Abstract
Background
Continence services in the UK have developed at different rates within differing care models, resulting in scattered and inconsistent services. Consequently, questions remain about the most cost-effective method of delivering these services.
Aim
To evaluate the impact of a new service led by a continence nurse practitioner compared with existing primary/secondary care provision for people with urinary incontinence and storage symptoms.
Design of study
Randomised controlled trial with a 3- and 6-month follow-up in men and women (n = 3746) aged 40 years and over living in private households (intervention [n = 2958]; control [n = 788]).
Setting
Leicestershire and Rutland, UK.
Method
The continence nurse practitioner intervention comprised a continence service provided by specially trained nurses delivering evidence-based interventions using pre-determined care pathways. They delivered an 8-week primary intervention package that included advice on diet and fluids; bladder training; pelvic floor awareness and lifestyle advice. The standard care arm comprised access to existing primary care including GP and continence advisory services in the area. Outcome measures were recorded at 3 and 6 months post-randomisation.
Results
The percentage of individuals who improved (with at least one symptom alleviated) at 3 months was 59% in the intervention group compared with 48% in the standard care group (difference of 11%, 95% CI = 7 to 16; P<0.001) The percentage of people reporting no symptoms or ‘cured’ was 25% in the intervention group and 15% in the standard care group (difference of 10%, 95% CI = 6 to 13, P = 0.001). At 6 months the difference was maintained. There was a significant difference in impact scores between the two groups at 3 and 6 months.
Conclusions
The continence nurse practitioner-led intervention reduced the symptoms of incontinence, frequency, urgency and nocturia at 3 and 6 months; impact was reduced; and satisfaction with the new service was high.
Keywords: costs and cost analysis, randomised controlled trials, urinary incontinence
INTRODUCTION
Continence services in the UK show considerable geographical variation: they remain fragmented and inconsistent, with differences in clinical input and lack continuity of care.1 Mode of service delivery is an important factor which affects patient outcomes.2 Recent policy changes in the UK have meant that continence services fall into the remit of primary care trusts through individual health improvement programmes.3 Ideally, services should be efficient and cost-effective, cross the primary/secondary care interface providing a seamless service for the individual who has sought help for these embarrassing symptoms.
There have been few systematic evaluations of service interventions for urinary symptoms.4-6 The Leicestershire Medical Research Council clinical intervention studies were conceived with the idea that consistent clinical care could be provided when delivered by a specially trained continence nurse practitioner following pre-determined care pathways.7-9
We conducted a randomised controlled trial to establish the clinical and cost-effectiveness, in both the short- and medium-term, of a new continence nurse practitioner-led service versus existing care for individuals reporting urinary symptoms. The study incorporated three further unpublished randomised controlled trials (reported elsewhere): a trial of topical oestrogen in post-menopausal women nested within the first 3 months of the continence nurse practitioner trial and two separate randomised controlled trials of treatments for detrusor overactivity and urodynamic stress incontinence nested between the 3 and 6 month outcomes.
METHOD
This was a randomised controlled trial with independent outcomes recorded at baseline, 3 and 6 months. The trial was undertaken in Leicestershire and Rutland, from April 1998 to September 2000. Men and women aged 40 years and over (n = 158 447) living in private households were randomly sampled by household from the Family Health Service Authority registers of participating general practices (71% of all practices in the counties participated). They were mailed an eight-page postal questionnaire about urinary symptoms developed for the study.10 Response rate was 57%. A detailed study into non-response demonstrated little non-response bias11 in the reporting of urinary symptoms.
Eligibility criteria
Eligibility criteria for entry to the trial were one or more of the following symptoms reported in the postal questionnaire: incontinence several times per month or more, or several times a year plus reported impact of symptoms on quality of life;12 frequency, hourly or more, or 2-hourly plus impact; nocturia, three times per night, or twice a night plus impact; or urgency, very strong or overwhelming, or strong with impact.
Individuals who met one or more of the eligibility criteria were sent a letter inviting them into the study (n = 29039, 32% of responders). Twenty-two per cent (n = 6207) of those mailed agreed to participate in a home interview that collected information on urinary symptoms, impact and general health. They were then invited to take part in the trial, provided they did not fit any of the exclusion criteria. Interviews were completed by 4385 individuals, of whom 3746 agreed to be randomised.
