Abstract
This study investigated the effects of familiarization and attention on event-related potential (ERP) correlates of recognition memory in infants. Infants 4.5, 6, or 7.5 months of age were either familiarized with 2 stimuli that were used during later testing or presented 2 stimuli that were not used later. Then, infants were presented with a recording of Sesame Street to elicit attention or inattention and presented with familiar and novel stimuli. A negative ERP component over the frontal and central electrodes (Nc) was larger in the preexposure familiarization group for novel- than for familiar-stimulus presentations, whereas the Nc did not differ for the group not receiving a familiarization exposure. Spatial independent components analysis of the electroencephelogram and “equivalent current dipole” analysis were used to examine putative cortical sources of the ERP components. The cortical source of Nc was located in areas of prefrontal cortex and anterior cingulate cortex.
Visual attention and recognition memory in infants are closely related. For example, infants demonstrate greater memory for events they were exposed to while in an attentive state than for events they were exposed to in an inattentive state (Frick & Richards, 2001; Richards, 1997). Events that have been partially encoded into memory or events that are novel elicit larger attention responses than those events that have been fully encoded (Bornstein, 1985; Fantz, 1961, 1963). Infants show larger orienting responses to novel events than to those events that are familiar to them. Studies examining visual attention and recognition memory simultaneously can provide insight into the overall cognitive activity involved in an organism’s adaptive responses to environmental information. The present study shows that attention-elicited event-related potential (ERP) responses differ as a function of the infant’s familiarity with the stimuli and suggests that these effects are mediated by the prefrontal cortex of the brain.
Several studies of infant recognition memory development have used the electroencephelogram (EEG) to measure ERPs related to recognition memory. ERPs are scalp voltage oscillations that are time locked with a specific physical or mental event (Fabiani, Gratton, & Coles, 2000; Picton et al., 2000). Courchesne, Ganz, and Norcia (1981) recorded ERP during an oddball procedure. They exposed 10 infants from 4 to 7 months of age to tachistoscopically presented slides of two unfamiliar female faces. One female face was presented on 88% of the trials (standard stimulus), and the other female face was presented on 12% of the trials (oddball stimulus). A negative component over the frontal and central electrodes with a latency of 700 ms, labeled Nc (“negative central”), was larger to the oddball stimulus than to the standard stimulus. A later occurring (latency = 1,360 ms) positive component followed both the infrequently and frequently presented stimuli. The authors concluded that the frequently presented stimulus was more familiar to the infants than the infrequently presented stimulus, and the differences in the Nc reflected the infants’ response to a novel stimulus. However, the conclusion that this differential response is based on the detection of novelty is confounded in this procedure. Both stimuli are novel at the beginning of testing. The frequent presentation of the standard stimulus is assumed to lead to familiarization of that stimulus relative to a lack of familiarization to the oddball stimulus. Therefore, the greater Nc amplitude found following presentations of the infrequent stimulus may be related to the infants’ detection of a low-probability event as opposed to the detection of a novel stimulus per se.
Nelson and Collins (1991, 1992) modified the oddball procedure to address the confounding of probability and familiarity. A familiarization phase was added. First, infants are exposed to repeated presentations of two different stimuli. Then, the participants are exposed to one of the familiar stimuli on 60% of the trials (frequent familiar), the other familiar stimulus on 20% of the trials (infrequent familiar), and novel-stimulus presentations on the remaining 20% of the trials (infrequent novel). The use of these three visual recognition memory (VRM) stimulus types allows for the assessment of differences in infants’ responses to presentation probability (frequent familiar vs. infrequent familiar) and of differences in the infants’ responses to novelty (infrequent familiar vs. infrequent novel). In contrast to the findings of greater amplitude Nc components following the infrequently presented stimulus reported from studies using the traditional oddball procedure, Nelson and Collins (1991, 1992) found no differences between the Nc component for any of the VRM stimuli for 4-, 6-, and 8-month-old infants. Nelson and Collins concluded that the Nc does not reflect detection of a novel stimulus but rather is indicative of a general orienting response. Later components of the ERP did differ between VRM stimuli for older infants. No clear conclusions could be drawn from the 4-month-old participants. Six-month-old infants’ response to the infrequent-familiar event took the form of a positive slow wave, whereas their response to the infrequent-novel event took the form of a negative slow wave. The negative slow wave was proposed to reflect novelty detection, whereas the positive slow wave was proposed to be associated with an updating of working memory for a stimulus that had previously been partially processed.
A close association between attention and the Nc component was shown in a recent study by Richards (2003a). Infants were tested at 4.5, 6, or 7.5 months of age. The modified-oddball procedure, developed by Nelson and Collins (1991, 1992), was used. Infants were presented with a recording of a Sesame Street movie. Heart rate changes elicited by the Sesame Street presentation were used to distinguish periods of time before attention was engaged (before heart rate deceleration), during sustained attentiveness (during heart rate deceleration), and during inattentiveness (after heart rate deceleration) (e.g., Casey & Richards, 1988; Richards, 1997; Richards & Casey, 1992). The VRM stimuli replaced the Sesame Street stimulus for a brief period of time, and ERPs were quantified in response to these stimuli. The Nc component did not differ for the three types of VRM stimuli but was significantly larger during periods of attention than during periods of inattentiveness. The Nc component during sustained attention increased in amplitude with age; Karrer and Ackles (1987, 1988) found a similar age effect with Nc amplitude increasing through 18 months of age. The late slow waves were similar for familiar and novel presentations for the 4.5-month-old infants, whereas the older infants (6- and 7.5-month-olds) displayed a negative late slow wave in response to the infrequent-novel stimulus and a positive slow wave in response to the infrequent-familiar stimulus. These late slow waves only occurred when the infants were attentive. Thus, the Nc component may reflect a general orienting response that is insensitive to stimulus novelty and probability, whereas the late slow waves are affected by attention status, novelty, and probability.
There are methodological differences among studies using the presentation of these brief visual stimuli that limit the interpretation of the Nc as an index of general orienting response that is insensitive to stimulus novelty and probability. First, initial studies using the oddball procedure included two stimuli that were novel at the beginning of the presentations (Courchesne, 1977; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995; Nikkel & Karrer, 1994). These studies reliably found the Nc component to be larger to the infrequently presented “oddball” stimulus than to the frequently presented “standard” stimulus. Second, studies using the modified-oddball procedure (Nelson & Collins, 1991, 1992; Richards, 2003a) first familiarized infants with two stimuli, one of which was then presented in the repeated brief presentations on 60% of the trials (frequent familiar), the other familiar stimulus on 20% of the trials (infrequent familiar), and novel, unrepeated stimuli on the remaining 20% of the trials (infrequent novel). In contrast to the studies that did not familiarize the infants with the stimuli, these studies report no difference in the amplitude of the Nc component to the frequent-familiar (standard) or infrequent-familiar (oddball) stimuli and no difference in the amplitude of this response to the novel stimuli. One explanation of the role of familiarization is that the orienting value is equated for the frequent-familiar and infrequent-familiar stimuli so that the Nc response, reflecting a larger orienting response to novel events, does not differ. However, this does not explain a lack of differential responding to the infrequent-novel stimuli. A third methodological difference is that in some studies, the infant is presented with stimuli with which he or she has become familiar outside of the laboratory context and with stimuli that are novel to the infant (e.g., de Haan & Nelson, 1997, 1999; see review, de Haan, Johnson, & Halit, 2003). For example, a mother’s face and a stranger’s face (or several stranger’s faces) presented equally often result in an Nc component that is larger to the familiar face than to the stranger face; the same is true for pictures of familiar objects and novel objects (de Haan & Nelson, 1997, 1999). Because the stimuli in these studies are presented equally often, stimulus probability is no longer an issue, and stimulus familiarity comes from an extensive exposure to the familiar faces or objects. One aim of this research was to examine the role of the familiarization procedure more directly by comparing the Nc response for stimuli to which familiarization exposure is given with stimuli that have no familiarization exposure. The discrepant findings in this area may be resolved by manipulating whether the infant receives a familiarization with the briefly presented stimuli prior to the brief presentation exposures.
An alternative account of the Nc response in this oddball procedure could rely on stimulus context. In the oddball procedure without familiarization, the standard stimulus is the “context” that changes infrequently. The oddball stimulus changes this context, and the Nc response is an orienting response to a new context. This would be consistent with the findings of Richards (2003a), in which all three VRM stimuli were presented against a “background” of Sesame Street and showed an equal and large Nc component for all three VRM stimulus types. Thus, the Nc may elicit an orienting response because of a change in context (standard to oddball, Sesame Street to any stimulus type) and be unrelated to familiarization. If this explanation is correct, then the manipulation of familiarity should result in equivalent Nc amplitudes to familiar and unfamiliar stimuli because both represent a change of context from the Sesame Street background.
The cortical sources of the ERP components may help distinguish the effects of attention-related ERP components and memory-related ERP components. Several reasons have been given to suggest that the Nc component represents a general orienting response more closely associated with attention than with recognition memory (Nelson & Collins, 1991, 1992; Richards, 2003a). Given the location of the Nc over the frontal scalp locations, one might expect that attention-sensitive areas in the prefrontal cortex may be involved in the generation of the Nc. The anterior cingulate and associated structures in the prefrontal cortex may play a role in this response. The anterior cingulate is part of the cingulate cortex—a paralimbic region of the brain that shares reciprocal connections with several subcortical, cortical, and limbic regions, for example, dorsolateral prefrontal cortex and the posterior parietal cortex (Cohen, 1993; Nelson & Dukette, 1998). Studies have shown that the anterior cingulate is involved in visual target detection and in the control or direction of attention (Casey et al., 1997; Goldman-Rakic, 1988). One would expect enhanced anterior cingulate activity to novel stimuli (no familiarization) or to the shift of attention from one context to another (standard to oddball, Sesame Street to any stimulus type). The finding that Nc increases in amplitude from 4 to 7.5 months of age (Richards, 2003a) is consistent with gains in voluntary attention that have been associated with further development of an executive attentional network comprising the anterior cingulate and areas of prefrontal cortex (Rothbart, Posner, & Rosicky, 1994). In distinction with the Nc component, the late slow wave components most likely reflect recognition memory processes (Nelson & Collins, 1991, 1992; Richards, 2003a). Structures within the medial temporal lobe have been assumed to underlie VRM (e.g., Nelson & Dukette, 1998). It is possible that novelty detection may be served by parietal activity and widely scattered activity in the prefrontal cortex. Thus, we may expect that the late slow wave activity would find its cortical sources in several areas of the cortex, including the temporal, parietal, and prefrontal cortex. A middle-latency negative component often found over occipital leads (labeled the occipital response) is another component of interest. The occipital response is insensitive to VRM stimulus type and is likely associated with early visual processing. Areas of visual cortex are plausible sources for the occipital response.
