Abstract
The effects of long-term supplementation with pharmacologic doses of zinc oxide on serum levels of zinc, lipids and hematocrit have not been studied systematically to date. Eleven Clinical Centers enrolled 4757 participants from 1992 to 1998 as part of the Age-Related Eye Disease Study (AREDS). Of these, 3640 participants, aged 55–80 y, who had early-to-late age-related macular degeneration (AMD) were randomly assigned to daily supplementation with or without 80 mg of zinc as zinc oxide plus 2 mg of copper as cupric oxide to study the effects of zinc supplementation on the progression to late AMD. This paper reports on the effect of a 5-y supplementation with zinc oxide and cupric oxide on serum zinc, copper, lipids, and hematocrit for 717 participants from three clinical centers. At the 5-y exam, the median increase in serum zinc levels for participants assigned to zinc formulations was 17% compared with a 2% increase for participants not assigned to zinc (P < 0.001). The differential effect on serum zinc was observed at 1 y and remained fairly constant over the 5-y period. After 5 y, no significant differences in changes in serum hematocrit, copper or lipids were found between participants assigned to formulations containing zinc and copper, and those assigned to formulations without zinc and copper. Estimates from a modified Block Food-Frequency Questionnaire suggest the AREDS population at baseline had a zinc intake from diet similar to that of the general population.
Keywords: AREDS, zinc oxide, cupric oxide, serum cholesterol, randomized trial, humans
Decreases in serum zinc levels and zinc/copper ratios are associated with aging, surgery and a number of disease states (1–12). Whether decreased zinc levels contribute to poor health in elderly people or disease, or are effects of the conditions, remains uncertain. Use of zinc supplements in free-living populations has been discouraged by some health professionals because doses greater than the Recommended Daily Allowance (RDA)4 of 15 mg of supplemental zinc over several months have been reported to be associated with impairment of immune response (13) and a decline in serum HDL (14). Zinc also acts as a copper antagonist, inhibiting absorption and mobilization, which can result in zinc-induced copper deficiency (15) and consequent anemia. Severe symptoms of copper deficiency have been reported to occur with chronic ingestion of zinc at ≥100–300 mg/d (16).
Zinc supplementation at a level of 80 mg/d of zinc sulfate was found in a short-term pilot study to reduce the risk of vision loss in persons with age-related macular degeneration (AMD) (17). Few studies have reported the effect of long-term supplementation with zinc on lipoprotein profiles or the risk of anemia. Whether long-term supplementation with zinc at moderately high doses increases serum zinc levels or leads to alterations of other serum levels is unclear. This is a particularly important issue because over half of the elderly U.S. population uses dietary supplements, and high doses of zinc are marketed in supplements purported to promote eye health.
Data collected as part of the Age-Related Eye Disease Study (AREDS) allow us to examine the effect of 5 y of daily, moderately high dose, oral zinc oxide supplementation on serum levels of zinc, copper, hematocrit and cholesterol in 717 elderly, relatively well-nourished participants with early-to-late signs of age-related macular degeneration. The clinical trial ended in 2001, and results were published in October 2001 (18,19).
SUBJECTS AND METHODS
Enrollment
Between 1992 and 1998, 11 clinical centers enrolled 4757 participants into clinical trials of AMD and cataract as part of AREDS. The 3640 participants with AMD, defined by the presence of drusen or retinal pigment epithelial abnormalities, were part of a factorial design evaluating the effect of both a zinc formulation and a vitamin/antioxidant formulation (Table 1) on progression to advanced AMD. To be eligible for enrollment, participants had to agree to stop using any supplements containing the study ingredients. To standardize supplemental nutrient intake among multivitamin supplementors, participants who wanted to take or continue to take a multivitamin were provided with Centrum (White-hall-Robins Healthcare, Madison, NJ), a multivitamin and mineral supplement containing RDA-type dosages. The study was double-masked, i.e., neither the participant nor the study’s clinicians were aware of the treatment assignment. The four treatment interventions were given as an oral total daily supplementation of antioxidants (500 mg of vitamin C, 400 iu of vitamin E, and 15 mg β-carotene), or zinc [80 mg of zinc as zinc oxide and 2 mg of copper as cupric oxide to prevent potential anemia (15)], or the combination of antioxidants and zinc, or placebo (containing no multivitamins or minerals). Compliance was measured at each clinic visit by estimated pill count from returned study medication bottles. Details of the study design have been reported elsewhere (20).
TABLE 1.