Exclusion criteria
Exclusion criteria determined at home interview were pregnancy, urinary fistula, pelvic malignancy and those currently receiving treatment for urinary symptoms (such as being on a waiting list for continence surgery).
How this fits in
Continence services in the UK are fragmented and inconsistent, and few individuals seek help for urinary symptoms.The costs of urinary symptoms to the NHS are substantial (estimates suggest that 1% of the NHS annual budget is spent on urinary symptoms for this age group). This is the first study to rigorously evaluate continence services across the primary/secondary care interface and to examine all bothersome storage symptoms including: frequency, urgency and nocturia as well as incontinence. This study describes the components of an effective continence service that could be adopted nationally.
Randomisation
Randomisation to the trial was undertaken by household, at a ratio of 4:1 in favour of the continence nurse practitioner. This allocation was necessary in order to ensure sufficient numbers in the nested trials for detrusor overactivity and urodynamic stress incontinence. The statistical programme SAS was used to generate the random allocation sequence and was implemented using sealed envelopes, numbered sequentially. Patients were enrolled into the study by trained interviewers at the end of the baseline home interview after exclusion criteria had been applied. Secure randomisation was ensured using centrally allocated sealed envelopes that were opened by the interviewer in the presence of the interviewee; patients were assigned to either the intervention arm or the standard care arm of the trial. It was not possible for the patient or interviewer to be blinded to the allocation group. This study was undertaken in accordance with the Medical Research Council guidelines for good clinical practice in clinical trials.13
The intervention
The intervention comprised a continence service provided by specially trained nurses delivering evidence-based interventions using pre-determined care pathways.7-9 A total of 21 nurses undertook the training programme. Each nurse recruited was a registered nurse with 2–10 years post-registration experience. The objective of the training module was to integrate theoretical and clinical skills to enable the nurses to independently assess, diagnose, treat and monitor individuals with urinary symptoms. The module enabled them to use discriminatory and analytical skills to undertake and interpret urodynamic tests. The module is described fully elsewhere.7 The continence nurse practitioners were able to cross traditional primary/secondary boundaries in the provision of care offering a seamless service which included urodynamic testing.
Standard care comprised access to general practice services and existing continence services in the area. During the study period, between 3 to 4 full-time continence advisors were in post. A team of 30–40 continence link nurses were identified (drawn largely from the district nursing team) in Leicestershire and Rutland. Individuals randomised to standard care were provided with a leaflet detailing how to access these services via the existing continence service or their general practice. Data were collected by home interview on: whether or not a healthcare professional was consulted; which health professional was consulted; what investigations were undertaken; and what treatments they received. A trained interviewer, independent of the intervention providers, collected this information.
In the intervention arm all patients were seen over an 8-week treatment period, with four planned visits. Each patient underwent a 1-hour assessment visit, in which a clinical history was taken, including the social and psychological impact of symptoms on their life. In addition, a physical examination was undertaken, including: mid-stream specimen of urine; estimation of post-void residual volume; blood pressure; body mass index; and in women, a vaginal examination. All patients were instructed to complete a 3-day urinary diary, and a 24-hour pad test. Patients were seen 1 week after provision of the diary and pad test, which were then reviewed and a treatment regime commenced. Treatments were offered to all patients and included advice on diet and fluids; bladder training; pelvic floor awareness and healthy eating; and treatment for candida and urinary tract infection where indicated. A further follow-up visit was scheduled when advice and therapy was re-enforced, before a final clinical assessment was undertaken. Individuals whose symptoms persisted after this primary intervention were offered urodynamic investigation before entry into randomised controlled trials specifically for urodynamic stress incontinence and detrusor overactivity (354 of the individuals were included in the 6-month analysis).
Outcome measures
An independent home interview was undertaken at baseline (just prior to the start of the intervention), at 3 months (after the primary care intervention period) and at 6 months (after any secondary care intervention). The primary outcome measure was improvement in one or more symptoms, of which cure (no symptoms) is a subset that was assessed using validated symptom severity questions,14 comprising the symptoms of incontinence, urgency, frequency and nocturia. Secondary outcome measures included: number of symptoms alleviated, a validated impact on quality of life scale12 (developed for the study — the scale ranged from 0 to 42, with 21 items and a maximum score of 2 on each item), patient perception of problem, cost-effectiveness and satisfaction with the service.
