Abstract
Objectives
To assess the ability of an Adjusted Clinical Group (ACG)-based morbidity measure to assess the overall health service needs of populations.
Data Sources/Study Setting
Three population-based secondary data sources: registration and health service utilization data from fiscal year 1995–1996; mortality data from vital statistics reports from 1996–1999; and Canadian census data. The study included all continuously enrolled residents in the universal health care plan in Manitoba.
Study Design
Using 60 small geographic areas as the units of analysis, we compared a population-based “ACG morbidity index,” derived from individual ACG assignments in fiscal year 1995–1996, with the standardized mortality ratio (ages <75 years) for 1996–1999. Key variables included a population-based socioeconomic status measure and age- and sex-standardized physician utilization ratios.
Data Extraction Methods
The ACGs were assigned based on the complement of diagnoses assigned to persons on physician claims and hospital separation abstracts. The ACG index was created by weighting the ACGs using average health care expenditures.
Principal Findings
The ACG morbidity index had a strong positive linear relationship with the subsequent rate of premature death in the small areas of Manitoba. The ACG index was able to explain the majority of the relationships between mortality and both socioeconomic status and physician utilization.
Conclusions
In Manitoba, ACGs are closely related to premature mortality, commonly accepted as the best single indicator for health service need in populations. Issues in applying ACGs in settings where needs adjustment is a primary objective are discussed.
Keywords: Case-mix adjustment, mortality, health status indicators, population, Canada, morbidity, health resources
Methods to quantify the burden of morbidity in populations and the resulting need for health care services have important applications in the management and financing of health systems. In the 1980s and 1990s, escalating health care costs across developed nations have led policymakers, administrators, and providers to seek strategies to maximize health gains with available budgets by streamlining delivery and reducing the provision of ineffective and unnecessary services. Central to this goal of organizational efficiency is aligning the delivery of services with what services are actually needed at the least expense. Measuring need for health care services is critical for the equitable allocation of resources and the elimination of disparities, which are primary social objectives in many health systems.(Judge and Mays 1994; Diderichsen, Varde, and Whitehead 1997; Newbold, Eyles, and Birch 1995). Since all health care needs cannot be met with finite resources, equitable allocation implies that patients with similar needs should receive similar bundles of services (horizontal equity) and that patients with greater needs should receive proportionately more services (vertical equity) (Carr-Hill 1994). As responsibility for organizing and funding health care is shifted from central governments to other organizations (such as local health authorities and primary care practices in Canada, the U.K., and Sweden), the search for more precise population-needs indicators has intensified. In the United States, the goal of eliminating disparities by 2010 has heightened interest in methods to assess population health status and care needs (Institute of Medicine 1999).
While “need” is a defining concept for the financing and organization of many health systems, it has proved difficult to operationalize for populations (Carr-Hill, Maynard, and Slack 1990; Culyer 1995). Need is a statement about the capacity to benefit from services that maintain, enhance, or prevent deterioration in health status for individuals or groups (Evans 1984) and is usually assessed by health care providers and patients in weighing the benefits, risks, and costs of various interventions. Characterization of a patient's condition or conditions is central to the definition of current service needs since the types of problems or illnesses largely determine the approaches that providers take in diagnosis, management, and follow-up. (Starfield 1998) However, because of the general lack of systematically collected morbidity data for populations, indirect measures of relative health care needs have been developed from demographics, mortality rates, and population surveys. Self-reported health status, aggregated from the individual to the population level, has been considered by some to be the “gold standard” for assessing the relative needs of populations (Eyles et al. 1991). Although potentially applied in some health systems to adjust funds (Hornbrook and Goodman 1995), this method has not been widely adopted because of the added data collection required. Age and sex are easily obtained and highly related to morbidity, yet they are considered unsatisfactory, as wide variations have been shown to exist after accounting for demographics (Carr-Hill and Sheldon 1992). Measures of social deprivation have been used as indirect measures of health care needs based on the association of social deprivation and morbidity (Mays and Bevan 1986; Townsend 1990; Eyles and Birch 1993). Mortality rates are also used as proxies for relative health needs in populations (particularly for acute care) based on the premise that high mortality reflects poor health status and greater health care needs. The premature mortality rate (deaths before the age of 75 years) is generally considered to be the best single proxy of overall population health needs that is currently available (U.S. General Accounting Office 1996; Roos and Mustard 1997; Carstairs and Morris 1989). The main rationale for using premature mortality comes from its high correlation with self-reported chronic sickness (Forster 1977; Brennan and Clare 1980) as well as poverty and unemployment (Mays 1989). In the United States, premature mortality (measured with years of potential life lost) was found to explain 36 percent of the cross-state differences in 18 indicators of population health status (including measures of disease incidence, low-weight births and childhood poverty), better than any other indicator (U.S. General Accounting Office 1996). Although premature mortality is not linked to all types of health service needs (e.g., maternity and preventive care needs), it is thought to be a reasonable indicator of overall need because of its relationship with those illnesses associated with sizeable and ongoing resource implications (Eyles et al. 1991). In the United Kingdom, the standardized mortality ratio (SMR) for persons <75 years of age has been used, in addition to demographics and other factors, to adjust for differential morbidity levels for funding hospitals and community health services since the 1970s (Mays 1995).