The present study used the modified oddball procedure, developed by Nelson and Collins (1991, 1992). Infants were assigned to a preexposure condition in which two stimuli were presented before the oddball procedure for familiarization (i.e., the typical modified oddball procedure) or to a control condition in which the infants were familiarized with two stimuli that were not seen later in the oddball procedure. We predicted differences in the late slow waves on the basis of familiarization condition. We used a cross-sectional design to test infants at 20, 26, and 32 weeks of age (i.e., 4.5, 6, and 7.5 months of age). Infants at these three testing ages show the Nc effect, but 26- and 32-week-olds demonstrate differential late slow wave responding on the basis of VRM stimulus type, whereas 20-week-olds do not (Nelson & Collins, 1991, 1992; Richards, 2003a). We expected to replicate these past findings. Scalp-recorded ERP was used to examine the Nc, late slow waves, and occipital response and the cortical sources of these components. A 128-channel recording system and independent components analysis (ICA) were used to estimate temporal-spatial components in the EEG, and cortical sources of the EEG were estimated with equivalent current dipole (ECD) analysis (Richards, 2003b, 2004, 2005). We predicted that areas of prefrontal cortex (including the anterior cingulate) would be associated with the Nc component, areas of medial temporal cortex and parietal cortex would be associated with late slow wave activity, and areas of visual cortex would be associated with the occipital response.
Method
Participants
Infants were recruited from the Columbia, South Carolina area. There were 66 infants sampled cross-sectionally at 20 (N = 22, M = 143.5 days, SD = 4.39; 11 boys and 11 girls), 26 (N = 22, M = 185.9 days, SD = 5.52; 15 boys and 7 girls), or 32 (N = 22, M = 227.5 days, SD = 3.36, 15 boys and 7 girls) weeks of age. The infants were full-term (birth weight greater than 2,500 g, gestational age at least 38 weeks, based on mother’s report of her last menstrual cycle) and in good health. The sample was drawn primarily from a White and middle-class population. An additional 12 infants were tested, who became fussy or sleepy during testing.
Apparatus
Each infant was held on a parent’s lap approximately 55 cm from a 29-in. (74-cm) color video computer monitor (NEC Multisync XM29) displayed at 1,280 horizontal and 1,024 vertical pixels. A neutral color material covered the surrounding area. A video camera was above the TV, and in an adjacent room, an observer judged the participant’s fixations on a TV monitor for controlling the experimental presentations. The video signal was digitized and stored in computer audio video interleaved files for later fixation judgments, and the frame numbers of the video recording at the experimental events were recorded.
The stimuli were a 2° blinking square, a Sesame Street movie (“Follow that Bird”), and computer-generated visual stimuli. The blinking square was used to attract fixation. The Sesame Street movie played on the center monitor without audio. The VRM stimuli were 16 computer-generated visual stimuli consisting of static black-and-white patterns (Frick & Richards, 2001; Richards, 1997, 2003a) presented in a 30° square centered on the monitor. Examples of VRM stimuli used include checkerboard, triangle, bulls-eye, and diamond-shaped patterns.
Procedure
The familiarization phase was designed to give the infant 20 s of exposure to two stimuli. A stimulus was presented until 5 s of accumulated looking time, as judged by an online observer. This stimulus was then replaced with the other stimulus for 5 s of accumulated looking time. This procedure was repeated four times for each stimulus, resulting in 20 s of accumulated looking time for each stimulus. Participants in the preexposure group were exposed to two stimuli that were used as frequent-familiar and infrequent-familiar stimuli in the VRM stimulus presentations, whereas the control-group participants were exposed to two stimuli that were not used in the VRM stimulus presentations. Thus, all VRM stimuli were novel at the onset of testing for the control group; however, two VRM stimuli were designated as frequent familiar and infrequent familiar.1 Twelve of the remaining stimuli served as the infrequent-novel stimuli for both groups.
The experimental trials consisted of the presentation of the Sesame Street movie and the frequent-familiar, infrequent-familiar, and infrequent-novel stimuli. Each experimental trial began with the presentation of the blinking square. When the infant was looking at the blinking square, one of the three VRM stimuli was presented. The stimulus was presented for 500 ms, followed by a blank screen for a 1.5- to 2.0-s interval. The stimulus was followed by the presentation of the Sesame Street movie. The movie has been found to elicit the various phases of attention and was used for this purpose. At intervals of approximately 3.5–6.0 s, the VRM stimuli were presented. This was done by replacing the ongoing Sesame Street movie with a VRM stimulus for 500 ms, followed by a 1.5- to 2.0-s blank screen, followed by the resumption of the Sesame Street movie. The VRM stimulus presentations were done only if the infant was judged to be looking at the monitor. This presentation period continued for 60 s, at which time the screen was blank for 5 s, and then the procedure was repeated.
The first VRM stimulus presentation on each experimental trial was equally divided between the three VRM stimuli. Overall, on 60% of the presentations, the frequent-familiar stimulus was presented, on 20% of the presentations, the infrequent-familiar stimulus was presented, and the remaining 20% of the presentations consisted of the infrequent-novel stimuli. This presentation sequence was accomplished by presenting three frequent-familiar, one infrequent-familiar, and one infrequent-novel stimulus randomly ordered in five-stimulus blocks. The trials were continued as long as the infants were not fussy in order to obtain as many trials as possible.
Looking Judgments
A single observer judged the infant’s fixation during the experiment in an adjacent room on a TV monitor to control the experimental protocol. The 60-s presentation periods were begun only when the infant was judged as looking at the blinking square. The Sesame Street recording was played for the entire 60 s regardless of infant looks. However, the VRM stimulus presentations were not done unless the online observer judged the infant to be looking at the monitor. Each session was also judged offline. Stimulus presentations were used only if the observer judged the infant to be looking at the VRM stimulus. In addition, trials in which infants were judged as looking away from the VRM stimulus within 2 s following stimulus onset were also eliminated from further analysis.
Recording of EEG and Segmenting of EEG
The EEG was recorded with the EGI (Electrical Geodesics Incorporated, Eugene, OR) 128-channel EEG recording system (Johnson et al., 2001; Tucker, 1993; Tucker, Liotti, Potts, Russell, & Posner, 1994). Sensor nets of different sizes were used, using one that most closely corresponded to the infant’s head circumference. Each sensor net is designed in a “geodesic” arrangement, with specific locations for the vertex, nasion, and ears. Placement of the net using these locations results in a consistent application of the locations for the other sensor net electrodes. The two EEG sensors below the infant’s eye were not used, and two of the electrodes were used for recording the electrocardiogram (ECG), resulting in 124-channel recordings. The EEG signal was referenced to the vertex, recorded with 20K amplification, at a sampling rate 250 Hz (4-ms samples), with bandpass filters set at 0.1–100 Hz, and with 100 Ω impedance.2 The vertex-referenced EEG was algebraically recomputed to an average reference. The placement of the net took about 5–10 min, during which time a second experimenter entertained the infants with toys, a child “busy box,” clown faces, and the like. The second experimenter also inspected the positioning of the net to ensure proper placement of the electrodes. Because the EGI system uses an electrolyte- and sponge-based application, the scalp was not abraded, making this a noncritical recording situation for human participants’ concerns (Pivik et al., 1993; Putnam, Johnson, & Roth, 1992).
The EEG recordings were inspected for artifacts (e.g., ΔEEG > 100 μV), poor recordings, or blinks. Individual channels or locations within trials were eliminated from the analyses if these occurred. Blinks were defined on the basis of a difference between the two electrodes on the sensor net on the outside canthii of the eye (1, 33; see Figure 1) and the two electrodes above the eye (8, 26; see Figure 1) and were defined as EOG changes >150 μV in the vertical direction.
Figure 1.
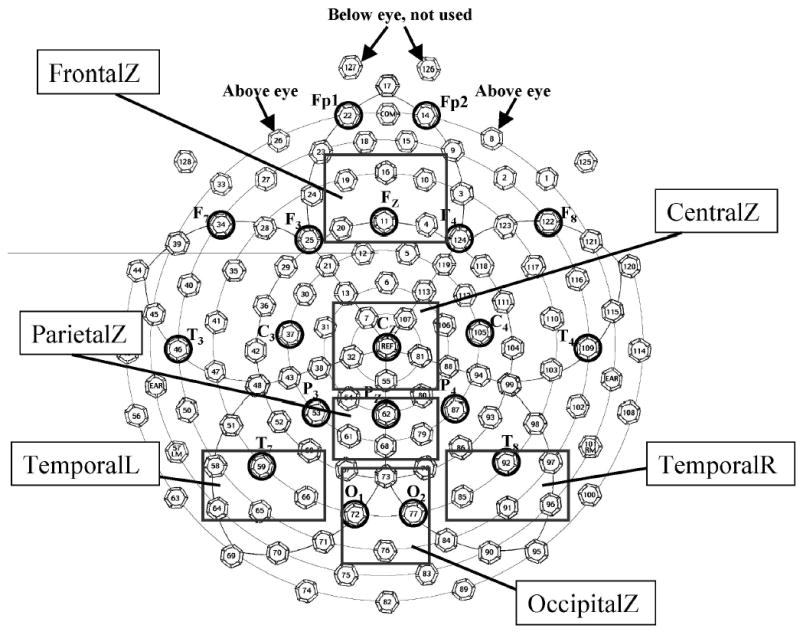
Sensor net layout. A schematic diagram of the 128-channel Electrical Geodesics Incorporated sensor net and the locations of the electrodes in the 10–20 system. The boxes indicate clusters of electrodes that were used in the analysis of the event-related potential components.