Sample size calculation
We aimed to recruit 5700 individuals which, allowing for 10% drop-out, would enable a minimum clinically significant difference in the proportion of individuals who improved in one or more of their symptoms of 10% (from 30% to 40%) to be detected at the 1% significance level with over 99% power. The power of the trial was designed to enable a sufficient number of patients to be recruited into the two further trials nested within the longer term follow-up of the intervention arm.
Statistical analysis
Effectiveness of the intervention was analysed by ‘intention to treat’. Groups were compared at both 3 and 6 months. Results were expressed as absolute differences between observed proportion of individuals with each symptom in the two groups and proportions of individuals who improved (together with corresponding 95% confidence interval [CI]). Satisfaction with services was reported descriptively. A χ2 test was used to test the difference in proportions and a t-test on the absolute difference in impact score from baseline was used for analysing impact score.
Economic evaluation
Questions on resource use were asked during the interview of a subset of responders. Questions included: contacts with health service providers in relation to urinary symptoms; and use of consumables, such as padding and prescription medicines. The costs of service provision by GPs, practice nurses, physiotherapists, occupational therapists and hospital doctors were taken from published national cost data.15 For the intervention service unit costs were estimated. All costs are for the year 2000–2001. The outcomes used to measure effectiveness were: mean number of symptoms alleviated at 3 and 6 months; and number of cases improved compared to baseline. The difference in the mean outcome at 3 and 6 months was calculated from the economic sub-sample, and cost-effectiveness was calculated by dividing the difference in the mean costs by the mean difference in outcome.
RESULTS
Of the 4385 individuals eligible to be randomised into the trial, 3746 (85.4%) agreed to participate and were randomised. Figure 1 shows the flow of patients through the study.
Figure 1.
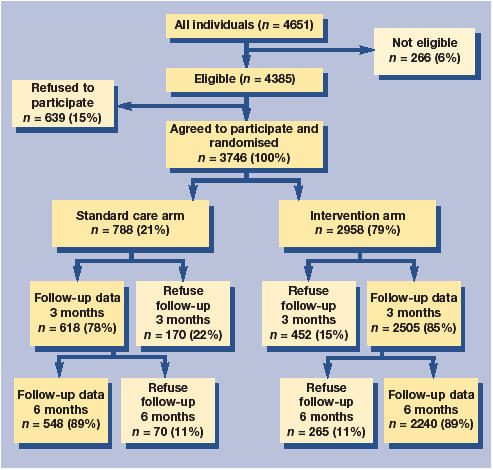
Progress of participants through trail.
Baseline characteristics of intervention, control (with and without follow-up data) and non-participant groups were compared for demographic variables, urinary symptoms and impact. Trial groups were found to be comparable at baseline (Table 1), but the non-participant group tended to be older, and to report fewer urinary symptoms and less impact from symptoms.
Table 1.
Baseline demographics and clinical characteristics.
| Randomised | |||
|---|---|---|---|
| Intervention | Standard care | Non-participants | |
| n = 2958 | n = 788 | n = 639 | |
| Age group (years) | |||
| 40–49 (%) | 578 (20) | 156 (20) | 73 (11) |
| 50–59 (%) | 838 (28) | 206 (26) | 130 (20) |
| 60–69 (%) | 825 (28) | 221 (28) | 188 (29) |
| 70–79 (%) | 560 (19) | 162 (21) | 165 (26) |
| ≥80 (%) | 157 (5) | 43 (6) | 83 (13) |
| Female (%) | 1838 (62) | 461 (59) | 316 (50) |
| Ethnic group white (%)a | 2797 (95) | 740 (94) | 593 (93) |
| Long-term illness (%) | 1140 (39) | 298 (38) | 261 (41) |
| Symptoms | |||
| Leakage (%)b | 2392 (82) | 618 (79) | 379 (59) |
| Frequency (%)c | 1563 (53) | 376 (48) | 187 (29) |
| Urgency (%)d | 1927 (65) | 529 (67) | 240 (38) |
| Nocturia (%)e | 1070 (36) | 285 (36) | 189 (30) |
| Impact Median impact score (IQR)f | 5 (2 to 10) | 4 (2 to 9) | 1 (0 to 3) |
| Self-report | |||
| Mild or no problem (%)g | 1560 (53) | 427 (54) | 530 (84) |
| Satisfied with current urinary symptoms for rest of life (%)h | 888 (30) | 270 (34) | 438 (69) |
IQR = interquartile range.
<1% missing (n = 5).
<1% missing (n = 27).
