Abstract
Rationale
The primary psychoactive constituent of marijuana, Δ9-THC, activates cannabinoid receptors, which are especially abundant in the frontal cortex and hippocampus. Acute marijuana smoking can disrupt working memory (WM) and episodic memory (EM) functions that are known to rely on these regions. However, the effects of marijuana on the brain activity accompanying such cognitive processes remain largely unexplored.
Objectives
To examine such effects on performance and neurophysiological signals of these functions, EEG recordings were obtained from ten subjects (5M, 5F) performing cognitive tasks before and after smoking marijuana (3.45% Δ9-THC) or a placebo. WM was assessed with a spatial N-back task, and EM was evaluated with a test requiring recognition of words after a 5–10 min delay between study and test.
Results
Marijuana increased heart rate and decreased global theta band EEG power, consistent with increased autonomic arousal. Responses in the WM task were slower and less accurate after smoking marijuana, accompanied by reduced alpha band EEG reactivity in response to increased task difficulty. In the EM task, marijuana was associated with an increased tendency to erroneously identify distracter words as having been previously studied. In both tasks, marijuana attenuated stimulus-locked event-related potentials (ERPs).
Conclusions
The results suggest that marijuana disrupted both sustained and transient attention processes resulting in impaired memory task performance. In subjects most affected by marijuana a pronounced ERP difference between previously studied words and new distracter words was also reduced, suggesting disruption of neural mechanisms underlying memory for recent study episodes.
Keywords: Marijuana, Delta-9-tetrahydrocannabinol, Working memory, Episodic memory, Event-related potentials
Introduction
The primary psychoactive constituent of marijuana, delta-9-tetrahydrocannabinol (Δ9-THC), binds to G-protein-coupled CB1 receptors that are found throughout the brain and are densely concentrated in the frontal lobes and medial temporal lobes (MTL) of the cerebral cortex (Gaoni and Mechoulam 1964; Devane et al. 1988; Herkenham et al. 1990). In humans, these areas are critical to sustained attention, working memory (WM) and episodic memory (EM) functions. Working memory refers to the ability to control attention in an effort to retain and manage active internal representations in the face of distracting influences (Baddeley and Hitch 1974; Goldman-Rakic 1987). This ability depends on the sustained activation of neurons in the frontal lobe, and recurrent interactions between frontal and posterior brain regions. Episodic memory refers to the ability to consciously remember a past study episode even after a distraction-filled delay, without continuous active maintenance of information between study and test (Tulving 1983, 2002). The plasticity required for maintenance of information in EM over a distraction-filled delay is critically dependent on the integrity of the hippocampal formation (Squire and Knowlton 1995, 2000), whereas episodic encoding and retrieval operations involve interactions between the MTL and the cortical regions that enable WM (Smith 1993; Brewer et al. 1998; Lee et al. 2000). Because WM and EM rely on brain regions dense in CB1 receptors, it is unsurprising that they tend to be disrupted in acute marijuana intoxication (Hampson and Deadwyler 1999).
Since the 1970s, numerous investigators have examined the acute effects of marijuana smoking in humans (see Earleywine 2002; Iversen 2000 for reviews). Although the results on myriad perceptual, psychomotor, and cognitive tasks have been equivocal, a consistent finding is impaired performance on relatively difficult tests of memory over periods of a few seconds to several minutes. Whereas the effects of marijuana smoking on brain function have been studied using EEG (Lukas et al. 1995; Solowij 1995), PET (Mathew et al. 1998; O’Leary et al. 2002), and MRI measures (Block et al. 2000; Mouzak et al. 2000), studies measuring brain activity during the performance of difficult memory tasks are still lacking. A recent review stated that “despite the obvious importance of the abundant CB1 receptors in the neocortex there have so far been few electrophysiological studies of their effects on neural activity” (Iversen 2003, p. 1258). To address this void, the current study investigated the effects of acute marijuana smoking on EEG and event-related potentials (ERPs) during performance of demanding memory tasks to examine the neurophysiological correlates of cognitive functions that rely specifically on brain areas in which cannabinoid receptors are abundant.
Working memory was assessed with a spatial “N-back” continuous performance task (Gevins et al. 1990). Neuroimaging studies have demonstrated that such tasks activate circuitry in the frontal lobes (Jonides et al. 1993; McCarthy et al. 1994), and patients with frontal lobe pathology show deficits on such tasks (McCallister et al. 2001; Perlstein et al. 2003). Furthermore, a large body of work has shown that both the ongoing EEG and stimulus-registered ERPs are sensitive to variations in the WM load imposed by this type of task (e.g. Gevins et al. 1996, 1997; McEvoy et al. 1998; Watter et al. 2001). The WM task was embedded in the delay period of a study-test word recognition memory paradigm to prevent overt rehearsal of the study list during that interval. Patients with hippocampal lesions are impaired on such recognition memory tests (Manns et al. 2003). ERPs during the test period of such tasks differ between words that are accurately recognized as having been previously studied and new word lures that did not appear in the study list (Rugg and Nagby 1989; Friedman 1990). Such scalp-recorded ERP differences are temporally associated with changes in synaptic and neuronal activity recorded directly from the hippocampus and other MTL structures (Smith et al. 1986; Heit et al. 1988), and are eliminated by lesions to the hippocampus in the language dominant hemisphere (Smith and Halgren 1989; Rugg et al. 1991). Such neurophysiological measures might therefore provide sensitive markers of changes in brain function and memory that accompany marijuana smoking. The present study tested the hypothesis that marijuana smoking would produce acute accuracy and reaction time deficits in WM and EM tasks, accompanied by changes in concomitant neurophysiological signals indicative of impaired memory and attention.