In the 1990s, a new class of case-mix tools have been developed that rely on diagnosis codes from administrative records to directly quantify overall requirements for resources based on diagnoses for individuals and, when aggregated, populations (Ellis et al. 1996; Starfield et al. 1991; Kronick, Dreyfus, and Zhou 1996). The Adjusted Clinical Group (ACG; formerly the Ambulatory Care Group) system is one such instrument that attempts to group a person's complement of diagnosis codes into broad categories of overall need for ambulatory and hospital resources (Weiner et al. 1991). As opposed to episode-of-care groupers, the ACGs account for a person's mix of illnesses that stretch across visits, facilities, and care providers for extended periods of time. Accounting for comorbidities is essential for population-based perspectives because illnesses are well known to cluster both within individuals and populations (Starfield 1998; Iezzoni 1994). ACGs have been applied in the private and public sectors to adjust payment rates for U.S. managed care providers (Weiner et al. 1998; Fowles et al. 1996; Weiner et al. 1996), adjust provider “economic profiles” (Salem-Schatz et al. 1994; Tucker et al. 1996; Green, Barlow, and Newman 1997; Verhulst, Reid, and Forrest 2001), and in health services research as a resource-based morbidity adjuster (Starfield et al. 1994; Briggs et al. 1995; Powe et al. 1996; Forrest and Reid 2001).
The main attraction of the ACG system for measuring differential health care needs relates to the fact that the data elements—age, sex, and diagnoses—are routinely collected in many health systems and are available for total populations. The principal disadvantage relates to the fact that diagnoses derived from physician recording are of uncertain quality and completeness. In many health systems, validity of the ACG system is threatened by coding that truncates diagnoses, the lack of secondary diagnoses, and the lack of mechanisms to monitor and improve diagnosis accuracy. The ability of the data to reflect the true population health care needs is also uncertain because diagnoses are generated only through encounters with health providers and thus their collection is clearly linked to issues of access to and use of health care services. For instance, persons without insurance or who reside in areas with an inadequate physician supply may be less likely to receive the appropriate diagnoses than those with insurance or who reside in areas with richer supplies. Little information is available to determine the susceptibility of the ACG system to these information biases.
Although the ACG system was designed to group diagnoses into categories of clinically defined need based on the opinions of expert clinicians buttressed by empirical analyses, validation has generally focused on how well they can account for individual and group-level variations in use and expenditures. In the United States, ACGs are able to explain more than 50 percent of same-year resource use by individuals and more than 20 percent if applied in the following year (Weiner et al. 1991). Similar predictive ability has been reported in both Canada (Reid et al. 2001) and Spain (Orueta et al. 1999). While these results suggest that the ACG system performs reasonably well in accounting for use and costs, little information is available to determine how well ACGs account for needs at either the individual or population level. Many nonneed factors are well known to influence service use including provider practice style (Roos and Roos 1994) and the supply of providers and technologies (Wennberg 2000). Therefore, before being applied confidently to direct funds to patient populations in response to their relative needs for care, other validation methods are required.
This article seeks to validate the ACG system as an indicator of relative health service needs for geographic populations in Manitoba, Canada. We chose the standardized mortality ratio (SMR) for ages <75 years as our criterion population needs indicator. In order to compare the nominal ACG categories to the SMR, we developed an “ACG morbidity index” for small areas by weighting the ACGs by their estimated costs, aggregating them to the population level, and age/sex standardizing the index. Manitoba is one of the few locations where researchers are able to simultaneously monitor an entire population's health and use of the health care system over long periods.
Methods
This study was based on the POPULIS database, which captures vital events, health service use trajectories, and other health-related data for all residents of Manitoba since the 1970s (Roos et al. 1995; Roos et al. 1999). Health care events are determined through the use of physician claims and discharge abstracts routinely collected to reimburse physicians and hospitals in Manitoba's universal insurance system (with first dollar coverage of physician and hospital services). Previous research has shown these administrative data to capture the vast majority of physician and hospital services in the province (Roos et al. 1983; Roos, Sharp, and Wajda 1989). The ACG-based needs indicator was created for 60 “physician service areas” (PSAs) by extracting demographic and diagnosis data for the geographic subpopulations from April 1995 to March 1996. The PSAs are analytic “catchment” areas formed by grouping neighboring communities based on where persons are most likely to obtain physician services (Roos et al. 1996). In the city of Winnipeg, catchment area boundaries are blurred and, thus, PSAs were formed by grouping neighborhoods into contiguous areas with similar median household incomes derived from census data.
The ACG morbidity index was created as follows: We assigned ACGs to the all Manitobans (i.e., each person was grouped into one of 82 ACG categories with the ACG software, version 4.1), weighted the assignments by the provincial mean expenditures in each ACG category as a proxy for relative illness, and aggregated the individual assignments to the PSA level. Both crude and age and sex-standardized ACG indices were calculated. The complete process is described below.
Assigning ACGs to Individuals and Creating ACG-specific Illness Weights
The ACG system uses a two-step process to assign persons a mutually exclusive ACG. First, persons are assigned from 0 to 32 Aggregated Diagnosis Groups (ADGs) based on the range of diagnoses they receive on ambulatory and inpatient records over a defined time period, typically one year (Johns Hopkins University School of Hygiene and Public Health 2000). The system developers grouped diagnoses into ADGs based on the opinions of expert physicians about the: (1) expected duration of illness, (2) expected disability and reduced longevity, (3) certainty of diagnosis, (4) etiology, and (5) expected need for specialist care and hospitalization. In the second step, the developers grouped ADGs into commonly occurring combinations called ACGs based on empirical analysis and clinical judgment. For our study, we assigned the 82 base ACGs to all continuously enrolled Manitobans using diagnoses from physician claims (excluding claims for laboratory and radiology services) and hospital separation abstracts during fiscal year 1995–1996. No changes were made to the ACG grouping algorithm. In Manitoba, one ICD-9 code is recorded on each physician claim and up to 16 ICD-9-CM diagnoses are entered on each hospital abstract. Since ACGs represent nominal groups, we used the average health care costs associated with each ACG across the province as relative illness weights for the ACGs. This method of weighting assumes that, on the whole, health services are expended in a fashion responsive of differential health needs. These weights are clearly not ideal since they are derived from actual service use and may not reflect the most desired standard of care. To assess the performance of different cost weights, we calculated weights for both “physician” and “total” services. Physician costs included all payments made to physicians (fees-for-services and equivalent fees for salaried physicians) from the provincial health plan relating to face-to-face visits or procedures. Total costs comprised both physician payments and estimated costs for acute care hospitalization and day surgery. Since Manitoba hospitals are globally budgeted, inpatient and day surgery costs associated with each ACG were estimated using refined diagnosis related groups (RDRGs) (Canadian Institute for Health Information 1994) and day procedure groups (DPGs), combined with Manitoba cost estimates (Jacobs et al. 1999). Because high-cost outliers can affect the stability of ACG cost estimates (Van Houten et al. 1998), we used “trimmed” averages by truncating outliers at three standard deviations above the mean.