AREDS AMD clinical trial design and participants with blood collection and follow-up, by treatment1
| Total | Placebo | Antioxidants | Zinc | Antioxidants plus zinc | |
|---|---|---|---|---|---|
| n | |||||
| Randomized | 3640 | 903 | 945 | 904 | 888 |
| Blood collected | 851 | 205 | 215 | 237 | 194 |
| 5-y follow-up | 717 | 166 | 181 | 202 | 168 |
AREDS, Age-Related Eye Disease Study; AMD, age-related macular degeneration.
Specimen collection and analysis
Three of the eleven AREDS clinical centers enrolled 868 participants between 55 and 80 y of age with early-to-late AMD, who were randomly assigned to supplements containing zinc or no zinc. A baseline zinc specimen was collected from 851 of these participants. This report is restricted to the 717 of these 851 participants with at least 5 y of follow-up data (Table 1). Participants consented to baseline and annual blood drawing to measure serum levels of antioxidants, zinc and copper as well as total and HDL cholesterol, triglycerides and hematocrit. Blood samples were sent to the AREDS Central Laboratory (the Centers for Disease Control in Atlanta) for measuring levels of lipids, antioxidants and zinc using methods described in the AREDS Manual of Operations (21). Serum copper and zinc are measured by inductively coupled plasma-atomic emission spectroscopy on a JY 70 plus Sequential and Simultaneous Inductively Coupled Plasma-Emission Spectrometer (Instruments SA, Edison, NJ). Serum (0.5 mL) is diluted with 4.5 mL of 0.1 mol/L HCl containing 1 mg/L yttrium. The sample is aspirated into an argon plasma whose temperature is ~8000°K. The intensity of copper is measured at the 324.754-nm wavelength and that of zinc is measured at the 213.856-nm wavelength. Total cholesterol was measured on the Abbott Spectrum Analyzer CCX (Abbott Laboratories, Diagnostics Division, Abbott Park, IL). In this procedure, cholesterol esters in serum are hydrolyzed to free cholesterol by cholesterol esterase. The cholesterol produced is oxidized by cholesterol oxidase in a reaction that results in the formation of hydrogen peroxide. Hydrogen peroxide reacts with 4-aminoantipyrine and phenol in the presence of peroxidase to yield a quinoneimine dye that absorbs at 500 nm. Serum HDL cholesterol was measured using the same procedure after precipitation of the other lipid fractions with a solution of dextran sulfate and magnesium.
Triglycerides were also measured with the Abbott Spectrum Analyzer CCX using a correction for free glycerol concentration. In this procedure, serum triglycerides are converted to glycerol and free fatty acids by lipoprotein lipase. The glycerol is then converted to glycerol-3-phosphate by glycerol kinase in the presence of ATP. The glycerol-3-phosphate is reacted with oxygen in the presence of glycerol-3-phosphate-oxidase to form hydrogen peroxide. This in turn forms a colored complex with aminoantipyrine and chlorophenol in the presence of peroxidase. The intensity of absorbance of the chromophore is directly proportional to the total triglyceride concentration in the sample and is measured spectrophotometrically at 500 nm.
Hematocrit was measured by each Clinical Center’s local laboratory at the participant’s initial visit and annually thereafter. LDL cholesterol was calculated using the Friedewald equation: LDL = total cholesterol − HDL − (triglyceride/5). The Block Food-Frequency Questionnaire (FFQ) was modified and validated (22), and then administered at baseline to all participants (21). Zinc intake was calculated by evaluating the nutrient content per 100 g of each food on the questionnaire. For example, the nutrient content of zinc per 100 g of oysters is 90.81 mg, whereas the nutrient content of zinc per 100 g of peas is 0.94 mg. All participants were asked at enrollment and 5 y later to report on use of concomitant medications, including lipid-lowering medications.
Statistical analysis
Changes in serum levels over time were tested using the SAS procedure MIXED (SAS Institute, Cary, NC) to perform separate repeated-measures analyses on serum zinc, HDL cholesterol, total cholesterol, calculated LDL cholesterol, triglycerides, copper and hematocrit. Primary covariates include time (discrete “year” values), assignment to a zinc formulation and baseline quartile of serum zinc. Additional covariate adjustments were for age, gender, and body mass index (BMI) and the interaction between the treatment assignments antioxidants and zinc. No significant interaction between administration of zinc and antioxidant vitamins was found for any of the outcome measures; this analysis and report considers only the contribution of trace metals, without regard to antioxidant supplementation.
