Abstract
The cortical sources of event-related potentials (ERP) were examined in a prosaccade and antisaccade task in college-age participants. The task included a cue that indicated the spatial location of the target, a cue that indicated the type of eye movement, or no cue. A principal component analysis and equivalent current dipole analysis showed that a peripheral spatial cue resulted in extrastriate activity localized in Brodmann’s area 19 whereas a central cue results in activity in areas 19 or 37. This extrastriate activity reflects an enhanced response to the target when attention was directed to that location. The presaccadic ERP activity primarily consisted of a contralateral positive potential and ipsilateral negative potential, localized in Brodmann’s areas 8, 10, and 11. The temporal proximity of this cortical activity and its relation to movement cueing suggests it reflects eye movement planning processes.
Descriptors: Presaccadic, Event-related potentials, Prosaccade-antisaccade, Cortical source analysis
The study of targeted eye movements in the prosaccade and antisaccade task has been used to understand the neural control of eye movements. The targeted task consists of the presentation of a target stimulus in one of two locations in opposite hemifields, and an eye movement is made either to the target (“prosaccade”) or away from the target to the location in the opposite hemifield (“antisaccade”). The antisaccade task, first introduced by Hallet (1978; also see Fischer & Weber, 1992), has been used in a wide variety of studies to examine visual attention, reflexive inhibition, eye movement characteristics, and neuropsychological status (see review by Everling & Fischer, 1998). Studies with animals (Funahashi, Chafee, & Goldman-Rakic, 1993; Schlag-Rey, Amador, Sanchez, & Schlag, 1997) and humans (Fox, Fox, Raichle, & Burde, 1985; O’Driscoll et al., 1995; Sweeney et al., 1996) have shown that areas of the frontal cortex, such as the supplementary eye fields (SEF), frontal eye fields (FEF), dorsolateral prefrontal cortex (DPC), and prefrontal cortex (PFC) are involved in the generation of prosaccade and antisaccade eye movements. Several studies have recorded scalp event-related potentials (ERP) prior to saccadic eye movements and show several types of presaccadic ERP activity related to prosaccade and antisaccade eye movements (Evdokimidis, Liakopoulos, Constantinidis, & Papageorgiou, 1996; Everling, Krappmann, & Flohr, 1997; Everling, Spantekow, Krappmann, & Flohr, 1998).
This article describes a study of college-age participants’ ERP activity in a prosaccade and antisaccade task using principal components analysis. Spatially coordinated ERP activity was analyzed in relation to the events occurring in the task, and cortical source analysis was used to infer the cortical areas controlling eye movements in this task.
Several studies have examined scalp-recorded EEG changes that are locked to the onset of the saccadic eye movements, “presaccadic ERP.” A negative ERP before an eye movement has been reported that begins up to 1 s prior to saccade onset and has its maximum values over the vertex (Becker, Hoehne, Iwase, & Kornhuber, 1973; Klostermann et al., 1994; Kurtzberg & Vaughan, 1980, 1982; Moster & Goldberg, 1990). A positive component (or slowly increasing positive wave) has been found about 30–300 ms prior to saccade onset and occurs primarily over parietal areas contralateral to the saccade direction (Becker et al., 1973; Csibra, Johnson, & Tucker, 1997; Kurtzberg & Vaughan, 1980, 1982; Moster & Goldberg, 1990). Finally, studies have reported a sharp positive spike potential in the presaccadic ERP over parietal scalp leads about 10 to 20 ms prior to saccade onset (Balaban & Weinstein, 1985; Becker et al., 1973; Csibra et al., 1997; Kurtzberg & Vaughan, 1980, 1982; Weinstein, Balaban, & Ver Hoeve, 1991). These potentials were larger, or more widespread, for saccades occurring to expected peripheral stimuli or predicted locations (Evdokimidis, Constantinidis, Gourtzelidis, Liakopoulos, & Papageorgiou, 1997; Evdokimidis, Liakopoulos, & Papageorgiou, 1991; Evdokimidis, Mergner, & Lucking, 1992; Kurtzberg & Vaughan, 1982; Thickbroom & Mastaglia, 1985) and larger for voluntary than for reflexive saccades (Balaban & Weinstein, 1985; Evdokimidis et al., 1991, 1992).