Creating the ACG Morbidity Indices
To create the ACG indices, Manitobans were first assigned the ACG cost weights developed from provincial expenditure data. A crude ACG morbidity index was created for each PSA by dividing the average weight for the PSA population by the overall provincial average. To account for the differential age structure of PSAs (and make the indices comparable to the standardized mortality ratio), we also calculated a standardized ACG index using the indirect method (Kahn and Sempos 1989). The standardized index was calculated by dividing the actual sum of the ACG cost weights for the PSA populations by the “expected” sum if provincial averages within twenty age and sex categories were applicable. The indices can be represented as follows:
 |
where:
njk=no. of users with ACG j and residing in PSA k
Nj=no. of users with ACG j in the province
Cj=sum of the observed costs for users with ACG j in the province
Wj=‘illness weight’ for ACG j=average provincial cost (trimmed) for users with ACG j=Cj/Nj
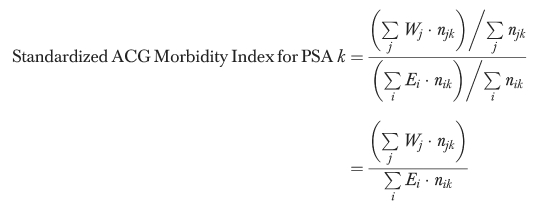 |
and also where:
njk=no. of users in age/sex stratum i and residing in PSA k
Nij=no. of users in age/sex stratum i with ACG j in the province
Ei=average ‘illness weight' for users in age/sex stratum i in the province
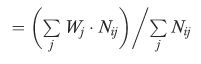 |
To examine the influence of using different cost weights and population restrictions, the morbidity indices were created using both physician and total costs and for the total and the aged <75 populations.
Premature Mortality and Other Variables
Four-year all-cause mortality rates and ratios (<75 years) were derived from Manitoba vital statistics death records and population counts from the POPULIS registry for calendar years 1996–1999 (Roos et al. 1999). Four years of data were necessary to stabilize the rates for the smaller PSAs. The crude premature mortality rate was calculated by dividing the number of deaths <75 years by the person-years of exposure. The denominator was estimated from Manitoba Health's registration files. The standardized mortality ratio (SMR) for persons <75 years was calculated using the Manitoba population as the standard. Variables reflecting the PSA size and demographics in fiscal year 1995–1996 were also examined, including the total population, the percentage female, mean age, the percentage of health system “nonusers” (i.e., persons without at least one physician visit, day surgery, and/or hospitalization), and the Socioeconomic Risk Index (SERI). The SERI is an aggregate index of socioeconomic status (SES) that combines census variables relating to the percentage aged 15–24 and 45–54 who were unemployed, the percentage of single parent female households, the proportion of the population aged 25–34 having graduated high school, the percentage of females participating in the labor force, and the average dwelling value (Frohlich and Mustard 1996; Mustard and Frohlich 1995). To examine how utilization of physicians relates to mortality and the ACG index, we also calculated indirectly standardized visit ratios (SVRs) for general/family practitioners (GP/FPs) and specialists based on number of face-to-face visits during fiscal year 1995–1996. The standardized ratios were created by dividing the total visits made by PSA residents by the number expected if they had the same age and sex-specific rates as did the overall provincial population.
The relationships between the SMR (<75 years), the ACG indices, and the visit ratios were explored using scatterplots and Pearson correlation coefficients. We also used ordinary least squares regression to examine the combined effects of the ACG index, SERI, and physician utilization on mortality. More specifically, the SMR (<75years) was modeled as a linear function of the following covariates: the standardized ACG morbidity index (created for the nonelderly with total weights), GP/FP and specialist SVRs, the proportion of health system nonusers, and the SERI. The SERI was added to examine the extent to which the ACG index captures the strong relationship that has been documented between a population's SES and its health (i.e., the lower the SES, the poorer the health) (Gissler, Rahkonen, and Jarvelin 1998; West 1997; Montgomery et al. 1996; Bor, Najman, and Anderson 1993). Similarly, the standardized visit ratios were added to examine if physician utilization is an independent predictor of population health and service need.