The quartile distributions of serum levels of zinc, copper, lipids and hematocrit were calculated using the SAS procedure UNIVAR-IATE. Baseline serum zinc is grouped into three categories defined by the upper, middle and lower quartiles; 25% of the distribution is less than the low quartile (Low), 50% between the low and high quartile (Middle 50%) and 25% greater than the high quartile (High). The percentage of change in serum zinc at 5 y by baseline zinc quartile group, and baseline cholesterol measures by baseline zinc quartile group, are represented by box plots. In a box plot, the horizontal line in the box is the median; the lower and upper edge of the box are the low and high quartile, respectively; the lower and upper vertical lines extend to approximately the 5th and 95th percentiles; the asterisks and circles indicate extreme and more extreme values, respectively. Off-center location of the median in the box or vertical lines of unequal length indicate an asymmetrical distribution.
RESULTS
Baseline characteristics of the 717 participants included in this analysis are provided in Table 2. Baseline characteristics were balanced by treatment assignment to zinc or no zinc formulations (data not shown). These participants were similar at baseline to the entire AREDS population enrolled in the AMD trial with respect to gender, age, BMI and reported zinc dietary intake (Table 2). The characteristics of the 134 participants without 5 y of followup were also similar (data not shown). The median age of the 717 participants at baseline was 69 y, 59% were female and median BMI was 26.6 kg/m2. The median daily zinc intake as estimated from the FFQ was 10.4 mg for men and 7.5 mg for women. The age-adjusted median zinc intake of the Third National Health and Nutrition Examination Survey (NHANES III) participants was similar, 10.3 mg for men and 7.8 mg for women (23). Only 13% of the participants in this analysis consumed at least the RDA (15.0 mg) of zinc by diet alone. Of the 717 participants examined, 41% were supplementing with zinc or a multivitamin/mineral for at least 1 y before joining the study, and 97% of this group chose to take Centrum. In addition, although not encouraged, an additional 25% who were not taking zinc supplements or a multivitamin/mineral before the start of the study chose to take Centrum. In all, 65% of participants chose to supplement with Centrum, from which they received 15 mg of zinc as zinc oxide. The choice to supplement with Centrum was similar across treatment groups. At 5 y, 79% of the participants took at least 75% of their study tablets.
TABLE 2.
Characteristics of participants with 5-y serum zinc measures vs. characteristics of all AREDS participants in the AMD clinical trial
| Characteristic | 5-y serum zinc | All participants |
|---|---|---|
| Total participants, n | 717 | 3640 |
| Women, % | 59 | 56 |
| Median age, y | 69 | 69 |
| Age range, y | 55–80 | 55–80 |
| Completed high school, % | 93 | 90 |
| Caucasian, % | 97 | 96 |
| Chose to take Centrum, % | 65 | 67 |
| Body mass index, kg/m2 | ||
| 25th percentile | 24.2 | 24.2 |
| Median | 26.6 | 26.8 |
| 75th percentile | 30.0 | 30.1 |
| Zinc intake1 | ||
| Total participants, n | 717 | 3635 |
| Intake,2 mg | 9.8 ± 0.2 | 9.4 ± 0.1 |
| 25th percentile | 6.3 | 5.9 |
| Median | 8.6 | 8.3 |
| 75th percentile | 11.8 | 11.4 |
Median baseline and 5-y serum levels, and percentage of change over 5 y are provided overall, as well as by gender, in Table 3 for each of the measures evaluated.
TABLE 3.