Recording and Quantification of the Electrocardiogram
The ECG was recorded with Ag-AgCl electrodes placed on the infant’s chest and digitized with the EGI system. The R-wave of the ECG was identified and interbeat intervals were computed as the interval between the occurrences of the R-wave. Three attention phases were defined. Stimulus orienting was defined as the period before a heart rate deceleration occurred, which usually lasted 2–5 s. Sustained attention was defined as beginning at the onset of a significant heart rate deceleration. A significant heart rate deceleration was defined as five successive beats with interbeat intervals each longer than the median of the five prestimulus beats (i.e., sustained attention; Richards, 1997, 2003a; Richards & Casey, 1991). Stimulus orienting and sustained attention were combined to define the attention condition. The inattention condition was defined as beginning when heart rate returned to its prestimulus level following a significant heart rate deceleration. The return of heart rate to its prestimulus level was defined as five beats with interbeat intervals shorter than the median of the five prestimulus beats (i.e., attention termination, or inattentiveness; Richards, 1997, 2003a; Richards & Casey, 1991). If the infant looked away for at least 2 s during the 60-s presentation periods, then the attention phases were defined again at the next look onset, beginning with another stimulus orienting phase.
Quantification of ERP and Cortical Source Analysis
ERP averages were done to create plots and topographical maps. These averages were made from the appropriate EEG segments. The ERP averages to stimulus onset were calculated from 50 ms before stimulus onset through 2 s after onset, and calculated as the difference scores from the prestimulus baseline. The EEG was averaged for individual infants for each attention phase (attention, inattention), VRM stimulus type (frequent familiar, infrequent familiar, infrequent novel), and electrode combination.
Three components have been consistently identified in previous work in this area. These components are the Nc component occurring approximately 350–800 ms poststimulus onset, an occipital response occurring with an approximate latency of 650–750 ms, and late slow waves occurring between 1,000 and 2,000 ms after stimulus onset (Courchesne et al., 1981; deHaan & Nelson, 1997 deHaan & Nelson, 1999; Nelson & Collins, 1991; Nelson & Salapatek, 1986; Richards, 2003a). The Nc component is typically located at frontal and central electrodes (i.e., Fz and Cz), the occipital response is found to occur at occipital sites (i.e., Oz, O1, O2), and the late slow waves are typically located at parietal and temporal sites (i.e., Pz, T3, T4, T5). We analyzed the mean data from clusters of electrodes of the EGI sensor net that corresponded to these regions. Figure 1 shows a schematic diagram of the sensor net used in the experiment, the approximate locations of the electrodes in the 10–20 system, and the clusters of electrodes that we analyzed. Nc latency and peak amplitude were analyzed from the intervals from 400 ms to 800 ms following stimulus onset from midline frontal (4, 10, 11, 16, 19, and 20; “FrontalZ”) and central (7, 32, 55, 81, and 107; “CentralZ”) electrode sites. For the occipital response, the ERP data for midline occipital electrodes (72, 73, 76, and 77; “OccipitalZ”) from the intervals from 650 ms to 850 ms following stimulus onset were analyzed. For the late slow waves occurring 1–2 s poststimulus onset, midline frontal (4, 10, 11, 16, 19, and 20; “FrontalZ”), parietal (61, 62, 68, and 79; “ParietalZ”), left temporal (58, 59, 65, and 66; “TemporalL”), and right temporal (85, 91, 92, and 97; “TemporalR”) sites were analyzed.
Topographical ERP scalp potential maps were calculated for the effects. For the topographical maps, the scalp potentials were plotted with interpolations using a third-order spherical spline technique (Nunez, 1990; Perrin, Bertrand, & Pernier, 1987; Perrin, Pernier, Bertrand, & Echallier, 1989). The topographical maps show the distribution of the scalp potentials averaged over a selected interval and help to visualize the ERP data shown in figures.
We give only a brief overview of the ICA and cortical sources analysis. The interested reader may refer to Richards (2003b, 2004b, 2005) for a detailed presentation of these methods. A spatial ICA was done on the EEG data in which the variables were the EEG channels and the observations were the millisecond intervals for which the EEG was sampled. The component weights resulting from the ICA represent the topographical information in the EEG and are similar to a set of topographic scalp maps (Johnson et al., 2001; Richards, 2003b, 2004, 2005). These component weights may be used with cortical source analysis. The ICAs were done separately on each participant’s data, using all the data from that participant in the ICA analysis, and using the first 20 of the 124 possible components. Using the first 20 components ensured that any components associated with significant experimental effects would be included in the analysis. The ICA components from all participants were clustered according to the similarity of the component loading weights.
We estimated the location of cortical sources from the ICA weights with ECD analysis. This analysis estimates a (a set of) dipole(s) located in the cortex representing a current source that generates the observed component weights. We chose only single-dipole ECD models. The ECDs were done by seeding the ECD analysis with a location defined by the average component loadings for that cluster. The ECD analysis was accepted only if the resulting dipole was in a location near the average cluster ECD. Thus, the ECDs coming from the component clusters were topographically similar (from clustering) and had similar ECD dipole locations. The activations of the component clusters were examined in relation to experimental events (experiment factors and temporal relation to stimuli onset).
Several aspects of the cortical source analysis relied on calculating the head shapes of individual participants (Richards, 2003b, 2005). A structural magnetic resonance imaging (MRI) recording was made for a 6-month-old participant, and skull/scalp landmarks were measured. The MRI recording was done with a 1.5T magnet using 3-mm axial slices and SE-T2-weighted images. An electrode placement map was generated for this individual on the basis of these head measurements and the known locations of the EGI electrodes. The same external head measurements were made for each participant in the present study. Electrode placement maps were generated for the participant by transforming the placement map from the individual with the MRI recording according to the head measurements of the infant participant. The individualized placement map was used for individual participants’ ECD analysis. This constrained the locations of the dipoles to a realistic topography on the basis of individual participant data. The coordinates of the ECDs for each participant were translated into the coordinate space of the MRI recording for the 6-month-old, and MRI plots were based on these coordinates. The locations also were translated into sagittal, coronal, and axial coordinates in the Talairach (Talairach & Tournoux, 1988) coordinate system. The MR Viewer (Source Signal Imaging, Inc., San Diego, CA) was used for the MRI displays.
Design for Statistical Analysis
The design for the study included the experimental factors of familiarization (2: preexposure, controls) and testing age (3: 20, 26, 32 weeks) as between-subjects factors, and attention phase (2: attention, inattention) and VRM stimulus type (3: frequent familiar, infrequent familiar, infrequent novel) as repeated-measures factors.3
The time-dependent changes in the ERP were analyzed in two ways. First, for the Nc and the occipital component, the amplitude and latency of the ERP at the time of the peak of the grand average was analyzed. These are the typical measures of the Nc as an ERP component and should therefore be comparable with past work in this area. Specifically, this was estimated as the maximum point of the ERP response occurring from 400 ms to 800 ms poststimulus onset for the Nc component and from 600 ms to 850 ms for the occipital component. The statistical tests for this measure used the error terms derived from the related intervals effects analyses, Scheffé-type methods to control for inflation of testwise error rate, and all significant tests that are reported occurred at p < .05. Second, the late slow waves were analyzed by examining mean ERP in 250-ms intervals from 1 s to 2 s following stimulus onset. Because we were interested in a changing pattern of responses over the period from 1,000 to 2,000 ms, we only examined main effects and interactions involving the intervals factor (4: 1,000–1,250 ms, 1,250–1,500 ms, 1,500–1,750 ms, and 1,750–2,000 ms).
Results
Three ERP components were identified. Figure 2 displays these components in the grand average ERP waveforms for the topographical locations that were analyzed for experimental effects. The Nc component occurring about 400–800 ms following stimulus onset is shown as a large negative ERP change located primarily in the frontal and central electrodes. The second ERP component was a large negative deflection at about 750 ms following stimulus onset, occurring primarily in the occipital electrodes. This change occurred as an additional negative potential to the ongoing Nc component and did not differ for the three stimulus types. Third, there were late slow waves in the period from about 1 s to 2 s following stimulus onset.
Figure 2.
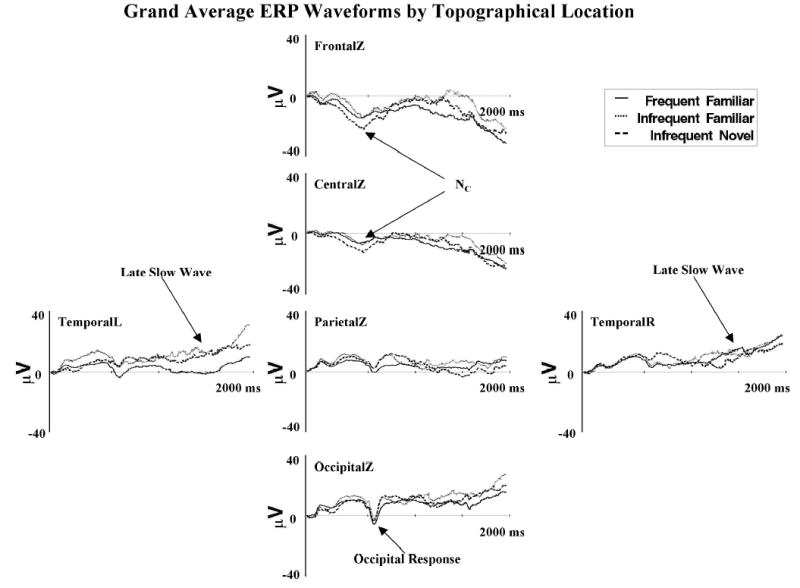
The grand average event-related potential (ERP) waveforms for each topographical location analyzed in the experimental analysis. The Nc (negative central), late slow waves, and occipital ERP components are indicated.