Materials and methods
Subjects
Ten casual marijuana smokers 23–31 years old (mean=26.7, 5M, 5F) participated in the study. Casual smokers were defined as those who reported smoking marijuana between once a month and once a week over the last year. Negative selection criteria included self-report of daily cigarette smoking, consumption of more than ten alcoholic drinks per week, family history of drug dependence, and prior habitual use of any illicit drug other than marijuana. Subjects were paid for their participation, and were given performance bonuses for fast and accurate responses. In accordance with principles of the Helsinki Declaration of 1964, the study protocol was reviewed by our Institutional Review Board, and all participants gave their informed consent prior to their inclusion in the study.
Recording days
Each subject participated in 1 training and 2 test days. On the training day, subjects learned the experimental tasks, became familiar with the recording procedures, and were administered the Wechsler Abbreviated Scale of Intelligence. Subjects were also taught the computerized smoking procedure described below, which they practiced by smoking an herbal cigarette containing no marijuana, tobacco, or nicotine. On test days, subjects performed warm-up blocks of the tasks, and then consumed a sandwich and a non-caffeinated beverage for lunch. After an electrode cap was applied, subjects participated in five recording intervals: one pre-smoking baseline interval, and four intervals that took place 0:20, 1:20, 2:20, and 3:20 h:min after smoking. It was postulated that the neurophysiological effects of marijuana smoking on WM and EM would follow the well-researched time course on physiological and behavioral measures, peaking in the first post-smoking recording interval and steadily dissipating over the next 3 h (Huestis et al. 1992; Joy et al. 1999).
Drug administration
The marijuana cigarettes were supplied by the National Institute on Drug Abuse, and were kept frozen and humidified overnight before use (Thomas et al. 1999). The active marijuana cigarettes contained 3.45±0.28% Δ9-THC and the placebo cigarettes contained 0.006±0.00% Δ9-THC, as assayed via gas–liquid chromatography by the Research Triangle Institute. Following the pre-smoking baseline interval, subjects smoked one cigarette containing active marijuana or placebo, counterbalanced across recording days and subjects. Holding the cigarette in their fingers, subjects took six puffs according to a paced, computerized procedure that was designed to be ecologically valid while standardizing smoking across subjects and sessions. On each puff, they inhaled for 1.5 s, held the smoke in their lungs for 8 s, exhaled, and then rested for 50.5 s before taking the next puff.
Tasks
In each recording interval, resting EEG was recorded for 90 s in both eyes-open and eyes-closed conditions, followed by two blocks of a task that combined WM and EM. Before each interval, blood pressure and pulse were recorded with a wrist blood pressure monitor, and subjects documented their subjective feelings on a 16-question visual analog scale and the Karolinska sleepiness scale (Torsvall and Åkerstedt 1988).
The EM–WM task had three parts, as illustrated in Fig. 1: word presentation (WP), working memory (WM), and word recognition (WR). In each part, subjects responded by pressing one of two response keys on a button box with the index or middle fingers of the right hand. In the WP task, a sequential list of 20 words was displayed on a computer monitor; half the words were presented in red and half in green. Each word was displayed for 500 ms, with an inter-stimulus interval (ISI) of 2000 ms. Subjects responded “red” or “green” to the display color of the words, and were instructed to remember each word and the color in which it was presented.
Fig. 1.
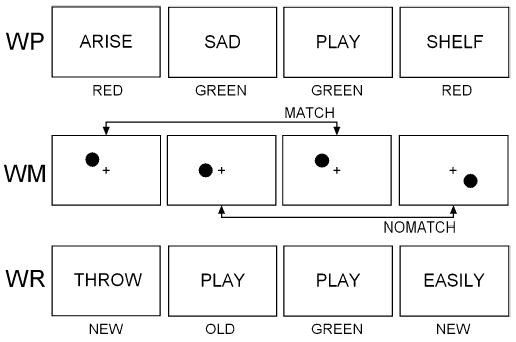
Schematic illustration of the memory task, with examples of stimuli and correct responses from the three parts: word presentation (WP), working memory (WM), and word recognition (WR)
Immediately following presentation of the word list, the WM task served the dual purpose of assessing working memory and providing a distraction-filled delay for the WR task that followed. In the spatial N-back WM task, a dot stimulus was displayed in one of six positions on each trial. In the high load version of the task (pictured), subjects had to decide whether the spatial location of the dot on each trial matched the location of the dot two trials before. Because which dot occurred two trials before changes with each passing trial, the high load version requires frequent manipulation of to-be-remembered items. In the low load version, subjects had to decide whether the location of the dot on each trial matched the location of the first dot in that block of 50 WM trials. Since the comparison stimulus does not change from trial to trial, the low load version requires relatively little manipulation of internal stimulus representations, and the maintenance of only a single stimulus position in WM. Subjects responded “match” or “no-match” on each trial. Each block consisted of 25 match and 25 no-match trials in random order, preceded by three practice trials. A fixation cross remained in the center of the screen throughout the task and all dots were presented at a 3 cm radius from the cross. Each dot subtended 0.95° of visual angle, and was presented for 300 ms with a mean ISI of 4500 ms (range 4000–5000 ms).
Episodic memory was assessed in the WR task, in which 40 words were displayed sequentially in white with response cues “old” and “new” underneath. Subjects indicated whether each word did or did not appear in the WP list displayed approximately 5 min earlier by responding old or new, respectively. Half the words were old and half were new, presented in random order with a 1500 ms ISI. Colored square cues appeared following an old response, and subjects then had to indicate whether that word was originally presented in red or in green in the WP study list.