<1% missing (n = 20).
<1% missing (n = 14).
<1% missing (n = 15).
12% missing (n = 451).
<1% missing (n = 16).
1% missing (n = 35).
Primary outcome
Urinary symptoms at 3 months. The percentage of individuals who improved (at least one symptom alleviated) at 3 months was 59% in the intervention group and 48% in the standard care group (difference of 11%, 95% CI = 7 to 16; P<0.001) (Table 2). At 3 months the proportion of people reporting no symptoms or ‘cured’ was 25% in the intervention group and 15% in the standard care group (difference of 10%, 95% CI = 6 to 13, P = 0.001) (Table 2). Individuals in the intervention group were less likely to report presence of urinary incontinence symptoms compared to the standard care group. Leakage was reported by 63% in the intervention group and 70% in the standard care group (difference of 7%, 95% CI = 3 to 11, P = 0.002). Similar reductions were also reported for frequency, urgency and nocturia (Table 2).
Table 2.
Number of individuals with each symptom and no symptoms at 3 and 6 months presented by randomisation group.
| Intervention | Standard care | ||||
|---|---|---|---|---|---|
| Total responders (n) | Individuals with symptom/event (n) | Total responders (n) | Individuals with symptom/event (n) | Difference (95% CI; P value) | |
| 3 months | |||||
| Leakage (several times per month) | 2483 | 1567 (63%) | 612 | 428 (70%) | −7% (−11 to −3; P = 0.002) |
| Frequency (≥ once hourly) | 2428 | 723 (30%) | 598 | 219 (37%) | −7% (−11 to −2; P = 0.001) |
| Urgency: very strong or overwhelming | 2503 | 819 (33%) | 618 | 248 (40% | −7% (−12 to −3; P = 0.001) |
| Nocturia (≥3 times per night) | 2502 | 497 (20%) | 617 | 164 (27%) | −7% (−11 to −3; P<0.001) |
| Improvement | 2378 | 1417 (60%) | 584 | 281 (48%) | 11% (7 to 16; P<0.001) |
| No symptoms (cure) | 2378 | 591 (25%) | 584 | 88 (15%) | 10% (6 to 13; P<0.001) |
| 6 months | |||||
| Leakage (several times per month) | 2235 | 1362 (61%) | 546 | 356 (65%) | −4% (−9 to 0; P = 0.066) |
| Frequency (≥once hourly) | 2231 | 539 (24%) | 545 | 182 (33%) | −9% (−14 to −5; P<0.001) |
| Urgency: very strong or overwhelming | 2236 | 682 (31%) | 546 | 228 (42%) | −11% (−16 to −7; P<0.001) |
| Nocturia (≥3 times per night) | 2236 | 420 (19%) | 547 | 133 (24%) | −6 % (−9 to −2; P = 0.004) |
| Improvement | 2201 | 1369 (62%) | 536 | 277 (52%) | 11% (6 to 15; P<0.001) |
| No symptoms (cure) | 2201 | 624 (28%) | 536 | 104 (19%) | 9% (5 to 13; P<0.001) |
Quality of life, reported ‘problem’ and satisfaction at 3 months. Absolute change from baseline in the overall impact on quality of life scale was calculated as −3.57 (standard error [SE] = 0.13) for the intervention group and −2.65 (SE = 0.25) for the standard care group resulting in a absolute difference between the groups of −0.92 (95% CI = −1.48 to −0.37, P = 0.001). At 3 months, 74% in the intervention group reported no problem or a mild problem compared to 68% in the standard care group (difference of 6%, 95% CI = 2 to 10, P = 0.003) (Table 3). Fifty-two per cent in the intervention group reported satisfaction with their current urinary symptoms for the rest of their life compared to 45% in the standard care group (difference 7%, 95% CI = 3 to 12, P = 0.001). Individuals who had had contact with either service during the follow-up period were asked to complete a service satisfaction questionnaire (91% of the intervention group and 81% in the standard care group). Of individuals in the intervention group, 97% were satisfied with the service in general compared with 77% in the standard care group (difference of 19%, 95% CI = 15 to 23).
Table 3.