ICA and Cortical Source Analysis
The EEG segments were analyzed with the ICA. The initial clustering resulted in 967 of the 1,320 possible ICA components being classified into eight groups. Table 1 contains the mean Talairach coordinates of each cluster, the magnitude of the dispersion, and the Brodmann and cortical areas represented by the ECDs. Figure 3A shows topographical maps of seven of the eight clusters, and Figure 4 shows one of the eight clusters. The clusters included a cluster over the midline frontal electrodes near the front of the scalp, a cluster over a large part of the frontal-central electrodes (see Figure 4), clusters located over the central-parietal electrodes and the parietal-occipital electrodes, a cluster located over the left temporal electrodes, and three clusters located over the occipital electrodes (right, center, left). These eight clusters accounted for 71% of the variance in the ICA projections. The rest of the components did not cluster together well and had idiosyncratic topographical patterns in the loading weights or idiosyncratic ECD locations.
Table 1.
Equivalent Current Dipole Information for the ICA Clusters
| Name | Saggital | Coronal | Axial | Mag. SDa | Brodmann area | Cortical area | n |
|---|---|---|---|---|---|---|---|
| Frontal pole | 5.3 | 58.1 | 6.0 | 15.5 | 10 | Superior frontal gyrus | 67 |
| Prefrontal | 9.4 | 42.9 | 16.4 | 23.0 | 8, 25, 47 | Inferior frontal, medial frontal, anterior cingulate | 389 |
| Central-parietal | −27.8 | −56.6 | 11.7 | 28.9 | 7 | Precuneus | 57 |
| Left temporal | −52.9 | −33.3 | −5.4 | 25.8 | 20, 21 | Inferior, middle temporal gyrus | 86 |
| Parietal occipital | −0.5 | −79.2 | 36.8 | 15.4 | 7, 19 | Cuneus, precuneus | 60 |
| Striate occipital (center) | 1.2 | −92.1 | −4.7 | 12.8 | 17, 18 | Lingual gyrus, cuneus | 117 |
| Extrastriate occipital (left) | −39.8 | −81.6 | 2.5 | 15.8 | 18, 19 | Middle occipital gyrus | 101 |
| Extrastriate occipital (right) | 27.7 | −85.2 | −8.0 | 20.4 | 18, 19 | Middle occipital gyrus | 44 |
Note. ICA = independent components analysis; Mag. = magnitude.
The magnitude of the cluster, calculated as the standard deviation (SD) of the lengths from the individual equivalent current dipoles to the cluster centroid, indicates the approximate radius of a sphere for locations within 1 standard deviation of the centroid. The scale of the coordinates is in millimeters and the coordinates are given in Talairach coordinates (Talairach & Tournoux, 1988).
Figure 3.
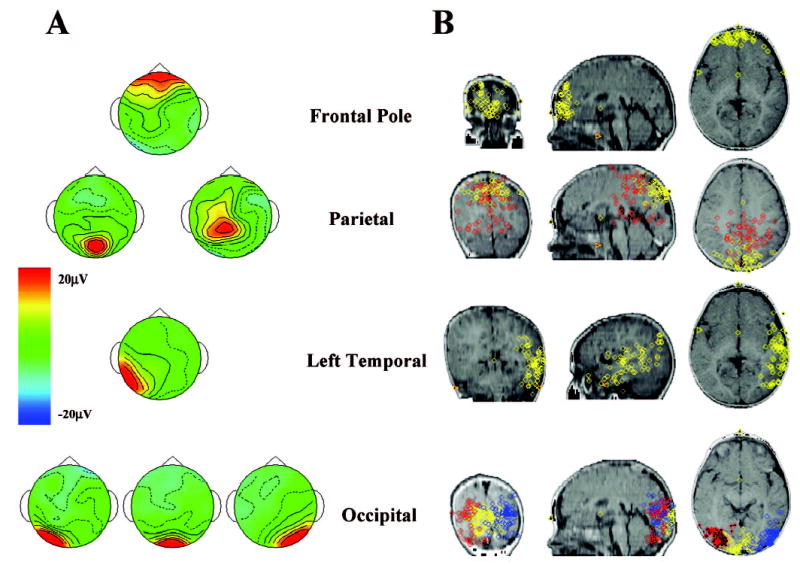
The independent components analysis (ICA) clusters and equivalent current dipole (ECD) locations. A: Topographical maps of the average ICA loadings for each cluster. B: The ECD locations are displayed on magnetic resonance imaging recordings, and each location represents an ICA from 1 individual. For the parietal cluster, the red circles represent ECDs for the central-parietal cluster, and the yellow circles represent ECDs for the parietal-occipital cluster. For the occipital clusters, the blue circles represent ECDs located in the right extrastriate occipital cluster, yellow circles represent center striate occipital, and red circles represent left extrastriate occipital.
Figure 4.
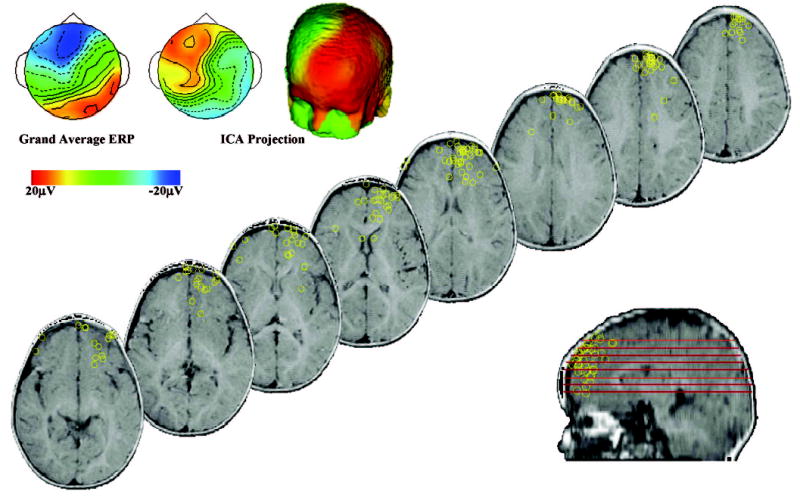
Prefrontal independent components analysis (ICA) cluster: Medial frontal gyrus (25), inferior frontal gyrus (47), anterior cingulate cortex (8) (Talairach coordinates: 9.4, 42.9, 16.4). The topographical map of the average ICA loadings are similar to the topographical map of the grand average event-related potential of the Nc (negative central) component. The equivalent current dipole locations are displayed as yellow circles on several magnetic resonance imaging slices, and each location represents an ICA from 1 individual.
The cortical source analysis of the ICAs was done using ECD analysis. Figure 3B shows the ECDs of individual components on MRI slices for each of the component clusters except the prefrontal cluster, which is shown in Figure 4. The cluster over the midline frontal electrodes had ECDs in the frontal pole, the superior frontal gyrus (Brodmann area [BA] 10). The central-parietal and parietal-occipital clusters had ECDs located in area 7 of the parietal lobe and in areas 7 and 19, respectively. The left temporal cluster had dipoles located in the inferior, middle, superior, and fusiform gyri (BA 20, 21, 22, 37, 38, 39) of the left temporal lobe. The occipital clusters had ECDs located in the occipital gyri (BA 17, 18, 19). Figure 4 shows a series of MRI slices for the cluster located over the frontal-central electrodes, with a wide scattering of ECDs located throughout areas of the prefrontal cortex, including the inferior (BA 47), medial (BA 25), and superior (BA 6) frontal gyri, and the anterior cingulate cortex (BA 8).
The Nc Component
ERP data
The ERP data from the intervals from 400 to 800 ms following stimulus onset were analyzed to determine the effects of age, familiarization, attention phase, and VRM stimulus type on the Nc component. The Nc latency and peak amplitude were analyzed separately for the FrontalZ and CentralZ electrode sites, with an Age (3: 20, 26, 32 weeks) × VRM Stimulus Type (3: frequent familiar, infrequent familiar, infrequent novel) × Attention Phase (2: attentive, inattentive) × Familiarization Condition (2: preexposure, controls) analysis of variance (ANOVA). No significant effects were found for Nc latency.
There were three significant effects for the peak Nc amplitude. First, there was a significant main effect of VRM stimulus type at frontal and central electrode sites, F(2, 118) = 4.78 and 4.75, respectively, p < .05. For the main effect of VRM stimulus type for FrontalZ electrodes, the amplitude of Nc following infrequent-novel (M = −50.25 μV) stimulus presentations was significantly greater than Nc amplitude following frequent-familiar (M = −35.91 μV) stimulus presentations, and the Nc peak amplitude for the infrequent-familiar stimulus presentation was between these (−43.49 μV). This effect was similar for the CentralZ electrode sites (peak Nc amplitudes of −34.56, −29.93, and −21.82 μV for infrequent-novel, infrequent-familiar, and frequent-familiar stimuli).
Second, there was an interaction between familiarization condition and VRM stimulus type at frontal electrodes, F(2, 118) = 4.07, p < .05. The differences found for the three VRM stimulus presentations just reported occurred primarily for the familiarization group. Figure 5 shows the ERP grand averages and topographical maps separately for the three VRM stimulus types and the two familiarization conditions. For the preexposure group, Nc amplitude was greater following infrequent-novel (M = −63.78 μV) stimulus presentations than presentations of frequent-familiar stimuli (M = −30.65 μV). No differences were found between infrequent-novel (M = −36.56 μV) and frequent-familiar (M = −41.12 μV) stimulus presentations for the control group. Additionally, Nc amplitude for infrequent-novel stimulus presentations was significantly greater for the preexposure group (−63.78 μV) than for the control group (−36.56 μV). No significant differences based on VRM stimulus type were found for the control group.
Figure 5.