Results
In 1995–1996, the size of the 60 physician service areas varied substantially from 1,100 persons in the isolated northern community of Churchill to 108,823 in Winnipeg North East. The distribution included 6 small PSAs with fewer than 2,000 persons, 33 with between 2,000–10,000, 11 with between 10,000–25,000, and 9 with greater than 25,000 residents. The 9 city of Winnipeg PSAs (all but one were in the largest category) accounted for almost 60 percent of Manitoba's 1.1 million population. Large age differences were apparent among PSAs ranging from a mean of 25 years in Island Lake to 45 years in Gilbert Plains (SD 5.0). Likewise, SES varied more than four-fold from a SERI of –1.05 in Winnipeg South West to 4.26 in Oxford House (SD 1.1; a higher index indicates lower SES). Between 1996 and 1999, there were 3.36 premature deaths per 1,000 per year in Manitoba; the SMRs for the PSAs ranged three-fold from 0.60 (Macdonald-Cartier) to 1.98 in the Winnipeg Inner Core (SD 0.20). In 1995–1996, 81.8 percent of Manitobans had at least one inpatient stay, ambulatory surgery, or physician encounter. The participation rate was similar among PSAs except for four sparsely populated and remote northern areas with particularly low user rates (<70 percent). Missing data partly explains this low use; these remote areas rely on federally funded nursing stations where encounter data are not captured. Manitobans made an average of 4.6 ambulatory physician visits per year overall—about 3.5 to generalists and 1.1 to specialists. While the standardized visit ratios for generalists were not dissimilar between the Winnipeg and non-Winnipeg PSAs (p = 0.61), we found large urban/rural differences in the use of specialists. All nine Winnipeg areas (including both the relatively affluent suburban neighborhoods and the relatively poor inner core) had specialist use ratios (1.25–1.65) that were significantly higher than any other Manitoba area (0.80–1.09).
To assign ACGs to the study population, an average of 3.62 unique diagnoses per person were extracted from the administrative data and submitted to the ACG software. [Readers are referred elsewhere for the distribution of ACGs and their related expenditures (Reid et al. 2001).] To create the ACG indices, the trimmed mean costs for each ACG (ranging from $53 to $2,579 for physician costs and from $54 to $24,112 for total costs) were assigned to individuals as illness weights and summed for each PSA. Using the total cost weights, the resulting standardized ACG index ranged from 0.80 to 1.60 (SD-0.20) across the PSAs. When limited to physician costs only, the variation across PSAs was reduced (range 0.82–1.30; SD-0.11).
Table 1 presents the Pearson correlation matrix for the standardized indices, the SERI, and the SMR. Figure 1 shows scatterplot between the SMR and the standardized ACG index (constructed with total costs for those younger than age 75). Overall, the standardized ACG indices were all highly correlated with the SMR (r = 0.80–0.87). The highest correlation was with the ACG index constructed with total cost weights and limited to those <75 years. The figure shows a linear relationship with some heteroskedadicity; no relationship with PSA size is evident. It is also noteworthy that the ACGs indices had higher correlation coefficients with the SMR than did either the SERI (r = 0.68) or the generalist physician visit ratio (r = 0.56). We found no significant bivariate relationship between specialist use and mortality, a finding consistent with prior research (Roos and Mustard 1997). As expected, the strong linear relationship between the SMR and the standardized ACG index was also reflected in a strong correlation between the crude mortality rate and the crude ACG index (r = 0.80).
Table 1.
Pearson Correlation Matrix
| Socioeconomic | Standardized ACG Morbidity Index | Standardized Visit Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMR | Risk Index | Total Costs | Physician Costs | |||||||
| (<75 yrs) | (S.E.R.I.) | All Ages | <75y only | All Ages | <75y only | GPs only | Specialists | |||
| SMR (<75 yrs) | 1.00 | 0.65 | 0.84 | 0.86 | 0.78 | 0.80 | 0.52 | −0.24 ns | ||
| S.E.R.I. | — | 1.00 | 0.66 | 0.68 | 0.56 | 0.57 | 0.39 | −0.47 | ||
| Standardized ACG | Total Cost | All Ages | — | — | 1.00 | 0.98 | 0.96 | 0.95 | 0.58 | 0.00 ns |
| <75y only | — | — | — | 1.00 | 0.96 | 0.97 | 0.62 | −0.01 ns | ||
| Morbidity Index | Physician | All Ages | — | — | — | — | 1.00 | 0.99 | 0.65 | 0.12 ns |
| Costs | <75y only | — | — | — | — | — | 1.00 | 0.67 | 0.10 ns | |
| Standardized | Visit Ratio | GPs only | — | — | — | — | — | — | 1.00 | −0.33 |
| Specialist | — | — | — | — | — | — | — | 1.00 | ||
Note: Excludes 4 PSAs with proportion of ‘users’ less than 70%. Correlation coefficients all statistically at p <0.01 except where marked ‘ns’.
Figure 1.
Scatterplot of ACG Morbidity Index and Standardized Mortality Ratio (<75 years), Manitoba Physician Service Areas (PSAs)
Table 2 presents the results of the least squares regression model that examines the combined effects of the standardized ACG index and the other variables on the SMR. Because of likely missing data for the four remote PSAs, they were excluded from the regression model. The final model achieved statistical significance, explaining 98 percent of the variation in the SMR. Residual analysis revealed two influential data points (studentized residuals >∣2∣) that were also excluded (Lynn Lake and Gillam). The model confirmed the direct, independent relationship between the ACG morbidity index and the rate of early death. The SERI also achieved statistical significance in the final model but the standardized visit ratios and proportion of nonusers did not. Two- and three-factor interaction terms were tested but were insignificant and dropped from the model.
Table 2.
Multiple Linear Regression for the Combined Effects of the ACG Morbidity Index, Socioeconomic Risk, and Physician Utilization on the SMR (<75 years), Manitoba Physician Service Areas
| Sum of Squares | SS | df | Mean Sq | F stat | P-value |
|---|---|---|---|---|---|
| Model | 712.62 | 5 | 180.4 | 695.4 | p <0.0001 |
| Error | 10.04 | 49 | 0.2 | ||
| Total | 722.67 | 54 | |||
| R2 | 0.98 |
| Parameter | Partial SS (Type III) | Beta | SE | P-value | |
|---|---|---|---|---|---|
| Standardized ACG Morbidity Index | 4.35 | 4.11 | 0.89 | <0.0001 | |
| Socioeconomic Risk Index (SERI) | 0.81 | 0.320 | 0.16 | 0.050 | |
| Standardized Visit Ratio – Specialists | 0.01 | −0.060 | 0.28 | NS | |
| Standardized Visit Ratio – GP/FPs | 0.09 | −0.390 | 0.57 | NS | |
| Proportion of Non-Users | 0.21 | −1.430 | 0.39 | NS |
Note: Four ‘low user’ PSAs (Churchill, Oxford House, Island Lake, and Burntwood Unorganized) and two influential data points (Lynn Lake and Gillam) excluded from final fitted model.