Number of individuals self reporting a mild problem or no problem and satisfaction with current urinary symptoms for rest of life at 3 and 6 months presented by randomisation group.
| Intervention | Standard care | ||||
|---|---|---|---|---|---|
| Total responders (n) | Individuals with symptom/event (n) | Total responders (n) | Individuals with symptom/event (n) | Difference (95% CI; P value) | |
| 3 months | |||||
| Mild or no problem | 2468 | 819 (74%) | 614 | 416 (68%) | 6% (2 to 10; P = 0.003) |
| Satisfied with current urinary symptoms for rest of life | 2498 | 1294 (52%) | 618 | 276 (45%) | 7% (3 to 12; P = 0.001) |
| 6 months | |||||
| Mild or no problem | 2181 | 1721 (79%) | 545 | 380 (70%) | 9% (5 to 13; P<0.001) |
| Satisfied with current urinary symptoms for rest of life | 2236 | 1428 (64%) | 546 | 289 (53%) | 11% (6 to 16; P<0.001) |
Outcomes at 6 months. The percentage of individuals who improved (with at least one symptom alleviated) at 6 months was 62% in the intervention arm compared with 52% in the standard care arm (difference 10%, 95% CI = 6 to 15, P = <0.001) (Table 2). At 6 months, the proportion of people reporting no symptoms or ‘cured’ was 28% in the intervention group and 19% in the standard care group (difference of 9%, 95% CI = 5 to 13, P = <0.001) (Table 2). Results at 6 months are similar to those reported at 3 months (Tables 2 and 3; Figures 2 and 3). with persistence of benefit remaining significant for all symptoms except leakage. The difference in self-reported problems and satisfaction with urinary symptoms increased.
Figure 2.
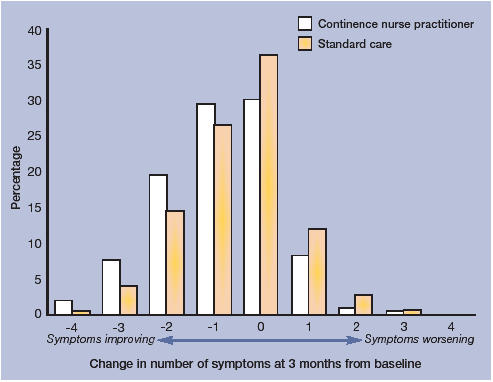
Number of symptoms alleviated at 3 months compared to baseline, presented by randomisation group.
Figure 3.
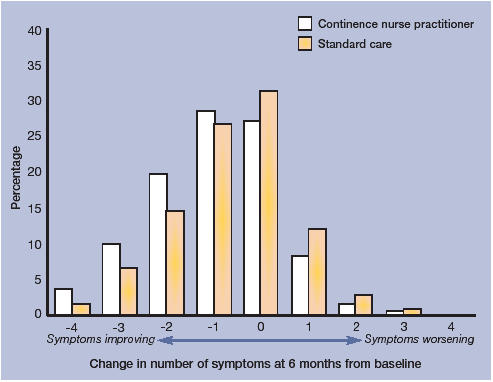
Number of symptoms alleviated at 6 months compared to baseline, presented by randomisation group.
At 6 months there was little change in impact (−4.21 (SE = 0.14) for the intervention group and −3.44 (SE = 0.29) for the standard care group), resulting in an absolute difference between the groups of −0.76 (95% CI = −1.37 to −0.15; P = 0.014).
Components of standard care compared to continence nurse practitioner care. Of those individuals randomised to standard care, 502 (81%) saw a healthcare professional about their urinary symptoms (compared to 2272 [91%] in the intervention arm). Of these, 380 (76%) saw a GP, 29 (6%) saw a hospital doctor, 188 (37%) saw a nurse (97 [19%] saw a continence nurse; 54 [11%] saw a practice nurse; and 37 [7%] a district nurse), and 6 (1%) saw a physiotherapist (an individual may have seen more than one healthcare professional).
Within standard care 208 (41%) of those who saw a healthcare professional were asked to provide a urine sample for testing, 79 (16%) underwent a bladder scan, 99 (20%) were asked to complete a urinary diary and six (1%) a pad test. In the intervention arm over 90% of participants were asked to complete these investigations.
In the standard care arm, 372 (74%) were given treatment or advice (compared to 95% in the intervention arm). Pelvic floor advice was given to 171 (34%) individuals, 43 (9%) received bladder training, 79 (16%) received medication, 41 (8%) were referred to the hospital for specialist care, 117 (23%) were given advice on fluid intake and 43 (8%) were told to return if their symptoms worsened.