Two task blocks were presented consecutively in each recording interval, with low/high load version of the WM alternating across blocks and counterbalanced across subjects. Within an interval, both task blocks used the same 20-word WP list but different recognition lists: the same 20 old words appeared in both WR lists, but the 20 new words were different. Subjects therefore had the opportunity to demonstrate learning by being tested twice on the same word list in each interval. In this way, the battery was designed to test the effects of marijuana on encoding of episodic information (WP task), sustained attention and working memory over a period of approximately 5–10 s (WM task), retrieval of episodic information presented 5–10 min previously (WR task), and learning (comparison of recognition memory between the first and the second time through a study list).
EEG recording
EEG was recorded from 40 scalp locations (FP1, FPZ, FP2, AF3, AFZ, AF4, FT9, FT7, FCZ, FT8, FT10, F9, F7, F3, FZ, F4, F8, F10, T7, T8, TP9, TP7, TP8, TP10, C3, CZ, C4, CPZ, P9, P7, P3, PZ, P4, P8, P10, POZ, O1, OZ, O2, IZ) referenced to linked mastoids. Potentials generated by eye movements and blinks were recorded by electrodes positioned above and at the outer canthus and superior orbital ridge of each eye, referenced against linked mastoids. Vertical eye movements and blinks were monitored with the superior orbital electrodes, and horizontal eye movements were monitored with the electrodes at the outer canthi. Electrode impedances were kept below 4 kΩ for the references and below 20 kΩ on all other channels. EEG signals were sampled at 256 Hz and band-pass filtered at 0.01–100 Hz. Automated artifact detection was followed by application of adaptive eye contaminant removal filters (cf. Du et al. 1994). The data were then visually inspected and data segments containing possible residual artifacts were eliminated from subsequent analyses.
Data analysis
Because topographic differences due to marijuana effects were generally not observed, analyses of power spectra bands and ERPs were conducted at the electrode site with maximum amplitude across the group of subjects. Background EEG power spectra were computed in the WP and WM tasks and the resting conditions by segmenting continuous data into 4-s epochs and computing fast Fourier transforms on 2 s windows with 50% overlap. In the WR task, 1-s windows with no overlap following the old or new response were used. The theta band was measured at 4–6 Hz and analyzed at AFZ. Alpha power was measured in 1-Hz intervals between 8 and 12 Hz, and was analyzed at PZ. Beta power was measured between 13 and 18 Hz, and was analyzed at CZ.
Unless otherwise noted, ERP analyses were restricted to trials on which the subject made a correct response. ERPs in all tasks were computed on epochs beginning 200 ms prior to stimulus onset and lasting 1400 ms, with amplitude measured relative to the 200 ms pre-stimulus baseline. In the WP task, N400 was measured as the minimum amplitude between 350 and 450 ms at channel Fz, and average slow wave amplitude was measured from 400 to 600 ms at CPz. In the WM task, the N100 peak was defined as the minimum of the negative-going peak occurring 110–250 ms after stimulus onset, measured at Pz. The P200 peak was defined as the maximum of the subsequent positive peak at FCz between 200 and 300 ms. The P300 peak was defined as the maximum of the largest positive peak at Pz following the N100–P200 complex, within a latency window of 270–450 ms. In the WR task, N100 and N400 peaks were measured, as was the area of a slow wave. The N100 peak was defined as the minimum of the negative-going peak at Oz occurring 90–220 after stimulus onset. N400 peak amplitude was measured as the minimum voltage between 300 and 450 ms at Cz, and slow wave amplitude was measured from 350 to 650 ms at CPz.
False alarm and hit rates were used to measure signal detection accuracy (d′) and response bias in the WR task (Snodgrass and Corwin 1988). Significance of marijuana effects was computed using repeated-measures analyses of variance (ANOVAs) with factors of drug, recording interval, and when applicable, task condition and trial type. Degrees of freedom values used in repeated-measures ANOVAs were adjusted when appropriate using the Greenhouse–Geisser correction technique to correct for violations of the sphericity assumption.
Results
Intoxication
Table 1 presents the effects of smoking placebo versus marijuana on variables measuring intoxication and behavior. Heart rate and subjective “highness” ratings are sensitive non-invasive indicators of Δ9-THC absorption (Chait and Pierri 1992). Although the THC content of the NIDA-issued cigarettes was low compared with marijuana commonly smoked for recreational purposes (ElSohly et al. 2000), these indicators provide evidence that an effective dose of marijuana was administered in the current study. Heart rate increased directly after smoking marijuana but not placebo [F(1,9)=62.35, P<0.001], then returned to near-baseline levels in subsequent post-smoking intervals. Highness rating also increased substantially after smoking marijuana [F(1,9)=43.4, P<0.001], and remained elevated over the next 3 h. Subjective ratings of stoned, impaired, enhanced sensations, and I feel the effects of the marijuana followed a similar pattern, whereas motivated to do well ratings were not affected by marijuana (P>0.10). Karolinska sleepiness ratings indicated that subjects got more tired over the course of the day [F(4,36)=3.53, P<0.05], independent of drug condition (F<1).
Table 1.