Discussion
To help policymakers steer resources to populations more efficiently and equitably, precise measures of an individual's or a group's relative capacity to benefit from health care resources are required. In Canada, as in other developed nations, such methods are gaining relevance with new applications of population-based funding (Hurley et al. 1999). Culyer suggests that ideal needs indicators should be clinically sensible, align with health system objectives, assess both vertical and horizontal equity, and link services to individuals at the level of the individual (Culyer 1995). The ACG system seeks to accomplish these tasks (and remain administratively feasible at the same time) by clustering morbidities into clinically meaningful categories of health care need based on the types of services that expert clinicians consider warranted. The ACGs also align with Culyer's criteria in that they link the full range of morbidities to expected resources and are person-specific by aggregating common combinations of morbidities for which people seek care. Thus, while ACGs are clearly not precise enough to assist with allocation decisions for individual patients, they arguably represent a significant advance in measuring health care need in populations. For policymakers and health administrators, they are particularly attractive because they use existing data and, as opposed to mortality rates, can potentially be applied to relatively small populations over short intervals (as short as six months). This feature is particularly important for populations that may be unstable and/or not defined by geography. However, even though they are closely aligned with the concept of need, previous validation has centered on how well ACGs explain variation in use and costs. While this information is vital to gauge how adjusters will function to stem risk selection (Dunn 1998), other validation approaches are necessary before they are applied where needs adjustment is the main objective (Hutchison et al. 1999).
Our study suggests substantial predictive validity in Manitoba for the standardized ACG morbidity index, a population-based morbidity and health care needs indicator derived from individual ACG assignments. The ACG index is closely related to the rate of early death in later years, a widely used and accepted measure of population health status and service need. There also appears to be a significantly stronger relationship between mortality and ACG index than between mortality and either physician utilization rates or the SERI, a previously validated measure of socioeconomic risk. The close linear association of the ACG index and premature mortality supports the validity of the needs-based ACG grouping algorithm. It also suggests that, despite the common use of truncated diagnoses and lack of secondary codes, the diagnoses in Manitoba are accurate and complete enough to account for substantial differences in illness at the population level. The accuracy of these data in the context of the ACGs broad illness groups is also supported by the ACGs ability to explain health care costs at the individual level (Reid et al. 2001). Because we used expenditures to weight the ACGs for illness burden and service need, the relationship between our index and mortality also implies that, on the whole, total expenditures (for acute care hospital and physician services) in Manitoba are responsive to need. This finding is consistent with prior research indicating that acute care use in Manitoba is highly correlated with other needs indicators including neighborhood income (Roos and Mustard 1997) and self-assessed health status (Newbold, Eyles, and Birch 1995). Our findings also suggest that, in Manitoba, the use of physician expenditures alone to weight the ACGs may underestimate the overall health care needs of relatively ill populations.
There are of course limits to using premature mortality as a gold standard of population health or need for medical care. Critics charge that premature mortality reflects past, not current or future needs (Carr-Hill and Sheldon 1992), ignores the impact of social circumstances (Carstairs and Morris 1989), underrepresents the needs of younger people (Palmer et al. 1979), and underrepresents needs without a significant risk of death such as nonserious conditions, mental illness, preventive care, and pregnancy (Forster 1977; Carr-Hill and Sheldon 1992). The latter poses a significant problem since many of these conditions hold significant resource implications. The ACGs and other diagnosis-based systems could theoretically better reflect overall need since they attempt to capture needs across the full spectrum of clinical conditions.
However, the significance of the SERI in the final regression model suggests that the ACGs may not fully capture the high illness burdens of disadvantaged populations. This assertion is plausible given that, in Manitoba, disadvantaged populations do not use physician services, particularly specialists, at rates that match their increased illness levels (Roos and Mustard 1997; Frohlich, Fransoo, and Roos 2001). Reduced access to specialists and diagnostic modalities in these populations likely result in less severe diagnosis recording and an underestimation of morbidity. This explanation also suggests that if ACGs were to be used for population-based needs adjustment, additional socioeconomic or area-based adjusters would likely be required. Alternatively, the significance of the SERI in the final model may suggest that for a given level of morbidity, populations with fewer social and economic resources experience higher mortality rates. To examine these and other possible explanations, more research is required to understand the interplay between ACGs and socioeconomic status in the specification of health service needs.
In assessing how well the ACGs are able to reflect health care needs, we view our research as a first but important step. In addition to premature mortality, comparisons with self-assessed health status and other health status indicators would be useful at both the individual and population levels. Also, research is required to assess the generalizability of our findings to settings without universal health insurance. In the United States, uninsured adults have greater unmet needs (Ayanian et al. 2000) and encounter greater barriers to care (Hafner-Eaton 1993) than do insured adults. Since coding of diagnoses may be incomplete or delayed in populations where significant barriers to access exist, the ACGs would likely underestimate their true health care needs.
A main drawback to using ACGs is the requirement for data systems that collect comprehensive diagnosis data from health care encounters. In jurisdictions where small numbers and/or long time periods are not an issue or where there are significant gaps in utilization or diagnosis data, premature mortality is likely the preferred needs indicator. As well, diagnosis-based needs assessment introduces the incentive for diagnosis “upcoding” when used for funding adjustment, which can limit the usefulness of this approach. Little empirical data is available, however, to assess the magnitude of this problem in the context of ACGs.