Cost-effectiveness results
Subjects were included in the analysis if full costs and number of symptoms alleviated could be calculated at both the 3- and 6-month home interview. This meant that of the 1045 responders who completed resource data, 905 (87%) were included in the economic analysis: 171 in the standard care arm and 734 in the intervention arm.
Resources considered by the cost-effectiveness analysis were: GP visits; other healthcare professional contacts; medications; aids and appliances; investigations; and hospital transport. The average costs incurred were similar for the intervention and standard care groups at both 3 and 6 months for aids and appliances, the main differences between the groups were for health professional visits and investigations (details of these costs are given online in Supplementary Table 4). The results of the cost-effectiveness analysis are given in Table 4. Both cost and cost-effectiveness are higher at 6 months for improvement and symptoms alleviated. In the second 3-month period, costs generated by the continence nurse practitioner service were similar to the first 3-month period but the overall difference in the mean number of symptoms alleviated remained the same. This gives an incremental cost per additional symptom alleviated that was greater at 6 months (£488) than at 3 months (£242) (Table 4).
Table 4.
Results of cost-effectiveness study.
| Costs of all NHS-related contacts | Benefits in terms of number of symptoms alleviated | ||||||
|---|---|---|---|---|---|---|---|
| Intervention | Standard care | Difference | Intervention | Standard care | Difference | Cost-effectiveness (£) | |
| First 3 months | £129 | £40 | £89 | 0.922 | 0.556 | 0.367 | 242 |
| (95% CI) | (122.82–134.78) | (30.78–49.34) | (77.65–99.82) | (0.835–1.010) | (0.383–0.728) | (0.166–0.567) | |
| Total 6-month period | £252 | £73 | £179 | 1.01 | 0.643 | 0.366 | 488 |
| (95% CI) | (234.66–268.53) | (53.07–92.88) | (152.41–204.83) | (0.914–1.105) | (0.46–0.826) | (0.149–0.583) | |
| Benefits in terms of cases improved | |||||||
| First 3 months | 0.610 | 0.497 | 0.113 | 783 | |||
| (95% CI) | (0.575–0.646) | (0.422–0.572) | (0.03–0.197) | ||||
| Total 6-month period | 0.6226 | 0.5146 | 0.1080 | 1654 | |||
| (95% CI) | (0.588–0.658) | (0.439–0.59) | (0.025–0.191) | ||||
DISCUSSION
The new service resulted in a statistically and clinically significant reduction in the urinary symptoms of incontinence, urgency, frequency and nocturia at both 3 and 6 months, with an approximate 10% difference in improvement and cure rates between the two groups. In addition, impact on the individual was significantly reduced in users of the new service and higher levels of patient satisfaction were achieved. This is the first study to show the effectiveness of services on storage symptoms (rather than incontinence) and impact on quality of life. The public health value of a 10% reduction in symptoms is substantial when applied to such a common problem.
Comparison with existing literature
Our findings are consistent with clinical trials of urinary incontinence in specific female groups,16,17 which show differences of 5–7% and reflect findings in smaller studies of less representative populations.18,6.
The key features of this new service were the educational preparation of the nurses,7 the adherence to consistent evidence-based protocols,9 high patient contact leading to continuous therapy reinforcement and motivation, use of nurse-led urodynamic investigation at the primary/secondary care interface, and contact between the continence nurse practitioner and a specialist medical team. The new service was compared to standard care within Leicestershire (where existing services are well established); this may have limited the differences between the groups. In addition, individuals randomised to the standard care arm were given information on how to approach and contact standard services enabling the same ease of access to services as was offered in the intervention arm. Eighty-one per cent of patients randomised to standard care contacted services; three-quarters of these saw a GP, 37% saw a nurse (although only half of these saw a specialist continence nurse).
An issue of key interest is the value of a symptom alleviated. The primary outcome of improvement does not capture all the potential economic benefits, (improvement in one or four symptoms would both be an improvement although clearly of differing degrees): we therefore included the number of symptoms alleviated to allow us to capture the degree of improvement. Our research indicates that a nurse-led service can alleviate symptoms at a cost of £242 per symptom over a 3-month period or £488 per symptom over a 6-month period. Whether this represents value for money depends on the value placed upon these symptom changes by individuals and decision makers. However, alternative standard drug therapy for storage symptoms would pose an open ended financial commitment of £20–30 per month for medication costs alone (£240–360 annually).