Effects of smoking placebo versus marijuana on mean±SEM subjective, autonomic, and memory task performance variables. Asterisks in post-smoking intervals 2–5 denote that the change from baseline interval 1 is significantly different between the placebo and marijuana conditions. Response times in ms; WM working memory; WR word recognition
| Recording interval
|
1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Hours since smoking | Pre-smoking | 0:20 | 1:20 | 2:20 | 3:20 | |
| Highness | Placebo | 2±1 | 6±2 | 3±1 | 2±1 | 2±1 |
| Rating | Marijuana | 1±0 | 60±12** | 53±10** | 33±7** | 20±6** |
| Heart | Placebo | 66±4 | 69±5 | 69±4 | 68±4 | 64±3 |
| Rate | Marijuana | 69±5 | 104±6** | 80±3 | 72±4 | 75±4 |
| WM low load | Placebo | 98.0±0.7 | 98.4±0.5 | 97.4±0.6 | 98.2±0.6 | 97.8±0.7 |
| % Correct | Marijuana | 98.4±0.5 | 99.0±0.4 | 98.8±0.4 | 95.4±2.4 | 98.4±0.7 |
| WM high load | Placebo | 96.8±1.0 | 97.4±0.5 | 98.2±0.7 | 98.0±0.8 | 97.2±1.0 |
| % Correct | Marijuana | 96.0±1.7 | 94.4±1.8* | 94.8±1.8 | 95.6±1.4 | 92.6±1.4 |
| WM low load | Placebo | 418±27 | 403±23 | 409±22 | 388±14 | 396±16 |
| Response time | Marijuana | 415±17 | 475±26* | 468±21* | 457±21 | 416±13 |
| WM high load | Placebo | 503±35 | 486±33 | 484±35 | 490±37 | 480±36 |
| Response time | Marijuana | 496±29 | 600±37** | 592±36* | 561±35 | 503±29 |
| WR old words | Placebo | 77.0±4.5 | 86.8±3.9 | 86.8±3.2 | 83.0±3.6 | 82.3±3.6 |
| % Correct | Marijuana | 79.5±3.5 | 77.8±4.5 | 84.3±3.4 | 84.3±3.2 | 82.5±3.4 |
| WR new words | Placebo | 84.8±2.5 | 85.5±2.5 | 84.3±3.5 | 84.5±3.8 | 86.3±2.6 |
| % Correct | Marijuana | 87.2±3.1 | 71.8±4.4 | 69.8±4.6** | 64.5±5.3* | 72.3±4.4 |
| WR old words | Placebo | 792±30 | 758±33 | 741±30 | 725±29 | 742±36 |
| Response time | Marijuana | 869±39 | 847±34 | 831±35 | 786±31 | 769±30 |
| WR new words | Placebo | 811±27 | 755±20 | 757±25 | 782±23 | 778±33 |
| Response time | Marijuana | 831±16 | 953±48** | 904±46 | 870±30 | 818±25 |
P < 0.05
P< 0.01
Performance
In the high load version of the WM task, responses were slower and less accurate overall than in the low load version (P<0.01 for all comparisons). Following marijuana smoking, accuracy decreased in the high [F(1,9)=6.98, P<0.05], but not the low load version P>0.10. Reaction time (RT) in the WM task increased after smoking marijuana [F(1,9)=16.15, P<0.01], and this response slowing did not differ between the two load levels (P>0.10). Marijuana smoking did not affect accuracy or speed of classifying words as red or green during the encoding WP task (P>0.05 for all comparisons). In the WR task, marijuana hampered the ability to discriminate between previously seen old words and unseen new words, as evidenced by a decrease in d′ (2.07±0.25 before versus 1.56±0.31 after) [F(1,9)=8.4, P<0.05]. Separate analyses of the two stimulus types indicate that the decrease was restricted mostly to new words (87.2±3.1% before versus 71.8±4.4% after), as subjects often claimed to recognize words that did not appear on the study list (made “false alarms”) after smoking marijuana. This decline in accuracy to new words after smoking marijuana first reached significance in Interval 3 [F(1,9)=15.12, P<0.01]. In contrast, accuracy to old words was not significantly affected by marijuana (79.5±3.5% before versus 77.8±4.5% after; P>0.05 for all comparisons). Similarly, RT to new words was longer after smoking marijuana [F(1,9)=15.32, P<0.01], whereas RT to old words was unaffected (P>0.05 for all comparisons).
Subjects learned more words after seeing the study list a second time, as overall WR accuracy was higher in the second (87.0±2.3%) than the first block within an interval (74.4±2.9%) [F(1,9)=79.71, P<0.001]. Reaction times were shorter in the second (776±25 ms) than the first block (841±28 ms) [F(1,9)=18.93, P<0.01]. Neither of these learning effects was affected by smoking marijuana, nor was memory for the original display color of the words (overall mean accuracy 69.1±3.3%; P>0.05 for all comparisons). In sum, marijuana had some deleterious effects on performance of the WM task, particularly on the more difficult version, and increased the tendency to falsely recognize new words as old in the word recognition task. Marijuana did not significantly affect source judgments (word color) or learning (the amount of performance improvement over repeated word list presentations).
EEG
EEG changes as a function of WM load after smoking placebo versus marijuana are shown in Fig. 2. Power in the theta range across the head was attenuated after smoking marijuana relative to placebo in all conditions of the WM, EM, and resting tasks (P<0.05 for all comparisons). Theta power over frontal midline cortex was generally larger in the high than the low load WM task [F(1,9)=6.06, P<0.05], whereas alpha power was generally smaller [F (1,9)=16.62, P<0.01]. This pattern of frontal theta increasing and alpha power decreasing with WM load during the N-back task is well established (e.g. Gevins et al. 1998; Gevins and Smith 2000; McEvoy et al. 2000; Ilan and Gevins 2001), and presumably reflects a sustained increase in the effort and attention allocated to task performance in response to the perceived demands of the more difficult task condition. Smoking marijuana disrupted the task difficulty effect in the alpha band, as the difference in alpha power between low and high load conditions decreased [F(1,9)=6.1, P<0.05]. Beta power decreased after marijuana smoking [F(1,9)=14.22, P<0.01], more so in the low load task than the high load task [F(1,9)=5.67, P<0.05].