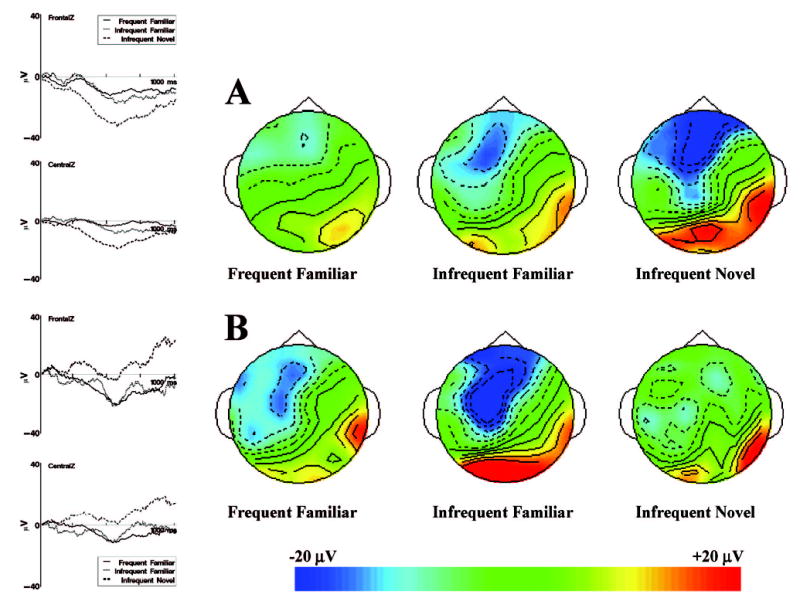
The Nc (negative central) component for the preexposure (A) and control (B) groups. The event-related potential (ERP) recording for 1 s following stimulus onset is shown for the FZ (FrontalZ) and CZ (CentralZ) electrodes for the preexposure group (A) and control group (B). The topographical scalp potential maps show the distribution of this component for the three memory stimulus types for the preexposure and control groups. The topographical maps represent an 80-ms average of the ERP for the Nc component at the maximum point of the ERP response. The data are plotted with a spherical spline interpolation algorithm and represent absolute amplitude of the ERP.
Third, there was an Age × Attention interaction for FrontalZ and CentralZ electrodes, F(2, 151) = 3.35 and 3.22, respectively, p < .05. At FrontalZ, the 20-week-olds demonstrated significantly greater amplitude Nc components during inattention (M = −59.23 μV) than during attention (M = −38.79 μV). Nc amplitude did not differ between attention and inattention for the two older age groups. The Nc amplitude during inattention for 20-week-olds (M = −59.23 μV) was greater in amplitude than Nc during inattention for 26- (−36.04 μV) and 32-week-olds (−45.44 μV), whereas no age differences were found on Nc amplitude during attention. The direction and significance of this interaction effect was similar at CentralZ electrodes.
ICA activations
We analyzed the activations of the ICA components for the Nc component. We examined the activations from 400 to 800 ms following stimulus onset for activity in the ECD clusters corresponding to the Nc component in the ERP.4 We analyzed the peak amplitude of the activation between 400 and 800 ms as well the means of the four 250-ms intervals from stimulus onset to 1,000 ms past stimulus onset. We examined these data with an Age (3) × VRM Stimulus Type (3) × Attention Phase (2) × Familiarization (2) ANOVA. For the prefrontal component (see Figure 4), the peak activation amplitude was significantly affected by VRM stimulus type, F(2, 110) = 7.74, p < .05; attention phase, F(1, 54) = 11.10, p < .05; and a VRM Stimulus Type × Attention Phase interaction, F(2, 87) = 6.88, p < .05. The activations for the frontal pole component cluster showed a significant VRM Stimulus Type × Attention Phase interaction, F(2, 42) = 5.31, p < .05. There were no significant effects of the experiment factors for the temporal, central-parietal and parietal-occipital, and occipital clusters.
The pattern of effects on the activations of the prefrontal component cluster paralleled the effects reported previously for the ERP data. Figure 6A and 6B show the activations for the first 1,000 ms for the prefrontal component. The activation for the frequent-familiar and infrequent-familiar stimuli (see Figure 6A) did not differ for attentiveness and inattentiveness phases. The activation of the component for the infrequent-novel stimulus (see Figure 6B) began at about 250 ms when the stimulus was presented during attention and was delayed until about 550 ms when the stimulus was presented during inattention. The pattern of this change was significantly different for the three VRM stimulus types (i.e., significant interaction of the intervals effect with VRM stimulus type), F(6, 336) = 2.57, p < .05, ɛ = .88. This effect parallels the larger Nc peak amplitude following novel-stimulus presentations found in the ERP data for frontal and central electrode sites (i.e., Figure 5). The pattern of effects for the frontal pole component cluster was similar. Figure 6C and 6D show the frontal pole activations. The response for the frequent-familiar and infrequent-familiar stimuli was similar in attention and inattention (see Figure 6C). The response to the infrequent-novel stimulus was similar during attention and inattention for the first 500 ms, followed by a large negative activation of the response during attention (see Figure 6D). This pattern of change was significantly different for this component cluster during attention and inattention over the three VRM stimulus types (significant interaction of intervals effect with VRM stimulus type and attention phase), F(6, 126) = 2.66, p < .05, ɛ = .88.
Figure 6.
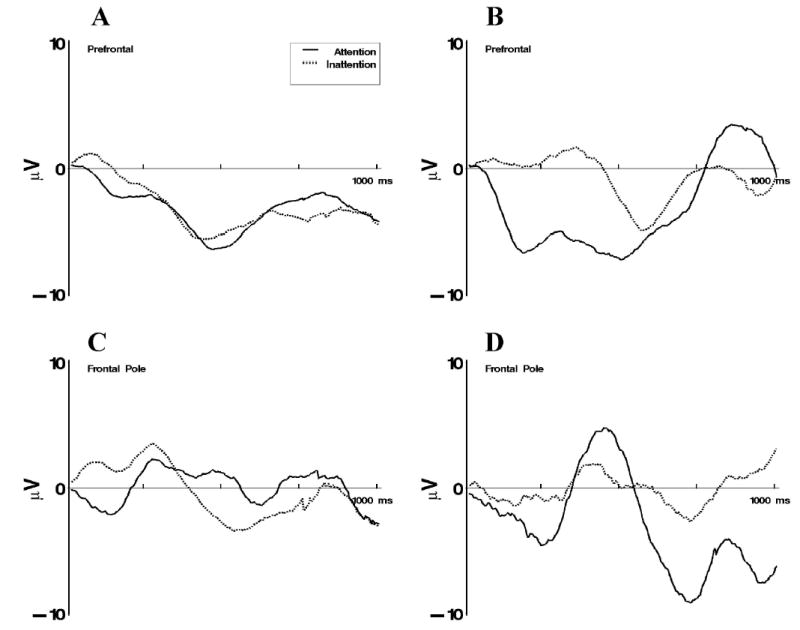
The independent components analysis activations for the frontal clusters for 1 s following stimulus onset. A: Display of combined responses to frequent-familiar and infrequent-familiar stimulus presentations separately for periods of attention and inattention. B: Display of responses for the prefrontal cluster to infrequent-novel stimulus presentations separately for periods of attention and inattention. C: Display of combined responses for the frontal pole cluster to frequent-familiar and infrequent-familiar stimulus presentations separately for periods of attention and inattention. D: Display of responses for the frontal pole cluster to infrequent-novel stimulus presentations separately for periods of attention and inattention.
We did not find any significant effects involving the familiarization condition for the peak amplitude of the activations of the component clusters. However, we examined some specific hypotheses on the basis of the pattern of ERP results. Similar to the ERP data, the peak amplitude of the activation of the prefrontal component cluster showed significant effects of attention, VRM stimulus type, and an interaction of these two factors, but only for the preexposure condition. The pattern of activation for this group was similar to the results just presented (i.e., Figure 6A and 6B). The control group did not show such effects. This was not the case for the frontal pole component cluster, whose pattern of significant effects held for both familiarization conditions.
The Occipital Response
ERP data
The ERP data for the OccipitalZ electrode sites from the intervals from 650 to 850 ms following stimulus onset were analyzed to determine the effects of the attention phase and VRM stimulus type on the large negative response that occurred primarily in the occipital leads. The ERP data from this interval were analyzed with an Age (3) × VRM Stimulus Type (3) × Attention Phase (2) × Familiarization Condition (2) ANOVA. There were no significant effects involving the experimental variables on the ERP data from this period.
ICA activations
The activations for the occipital cluster were analyzed in the period from 650 to 850 ms following stimulus onset to determine whether any experiment effects occurred in the occipital component clusters. The peak amplitude from this epoch was analyzed with an Age (3) × VRM Stimulus Type (3) × Attention Phase (2) × Familiarization Condition (2) ANOVA. As with the ERP data, there were no significant effects of the experimental variables on the activation for the occipital component clusters during this period.
The Late Slow Wave
ERP data
The ERP data from the intervals from 1 s to 2 s following stimulus onset were analyzed as the late slow waves in the ERP response. The ERP data from this interval were divided into four 250-ms intervals. The mean ERP data from these intervals were analyzed with an Age (3) × VRM Stimulus Type (3) × Attention Phase (2) × Familiarization Condition (2) × Intervals (4) mixed ANOVA, with intervals serving as a repeated factor, separately for FrontalZ, ParietalZ, TemporalL, and the TemporalR electrodes. No significant effects were found for ParietalZ or TemporalL electrodes. There was a significant three-way VRM Stimulus Type × Attention × Intervals interaction for FrontalZ, F(6, 435) = 2.94, p < .05. A significant three-way VRM Stimulus Type × Attention × Intervals interaction was also observed at TemporalR, F(6, 435) = 3.52, p < .05. Figure 7 shows spatiotemporal topographical maps separately for the three VRM stimulus types. It may be seen in this figure that there was a slow negative wave for all three VRM stimulus types, but the negative slow wave occurring over the frontal electrode sites began more quickly and was more negative for the infrequent-novel stimuli.
Figure 7.
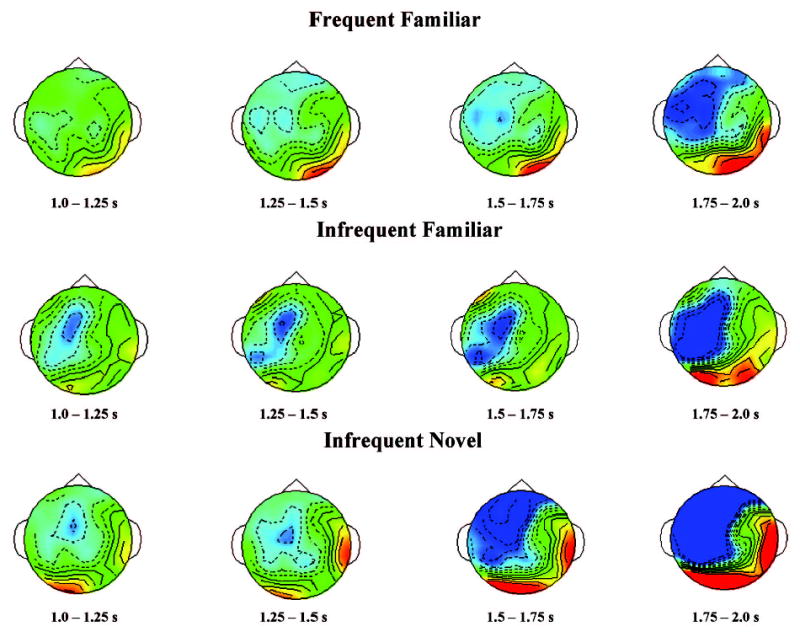
Topographical event-related potential scalp potential maps representing the late slow wave for the three memory stimulus types during attention. The topographical maps are displayed for 250-ms intervals from 1 to 2 s following stimulus presentation.