Presaccadic ERP activity specific to the antisaccade task has been examined (Brickett, Weinberg, & Davis, 1984; Evdokimidis et al., 1996; Everling et al., 1997; Everling, Spantekow, et al., 1998; Klein, Heinks, Andresen, Berg, & Moritz, 2000). Evdokimidis et al. (1996) used a mixed-choice paradigm (prosaccade, antisaccade, and catch trial targets). They found no difference in the presaccadic negative potential for prosaccade and antisaccade trials, but the presaccadic positive potential began earlier for prosaccade trials and resulted in more positive presaccadic ERP on those trials. Everling et al. (1996, 1997) used a block design in which prosaccade and antisaccade trials were given in separate blocks. The presaccadic negative potential had a larger amplitude and more widespread scalp distribution on the blocks in which the antisaccade eye movements were made than in the blocks in which the prosaccade eye movements were made. The presaccadic positive potential and spike potential were larger in the prosaccade blocks than in the antisaccade blocks, but this difference was much smaller in magnitude and only at two electrodes (C3, C4 for presaccadic positive potential, and Pz, P4 for spike potential). Klein et al. (2000) used a warning stimulus that gave a cue indicating the type of upcoming movement (prosaccade or antisaccade) and an imperative stimulus that showed where the eye movement should occur. They found a negative presaccadic potential during the warning interval preceding the onset of the imperative stimulus that was larger on the antisaccade trials than on the prosaccade trials.
The studies of presaccadic ERP may be compared indirectly to neuroimaging (PET or fMRI) studies of brain activity for prosaccadic and antisaccadic eye movements. The blocked design used by Everling et al. (1996, 1997) is similar to the blocked subtraction used in the PET studies of prosaccade and antisaccade eye movements (e.g., O’Driscoll et al., 1995; Sweeney et al., 1996). The frontal-central distribution of the presaccadic negative potential may be analogous to the frontal (SEF, FEF) and prefrontal (DPC, ventromedial or ventrolateral PFC) cortical activity found in neuroimaging studies. The posterior (primarily parietal) scalp distribution of the presaccadic positive potential would be most closely related to the activity found in the superior parietal lobes in neuroimaging studies (O’Driscoll et al., 1995; Sweeney et al., 1996; see Everling & Fischer, 1998). The time course of the presaccadic negative potential undermines its importance in the actual eye movement. In many studies, the negative presaccadic potential begins from 500 to 1,000 ms before saccade onset (Evdokimidis et al., 1992, 1996, 1997; Everling et al., 1996; Klein et al., 2000; Klostermann et al., 1994; Moster & Goldberg, 1990). Therefore, given reaction times to the prosaccade or antisaccade target of 400 to 500 ms, the negative presaccadic activity precedes the target onset (e.g., Evdokimidis et al., 1996; Everling et al., 1996; Klein et al., 2000). Thus, in the mixed-choice paradigm, this potential could not distinguish between prosaccade and antisaccade eye movements (Evdokimidis et al., 1996). In the antisaccade trials in the ERP blocked design (Everling et al., 1996) or the PET blocked design (e.g., O’Driscoll et al., 1995; Sweeney et al., 1996), this activity may represent a response set for antisaccade eye movements or preparatory cognitive processes. In this sense, these frontal areas may represent the inhibition of preparatory prosaccade-related neural activity or stimulus-directed neural activity and the enhancement of the fixation in the center location (Everling, Dorris, Klein, & Munoz, 1999; Everling, Dorris, & Munoz, 1998), rather than the programming of the saccade away from the target to the opposite field.
The primary goal of this study was to investigate the neural control of prosaccade and antisaccade eye movements using presaccadic ERP recording. This was done by using high-density EEG recording (128 channels) during a targeted eye movement task, estimating the cortical sources of the EEG with current dipole analysis, and relating the EEG and cortical sources to the events occurring in the task. The PET studies of the antisaccade activity used blocked-trials designs and imaged neural activity over long intervals and over many trial presentations. This makes it impossible to determine the temporal relation of these cortical areas to the components of the task (target preparation, target onset, eye movement preparation, and eye movement; Everling & Fischer, 1998). Prior studies in this task using presaccadic ERP activity (Brickett et al., 1984; Evdokimidis et al., 1996; Everling et al., 1997; Everling, Spantekow, et al., 1998; Klein et al., 2000) used a limited number of electrodes (12, 19, 25, or 32 electrodes in the 10–20 system). These studies produced topographical scalp potential maps and interpreted this scalp activity as being generated by cortical sources such as the SEF. In the present study, college-age participants were tested in a targeted procedure in which targets indicated to the participant to make a prosaccade eye movement toward the target, an antisaccade eye movement away from the target, or no eye movement. The ERP activity related to target onset and to saccade onset was studied with principal components analysis to identify “principal components” (PC) in the spatial electrode array (Picton et al., 2000; Spencer, Dien, & Donchin, 1999). The relation of the PCs to experiment events was examined with PC scores computed in the temporal domain. The PC analysis was followed by cortical source analysis (“brain electrical source analysis,” “equivalent current dipole analysis”; Huizenga & Molenaar, 1994; Scherg, 1990, 1992; Scherg & Picton, 1991) to estimate cortical dipoles that may generate the observed presaccadic ERP activity shown in the PC analysis.