Conclusions
For health policymakers, managers, and researchers, ACGs represent an alternative and potentially useful way to measure needs for health care in populations. Their main advantage lies in the fact that the data are already collected in many health systems. In comparison to premature mortality, ACGs also have the advantage that they are clinically sensible and can be specified over shorter intervals. For health care systems such as Canada where equity is a main concern, our study provides evidence to support their consideration in population-based resource allocation. Our research also suggests, however, that additional socioeconomic adjusters may be necessary.
Acknowledgments
We wish to acknowledge Barbara Starfield, M.D., M.P.H., Christopher Forrest, M.D., Ph.D., Jonathan Weiner, Dr. P.H., Samuel Sheps, M.D., M.Sc., and Leslie Roos, Ph.D., for reviewing previous versions of the manuscript.
Footnotes
This research was supported as part of one of six projects undertaken by the Manitoba Centre for Health Policy each year under contract with Manitoba Health. The results and conclusions are those of the authors and no official endorsement by the Government of Manitoba was intended or should be implied.
References
- Ayanian JZ, Weissman JS, Schneider EC, Ginsburg JA, Zaslavsky AM. “Unmet Health Needs of Uninsured Adults in the United States.”. Journal of the American Medical Association. 2000;284(16):2061–9. doi: 10.1001/jama.284.16.2061. [DOI] [PubMed] [Google Scholar]
- Bor W, Najman M, Anderson M. “Socioeconomic Disadvantage and Child Morbidity: An Australian Longitudinal Study.”. Social Science and Medicine. 1993;36:1053–61. doi: 10.1016/0277-9536(93)90123-l. [DOI] [PubMed] [Google Scholar]
- Brennan ME, Clare PH. “The Relationship between Mortality and Two Indicators of Morbidity.”. Journal of Epidemiology and Community Health. 1980;34:134–8. doi: 10.1136/jech.34.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs LW, Rohrer JE, Ludke RL, Hilsenrath PE. “Geographic Variation in Primary Care Visits in Iowa.”. Health Services Research. 1995;30:657–71. [PMC free article] [PubMed] [Google Scholar]
- Canadian Institute for Health Information. Resource Intensity Weights. Ottawa ON: Canadian Institute for Health Information; 1994. [Google Scholar]
- Carr-Hill RA. “Efficiency and Equity Implications of the Health Care Reforms.”. Social Science and Medicine. 1994;39:1189–201. doi: 10.1016/0277-9536(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Carr-Hill RA, Maynard A, Slack R. “Morbidity Variation and RAWP.”. Journal of Epidemiology and Community Health. 1990;44:271–3. doi: 10.1136/jech.44.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr-Hill R, Sheldon T. “Rationality and the Use of Formulae in the Allocation of Resources to Health Care.”. Journal of Public Health Medicine. 1992;14:117–26. [PubMed] [Google Scholar]
- Carstairs V, Morris R. “Deprivation, Mortality and Resource Allocation.”. Community Medicine. 1989;11:364–72. [PubMed] [Google Scholar]
- Culyer AJ. “Need: The Idea Won't Do—but We Still Need It.”. Social Science and Medicine. 1995;40:727–30. doi: 10.1016/0277-9536(94)00307-f. [DOI] [PubMed] [Google Scholar]
- Diderichsen F, Varde E, Whitehead M. “Resource Allocation to Health Authorities: The Quest for an Equitable Formula in Britain and Sweden.”. British Medical Journal. 1997;315:875–8. doi: 10.1136/bmj.315.7112.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn DL. “Application of Health Risk Adjustment: What Can Be Learned from Experience to Date?”. Inquiry. 1998;35:132–47. [PubMed] [Google Scholar]
- Ellis RP, Pope GC, Iezzoni LI, Ayanian JZ, Bates DW, Ash A. “Diagnosis-Based Risk Adjustment for Medicare Capitation Payments.”. Health Care Financing Review. 1996;3 [PMC free article] [PubMed] [Google Scholar]
- Evans R. Strained Mercy: The Economics of Canadian Health Care. Toronto: Butterworths; 1984. p. 17. [Google Scholar]
- Eyles J, Birch S. “A Population Needs-based Approach to Health-Care Resource Allocation and Planning in Ontario: A Link between Policy Goals and Practice?”. Canadian Journal of Public Health. 1993;84:112–7. [PubMed] [Google Scholar]
- Eyles J, Birch S, Chambers S, Hurley J, Hutchison B. “A Needs-based Methodology for Allocating Health Care Resources in Ontario, Canada: Development and an Application.”. Social Science and Medicine. 1991;33:489–500. doi: 10.1016/0277-9536(91)90331-6. [DOI] [PubMed] [Google Scholar]
- Forrest CB, Reid RJ. “Prevalence of Health Problems and Primary Care Physicians' Specialty Referral Decisions.”. Journal of Family Practice. 2001;50:427–32. [PubMed] [Google Scholar]
- Forster DP. “Mortality, Morbidity, and Resource Allocation.”. Lancet. 1977;1:997–8. doi: 10.1016/s0140-6736(77)92291-7. [DOI] [PubMed] [Google Scholar]
- Fowles J, Weiner JP, Knudson D, Fowler E, Tucker AM, Ireland M. “Taking Health Status into Account When Setting Capitation Rates: A Comparison of Risk-Adjustment Methods.”. Journal of the American Medical Association. 1996;276:1316–21. [PubMed] [Google Scholar]
- Frohlich N, Fransoo R, Roos NP. Indicators of Health Status and Health Service Use for the Winnipeg Regional Health Authority. Winnipeg: Manitoba Centre for Health Policy and Evaluation; 2001. [Google Scholar]