At frontal sites, late slow waves differed significantly between attentive and inattentive periods for infrequent-novel stimulus presentations. A clear negative slow wave was observed following novel-stimulus presentations during attention, whereas the slow wave following novel-stimulus presentations during inattention showed little change across intervals (see Figure 8A). Additionally, the late slow wave following frequent-familiar presentations differed significantly from the infrequent-familiar presentations during attention. Figure 8B demonstrates that infrequent-familiar presentations elicited a positive-going slow wave, whereas frequent-familiar presentations elicited a negative slow wave.
Figure 8.
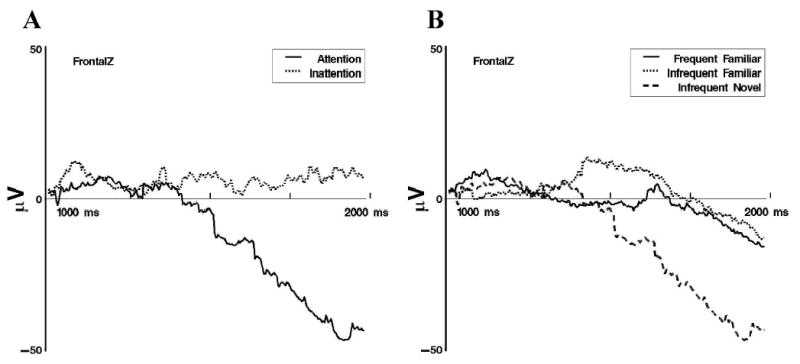
Late slow wave event-related potentials at FrontalZ from 1 to 2 s following stimulus onset. A: Display of differences in responding following infrequent-novel stimulus presentations during attention and inattention. B: Display of responses to the three memory stimulus types during attention.
At right temporal electrodes, late slow waves differed significantly between attentive and inattentive periods for infrequent-familiar stimulus presentations. As illustrated in Figure 9A, a positive slow wave occurred during attention, whereas little change occurred during inattention. Late slow waves differed significantly for infrequent-familiar and infrequent-novel stimulus presentations, but only during attention. The negative- then positive-going slow wave elicited by infrequent-novel presentations stood in contrast to the positive slow wave elicited by infrequent-familiar presentations during attention (see Figure 9B).
Figure 9.
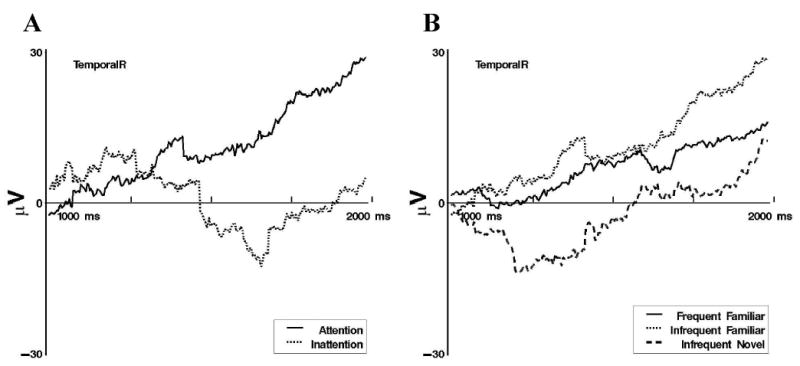
Late slow wave event-related potentials at TemporalR from 1 to 2 s following stimulus onset. A: Display of differences in responding following infrequent-familiar stimulus presentations during attention and inattention. B: Display of responses to the three memory stimulus types during attention.
ICA activations
The activations of the ICA component clusters were analyzed from 1 to 2 s following stimulus onset, corresponding to the time of the late slow wave in the ERP data. As with the ERP data, we examined means for the four 250-ms intervals between 1,000 and 2,000 ms following stimulus onset. The frontal pole and prefrontal component clusters were first examined. There were no significant statistical effects involving the experimental factors for the frontal pole component cluster. There were several significant effects for the activations of the prefrontal cluster, but we emphasize only those consistent with the findings in the ERP data. The VRM Stimulus Type × Attention × Intervals interaction was not statistically significant (F < 1.0), but the Attention × VRM Stimulus Type interaction was significant, F(2, 91) = 3.42, p < .05. Figure 10A shows the activation for the prefrontal component cluster in response to the frequent-familiar and infrequent-familiar stimulus separately for attention and inattention. There was no difference between the activation in these VRM stimulus types in attention or inattention. Alternately, there was a large negative activation of the component cluster during attention for the infrequent-novel stimulus compared with the same VRM stimulus type presented during inattentiveness (see Figure 10B). This effect parallels the effect found for the FrontalZ ERP data. We did not find a difference between the late slow wave activity for the frequent-familiar and infrequent-familiar data.
Figure 10.
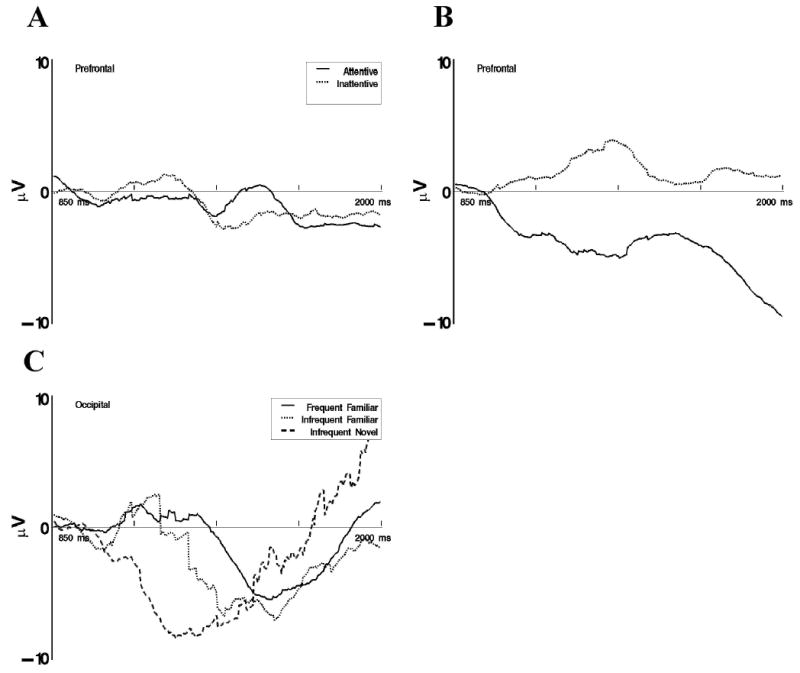
Late slow wave independent components analysis activations from 1 to 2 s following stimulus onset. A: Display of the prefrontal cluster responses to frequent-familiar and infrequent-familiar stimuli separately for attention and inattention. B: Display of prefrontal cluster responses to infrequent-novel stimuli separately for attention and inattention. C: Display of occipital cluster responses separately for the three memory stimulus types.
The clustering analysis of the ICA components did not result in a cluster comparable to the TemporalR electrodes. The activations in the period from 1 to 2 s were analyzed for the occipital component clusters, parietal component clusters, and left temporal cluster. There were no significant effects on the activations coming from this period for the left temporal cluster. The temporal pattern of the activations for the occipital cluster differed over the late epoch for the three VRM stimulus types, F(6, 300) = 2.56, p < .05, ɛ = .85. Figure 10C shows the activations of the occipital component cluster for the three VRM stimulus types. There was a negative activation of this component cluster for all three VRM stimulus types, but it occurred more quickly for the infrequent-novel and infrequent-familiar than for the frequent-familiar stimulus. The activations for the parietal component clusters had a significant interaction between the intervals factor, attention phase, VRM stimulus type, and age, F(12, 159) = 2.21, p < .05, ɛ = .96. We do not report the pattern of effects for that analysis. Several of the post hoc tests resulted in incomplete data in some of the cells and unusual outliers. Also, this effect did not have a comparable effect in the ERP data.
Discussion
The present study investigated the effects of attention, age, familiarization, and VRM stimulus type on electrophysiological correlates of recognition memory in infants. One goal of the research was to examine the role of the familiarization on the Nc and late slow wave activity responses to briefly presented visual stimuli, vis-à-vis the infant’s attentional state at the time of presentation. A second goal was to determine whether the cortical sources of the ERP components would distinguish the effects of attention-related and memory-related ERP components.
The Nc Component
There was a large effect of the familiarization procedure on the Nc response to the familiar and novel stimuli. The preexposure group receiving the familiarization presentations had a larger amplitude Nc ERP component to the novel-stimulus presentations than to either of the familiar-stimulus presentations (frequent familiar, infrequent familiar). The control group familiarized with stimuli not used in testing showed equivalent Nc ERP responses to the three VRM stimulus types (frequent-familiar, infrequent-familiar, infrequent-novel stimulus). Similar to the ERP data, the peak amplitude of the activation of the prefrontal component cluster showed significant effects of attention, VRM stimulus type, and an interaction of these two factors, but only for the preexposure condition. This effect on Nc was due to stimulus novelty rather than to stimulus probability because the Nc component to the frequently presented and infrequently presented familiar stimuli was similar, whereas the Nc component was larger to the infrequent-novel than to the infrequent-familiar stimulus.