Median baseline and 5-y serum chemistry and median % changes for Age-Related Eye Disease Study (AREDS) participants by zinc assignment and gender
| Women
|
Men
|
Total
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline median | 5-y median | Median %change | Baseline median | 5-y median | Median %change | Baseline median | 5-y median | Median %change | |
| Zinc, μmol/L* | ||||||||||
| Zinc | 370 | 12.5 | 14.7 | 17* | 13.0 | 14.7 | 14* | 12.7 | 14.7 | 17* |
| No zinc | 347 | 12.4 | 12.9 | 2 | 12.9 | 13.5 | 3 | 12.5 | 13.2 | 2 |
| Copper, μmol/L | ||||||||||
| Zinc | 370 | 20.5 | 20.5 | 0 | 16.1 | 15.7 | −1 | 18.3 | 17.8 | −1 |
| No zinc | 345 | 20.6 | 21.2 | 1 | 16.1 | 15.8 | 0 | 18.4 | 18.4 | 1 |
| Hematocrit | ||||||||||
| Zinc | 369 | 0.40 | 0.40 | 0 | 0.44 | 0.44 | −2 | 0.42 | 0.41 | 0 |
| No zinc | 345 | 0.41 | 0.41 | 0 | 0.44 | 0.44 | −2 | 0.42 | 0.42 | 0 |
| Total cholesterol, mmol/L | ||||||||||
| Zinc | 370 | 5.9 | 5.7 | −4 | 5.5 | 5.3 | −4 | 5.8 | 5.5 | −4 |
| No zinc | 347 | 5.9 | 5.8 | −2 | 5.4 | 5.1 | −3 | 5.6 | 5.5 | −2 |
| Triglycerides, mmol/L | ||||||||||
| Zinc | 370 | 1.5 | 1.5 | −2 | 1.5 | 1.5 | −3 | 1.5 | 1.5 | −2 |
| No zinc | 347 | 1.4 | 1.5 | 6 | 1.5 | 1.5 | −7 | 1.4 | 1.5 | 2 |
| HDL cholesterol, mmol/L | ||||||||||
| Zinc | 364 | 1.5 | 1.5 | 1 | 1.1 | 1.1 | −2 | 1.3 | 1.3 | 0 |
| No zinc | 344 | 1.5 | 1.5 | 0 | 1.0 | 1.1 | 4 | 1.3 | 1.3 | 1 |
| LDL cholesterol,1 mmol/L | ||||||||||
| Zinc | 364 | 3.7 | 3.4 | −6 | 3.5 | 3.3 | −5 | 3.7 | 3.3 | −6 |
| No zinc | 344 | 3.6 | 3.4 | −4 | 3.5 | 3.2 | −6 | 3.5 | 3.3 | −5 |
Calculated: LDL = total cholesterol − HDL − (triglycerides/5).
P < 0.01 for comparison between Zinc and No Zinc groups at y 5.
Serum zinc
The baseline median serum zinc value was higher for men than women, and not different between participants in the zinc and no zinc treatment groups (Table 3). Figure 1 shows the mean and standard error of serum zinc levels over 5 y by zinc assignment. At 1-y, zinc levels in the zinc supplementation group had increased by a median of 17% and this increase was essentially unchanged in subsequent years. An analysis of the change in serum zinc over time, adjusted for baseline zinc quartile, age, gender and BMI, found a difference in the change in serum zinc between the zinc-assigned group and the no zinc group (P < 0.001).
FIGURE 1.
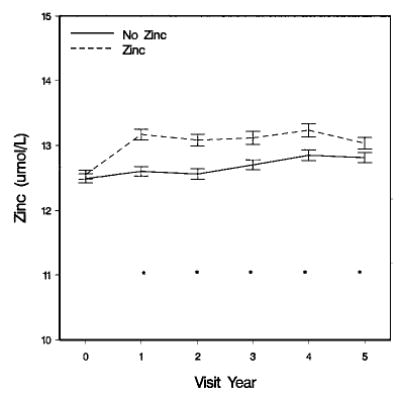
Serum zinc at baseline and follow-up, by zinc group assignment of Age-Related Eye Disease Study (AREDS) participants. Values are means ± sem, n = 717. *Groups differ (P < 0.01)
The distribution of change in zinc levels at 5 y by baseline zinc quartile and gender for individuals randomly assigned to receive supplements with or without zinc are provided in Figures 2A (women) and 2B (men). After 5 y, participants assigned to zinc supplementation had a median percentage of change in serum zinc of +25% in females and +28% in males in the low group, +15% and +13% in the middle group, and +13% and −3% in the high group. By contrast, the corresponding changes for those not assigned to zinc supplements were +8% and +10% in the low group, +2% and +7% in the middle group, and −4% and −7% in the high group for women and men, respectively. At enrollment, 65% of participants chose to use daily Centrum containing 15 mg of zinc as zinc oxide. Use of Centrum did not significantly affect blood levels in either the zinc or no zinc groups (data not shown). Within the zinc-assigned group, there was a moderate interaction between baseline zinc level and time (P = 0.14), with participants beginning with higher zinc levels experiencing less of an increase over time than participants beginning with lower levels.
FIGURE 2.