A second goal of the study was to examine components used in carrying out this task by separating ERP activity occurring in preparation to the target, in response to the target, and prior to the saccade. These issues have been a major difficulty in interpreting the results of prior studies of presaccadic ERP in the prosaccade and antisaccade task. This was accomplished partially by examining target-related EEG segments separately from saccade-related EEG segments. In this way target-related ERP activity (and its corresponding cortical sources) may be distinguished from saccade-related ERP activity (and its corresponding cortical sources). The study was designed as a mixed-choice trials design (Evdokimidis et al., 1996) that allowed response preparation on a trial-by-trial basis (cf. Klein et al., 2000). A movement cue condition consisted of a color cue that indicated the type of the upcoming eye movement (prosaccade, antisaccade, no eye movement; see Klein et al., 2000). This allowed movement response preparation possible previously only in the ERP blocked design (Everling et al., 1996) or the PET blocked design (e.g., O’Driscoll et al., 1995; Sweeney et al., 1996). Thus, preparatory responses would be unique to the trial rather than to the block and response sets in the block would not occur. Spatial cue conditions (peripheral or central) were given that separated the contribution of location response preparation independent of movement response preparation. These two conditions separate the preparatory processes due to movement-type response set from the processes due to location-type response set. Finally, a no-cue condition closely mimicked the mixed-trial designs used previously (e.g., Evdokimidis et al., 1996).
Method
Participants
Participants were 50 undergraduate students at the University of South Carolina and were recruited from the human participant pool in the Department of Psychology. The median age of the participants was 20 years, 9 months. The participants were randomly assigned to one of five cueing conditions, with 10 participants per cueing condition. The ages of the participants were not significantly different for the five cueing conditions. There were 33 women and 17 men. The research was approved by the Institutional Review Board for the Use of Human Subjects and informed consent to participate in the study was obtained from each participant.
Apparatus and Stimuli
Each participant sat in a comfortable chair approximately 75 cm from a 29-in. color video computer monitor (NEC Multisync XM29) displaying at 1,280 horizontal and 1,024 vertical pixels. The stimuli were presented in a 2.6° square area, located in the center or 10° to the right or left of center. The pretarget stimuli consisted of a square outline at each of the three areas. The squares to the right and left of center were outlines and the center square had 9 internal outline squares in a 3 × 3 pattern. At the target onset, the center square was removed, a target replaced one of the squares in the periphery, and the other peripheral square remained. The targets consisted of a solid triangle, a checkerboard pattern, or a four-point star, presented within the bounds of the 2.6° square area. The peripheral spatial cue consisted of a 1° solid blinking square in the right or left target location prior to the pretarget interval. The central spatial cue consisted of presenting a solid pattern in 1 of the 9 internal outline squares in the pretarget center stimulus. The movement cue consisted the center square outline being in green, red, or white.
Procedure
The participant sat in the chair and the viewing area facing the television monitor. The participant was informed that this was a study of the brain control of eye movements and was given instructions and practice in the procedure. The pretarget stimuli were presented for 2 s, followed by the presentation of the target for 2.5 s, followed by an interstimulus interval varying randomly from 1 s to 3 s. The participants were instructed to fixate on the center square during the pretarget period, to make an eye movement toward the checkerboard target when it appeared (prosaccade), away from the triangle target to the opposite outline square (antisaccade), or to keep the eyes fixated in the center location when the four-point star appeared (catch trial). The stimuli were presented continuously in 5-min blocks, allowing about 40 presentations per block. Six 5-min blocks were done.