Fig. 2.
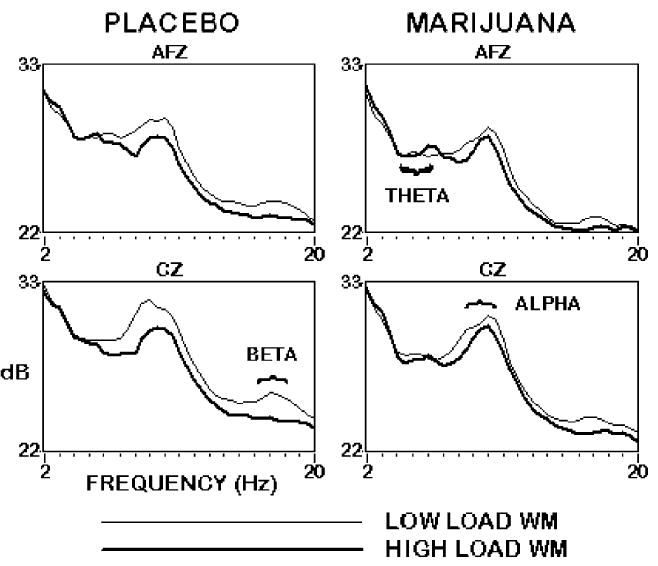
EEG spectral power at anterior midline frontal (AFz) and central (Cz) sites in the low load (light line) and high load (dark line) versions of the Working Memory task in the placebo (left) and marijuana (right) conditions, 20 min after smoking. Marijuana attenuated the task difficulty effect in the alpha band, and decreased power in the theta and beta bands for both load levels. n=10 subjects
The primary EEG finding in the placebo condition of the word recognition task was greater power during the one second following a correct response of “new” relative to a response of “old.” This power difference was most evident parietally, over a wide frequency range of approximately 4–20 Hz [F(1,9)=72.16, P<0.001]. The reduced power following old responses may reflect consideration of source color, or other preparation for the color decision that follows only old responses. Marijuana smoking disrupted this relationship, as EEG power tended to decrease in the theta and beta bands but increase in the alpha band, regardless of whether an old or a new response was made. Whereas this old/new difference was robust over the 4–20 Hz range after placebo, it was not observed after smoking marijuana, resulting in a drug×trial type×interval interaction [F(4,36) =4.1, P<0.05].
ERPs
In the baseline and placebo conditions, ERPs in the WM task had the typical morphology described in detail elsewhere (e.g. Gevins et al. 1996; Watter et al. 2001). After smoking marijuana, ERP amplitude decreased for N100 [F(1,9)=13.50, P<0.01], P200 [F(1,9)=15.77, P<0.01], and P300 [F(1,9)=17.44, P<0.01]. The P300 amplitude decrease following marijuana smoking was larger in the low than the high load WM condition [F(1,9) =5.2, P<0.05]. ERP latencies were not significantly affected by marijuana smoking.
In the WP task, a frontal N400 peak was more pronounced and larger the first time through the study list [F(1,9)=31.14, P<0.001], whereas a centro-parietal positive slow wave was larger the second time through the list [F(1,9)=75.63, P<0.001]. These list repetition effects did not differ between marijuana and placebo (P>0.05 for all interactions), but slow wave amplitude decreased after smoking marijuana [F(1,9)=24.82, P<0.01].
An ERP effect commonly observed during verbal episodic recognition memory paradigms is the “old–new effect” or “memory-evoked shift”: a broad, positive shift of the ERP elicited by old words relative to new words, beginning around 300 ms after word onset, and lasting for 300–400 ms (Rugg and Nagby 1989; Friedman 1990). The effect typically comprises a reduction in amplitude of a negative peak in the ERP waveform between about 300 and 450 ms following onset of old words relative to new words (Smith et al. 1986; Halgren and Smith 1987), superimposed on a broader, parietal-distributed positive slow wave that is enhanced for old words (Smith and Halgren 1989; Smith and Guster 1993). These effects were observed in the current experiment. As illustrated in the placebo condition of Fig. 3, a broad slow wave, maximal centroparietally 350–650 ms after word onset, was larger to correctly recognized old words than to correctly rejected new words [F(1,9)=30.89, P<0.001]. This slow wave was preceded by a centrally maximum “N400” negative potential peaking at approximately 350 ms that was more negative to new words than to old words [F(1,9) =50.41, P<0.001]. As was the case in the WM and WP tasks, marijuana smoking tended to attenuate ERP amplitudes in the WR task, an effect observed for the slow wave [F(4,36)=3.79, P<0.05], but not for N400. Although this slow wave amplitude attenuation did not interact with the magnitude of the old/new word difference in the subject group as a whole, examination of individual subjects suggested that those who became most intoxicated evinced a marked reduction in the memory-evoked shift after smoking marijuana, as discussed below.
Fig. 3.