Previous studies in this area have produced inconsistent results regarding the effects of experience on Nc amplitude, and some of these effects are inconsistent with our findings. Studies that did not use a familiarization phase found that infants demonstrate greater amplitude Nc to the infrequently presented, rare (oddball) stimulus than to the frequently presented (standard) stimulus (Courchesne, 1977; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995; Nikkel & Karrer, 1994). Our results differed from these studies. The control condition in which the stimuli in the familiarization phase were not used in the brief stimulus presentations resulted in equivalent Nc component amplitude for the frequent-familiar, infrequent-familiar, and infrequent-novel stimuli. Studies using a familiarization phase have found Nc components of equivalent amplitude to the three VRM stimulus types (Nelson & Collins, 1991, 1992; Richards, 2003a). This finding has been interpreted as indicating that the Nc component reflects a general orienting response or processing of a contextual shift that is insensitive to stimulus novelty and probability. However, the present study found a larger Nc response to the infrequently presented novel stimuli compared with the two familiarized stimuli. This finding is consistent with a novelty-detection function for the processes generating the Nc ERP component and indicates that familiarization has an effect on Nc. However, studies using stimuli that infants are highly familiar with (e.g., pictures of a mother’s face or a favorite toy) have shown that infants demonstrate greater amplitude Nc to these familiar (and meaningful) stimuli than to unfamiliar stimuli (de Haan & Nelson, 1997, 1999). Thus, Nc amplitude may be greater to the stimulus that elicits the greatest attentional response regardless of novelty versus familiarity or frequency of presentation. A study with a behavioral measure of attention and recognition memory embedded within the modified oddball paradigm would address this possibility at the behavioral and electrophysiological level.
Richards (2003a) found that Nc amplitude increased with age during attention. Age interacted with attention in the present study; a simple effect was found with 20-week-olds demonstrating greater Nc during inattention than during attention. The presentation of a stimulus during inattention may elicit an obligatory orienting response for younger infants that decreases with age. Richards (2003a) also found that the amplitude of the Nc component was significantly larger during periods of attention than during periods of inattentiveness. The Age × Attention interaction found in the present study is inconsistent with this finding and may warrant replication. No main effect of attention was found in the ERP analysis of Nc in the present study. Although the ERP component amplitude was not significantly affected by the attention factor, the activation of the frontal ICA component clusters did show an effect of the attention level of the infant. For the prefrontal and frontal pole ICA clusters, the response to novel-stimulus presentations was significantly greater during attention than during inattention, and the response to familiar-stimulus presentations did not differ for attention and inattention. The temporal pattern of this response was illuminating. The prefrontal cluster showed the effects of attention as a large negative activation occurring beginning at 250 ms following stimulus onset, whereas the activation of the frontal pole cluster occurred beginning at about 500 ms after stimulus onset. This indicates that the Nc component commonly found in ERP studies may reflect combined activity from separate brain areas that are involved in different aspects of information processing, with these different processes being affected by attentional state.
The ICA and cortical source localization analyses provide insight into possible cortical sources of the Nc component. The prefrontal ICA component cluster accounted for the most variance in the data and was located over left frontal and midline frontal electrodes, with an activation pattern similar to the Nc ERP waveform. The experimental effects on this ICA cluster were consistent with experimental effects found in the ERP analysis of the Nc component. The ECD analysis of this ICA cluster revealed dipoles in the inferior and medial frontal gyri and the anterior cingulate cortex. Studies using positron emission topography (PET) and functional MRI have found activation of frontal cortex and the anterior cingulate in tasks related to attention and recognition memory (e.g., Coull, Frith, Frackowiak, & Grasby, 1996; Haxby, Ungerleider, Horwitz, Maisog, Rapoport, & Grady, 1996; Klingberg & Roland, 1998; Owen, Milner, Petrides, & Evans, 1996; Rugg, Fletcher, Chua, & Dolan, 1999; Slotnick, Moo, Segal, & Hart, 2003). The anterior cingulate shares reciprocal connections with regions involved in voluntary attention and object recognition and is involved in visual target detection and the control or direction of attention (Casey et al., 1997; Cohen, 1993; Goldman-Rakic, 1988; Nelson & Dukette, 1998). Thus, the greater amplitude Nc to novel stimuli found in the present study is consistent with the finding that ECDs were located in the anterior cingulate and frontal cortex.
The ICA and cortical source localization analyses also may resolve the inconsistency in the ERP findings of this study and previous similar studies. The ICA cluster located over the frontal pole also demonstrated similar experimental effects to the Nc ERP component; however, familiarization condition had no effect on activity in this cluster. This may explain the inconsistency between the Nc ERP findings from the present study when compared with past studies in this area (e.g., Nelson & Collins, 1991, 1992; Richards, 2003a). Nelson and Collins (1991, 1992) and Richards (2003a) used fewer electrodes (referenced to different electrode sites) for EEG recordings and may have only registered activity analogous to the frontal pole ICA cluster, which was insensitive to familiarization. In contrast, the present study used high-density EEG and thus registered a more widespread cortical activity that was associated with Nc. The prefrontal cluster was affected by familiarization condition, whereas the second cluster (frontal pole) was not affected by familiarization condition. Dipoles from the frontal pole ICA cluster were located in midline areas of the superior frontal gyrus. However, it is important to note that Nelson and Collins (1991, 1992) used faces as stimuli, and this could also account for the inconsistency between their findings and the findings of the present study.
The Late Slow Waves
In Richards (2003a), the late slow waves differed for the frequent-familiar, infrequent-familiar, and infrequent-novel stimuli primarily during attention. The present study affirms those results (e.g., Figures 8, 9, and 10). In the late slow wave ERP analysis, there was a significant interaction between attention and VRM stimulus type at frontal and right temporal electrode sites. At frontal electrodes, infants demonstrated a negative slow wave following novel-stimulus presentations during attention that was not seen during periods of inattention. At temporal electrodes in the right hemisphere, infants demonstrated a positive slow wave following infrequent-familiar stimulus presentations during attention but not during inattention. Consistent with these findings, there was a significant interaction between attention and VRM stimulus type on the prefrontal ICA cluster activations during the period of the late slow waves. Nelson and colleagues (de Haan & Nelson, 1997; Nelson & Collins, 1991, 1992) have proposed that the negative slow wave reflects detection of novelty, and the positive slow wave reflects memory updating for a partially encoded (i.e., infrequent familiar) stimulus and suggests cortical locations for these processes. The prefrontal ICA cluster demonstrated significant effects following novel-stimulus presentations during attention consistent with slow wave components of the ERP analysis. Given the cortical sources of this component cluster, this implies that the prefrontal cortex is involved in novelty detection. This is reflected both in the Nc and the negative late slow wave. The prefrontal cortex is most likely part of an integrated network of areas involved in novelty detection; other areas involved in this network may include subcortical structures (e.g., the hippocampus). The positive slow wave found in response to the infrequent-familiar stimulus implies that the updating of memory for a partially encoded stimulus occurs in temporal cortical areas.
Discussion of ICA and Cortical Source Analysis
A distinct advantage of the analysis in the present article involves the use of ICA and the cortical source analysis. ICA of EEG decomposes the variance produced by the simultaneous activation of discrete cortical areas in observed electrical activity via its cooccurrence in the spatial coordinates of the electrodes. Analysis of the activations of the components may be better than analyzing single electrode EEG or multiple electrode EEG. In the present study, the distinction of the prefrontal ICA cluster and the frontal pole cluster revealed a different time course of activation for the midlatency scalp activity than analyzing the ERP component (Nc). It also showed this time course was sensitive to the influence of attentive state (see Figure 6), whereas this effect was not significant in the ERP analysis. However, the cortical sources of ICA clusters inferred from equivalent current dipole analyses should be seen as tentative because there are some unresolved issues with this approach. The traditional model for cortical source analysis is based on parameters for use with adult participants. The models are based on impedance values for cortical matter, skull, and scalp of adult participants. Adult values of impedance are higher than those in infants. The use of adult impedance values with infant participants may have the effect of inferring the source of the electrical current on infant participants as being deeper in the cortex than where it actually occurred. The ideal source analysis technique uses anatomical data from individual participants (i.e., structural MRI). In this study, we used a structural MRI from a single 6-month-old participant and generated an electrode placement map on the basis of this individual’s head measurements. This placement map was then transformed to match the head measurements of each participant, and these transformed placement maps were used for each participant’s ECD analysis. This technique served to constrain the locations of the dipoles to a realistic topography, individualized for each participant; however, obtaining structural MRIs for each individual infant would be ideal. Finally, the Talairach (Talairach & Tournoux, 1988) coordinate system may be problematic for use in infant studies because a precise relation between the Talairach and infant coordinate space is undetermined at this time. Because of these present limitations, we believe it is appropriate to limit conclusions regarding cortical sources of infant ERP components to broad areas within the cortex. Notwithstanding these problems, however, we view the localization of the cortical sources of these ERP components as a great advance in the study of the ERP components of infant recognition memory.
Conclusion
The present study examined the effects of familiarization and attention on electrophysiological correlates of infant recognition memory. Infants demonstrated greater amplitude Nc following novel-stimulus presentations when compared with familiar-stimulus presentations. Infants exposed to the familiar stimuli during a familiarization phase primarily accounted for this effect. This finding is inconsistent with previous studies in this area using a familiarization phase (Nelson & Collins, 1991, 1992; Richards, 2003a); however, the results of the cortical source analysis may shed light on this inconsistency. One component cluster, with dipoles located in the frontal pole of the prefrontal cortex, was topographically and temporally similar to the Nc component but did not show an effect of the familiarization procedure on the time course of its activation. A second component cluster, with dipoles located in prefrontal cortex (inferior and medial frontal gyri and the anterior cingulate), showed the effects of the familiarization condition. It is possible that previous studies in this area (Nelson & Collins, 1991, 1992; Richards, 2003a) measured EEG activity that was heavily influenced by areas within the superior frontal gyrus that are not affected by familiarization.