Each participant received two cueing procedures in alternating blocks: (1) No cue or peripheral spatial cue: The no cue procedure consisted of the presentation of the pretarget and target stimuli without a cue. The peripheral cue procedure consisted of the presentation of the solid blinking square for 500 ms in the right or left location where the target would appear. (2) No cue or central cue: The central cue procedure consisted of the solid pattern in the square at the middle-right or middle-left used to indicate an upcoming target in the right or left location, respectively, and remained during the pretarget interval. (3) No cue or movement cue: The movement cue procedure consisted of the presentation of the center square in the color green for an upcoming prosaccade target, in the color red for an antisaccade target, and in the color white for a catch trial. (4) Peripheral cue or peripheral-movement combination: The peripheral-movement procedure was a combination of a peripheral and a movement cue, indicating in advance the type of eye movement and location of the target, thus allowing full planning of the eye movement prior to target onset. (5) Central cue or central-movement combination: The central-movement procedure was a combination of the central cue and the movement cue. However, saccade latencies were so fast for the peripheral-movement and the central-movement procedures (e.g., about 275 ms) and some of the ERP components were in the 200-ms range, so the ERP from the combination procedures were not analyzed.
Recording of EEG and Segmenting of EEG for ERP
The electroencephalogram (EEG) was recorded with the EGI (Electrical Geodesics Incorporated, Eugene, OR) 128-channel EEG recording system (Tucker, 1993; Tucker, Liotti, Potts, Russell, & Posner, 1994). The EGI sensor net’s anatomically marked locations were used to position the electrodes and the elasticity of the connections between pedestals positioned the other electrodes in fixed locations on the scalp. The electrode recordings were adjusted to record with impedances below 100 kΩ.1 The EEG signal was referenced to the vertex, recorded with 20K amplification, at a sampling rate 250 Hz (4-ms samples) with band-pass filters set at 0.1 to 100 Hz. The vertex-referenced EEG was algebraically recomputed to an average reference.
The segmenting of the EEG for the ERP was done with respect to the target onset and the saccade onset. For the ERP segments, the electrooculogram (EOG) was defined as the difference between electrical potential at two electrodes near the outer canthii of eyes. Saccades were identified in the EOG recording with an algorithm based on a third-order differentiation of the raw EOG signal (Matsuoka & Harato, 1983; Matsuoka & Ueda, 1986; Richards & Hunter, 1997) and each identified saccade was visually inspected. Only trials with correct saccades (toward prosaccade target, away from antisaccade target, or no saccade on catch trial) were included. The stimulus-locked segments used the stored information about target onset time to extract the EEG data from 1,000 ms before to 200 ms after stimulus onset, excluding trials with eye movements during this period. The lateral leads in the target onset segments were reversed across sides so that the target location was always represented on the left side of the head. The response-locked segments extracted the EEG data from the onset of the target until the onset of the saccade identified in the EOG signal. These segments varied in length depending on the latency of the eye movement toward or away from the target. The lateral leads in the presaccadic segments were reversed across sides for half of the trials so that all eye movements were represented as moving to the left side. Postsaccadic segments were extracted from the EEG from 50 ms prior to saccade onset to 200 ms following saccade onset. These segments were used for display purposes only and were not analyzed. The participants were instructed to blink during the interstimulus interval. Blinking was measured with the difference between electrical activity above and below the eye and trials with blinks during the segment being analyzed were not used in the analysis.
Principal Components Analysis
The ERP activity related to target onset and to saccade onset was analyzed with principal components analysis to identify principal components in the spatial electrode array (Picton et al., 2000; Spencer et al., 1999).2 The “variables” for the analysis were the 128 electrodes, “observations” were the sequence of 4 ms (250 Hz) samples from the ERP segments, and the variable values were the EEG values at that point in time for that channel. The weights for a single PC represent the topographically organized co-occurrence of activity in the 128 electrodes in the ERP segments. The PCs were done separately for each participant, and separately for the stimulus-locked and response-locked ERP segments. The 20 PCs with the largest eigenvalue for each individual were used in the analyses. A clustering strategy identified similar PCs across participants. There were three steps in this analysis. First, the data from 2 participants from each of the five testing conditions were chosen to “seed” the clusters. The 20 PCs of these participants were clustered, with clusters being defined as the minimum distance between the individual PC clusters. The 10 participants’ PCs were uniquely assigned to the 20 clusters. Second, the PCs for each individual were reassigned to the 20 clusters by removing an individual’s data from the clusters, recalculating each centroid for the 20 PC clusters, and assigning the 20 PCs of the current individual to the cluster with the minimum distance between the centroid and the PC. This was done iteratively until the PCs for all individuals were assigned to the same clusters on subsequent steps. These clusters were visually inspected and were modified to remove obvious outliers. The third step accepted the clusters from the second step as the fixed clusters for the study. The PCs from the remaining 40 participants were classified into these groups based on the minimum distance between the centroid of the group and the PCs of the individuals.