- Frohlich N, Mustard C. “A Regional Comparison of Socioeconomic and Health Indices in a Canadian Province.”. Social Science and Medicine. 1996;42:1273–81. doi: 10.1016/0277-9536(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Gissler M, Rahkonen O, Jarvelin MR. “Social Class Differences in Health until the Age of Seven Years among the Finnish 1987 Birth Cohort.”. Social Science and Medicine. 1998;46:1552. doi: 10.1016/s0277-9536(98)00013-6. [DOI] [PubMed] [Google Scholar]
- Green B, Barlow J, Newman C. “Ambulatory Care Groups and the Profiling of Primary Care Physician Resource Use: Examining the Application of Case-Mix Adjustments.”. Journal of Ambulatory Care Management. 1997;19:90–7. doi: 10.1097/00004479-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Hafner-Eaton C. “Physician Utilization Disparities between the Uninsured and Insured: Comparisons of the Chronically Ill, Acutely Ill, and Well Nonelderly Populations.”. Journal of the American Medical Association. 1993;83:787–92. [PubMed] [Google Scholar]
- Hornbrook MC, Goodman MJ. “Assessing Relative Health Plan Risk with the RAND-36 Health Survey.”. Inquiry. 1995;32:56–74. [PubMed] [Google Scholar]
- Hurley J, Hutchison B, Giacomini M, Birch S, Dorland J, Reid R, Pizzoferrato G. Policy Considerations in Implementing Capitation for Integrated Health Systems. Ottawa ON: Canadian Health Services Research Foundation; 1999. [Google Scholar]
- Hutchison B, Hurley J, Reid R, Dorland J, Birch S, Giacomini M, Pizzoferrato G. Capitation Formulae for Integrated Health Systems: A Policy Synthesis. Ottawa ON: Canadian Health Services Research Foundation; 1999. [Google Scholar]
- Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Ann Arbor MI: Health Administration Press; 1994. [Google Scholar]
- Institute of Medicine. Leading Health Indicators for Healthy People 2010. Washington DC: National Academy Press; 1999. [Google Scholar]
- Jacobs P, Shanahon M, Roos N, Farnsworth M. Cost List for Manitoba Health Services. Winnipeg: Manitoba Centre for Health Policy and Evaluation; 1999. [Google Scholar]
- Johns Hopkins University School of Hygiene and Public Health. The Johns Hopkins University Case-Mix Adjustment System: Documentation for PC-DOS and UNIX, Version 4.1. Baltimore MD: Johns Hopkins University; 1988. [Google Scholar]
- Johns Hopkins University School of Hygiene and Public Health. ACG Software Documentation and Users Manual (version 4.5) Baltimore MD: Johns Hopkins University; 2000. “The ACG Assignment Process.”; pp. 27–46. [Google Scholar]
- Judge K, Mays N. “Allocating Resources for Health and Social Care in England.”. British Medical Journal. 1994;308:1363–6. doi: 10.1136/bmj.308.6940.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn HA, Sempos CT. Statistical Methods in Epidemiology. New York: Oxford University Press; 1989. [Google Scholar]
- Kronick RT, Dreyfus LL, Zhou Z. “Diagnostic Risk Adjustment for Medicaid: The Disability Payment System.”. Health Care Financing Review. 1996;17:7–33. [PMC free article] [PubMed] [Google Scholar]
- Mays N. “NHS Resource Allocation after the 1989 White Paper: A Critique of the Research for the RAWP Review.”. Community Medicine. 1989;11:173–86. doi: 10.1093/oxfordjournals.pubmed.a042466. [DOI] [PubMed] [Google Scholar]
- Mays N. “Geographical Resource Allocation in the English National Health Service 1971–94: The Tension between Normative and Empirical Approaches.”. International Journal of Epidemiology. 1995;24:96–102. doi: 10.1093/ije/24.supplement_1.s96. [DOI] [PubMed] [Google Scholar]
- Mays N, Bevan G. Resource Allocation in the Health Service, Occasional Paper in Social Administration 81. London: Bedford Square Press; 1986. [Google Scholar]
- Montgomery LE, Kiely JL, Pappas G. “The Effects of Poverty, Race, and Family Structure on U.S. Children's Health: Data from the NHIS, 1978 through 1980 and 1989 through 1991”. American Journal of Public Health. 1996;86:1405. doi: 10.2105/ajph.86.10.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard CA, Frohlich N. “Socioeconomic Status and the Health of the Population.”. Medical Care. 1995;33(supplement):DS43. doi: 10.1097/00005650-199512001-00007. [DOI] [PubMed] [Google Scholar]
- Newbold BK, Eyles J, Birch S. “Equity in Health Care: Methodological Contributions to the Analysis of Hospital Utilization in Canada.”. Social Science and Medicine. 1995;40:1181–92. doi: 10.1016/0277-9536(94)00229-m. [DOI] [PubMed] [Google Scholar]
- Orueta JF, Lopez-DeMunain J, Baez K, Airzaguena JM, Aranguren JL, Pedrerae E. “Application of Ambulatory Care Groups in the Primary Care of a European National Health Care System—Does It Work?”. Medical Care. 1999;37:238–48. doi: 10.1097/00005650-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Palmer S, West P, Patrick D, Glynn M. “Mortality Indices in Resource Allocation.”. Community Medicine. 1979;1:275–81. doi: 10.1007/BF02549239. [DOI] [PubMed] [Google Scholar]
