflex in humans. Erlbaum; Hillsdale, NJ: 1979. pp. 137–167. [Google Scholar]
- Haith MM. The response of the human newborn to visual movement. Journal of Experimental Child Psychology. 1966;3:235–243. doi: 10.1016/0022-0965(66)90067-1. [DOI] [PubMed] [Google Scholar]
- Haith MM, Benson JB. Infant cognition. In: Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2. Cognition, perception, and language. 5th Wiley; New York: 1998. pp. 199–254. [Google Scholar]
- Hayne H. Infant memory development: Implications for childhood amnesia. Developmental Review. 2004;24:33–73. [Google Scholar]
- Hicks JM, Richards JE. The effects of stimulus movement and attention on peripheral stimulus localization by 8- to 26-week-old infants. Infant Behavior and Development. 1998;21:571–589. [Google Scholar]
- Hunter MA, Koopman A. Interobserver agreement and reliability of infant visual fixation data. Infant Behavior and Development. 1990;13:109–116. [Google Scholar]
- Hunter SK, Richards JE. Peripheral stimulus localization by 5- to 14-week-old infants during phases of attention. Infancy. 2003;4:1–25. doi: 10.1207/S15327078IN0401_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski JJ, Rose SA. The distribution of visual attention in infants. Journal of Experimental Child Psychology. 1997;65:127–140. doi: 10.1006/jecp.1996.2363. [DOI] [PubMed] [Google Scholar]
- Jankowski JJ, Rose SA, Feldman JF. Modifying the distribution of attention in infants. Child Development. 2001;72:339–351. doi: 10.1111/1467-8624.00282. [DOI] [PubMed] [Google Scholar]
- Kellman PJ, Banks MS. Infant visual perception. In: Kuhn D, R. S. Siegler, editors. Handbook of child psychology: Vol. 2. Cognition, perception, and language. 5th Wiley; New York: 1998. pp. 103–146. [Google Scholar]
- Kellman PJ, Spelke E. Perception of partly occluded objects in infancy. Cognitive Psychology. 1983;15:483–524. doi: 10.1016/0010-0285(83)90017-8. [DOI] [PubMed] [Google Scholar]
- Khaneman D. Prentice-Hall; Englewood Cliffs, NJ: 1973. Attention and effort. [Google Scholar]
- Lansink JM, Mintz, Richards JE. The distribution of infant attention during object examination. Developmental Science. 2002;3:163–170. [Google Scholar]
- Lansink JM, Richards JE. Heart rate and behavioral measures of attention in 6-, 9-, & 12-month-old infants during object exploration. Child Development. 1997;68:610–620. [PubMed] [Google Scholar]
- Lewis M, Kagan J, Campbell H, Kalafat J. Cardiac response as a correlate of attention in infants. Child Development. 1966;37:63–71. [Google Scholar]
- Mayes L, Kessen W. Maturational changes in the measures of habituation. Infant Behavior and Development. 1989;12:437–456. [Google Scholar]
- Maikranz JM, Colombo J, Richman WA, Frick JE. Autonomic correlates of individual differences in sensitization and look duration during infancy. Infant Behavior and Development. 2000;23:137–151. [Google Scholar]
- McCall RB. What process mediates prediction of childhood IQ from infant habituation and recognition memory? Speculations on the roles of inhibition and rate of information processing. Intelligence. 1994;10:251–263. [Google Scholar]
- Oakes LM, Tellinghuisen DJ. Examining in infancy: Does it reflect active processing? Developmental Psychology. 1994;30:748–756. [Google Scholar]
- Posner MI. Attention in cognitive neuroscience: An overview. In: Gazzaniga M, editor. The cognitive neurosciences. MIT Press; Cambridge, MA: 1995. pp. 615–624. [Google Scholar]
- Posner MI, Raichle ME. Images of mind. W. H. Freeman; New York: 1994. [Google Scholar]
- Reynolds GD, Richards JE. Infant heart rate: A developmental psychophysiological perspective. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology. Cambridge Press; in press. [Google Scholar]
- Richards JE. The development of sustained attention in infants from 14 to 26 weeks of age. Psychophysiology. 1985;22:409–416. doi: 10.1111/j.1469-8986.1985.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Development and stability in heart-rate-defined visual sustained attention in 14-, 20-, and 26-week-old infants. Psychophysiology. 1989;26:422–430. doi: 10.1111/j.1469-8986.1989.tb01944.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Effects of attention on infants' preferences for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997a;33:22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards JE. Peripheral stimulus localization by infants: Attention, age, and individual differences in heart rate variability. Journal of Experimental Psychology: Human Perception and Performance. 1997b;23:667–680. doi: 10.1037//0096-1523.23.3.667. [DOI] [PubMed] [Google Scholar]