The “principal component score” was calculated from each PC at each point in the sequence of the ERP segments. This sequence represents the “temporal activation” (Makeig, Bell, Jung, & Sejnowski, 1996; Makeig, Jung, Bell, Ghahremani, & Sejnowski, 1997) of the PC and was analyzed in conjunction with experimental factors. The PC weights and activations were multiplied to form “projections” of the PCs from the PC space back into the electrode space. The projections with a limited number of components represent the activity of the selected PCs plotted in the metric of the original electrode recording. Topographical maps of cortical surface potentials were done based on the PC analysis. The data for the topographical maps came from the PC loadings (eigenvectors) or from the projections of the PC into the electrode space. For the PC loadings, a single topographical map represents the spatial distribution of the component across the entire ERP segment that was analyzed and thus is independent of the experimental factors. For the PC projections, a single topographical map represents the spatial distribution of the component at a specific point in time. Spatiotemporal displays were made of the PC projections by creating multiple topographical maps over specified periods of time in relation to experiment events. The EMSE computer program (Source Signal Imaging, San Diego, CA) was used to make the topographical maps, which consist of a spherical spline interpolation (Perrin, Pernier, Bertrand, & Echallier, 1989) shown in a radial projection (Perrin, Bertrand, & Pernier, 1987).
Cortical Source Analysis
The PC analyses (spatial topography, activation, and experimental differences) were followed by cortical source analysis (brain electrical source analysis, equivalent current dipole analysis; Huizenga & Molenaar, 1994; Scherg, 1990, 1992; Scherg & Picton, 1991). The number of dipoles generating surface recorded activity is limited, and the number of reasonable dipole parameters is limited by the rank of the covariance matrix of the data. An estimate of this rank is the number of “significant” components in the PC analysis (Mosher, Lewis, & Leahy, 1992). This number of reasonable dipole parameters may be found by using a limited set of PCs, ordered by the size of the “eigenvalue” (i.e., percent of variance of data matrix), with traditional methods used for accepting the number of significant PCs in the data (e.g., “scree test”; Sharma, 1996). I assumed that the weights (eigenvector weights) relating the electrodes to a component for a single PC represent topographically organized co-occurrence of activity on the scalp. Therefore, I approached the source analysis problem by estimating a single dipole (location and moment parameters) for loading weights for a single PC derived from the segmented EEG data (Achim, Richaer, & Saint-Hilaire, 1988; Maier, Dagnelie, Spekreijse, & van Dijk, 1987; also cf. Mosher et al., 1992). This single dipole analysis was done on the PCs obtained from the raw event-segmented EEG data for individual participants. The EMSE computer program was used to obtain equivalent current dipoles for the PCs.
Several aspects of the current analysis relied on individual participant data (Richards, 2002). A structural MR recording was made for one individual and scalp/skull landmarks were measured. An electrode placement map was generated for this individual based on these head measurements and the known locations of the EGI electrodes. For each participant in the study, the same external head measurements were made (e.g., nasion-inion diameter and circumference). Electrode placement maps were generated for the participant by transforming the placement map from the individual with the MR recording according to the head measurements of the participant. These electrode placement maps were used in the EMSE computer program with data from the individual participant to identify current dipole sources for that individual for component loadings or projections. This constrained the locations of the dipoles to a realistic topography based on individual participant data. The locations were then translated into saggital, coronal, and axial coordinates in the Talairach (Talairach & Tournoux, 1988) coordinate system. These coordinates provided a location for the dipole based on the individual participant head size and shape translated into the standardized coordinate system. The resulting coordinates were plotted on the Talairach atlas maps and on the MR recording for the individual in this study. The individual participant coordinates were then used in group averages and dispersions, MR plotting, and analysis of the cortical source locations.