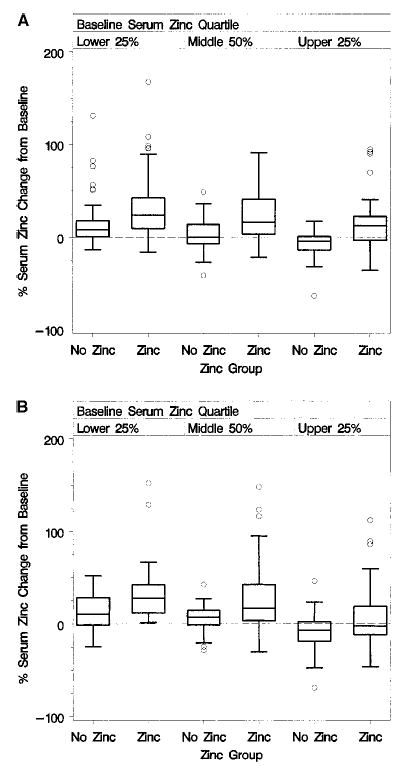
Box plots of the percentage of serum zinc change at 5 y, by zinc group assignment and baseline serum zinc quartile of female (panel A) and male (panel B) Age-Related Eye Disease Study (AREDS) participants.
Copper and hematocrit
Serum copper levels and hematocrit were not affected by the zinc plus copper supplements (P ≥ 0.10). The median percentage of change in copper measures over 5 y was a decrease of 1% for the zinc-supplemented group and a 1% increase in the no zinc group. The median percentage of change in hematocrit was 0% for both treatment groups (Table 3).
Total cholesterol, triglycerides, HDL cholesterol, calculated LDL cholesterol
Total cholesterol, triglycerides, HDL cholesterol and calculated LDL cholesterol serum levels were not significantly affected by zinc supplementation (P > 0.25). The median total cholesterol decreased after 5 y by 4% and 2% for the zinc and no zinc groups, respectively, whereas the median triglyceride level decreased by 2% for the zinc group and increased by 2% for the no zinc group. HDL cholesterol level median change was 0% for the zinc group and +1% for the no zinc group (Table 3). The median change in calculated LDL cholesterol levels was a decrease of 6% for the zinc group and a decrease of 5% for the no zinc group.
Baseline serum zinc levels were significant predictors of baseline lipid measures. Lower baseline serum zinc levels were associated with lower levels of total cholesterol (P < 0.001, Fig. 3) and triglycerides (P < 0.001, Fig. 4), with higher levels of HDL cholesterol (P < 0.001, Fig. 5) and with lower levels of calculated LDL cholesterol (P < 0.001, Fig. 6). No difference between treatment groups was observed in the change in use of cholesterol-modifying medications between baseline and 5 y (P > 0.15). An analysis restricted to participants who never took cholesterol-lowering medications found no increases in total cholesterol, triglycerides, HDL cholesterol or LDL cholesterol for the zinc and no zinc groups over 5 y (P > 0.09). Differences between zinc and no zinc groups in median percentage of change did not exceed 2%.
FIGURE 3.
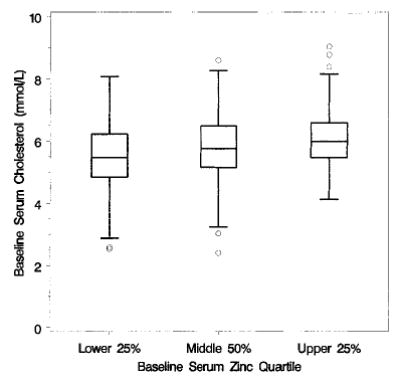
Box plots of baseline cholesterol, by baseline zinc quartile of Age-Related Eye Disease Study (AREDS) participants; n = 717.
FIGURE 4.
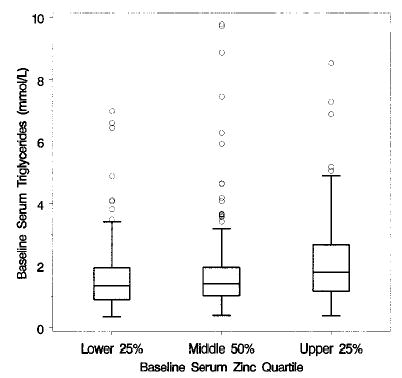
Box plots of baseline triglycerides, by baseline zinc quartile of Age-Related Eye Disease Study (AREDS) participants; n = 717.
FIGURE 5.