Strengths and limitations of this study
As the study was evaluating a complex intervention,19 several interventions had to be assessed together. Sensitivity analysis was performed that investigated the effects of removing patients from the nurse trial who were also involved in the other trials (Supplementary Tables 1–4). Including or excluding these trial groups did not affect clinical outcomes regardless of whether all groups were excluded or only those in the control arms, but it did reduce the estimate of costs and cost-effectiveness, particularly at 6 months; providing a 3-month service in isolation would appear to be a more cost-effective service. However, this does not provide a true picture as those individuals who enter the nested trials after 3 months would tend to be those with more serious symptoms or who had benefited the least from the primary interventions. Development of a method to identify those who are unlikely to respond to primary interventions and ‘fast-track’ them directly to secondary care, may offer a more cost-effective treatment strategy.
The strengths of this pragmatic trial were; it was a large population-based study with a randomised controlled design, the proportion of available follow-up data was high and it used a realistic package of urinary storage symptoms, rather than incontinence alone.
A limitation of the study is that the cost-effectiveness analysis only covers a 6-month period. This does not value any costs and benefits of the service that occur outside this time frame. For example, further costs may occur in the standard care arm at a later stage. The new service requires a large up-front expenditure; the value of the benefits obtained from this will depend on their persistence over time. One of the key features of the new service was to speed up service provision and minimise waiting times, by doing so short-term costs would increase but symptoms would be alleviated more speedily. It is also important to recognise that the research component of the new service imposes higher initial start-up costs and longer consultation times than a service set up in normal clinical practice. Over time, therefore this cost may diminish. While we did not recruit the planned number of individuals, the absolute levels of improvement were higher than originally anticipated. The total number (2784) of patients with follow-up data at 6 months, would nevertheless enable a minimum clinically significant difference of 10% (from 50–60%) to be detected at the 1% significance level with over 95% power.
Implications for future research and clinical practice
The nature of the continence nurse practitioner-led service is both feasible and appropriate for wider implementation, making the results of this trial directly applicable to clinical care provision. We focused on men and women aged 40 years and over living in private households; notably, elderly people in residential care were not included, largely due to their need for specific interventions. They constitute an important priority group for future study. Also, those with mental health problems or learning disabilities present additional challenges. It should, however, be possible to adapt the new service to target these groups. In addition, it may be possible to augment the differences seen between the two arms by implementing more targeted lifestyle interventions to take account of comorbidities such as diabetes, and by addressing nutritional status.20,21
Implementation of this new service is effective in the reduction of symptoms of incontinence, frequency, urgency and nocturia in men and women aged 40 years and over. Management of such a service is critical. The role of the nurse consultant22 to lead such a service combining clinical expertise, high level training and leadership in the provision of integrated continence services may offer a blueprint for future implementation. A study is currently being undertaken to evaluate the long-term outcome and cost-effectiveness of the new service.
Acknowledgments
In addition to the authors the Leicestershire MRC Incontinence Study Team comprised: Usman Azam, Helen Dallosso, Madeleine Donaldson, Lesley Harris, Tom Hayward, Jennie Lucas, Peter Marsh, Gurminder Matharu, Fiona Mensah, Sarah Perry and Chris Sanders. We thank the individuals who participated in the trial and the nursing staff involved in the programme, particularly Christine Rippin.