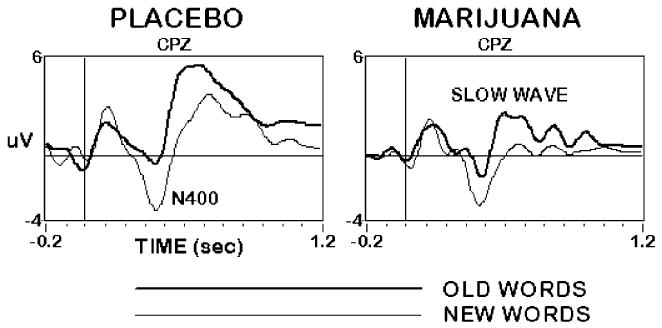
Event-related potentials at the midline centro-parietal (CPz) site during the Word Recognition task, 20 min after smoking placebo (left) and marijuana (right). The N400 was larger to new words (light line), whereas the positive slow wave was larger to old words (dark line). Overall slow wave amplitude decreased after smoking marijuana, but old/new word differences were not significantly affected. n=10 subjects
Discussion
Novel evidence of marijuana interfering with neurophysiological signals of WM and EM was observed in the current study. Across all subjects, marijuana smoking had a number of global neurophysiological effects. ERP components thought to reflect stages of memory encoding, manipulation, and retrieval, such as the slow waves in the WP and WR tasks and the P300 in the WM task, decreased in amplitude after marijuana smoking. Such decreases suggest that less transient attention was devoted to processing the colors, dots, and words during performance of the various tasks. EEG activity in the theta band decreased in both passive resting and active task conditions, consistent with the increased autonomic response to marijuana smoking indicated by the large increase in heart rate. Consistent with previous studies (Fant et al. 1998; Wachtel et al. 2002), drowsiness ratings did not differ between marijuana and placebo conditions. These findings suggest that the global neurophysiological effects of marijuana were not a byproduct of increased sedation. Rather, marijuana intoxication appears to have interfered with subjects’ ability or will to keep their attention focused on performing the various repetitious tasks they were asked to do. Indeed, subjects reported being intoxicated and experiencing “enhanced sensations” after smoking marijuana, and may have had difficulty staying focused on the tedious memory tasks at the expense of other more compelling sensations, perceptions, thoughts, and feelings competing for their attention.
The behavioral results suggest that marijuana intoxication affected certain memory functions more than others. Reaction time increased in both load levels of the WM task, but accuracy decreased only in the high load condition, suggesting that marijuana diminished the ability to sustain representations in working memory. Consistent with previous reports of decreased memory for material learned while intoxicated (Miller et al. 1978; Hooker and Jones 1987), the ability to distinguish old from new words in the WR task was diminished after marijuana smoking. Specifically, behavioral responses to non-studied new word lures were slower and less accurate after marijuana smoking, whereas responses to old words were comparatively unaffected. Intoxicated subjects tended to respond “old” more often, even to new words, possibly because of an increased sense of familiarity to novel items. This increased frequency of classifying new words as old is consistent with reports of higher false alarms, or “intrusion errors” during free recall after marijuana smoking (Miller et al. 1977). The incidence of such errors following marijuana smoking tends to increase when subjects are exposed to distracting intervening stimuli, in this case, a spatial WM task, between study and test (Iversen 2000). Because relatively common English words were used as stimuli in the recognition task, even the new words had some stable level of familiarity. Subjects may have had trouble judging whether these familiar words had been part of the “learning episode” (Engelkamp and Zimmer 2001) while under the influence of marijuana, leading to a disproportionate number of old responses. This interpretation is supported by the reduced N400s to new words observed in some subjects, which may reflect a diminished sense of a novelty in the intoxicated state.
Previous studies have reported transient increases in alpha and decreases in beta power in simple or passive tasks following acute marijuana use (Fink 1976; Bauer 2001; Struve et al. 2003). In the current study, beta and theta power decreased in resting and task conditions after marijuana smoking, but a general increase in alpha power was not observed. In the WM task, marijuana reduced alpha band EEG reactivity in response to increased task difficulty. Examination of individual subjects suggests that the well-established variability between people in subjective and physiological reactions to marijuana smoking (Azorlosa et al. 1992; O’Brien 1996; Kirk et al. 1998) may modulate its neurophysiological effects. Although the small number of subjects involved precludes meaningful statistical comparisons, these data are suggestive of a relationship between degree of marijuana intoxication and the neurocognitive consequences of the drug. Whereas the difference in alpha band power traditionally observed between easy and difficult WM task conditions decreased after marijuana smoking across the entire subject sample, the cause of this reduction seemed to depend on how intoxicated an individual became after smoking marijuana. For those subjects who were affected most by marijuana smoking (in terms of increased heart rate and subjective rating of intoxication), the reduced task difficulty effect resulted from decreased alpha power, primarily in the low load condition. In contrast, for subjects who had relatively muted responses to marijuana smoking the reduced task difficulty effect resulted from increased alpha power, primarily in the high load condition. Because alpha reduction (“desynchronization”) is interpreted as an electrophysiological manifestation of cortical activation (Gevins and Schaffer 1980; Niedermeyer 1993; Pfurtscheller et al. 1996), such changes in alpha power suggest that the amount of effort subjects devoted to task performance after marijuana smoking may have been related to their level of intoxication. Subjects who felt strongly intoxicated after smoking marijuana may have expended more effort to maintain task performance, as evidenced by decreased alpha power. Subjects who experienced a more subdued response to marijuana may have felt such a strategy unnecessary and instead tended to devote less sustained attention to the task after smoking, as evidenced by increased alpha power. Despite the different strategies, both types of subjects exhibited decreased performance in the WM task, and smaller task load differences on alpha band power after smoking marijuana.