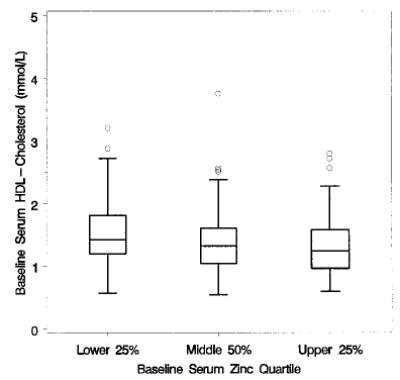
Box plots of baseline HDL cholesterol, by baseline zinc quartile of Age-Related Eye Disease Study (AREDS) participants; n = 708.
FIGURE 6.
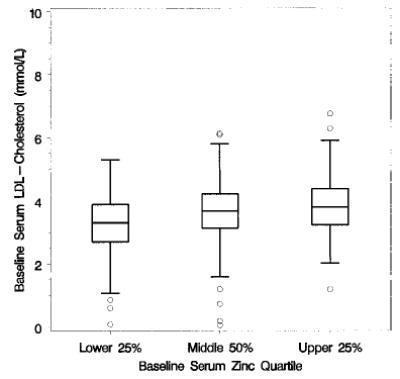
Box plots of baseline [calculated] LDL cholesterol, by baseline zinc quartile of Age-Related Eye Disease Study (AREDS) participants; n = 708.
DISCUSSION
Baseline median zinc dietary intake levels of AREDS participants are similar to those of the NHANES III population. Serum levels of zinc and copper are not available for NHANES III, but median zinc concentrations from NHANES II of ~13 μmol/L each for women and men, respectively (24), are similar to the median values observed for the AREDS population of 12–13 μmol/L. The similarity of these cohorts with respect to intake and serum levels suggests that the effect on serum levels of the AREDS formulation observed in this study may be similar to values that would be obtained with these formulations in the general population.
Despite homeostatic mechanisms that regulate zinc absorption, secretion and redistribution, previous studies have shown that dietary zinc supplementation increases plasma zinc levels (25,26). Similarly, we found that daily supplementation with 80 mg of zinc as zinc oxide combined with 2 mg of copper as cupric oxide resulted in a median increase in zinc levels of 17% after 5 y of supplementation in a cohort for which high adherence to study medications was estimated to be present in 79% of the group. This increase is much greater than the 2% median increase in the group not receiving zinc. The median increase in serum zinc levels in the group not receiving zinc may be due in part to supplementation by 65% of participants with daily Centrum, which contains 15 mg of zinc as zinc oxide.
Changes in mean serum zinc levels from baseline were uniform over the period from 1 through 5 y of follow-up. Baseline zinc intake and serum concentrations were higher in men than women, and the percentage of increase in serum zinc over the study period decreased with an increase in the baseline zinc level. Overall, the percentages of change in zinc levels with supplementation were similar in men and women. Within each quartile of baseline zinc at the 5-y visit, the difference in the median percentage of change in serum zinc between the zinc group and no zinc group was similar, except for the high baseline serum zinc quartile for men. In this group, the difference in the median percentage of change was only 4%. Whether this finding is a result of a combination of bioregulation preventing increase beyond threshold levels and regression to the mean requires further study.
The combination of 80 mg of zinc as zinc oxide and 2 mg of copper as cupric oxide increased serum zinc, but had no effect on copper levels. The absence of an effect on copper levels is consistent with the findings of an earlier study reporting that supplementation with 150 mg/d of zinc alone (~10 times the RDA) in healthy volunteers had no effect on plasma copper levels over 6 wk, suggesting no effect of even this relatively high zinc intake on copper transport (27). Long-term effects on hematopoiesis were not investigated in that study, but other toxicities were observed, including headaches, abdominal cramps, nausea, loss of appetite and vomiting. A summary of adverse experiences occurring in the AREDS population was described as part of the primary results from the clinical trial, reported in the fall of 2001 (18,19).
The effect of zinc levels on lipids has been studied in cross-sectional studies (3,9,28). Between 1987 and 1990, fasting serum zinc and serum lipid levels were examined in individuals aged 22–80 y (28). In that study, serum zinc levels, particularly those above the highest quintile, were found to be associated with higher levels of total serum cholesterol, LDL cholesterol and triglycerides. No association was found between zinc and HDL cholesterol. Baseline serum zinc in AREDS was positively associated with total cholesterol, calculated LDL cholesterol and triglycerides, and negatively with HDL cholesterol levels. Zinc supplements in AREDS did result in increases in zinc levels but no associations were found with lipids or hematocrit over 5 y of follow-up. The effect of increased zinc levels over longer periods may still be a concern.