All age groups demonstrated differences in Nc and late slow wave responses on the basis of VRM stimulus type. This indicates that by 20 weeks (4.5 months) of postnatal age, infants are able to demonstrate responsiveness to novelty (i.e., recognition memory) at the cortical level with 20 s of preexposure to a familiar stimulus. This is consistent with behavioral studies showing that 3.5-month-olds (16-week-olds) require 30 s of familiarization to demonstrate novelty preferences, whereas 6-month-olds (26-week-olds) demonstrate novelty preferences following 20 s of familiarization (e.g., Courage & Howe, 2001; Richards, 1997; Rose, 1983; Rose, Gottfried, Melloy-Carminar, & Bridger, 1982). It is important to note that infants in the present study received repeated, brief exposures to the familiar stimuli during testing in addition to 20 s of familiarization. Additionally, behavioral measures reflect the final outcome of cognitive processing, whereas ERP measures may better reflect neural operations occurring during the recognition memory process (de Haan & Nelson, 1997). Thus, differences in cortical responding to novelty may be expected prior to behavioral differentiation of novelty. However, the present findings combined with previous behavioral findings (Richards, 1997) suggest the ability to demonstrate recognition memory with relatively brief periods of familiarization (i.e., 20 s) emerges early in postnatal development and is present by 20 weeks of age.
Attention was an important mediator of the ERP components found in this study. This was true for the Nc ERP component, particularly for the two ICA component clusters representing the Nc scalp activity. Similarly, late slow waves in the ERP that reflected different responses to the frequent-familiar, infrequent-familiar, and infrequent-novel stimuli, and which therefore show a discrimination between stimulus probability (frequent and infrequent familiar) and stimulus novelty (infrequent familiar and novel stimuli), occurred primarily during attention. These results replicate Richards (2003a) and illustrate the strong relationship between attention and recognition memory in early cognitive development.
Acknowledgments
This research was supported by National Institute of Child Health and Human Development Grant R01-HD18942 and by Major Research Instrumentation Award BCS-9977198 from the National Science Foundation. We thank Michael Stevens, Brittany Mallin, and Bill Campbell for their aid in testing participants, data editing, and analysis. We acknowledge April Benasich and workers at the Infancy Studies Laboratory at Rutgers University for the infant MRI, for which acquisition was supported by the Elizabeth H. Solomon Center for Neurodevelopmental Research.
Footnotes
The frequently presented stimulus was labeled frequent familiar and the infrequently presented stimulus was labeled infrequent familiar. However, for the control group, these stimuli were novel stimuli that had not been previously presented and were novel at the beginning of the stimulus presentation.
The choice of 100 kΩ as the maximum impedance value was based on the high input impedance of the EGI amplifiers (see Richards, 2003b, 2004). These amplifiers have an input impedance of about 200 MΩ, so given the recommendation of interelectrode impedances being at least 1% of amplifier input impedance (e.g., 10 kΩ for 10 MΩ amplifier; Picton et al., 2000), 100 kΩ is appropriate for this amplifier. Ferree, Luu, Russell, and Tucker (2001) estimate that for this amplifier system a 50 kΩ preparation would lead to a maximum 0.025% signal loss, and therefore the current levels should lead to no more than a 0.050% signal loss. They found no discernible signal loss with electrode preparations at about 40 kW.
The ANOVAs for the analyses were done with a general linear models approach using nonorthogonal design because of the unequal distribution of the number of trials in the cells of the Stimulus Types × Familiarization Type × Attention Phases × Participants factorial design (see Hocking, 1985; Searle, 1971, 1987). The sums of squares (hypothesis and error) for the nested effects in the design were estimated using “participants” as a class and nesting repeated measures (attention phase, stimulus type, familiarization type) within this class variable. The “PROC GLM” of SAS was used for the computations.
There were only enough clusters for all participants and factors for the prefrontal component cluster and the left temporal cluster to analyze the cluster by itself. So, the two frontal clusters were tested together (prefrontal and frontal pole), the two parietal clusters together (central-parietal and parietal occipital), and the three occipital clusters were analyzed together.
References
- Bornstein, M. C. (1985). Habituation of attention as a measure of visual information processing in human infants: Summary, systematization, and synthesis. In G. Gottlieb & N. A. Krasnegow (Eds.), Measurement of audition and vision in the first year of postnatal life: A methodological overview (pp. 253–300). Norwood, NJ: Ablex Publishing.
- Casey BJ, Richards JE. Sustained visual attention measured with an adapted version of the visual preference paradigm. Child Development. 1988;59:1514–1521. [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997;30:61–69. [PubMed] [Google Scholar]
- Cohen, R. A. (1993). The neuropsychology of attention New York: Plenum Press.
- Coull JT, Frith CD, Fackowiak RSJ, Grasby PM. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Courage ML, Howe ML. Long-term retention in 3.5-month-olds: Familiarization time and individual differences in attentional style. Journal of Experimental Child Psychology. 2001;79:271–293. doi: 10.1006/jecp.2000.2606. [DOI] [PubMed] [Google Scholar]
- Courchesne E. August). Event-related brain potentials: Comparison between children and adults. Science. 1977;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Fabiani, M., Gratton, G., & Coles, M. G. H. (2000). Event-related brain potentials: Methods, theory, and applications. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 53–84). New York: Cambridge University Press.
- Fantz RL. The origin of form perception. Scientific American. 1961;204:66–72. doi: 10.1038/scientificamerican0561-66. [DOI] [PubMed] [Google Scholar]
- Fantz RL. Pattern vision in newborn infants. Science. 1963;140:296–297. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Journal of Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Frick J, Richards JE. Individual differences in recognition of briefly presented visual stimuli. Infancy. 2001;2:331–352. doi: 10.1207/S15327078IN0203_3. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annual Review of Neuroscience. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SL, Grady CL. Face encoding and recognition in the human brain. Proceeding of the National Academy of Sciences, USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking, R. R. (1985). The analysis of linear models Monterey, CA: Brooks/Cole.
- Johnson MH, de Haan M, Oliver A, Smith W, Hatzakis H, Tucker LA, Csibra G. Recording and analyzing high-density event-related potentials with infants using the Geodesic sensor net. Developmental Neuropsychology. 2001;19:295–323. doi: 10.1207/S15326942DN1903_4. [DOI] [PubMed] [Google Scholar]
- Karrer, R., & Ackles, P. K. (1987). Visual event-related potentials of infants during a modified oddball procedure. In R. Johnson, J. W. Rohrbaugh, & R. Parasuraman (Eds.), Current trends in event-related potential research (pp. 603–608). Amsterdam: Elsevier Science. [PubMed]
- Karrer, R., & Ackles, P. K. (1988). Brain organization and perceptual/cognitive development in normal and Down’s syndrome infants: A research program. In P. Vietze & H. G. Vaughan, Jr. (Eds.), The early identification of infants with developmental disabilities (pp. 210–234). Philadelphia: Grune & Stratton.
- Karrer R, Monti LA. Event-related potentials of 4–7 week-old infants in a visual recognition memory task. Electroencephalography and Clinical Neurophysiology. 1995;94:414–424. doi: 10.1016/0013-4694(94)00313-a. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Roland PE. Right-prefrontal activation during encoding, but not retrieval, in a non-verbal paired-associates task. Cerebral Cortex. 1998;8:73–79. doi: 10.1093/cercor/8.1.73. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19:105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nelson, C. A., & Dukette, D. (1998). A cognitive neuroscience perspective on the relation between attention and memory development. In J. E. Richards (Ed.), Cognitive neuroscience of attention: A developmental perspective (pp. 327–362). Hillsdale, NJ: Erlbaum.
- Nelson CA, Salapatek P. Electrophysiological correlates of infant recognition memory. Child Development. 1986;57:1483–1497. [PubMed] [Google Scholar]
- Nikkel L, Karrer R. Differential effects of experience on the ERP and behavior of 6-month-old infants: Trends during repeated stimulus presentation. Developmental Neuropsychology. 1994;10:1–11. [Google Scholar]
- Nunez PL. Localization of brain activity with electroencephalography. Advances in Neurology. 1990;54:39–65. [PubMed] [Google Scholar]
- Owen AM, Milner B, Petrides M, Evans AC. Memory for object features vs. memory for object location: A positron-emission tomography study of encoding and retrieval processes. Proceedings of the National Academy of Sciences, USA. 1996;93:9212–9217. doi: 10.1073/pnas.93.17.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Scalp current density mapping: Value and estimation from brain data. IEEE Transactions on Biomedical Engineering. 1987;34:283–288. doi: 10.1109/tbme.1987.326089. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–588. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Putnam LE, Johnson R, Roth WT. Guidelines for reducing the risk of disease transmission in the psychophysiology laboratory. Psychophysiology. 1992;29:127–141. doi: 10.1111/j.1469-8986.1992.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997;33:22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003a;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Cortical sources of event-related potentials in the prosaccade and antisaccade task. Psychophysiology. 2003b;40:878–894. doi: 10.1111/1469-8986.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Recovering cortical dipole sources from scalp-recorded event-related potentials using component analysis: Principal component analysis and independent component analysis. International Journal of Psychophysiology. 2004;54:201–220. doi: 10.1016/j.ijpsycho.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing cortical sources of event-related potentials in infants’ covert orienting. Developmental Science. 2005;8:255–278. doi: 10.1111/j.1467-7687.2005.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Richards, J. E., & Casey, B. J. (1992). Development of sustained visual attention in the human infant. In B. A. Campbell, H. Hayne, & R. Richardson (Eds.), Attention and information processing in infants and adults (pp. 30–60). Mahwah, NJ: Erlbaum.
- Rose SA. Differential rates of visual information processing in full-term and preterm infants. Child Development. 1983;54:1189–1198. [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar PM, Bridger WH. Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology. 1982;18:704–713. [Google Scholar]
- Rothbart MK, Posner MI, Rosicky J. Orienting in normal and pathological development. Development and Psychopathology. 1994;6:635–652. [Google Scholar]
- Rugg MD, Fletcher PC, Chua PML, Dolan RJ. The role of prefrontal cortex in recognition memory for source: An fMRI study. NeuroImage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Searle, S. R. (1971). Linear models New York: Wiley.
- Searle, S. R. (1987). Linear models for unbalanced data New York: Wiley.
- Slotnick SD, Moo LR, Segal JB, Hart JH., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain NY: Thieme Medical Publishers.
- Tucker DM. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Liotti M, Potts GF, Russell GS, Posner MI. Spatiotemporal analysis of brain electrical fields. Human Brain Mapping. 1994;1:134–152. [Google Scholar]