Analyses of individual subjects also suggested that degree of intoxication following marijuana smoking influenced neurophysiological signals of EM. Subjects who became most intoxicated by marijuana tended to have a liberal response bias after smoking, often responding “old” to new words, leading to a larger increase in false alarm rate than what was observed in less intoxicated subjects. The most intoxicated subjects also had a more dramatic reduction in ERP amplitude in the WR task, and their old/new ERP differences were essentially eliminated. The similar neurophysiological responses to old and new words may help explain why these subjects had little success differentiating the two word types from each other after marijuana smoking. Reduced old/new ERP differences have been observed in patients with hippocampal damage (Smith and Halgren 1989; Rugg et al. 1991), and high rates of false recognition have been observed to accompany right frontal lobe damage (Schacter et al. 1996; Curran et al. 1997). The EM effects observed in the most intoxicated subjects in the current experiment might therefore reflect a temporary, pharmacological disruption of memory mechanisms dependent on MTL and frontal cortical regions.
In conclusion, difficulty maintaining a coherent train of thought because of the intrusion of irrelevant information is part of the “high” that accompanies acute marijuana smoking. These intrusions may result from activated CB1 receptors disrupting the selective filtering of information into what is relevant and what should be “forgotten” (Terranova et al. 1996; Pollan 2001; Varvel and Lichtman 2002). Such disruption may interfere with the temporal organization of information required in WM and EM. The results presented here suggest that subjects who became most intoxicated after marijuana smoking had difficulty manipulating information in WM and discriminating previously studied words from those that merely seemed familiar. As they are based on a small number of subjects, the individual difference findings presented here should be considered preliminary and suggestive. Further research with more subjects and additional Δ9-THC doses would help clarify the association between individual differences in intoxication, neurophysiological changes in signals of WM and EM following marijuana smoking, and polymorphisms in the CB1 receptor gene that have been shown to affect cannabinoid-modulated reward pathways and ERP amplitudes (Comings et al. 1997; Johnson et al. 1997; Schmidt et al. 2002). The availability of CB1-specific ligands may also prove useful in understanding the mechanisms of these memory changes.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse. We gratefully acknowledge the dedicated effort of Elizabeth Nichols during data acquisition and analysis, the contributions of Patrick Sullivan and An Jiang in developing the software used to collect and analyze the neurophysiological data, and Dr. Linda McEvoy for scientific advice and guidance. The experiment complied with the current laws of the United States of America.
References
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–122. [PubMed] [Google Scholar]
- Baddeley A, Hitch G (1974) Working memory. In: Bower G (ed) Recent advances in learning and motivation. Academic, New York, pp 47–90
- Bauer LO (2001) Electroencephalographic studies of substance use and abuse. In: Kaufman MJ (ed) Brain imaging in substance abuse: research, clinical, and forensic applications. Humana, Totowa, New Jersey, pp 77–112
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Chait LD, Pierri J (1992) Effects of smoked marijuana on human performance: a critical review. In: Murphy L, Bartke A (eds) Marijuana/cannabinoids: neurobiology and neurophysiology. CRC, Boca Raton, Fla., pp 387–424
- Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray J. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2:161–168. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- Curran T, Schacter DL, Norman KA, Galluccio L. False recognition after a right frontal lobe infarction: memory for general and specific information. Neuropsychologia. 1997;35:1035–1049. doi: 10.1016/s0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Du W, Leong HM, Gevins AS (1994) Ocular artifact minimization by adaptive filtering. Proceedings of the Seventh IEEE SP Workshop on Statistical Signal and Array Processing, Quebec City, Canada, pp 433–436
- Earleywine M (2002) Understanding marijuana. Oxford University, Oxford
- ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF., III Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J Forens Sci. 2000;45:24–30. [PubMed] [Google Scholar]
- Engelkamp J, Zimmer H. Categorical and order information in free recall of action phrases. Psicologica. 2001;22:71–96. [Google Scholar]
- Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998;60:777–784. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- Fink M. Effects of acute and chronic inhalation of hashish, marijuana, and delta 9-tetrahydrocannabinol on brain electrical activity in man: evidence for tissue tolerance. Ann N Y Acad Sci. 1976;282:387–398. doi: 10.1111/j.1749-6632.1976.tb49911.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biol Psychol. 1990;30:61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Gevins AS, Schaffer RE. A critical review of electroencephalographic EEG correlates of higher cortical functions. Crit Rev Bioeng. 1980;4:113–164. [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex. 2000;10:829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Bressler SL, Cutillo BA, Illes J, Miller JC, Stern J, Jex HR. Effects of prolonged mental work on functional brain topography. Electroencephalogr Clin Neurophysiol. 1990;76:339–350. doi: 10.1016/0013-4694(90)90035-i. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Smith ME, Le J, Leong H, Bennett J, Martin N, McEvoy L, Du R, Whitfield S. High resolution evoked potential imaging of the cortical dynamics of human working memory. Electroencephalogr Clin Neurophysiol. 1996;98:327–348. doi: 10.1016/0013-4694(96)00288-x. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum Fact. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P (1987) Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle V (eds) Handbook of physiology, the nervous system—higher functions of the brain. American Physiological Society, Bethesda, Md., pp 373–417