A study in atherosclerotic men reported a decreased Zn/Cu ratio and a high correlation between copper levels and both total and LDL cholesterol (3). In another study, decreased dietary zinc and decreased plasma zinc levels were reported to be associated with coronary artery disease and diabetes, as well as with the coronary artery disease risk factors of hypertension and hypertriglyceridemia (9). The results of these two studies contradict the notion that higher zinc levels predispose to coronary heart disease by elevating LDL cholesterol and triglycerides. In AREDS, we found lipid metabolism, as indicated by serum measurements of total cholesterol, calculated LDL cholesterol, triglycerides and HDL cholesterol, to be unchanged by moderately high, long-term supplementation with zinc plus copper. Differences in lipid levels may have been masked by the initiation of cholesterol-modifying medications, but no difference between the zinc and no zinc treatment groups was found in the frequency of new onset use of cholesterol-modifying medications at 5 y. Furthermore, no changes in lipid levels were observed among those who had never used lipid-lowering medications. Our findings suggest that the positive associations between baseline serum zinc levels and lipids found in AREDS and in cross-sectional studies may not necessarily reflect a causal relationship between zinc and these measures, but may rather result from zinc being a surrogate for unknown factors that influence serum cholesterol.
In conclusion, 5 y of daily, oral supplementation with 80 mg of zinc as zinc oxide and 2 mg of copper as cupric oxide in AREDS participants did not influence the laboratory parameters of greatest health concern, namely, hematopoiesis as measured by hematocrit, and lipid metabolism as determined from total cholesterol, HDL cholesterol, calculated LDL cholesterol and triglyceride levels. Because zinc plays a role in many major metabolic pathways and is a constituent of many enzymes, there could be adverse or beneficial effects of oral zinc supplementation on physiologic endpoints that we did not investigate.
Footnotes
Supported by contracts from the National Eye Institute, National Institutes of Health, with additional support from Bausch and Lomb, Inc.
Abbreviations used: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; BMI, body mass index; FFQ, food-frequency questionnaire; NHANES, National Health and Nutrition Examination Survey; RDA, Recommended Daily Allowance.
References
- 1.Forsleff L, Schauss AG, Bier ID, Stuart S. Evidence of functional zinc deficiency in Parkinson’s disease. J Altern Complement Med. 1999;5:57–64. doi: 10.1089/acm.1999.5.57. [DOI] [PubMed] [Google Scholar]
- 2.Ishihara N, Yuzawa M, Tamakoshi A. Antioxidants and angiogenetic factor associated with age-related macular degeneration (exudative type) Nippon Ganka Gakkai Zasshi [Acta Soc Ophthalmol Jpn] 1997;101:248–251. [PubMed] [Google Scholar]
- 3.Iskra M, Patelski J, Majewski W. Concentrations of calcium, magnesium, zinc and copper in relation to free fatty acids and cholesterol in serum of atherosclerotic men. J Trace Elem Electrolytes Health Dis. 1993;7:185–188. [PubMed] [Google Scholar]
- 4.Mezzetti A, Pierdomenico SD, Costantini F, Romano F, DeCesare D, Cuccurullo F, Imbastaro T, Riario-Sforza G, DiGiacomo F, Zuliani G, Fellin R. Cooper/zinc ratio and systemic oxidant load: effect of aging and aging-related degenerative diseases. Free Radic Biol Med. 1998;25:676–681. doi: 10.1016/s0891-5849(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 5.Vilanova A, Gutierrez C, Serrat N, Raga X, Paternain JL. Metallothionein, zinc, and copper levels: relationship with acute myocardial infarction. Clin Biochem. 1997;30:235–238. doi: 10.1016/s0009-9120(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 6.Papadopol V, Palamaru I, Tcaciuc C, Sapunaru E, Iacob E, Rusu L. The relationship between zinc, copper and lipid metabolic indicators in adults. Rev Med-Chir Soc Med Nat Iasi. 1995;99:121–127. [PubMed] [Google Scholar]
- 7.Peretz A, Neve J, Jeghers O, Pelen F. Zinc distribution in blood components, inflammatory status, and clinical indexes of disease activity during zinc supplementation in inflammatory rheumatic diseases. Am J Clin Nutr. 1993;57:690–694. doi: 10.1093/ajcn/57.5.690. [DOI] [PubMed] [Google Scholar]