Supplementary information
Additional information accompanies this article at http://www.rcgp.org.uk/journal/index.asp
Funding body
Medical Research Council (UK) (G9410491). Nicola J Cooper was funded by University Hospitals of Leicester (UHL) NHS Trust. David A Turner was funded by Trent Institute for Health Services Research
Ethics committee
Leicestershire Ethics Committee (3650)
Competing interests
None
REFERENCES
- 1.Milne JL, Moore KN. An exploratory study of continence care services worldwide. Int J Nurs Stud. 2003;40:235–247. doi: 10.1016/s0020-7489(02)00082-2. [DOI] [PubMed] [Google Scholar]
- 2.The Royal College of Physicians. Incontinence. Causes, management and provision of services. A Working Party of the Royal College of Physicians. J R Coll Physicians Lond. 1995;29:272–274. [PMC free article] [PubMed] [Google Scholar]
- 3.The Department of Health. The new NHS: modern, dependable. London: The Stationary Office; 1997. [Google Scholar]
- 4.Roe B, Wilson K, Doll H, Brooks P. An evaluation of health interventions by primary health care teams and continence advisory services on patient outcomes related to incontinence: volume 1, main report. Oxford: Health Services Research Unit of the University of Oxford; 1996. [Google Scholar]
- 5.Shields N, Thomas C, Benson K, et al. Development of a community nurse-led continence service. Br J Nurs. 1998;7(14):824–830. doi: 10.12968/bjon.1998.7.14.5634. [DOI] [PubMed] [Google Scholar]
- 6.Borrie MJ, Bawden M, Speechley M, Kloseck M. Interventions led by nurse continence advisors in the management of urinary incontinence: a randomised controlled trial. CMAJ. 2002;166:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 7.Williams K, Assassa RP, Smith NKG, et al. Educational preparation for specialist practice in continence care. Br J Nurs. 1999;8:1198–1207. doi: 10.12968/bjon.1999.8.18.6481. [DOI] [PubMed] [Google Scholar]
- 8.Williams KS, Assassa RP, Smith NKG, et al. Development, implementation and evaluation of a new nurse-led continence service: a pilot study. J Clin Nurs. 2000;9:566–5673. doi: 10.1046/j.1365-2702.2000.00386.x. [DOI] [PubMed] [Google Scholar]
- 9.Williams KS, Assassa RP, Smith N, et al. Good practice in continence care: development of a nurse-led service. Br J Nurs. 2002;11:548–559. doi: 10.12968/bjon.2002.11.8.10164. [DOI] [PubMed] [Google Scholar]
- 10.Perry S, Shaw C, Assassa P, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. Public Health Med. 2000;22:427–434. doi: 10.1093/pubmed/22.3.427. [DOI] [PubMed] [Google Scholar]
- 11.Dallosso HM, Matthews RJ, McGrother CW, et al. An investigation into nonresponse bias in a postal survey on urinary symptoms. BJU. 2003;91:631–636. doi: 10.1046/j.1464-410x.2003.04172.x. [DOI] [PubMed] [Google Scholar]
- 12.Shaw C, Matthews RJ, Perry SI, et al. Validity and reliability of a questionnaire to measure the impact of lower urinary tract symptoms on quality of life: the Leicester Impact Scale. Neurourol Urodyn. 2004;23:229–236. doi: 10.1002/nau.20017. [DOI] [PubMed] [Google Scholar]
- 13.Medical Research Council. MRC guidelines for good practice in clinical trials. Medical Research Council: London, 1998 http://www.mrc.ac.uk/pdf-ctg.pdf (accessed 4 August 2005)
- 14.Shaw C, Matthews RJ, Perry SI, et al. Validity and reliability of an interviewer-administered questionnaire to measure the severity of lower urinary tract symptoms of storage abnormality: the Leicester Urinary Symptom Questionnaire. BJU Int. 2002;90:205–215. doi: 10.1046/j.1464-410x.2002.02893.x. [DOI] [PubMed] [Google Scholar]
- 15.Netten A, Curtis L. Unit costs of health and social care 2000. Canterbury: Personal Social Services Research Unit, University of Kent; 2000. [Google Scholar]
- 16.Glazener CMA, Herbison GP, Wilson PD, et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial. BMJ. 2001;323:593–596. doi: 10.1136/bmj.323.7313.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiarelli P, Cockburn J. Promoting urinary continence in women after delivery: randomised controlled trial. BMJ. 2002;324:1241–1244. doi: 10.1136/bmj.324.7348.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien J, Austin M, Sethi P, O'Boyle P. Urinary incontinence: prevalence, need for treatment, and effectiveness of intervention by nurse. BMJ. 1991;303:1308–1312. doi: 10.1136/bmj.303.6813.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to explore health. BMJ. 2000;321:694. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92:69–77. doi: 10.1046/j.1464-410x.2003.04271.x. [DOI] [PubMed] [Google Scholar]
- 21.Dallosso H, Matthews R, McGrother C, Donaldson M. Diet as a risk factor for the development of stress urinary incontinence: a longitudinal study in women. Eur J Clin Nutr. 2004;58(6):920–926. doi: 10.1038/sj.ejcn.1601913. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health. Nurse, midwife and health visitor consultants: establishing posts and making appointments. London: The Stationery Office (Health Service Circulars); 1999. http://www.dh.gov.uk/assetRoot/04/01/22/27/04012227.pdf (accessed 4 August 2005) [Google Scholar]