- Richards JE. Attention in young infants: A developmental psychophysiological perspective. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neurosciences. MIT Press; Cambridge, MA: 2001. pp. 321–338. [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. The development of sustained attention in infants. In: Posner M, editor. Cognitive Neuroscience of Attention. Guilford Press; New York: 2004. pp. 342–356. [Google Scholar]
- Richards JE, Anderson DR. Attentional inertia in children's extended viewing of television. Advances in Child Development and Behavior. 2004;32:163–212. doi: 10.1016/s0065-2407(04)80007-7. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Development of sustained visual attention in the human infant. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults. Erlbaum; Hillsdale, NJ: 1992. pp. 30–60. [Google Scholar]
- Richards JE, Cronise K. Extended visual fixation in the early preschool years: Look duration, heart rate changes, and attentional inertia. Child Development. 2000;71:602–620. doi: 10.1111/1467-8624.00170. [DOI] [PubMed] [Google Scholar]
- Richards JE, Gibson TL. Extended visual fixation in young infants: Look distributions, heart rate changes and attentional inertia. Child Development. 1997;68:1041–1056. [PubMed] [Google Scholar]
- Richards JE, Hunter SK. Peripheral stimulus licalization by infants with eye and head movements during visual attention. Vision Research. 1997;37:3021–3935. doi: 10.1016/s0042-6989(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Richards JE, Lansink JM. Distractibility during visual fixation in young infants: The selevtivity of attention. In: Rovee-Collier C, editor. Advances in infancy research. Vol. 13. Ablex Publishing Co; Norwood, NJ: 1998. pp. 407–444. [Google Scholar]
- Richards JE, Turner ED. Extended visual fixation and distractibility in children from six to twenty-four months of age. Child Development. 2001;72:963–972. doi: 10.1111/1467-8624.00328. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Predicting IQ and specific cognitive abilities at 11 years from infancy measures. Developmental Psychology. 1995;31:685–696. [Google Scholar]
- Rose SA, Feldman JF. Memory and speed: Their role in the relation of infant information processing to later IQ. Child Development. 1997;68:630–641. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1st year of life: A longitudinal study of preterm and full-term infants. Developmental Psychology. 2002;38:895–902. doi: 10.1037//0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24:74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Wallace IF. Infant information processing in relation to six-year cognitive outcome. Child Development. 1992;63:1126–1141. [PubMed] [Google Scholar]
- Ruff HA, Capozzoli MC. Development of attention and distractibility in the first 4 years of life. Developmental Psychology. 2003;39:877–890. doi: 10.1037/0012-1649.39.5.877. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Lawson KR. Development of sustained, focused attention in young children during free play. Developmental Psychology. 1990;26:85–93. [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development. Oxford; New York: 1996. [Google Scholar]
- Ruff HA, Saltarelli LM. Exploratory play with objects: Basic cognitive processes and individual differences. In: Bornstein M, O'Reilly A, editors. New directions for child development: Vol. 59. The role of play in the development of thought. Josey-Bass; San Francisco, CA: 1993. pp. 5–15. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Saltarelli LM, Capozzoli M, Dubiner K. The differentiation of activity in infants' exploration of objects. Developmental Psychology. 1992;28:851–861. [Google Scholar]
- Shaddy DJ, Colombo J. Developmental changes in infant attention to dynamic and static stimuli. Infancy. 2004;3:355–365. [Google Scholar]
- Sigman M, Cohen SE, Beckwith L. Why does infant attention predict adolescent intelligence? Infant Behavior and Development. 1997;20:133–140. [Google Scholar]
- Sokolov E. Perception and the conditioned reflex. The Pergamon Press; New York, NY: 1963. [Google Scholar]