- 8.Reunanen A, Knekt P, Marniemi J, Maki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50:431–437. [PubMed] [Google Scholar]
- 9.Singh RB, Niaz MA, Rastogi SS, Bajaj S, Gaoli Z, Shoumin Z. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr. 1998;17:564–570. doi: 10.1080/07315724.1998.10718804. [DOI] [PubMed] [Google Scholar]
- 10.Zoli A, Altomonte L, Caricchio L, Galossi A, Mirone L, Ruffini MP, Magaro M. Serum zinc and copper in active rheumatoid arthritis: correlation with interleukin 1 beta and tumour necrosis factor alpha. Clin Rheumatol. 1998;17:378–382. doi: 10.1007/BF01450895. [DOI] [PubMed] [Google Scholar]
- 11.Hallbook T, Hedelin H. Zinc metabolism and surgical trauma. Br J Surg. 1977;64:271–273. doi: 10.1002/bjs.1800640412. [DOI] [PubMed] [Google Scholar]
- 12.Lindeman RD, Bottomley RG, Cornelison RL, Jacobs LA. Influence of acute tissue injury on zinc metabolism in man. J Lab Clin Med. 1972;79:452–460. [PubMed] [Google Scholar]
- 13.Chandra RK. Excessive intake of zinc impairs immune responses. J Am Med Assoc. 1984;252:1443–1446. [PubMed] [Google Scholar]
- 14.Hooper PL, Visconti L, Gary PJ, Johnson GE. Zinc lowers high-density lipoprotein cholesterol levels. J Am Med Assoc. 1980;244:1960–1961. [PubMed] [Google Scholar]
- 15.Patterson WP, Winkelmann M, Perry MC. Zinc-induced copper deficiency: mega-mineral sideroblastic anemia. Ann Intern Med. 1985;103:385–386. doi: 10.7326/0003-4819-103-3-385. [DOI] [PubMed] [Google Scholar]
- 16.Berdanier, C. D. (1998) Advanced Nutrition: Micronutrients. CRC Press, Boca Raton, FL.
- 17.Newsome DA, Swartz M, Leone NC, Elston RC, Miller E. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106:192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- 18.AREDS Research Group. The Age-Related Eye Disease Study (AREDS): a randomized, placebo-controlled, clinical trial of high-dose supplementation with Vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS Report No 8 Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AREDS Research Group. The Age-Related Eye Disease Study (AREDS): a randomized, placebo-controlled, clinical trial of high-dose supplementation with Vitamins C and E and beta carotene for age-related cataract and vision loss. AREDS Report No 9 Arch Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AREDS Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS Report No 1 Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AREDS Research Group. (2000) AREDS Manual of Operations. Rockville, MD. AREDS Coordinating Center, The EMMES Corporation, aredspub@emmes.com
- 22.Kurinij, N., Gensler, G. & Milton, R. for the Age-Related Eye Disease Study Group. (May 1998) Evaluation of a food frequency questionnaire in a randomized clinical trial of eye diseases. International Conference on Dietary Assessment Methods, Papendal, Arnhem, The Netherlands.
- 23.Alaimo K, McDowell MA, Briefel RR, Bischof AM, Caughman CR, Loria CM, Johnson CL. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data. 1994;258:1–28. [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services (1983) Hematological and nutritional biochemistry reference data for persons 6 months-74 years of age: United States, 1976–80. Data from the National Health Survey Series II, No. 232 (Tables 56–59). Vital Health Stat., DHHS Publication no. (PHS) 83–1682. [PubMed]
- 25.Black MR, Medeiros DM, Brunett E, Welke R. Zinc supplement and serum lipids in young adult white males. Am J Clin Nutr. 1988;47:970–975. doi: 10.1093/ajcn/47.6.970. [DOI] [PubMed] [Google Scholar]
- 26.Fischer PWF, Giroux A, L’Abbe MR. Effect of zinc supplementation on copper status in adult man. Am J Clin Nutr. 1984;40:743–746. doi: 10.1093/ajcn/40.4.743. [DOI] [PubMed] [Google Scholar]
- 27.Samman S, Roberts DC. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. Med J Aust. 1987;146:246–249. doi: 10.5694/j.1326-5377.1987.tb120232.x. [DOI] [PubMed] [Google Scholar]
- 28.Hiller R, Seigel D, Sperduto RD, Blair N, Burton TC, Farber MD, Gragoudas ES, Gunter EW, Haller J, Seddon JM. Serum zinc and serum lipid profiles in 778 adults. Ann Epidemiol. 1995;5:490–496. doi: 10.1016/1047-2797(95)00066-6. [DOI] [PubMed] [Google Scholar]