- Powe NR, Weiner J, Starfield B, Stuart ME, Baker A, Steinwachs D. “Systemwide Performance in a Medicaid Program: Profiling the Care of Patients with Chronic Illness.”. Medical Care. 1996;34:798–810. doi: 10.1097/00005650-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Reid RJ, MacWilliam L, Verhulst L, Roos N, Atkinson M. “Performance of the ACG Case-Mix System in Two Canadian Provinces.”. Medical Care. 2001;36:86–99. doi: 10.1097/00005650-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Roos LL, Roos NP, Cageorge SM, Nichol JP. “How Good Are the Data? Reliability of One Health Care Data Bank.”. Medical Care. 1983;20:266–76. doi: 10.1097/00005650-198203000-00003. [DOI] [PubMed] [Google Scholar]
- Roos LL, Sharp SM, Wajda A. “Assessing Data Quality: A Computerized Approach.”. Social Science and Medicine. 1989;28:175–82. doi: 10.1016/0277-9536(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Roos NP, Black C, Roos LL, Frohlich N, DeCoster C, Mustard C, Brownell MD, Shanahan M, Fergusson P, Toll F, Carriere KC, Burchill C, Fransoo R, MacWilliam L, Bogdanovich B, Frieson D. “Managing Health Services: How the Population Health Information System (POPULIS) Works for Policymakers.”. Medical Care. 1999;37:JS27–41. doi: 10.1097/00005650-199906001-00007. [DOI] [PubMed] [Google Scholar]
- Roos NP, Black CD, Frohlick N, DeCoster C, Cohen MM, Tataryn DJ, Mustard CA, Toll F, Carriere KC, Burchill CA, MacWilliam L, Bogdanovich B. “A Population-based Health Information System.”. Medical Care. 1995;33:DS13–20. doi: 10.1097/00005650-199512001-00005. [DOI] [PubMed] [Google Scholar]
- Roos NP, Fransoo R, Bogdanovich B, Frohlich N, Carrier KC, Patton D, Wall R. Needs-based Planning for Manitoba's Generalist Physicians. Winnipeg: Manitoba Centre for Health Policy and Evaluation; 1996. [Google Scholar]
- Roos NP, Mustard CA. “Variation in Health and Health Care Use by Socioeconomic Status in Winnipeg, Canada: Does The System Work Well? Yes and No.”. Milbank Quarterly. 1997;75:89–111. doi: 10.1111/1468-0009.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos NP, Roos LL. “Small Area Variations, Practice Style and Quality of Care.”. In: Evans RG, Barer M, Marmor T, editors. Why are Some People Healthy and Others Not? The Determinants of Health of Populations. New York: Aldine de Gruyter; 1994. pp. 231–52. [Google Scholar]
- Salem-Schatz S, Moore G, Rucker M, Pearson SD. “The Case for Case-Mix Adjustment in Practice Profiling.”. Journal of the American Medical Association. 1994;272:871–4. [PubMed] [Google Scholar]
- Starfield B. Primary Care: Balancing Health Needs, Services and Technology. New York: Oxford University Press; 1998. “Morbidity in Primary Care.”. [Google Scholar]
- Starfield B, Powe NR, Weiner J, Stuart ME, Steinwachs D, Scholle S, Gerstenberger A. “Costs Versus Quality in Different Types of Primary Care Settings.”. Journal of the American Medical Association. 1994;272:1903–8. [PubMed] [Google Scholar]
- Starfield B, Weiner J, Mumford L, Steinwachs D. “Ambulatory Care Groups: A Categorization of Diagnoses for Research and Management.”. Health Services Research. 1991;26:53–74. [PMC free article] [PubMed] [Google Scholar]
- Townsend P. Deprivation and Ill Health: New Scientific Evidence and the Implications for Policy. Southampton UK: Institute for Health Policy Studies; 1990. [Google Scholar]
- Tucker AM, Weiner J, Honigfeld S, Parton RA. “Profiling Primary Care Physician Resource Use: Examining the Application of Case Mix Adjustment.”. Journal of Ambulatory Care Management. 1996;19:60–80. doi: 10.1097/00004479-199601000-00006. [DOI] [PubMed] [Google Scholar]
- U.S. General Accounting Office. A Health Status Indicator for Targeting Federal Aid to States. Washington DC: U.S. General Accounting Office; 1996. [Google Scholar]
- Van Houten HK, Naessens JM, Evans RW, Rademacher DM. Paper presented at Association for Health Services Research Annual Meeting. Chicago IL: 1998. “Abstract. Assessment of Alternative Outlier Adjustments in the Creation of ACG Weights.”. [Google Scholar]
- Verhulst LB, Reid RJ, Forrest CB. “Hold It My Patients Are Sicker! The Importance of Case-Mix Adjustment to Practitioner Profiles in British Columbia.”. British Columbia Medical Journal. 2001;43:344–9. [Google Scholar]
- Weiner J, Dobson A, Maxwell S, Coleman K, Starfield B, Anderson G. “Risk-Adjusted Medicare Capitation Rates Using Ambulatory and Inpatient Diagnoses.”. Health Care Financing Review. 1996;17:77–99. [PMC free article] [PubMed] [Google Scholar]
- Weiner J, Starfield B, Steinwachs D, Mumford L. “Development and Application of a Population-Oriented Measure of Ambulatory Care Case-Mix.”. Medical Care. 1991;29:452–72. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Weiner J, Tucker AM, Collins AM, Fakhraei H, Lieberman R, Abrams C, Trapnell DG, Folkmer JG. “The Development of a Risk-Adjusted Capitation Payment System for Medicaid MCOs: The Maryland Model.”. Journal of Ambulatory Care Management. 1998;21(4):29–52. doi: 10.1097/00004479-199810000-00003. [DOI] [PubMed] [Google Scholar]
- Wennberg G. The Dartmouth Atlas of Health Care in the United States 1999. Chicago IL: American Hospital Association; 2000. [Google Scholar]
- West P. “Health Inequalities in the Early Years: Is There Equalization in Youth?”. Social Science and Medicine. 1997;44:833–58. doi: 10.1016/s0277-9536(96)00188-8. [DOI] [PubMed] [Google Scholar]