- Halgren E, Smith ME. Cognitive evoked potentials as modulatory processes in human memory formation and retrieval. Hum Neurobiol. 1987;6:129–139. [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Heit G, Smith ME, Halgren E. Neural encoding on individual words and faces by the human hippocampus and amygdala. Nature. 1988;333:773–775. doi: 10.1038/333773a0. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology. 1987;91:20–24. doi: 10.1007/BF00690920. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther. 1992;52:31–41. doi: 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Gevins A. Prolonged neurophysiological effects of cumulative wine drinking. Alcohol. 2001;25:137–152. doi: 10.1016/s0741-8329(01)00191-4. [DOI] [PubMed] [Google Scholar]
- Iversen LL (2000) The science of marijuana. Oxford University, New York
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Johnson JP, Muhleman D, MacMurray J, Gade R, Verde R, Ask M, Kelley J, Comings DE. Association between the cannabinoid receptor gene (CNR1) and the P300 event-related potential. Mol Psychiatry. 1997;2:169–171. doi: 10.1038/sj.mp.4000246. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun M. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Joy JE, Watson SJ Jr, Benson JA Jr (1999) Marijuana and medicine: assessing the science base. National Academy, Washington D. C. [PubMed]
- Kirk JM, Doty P, de Wit H. Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;59:287–293. doi: 10.1016/s0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- Lee AC, Robbins TW, Owen AM. Episodic memory meets working memory in the frontal lobe: functional neuroimaging studies of encoding and retrieval. Crit Rev Neurobiol. 2000;14:165–197. [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Benedikt R. Electroencephalographic correlates of marihuana-induced euphoria. Drug Alcohol Depend. 1995;37:131–140. doi: 10.1016/0376-8716(94)01067-u. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE. Cerebellar activity and disturbed time sense after THC. Brain Res. 1998;797:183–189. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- McCallister TW, Sparling MB, Flashman LA, Gueirn SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman-Rakic P, Shulman RG. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Gevins A. Dynamic cortical networks of verbal and spatial working memory: effects of memory load and task practice. Cereb Cortex. 1998;8:563–574. doi: 10.1093/cercor/8.7.563. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Gevins A. Test–retest reliability of cognitive EEG. Clin Neurophysiol. 2000;111:457–463. doi: 10.1016/s1388-2457(99)00258-8. [DOI] [PubMed] [Google Scholar]
- Miller LL, McFarland D, Cornett TL, Brightwell D. Marijuana and memory impairment: effect on free recall and recognition memory. Pharmacol Biochem Behav. 1977;7:99–103. doi: 10.1016/0091-3057(77)90191-5. [DOI] [PubMed] [Google Scholar]
- Miller L, Cornett T, McFarland D. Marijuana: an analysis of storage and retrieval deficits in memory with the technique of restricted reminding. Pharmacol Biochem Behav. 1978;8:327–332. doi: 10.1016/0091-3057(78)90065-5. [DOI] [PubMed] [Google Scholar]
- Mouzak A, Agathos P, Kerezoudi E, Mantas A, Vourdeli-Yiannakoura E. Transient ischemic attack in heavy cannabis smokers—how “safe” is it? Eur Neurol. 2000;44:42–44. doi: 10.1159/000008191. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E (1993) The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F (eds) Electroencephalography. Basic principles, clinical applications, and related fields. Williams and Wilkins, Baltimore, pp 131–152
- O’Brien CP (1996) Drug addiction and drug abuse. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw-Hill, New York, pp 557–577
- O’Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Pollan M (2001) The botany of desire. Random House, New York
- Rugg MD, Nagby ME. Event-related potentials and recognition memory for words. Electroencephalogr Clin Neurophysiol. 1989;72:395–406. doi: 10.1016/0013-4694(89)90045-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Roberts RC, Potter DD, Pickles CD, Nagy ME. Event-related potentials related to recognition memory. Effects of unilateral temporal lobectomy and temporal lobe epilepsy. Brain. 1991;114:2313–2332. doi: 10.1093/brain/114.5.2313. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: a case study. Neuropsychologia. 1996;34:793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition judgments. J Cognit Neurosci. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Smith ME, Guster K. Decomposition of recognition memory event-related potentials yields target, repetition, and retrieval effects. Electroencephalogr Clin Neurophysiol. 1993;86:335–343. doi: 10.1016/0013-4694(93)90046-x. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Dissociation of recognition memory components following temporal lobe lesions. J Exp Psychol [Learn Mem Cognit] 1989;15:50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]
- Smith ME, Stapleton JM, Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr Clin Neurophysiol. 1986;63:145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol [Gen] 117 [DOI] [PubMed]
- Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sci. 1995;56:2119–2126. doi: 10.1016/0024-3205(95)00197-e. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton BJ (1995) Memory, hippocampus, and brain systems. In: Gazzaniga MS (ed) The cognitive neurosciences. MIT, Cambridge, Mass., pp 825–837
- Squire LR, Knowlton B (2000) The medial temporal lobe, the hippocampus, and the memory systems of the brain. In: Gazzaniga MS (ed) The new cognitive neurosciences. MIT, Cambridge, Mass.
- Struve FA, Manno BR, Kemp P, Patrick G, Manno JE. Acute marihuana (THC) exposure produces a “transient” topographic quantitative EEG profile identical to the “persistent” profile seen in chronic heavy users. Clin Electroencephalogr. 2003;34:75–83. doi: 10.1177/155005940303400206. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology. 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Parker VL, Caddell LW, Jones LV, Sabharwal SK, McDaniel AI, Keimowitz AR, Scheffler NM, Hart ED, Mitchell JM, Davis KHJ (1999) Composition and stability of a standard marihuana cigarette. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S (eds) Marihuana and medicine. Humana, Totowa, New Jersey, pp 137–143
- Torsvall L, Åkerstedt T. Extreme sleepiness: quantification of EOG and spectral EEG parameters. Int J Neurosci. 1988;38:435–441. doi: 10.3109/00207458808990704. [DOI] [PubMed] [Google Scholar]
- Tulving E (1983) Elements of episodic memory. Oxford University, New York
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;23:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB(1) receptor knockout mice in the morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Watter S, Geffen GM, Geffen LB. The n-back as a dual-task: P300 morphology under divided attention. Psychophysiology. 2001;38:998–1003. doi: 10.1111/1469-8986.3860998. [DOI] [PubMed] [Google Scholar]