Results
Saccade Latency
The onset of the saccade from the center fixation square to the targeted square was analyzed to determine if the prosaccade and antisaccade latencies were affected by the cueing procedures. The latency (in milliseconds) was analyzed with a Procedure (6: no cue, peripheral cue, central cue, movement cue, peripheral-movement combination, central-movement combination) × Movement Type (prosaccade, antisaccade) repeated measures ANOVA.3 As expected, there was a main effect of the movement type on the saccade latency, F(1,49) = 38.08, p <.001. The latency to make a prosaccade was smaller than the latency to make an antisaccade (Ms = 385.76 ms [SE = 2.068] and 441.43 ms [SE = 2.434], respectively).
There were statistically significant effects of the cueing procedure, F(5,44) = 61.71, p <.001, and an interaction between the cueing procedure and the saccade movement type, F(5,49) = 4.38, p = .002. Table 1 contains the descriptive statistics for the prosaccade and antisaccade latencies for the cueing procedures. The no-cue and both spatial-cue procedures had longer antisaccade latencies than prosaccade latencies, and these three procedures did not differ in the prosaccade–antisaccade latency difference. The movement cue procedure, in which the type of movement but not the location of the target was cued, also differed between prosaccade and antisaccade latencies, but this difference was smaller than in the prior conditions. The combination procedures, in which the direction and type of movement could be determined from the cues, did not differ in the prosaccade and antisaccade latencies, had the shortest latencies of the six procedures, and did not differ between themselves in the saccade latencies.
Table 1.
Descriptive Statistics for Saccade Latencies for the Cueing Procedures
| N | M | SD | Mediana | |
|---|---|---|---|---|
| No cue | ||||
| Prosaccade | 1,477 | 413.79 ms | 125.52 | 420 ms |
| Antisaccade | 1,379 | 489.70 ms | 136.09 | 488 ms |
| Peripheral spatial cue | ||||
| Prosaccade | 881 | 419.08 ms | 113.57 | 430 ms |
| Antisaccade | 863 | 475.56 ms | 140.80 | 479 ms |
| Central spatial cue | ||||
| Prosaccade | 854 | 424.53 ms | 138.57 | 424 ms |
| Antisaccade | 824 | 495.68 ms | 149.17 | 497 ms |
| Movement cue | ||||
| Prosaccade | 457 | 303.31 ms | 112.58 | 284 ms |
| Antisaccade | 452 | 342.27 ms | 113.06 | 322 ms |
| Combination peripheral-movement cue | ||||
| Prosaccade | 408 | 269.47 ms | 149.32 | 258 ms |
| Antisaccade | 386 | 285.10 ms | 153.91 | 278 ms |
| Combination central-movement cue | ||||
| Prosaccade | 441 | 289.00 ms | 129.75 | 276 ms |
| Antisaccade | 418 | 301.30 ms | 142.89 | 296 ms |
The median was calculated as the average of the per-subject median.
Grand Average ERP
Topographical maps were constructed from the grand average ERP across the cue procedures and eye movement types. These maps were done to display the traditional stimulus-locked and response-locked ERP changes occurring in this task. Figure 1 shows the pretarget ERP from 1 s before target onset to target onset and the presaccadic ERP from about 184 ms prior to saccade onset through 32 ms of saccade onset, collapsed across all conditions. A clear bilateral negative potential occurred prior to the target onset that was maximal in the frontal-central scalp leads (top panels). This activity was very similar to the negative presaccadic potential activity found in studies of saccade eye movement. The presaccadic ERP activity showed the presaccadic positive potential located over the parietal scalp areas beginning about 80 ms prior to saccade onset and becoming maximal immediately prior to saccade onset (Figure 1, bottom panels). Figure 2 shows ERP data plotted for several frontal-central electrodes with the pretarget data, and several parietal-occipital electrodes for the presaccade data. The negative presaccadic activity over the frontal-central may be seen across the frontal-central leads. The positive presaccadic potential shown in Figure 1 (bottom panels) occurring immediately before saccade onset appears to be in Figure 2 the “spike potential.” This may be seen primarily in the central parietal leads, and somewhat in leads contralateral to the eye movement. In addition to these potentials typically found in studies of presaccadic ERP, there was a presaccadic positive ERP component that was contralateral to the eye movement in the frontal electrodes that began about 180 ms before saccade onset and peaked at about 75 ms prior to saccade onset (seen in Figure 1; not illustrated in Figure 2). This was accompanied by a negative ERP component that was ipsilateral to the eye movement but which peaked later in the interval. Finally, the eye movement toward the target (prosaccade) or away from the target (antisaccade) may be seen in the lower right panel of Figure 1 as a large electrical potential change in ipsilateral and contralateral frontal leads, due to the electrooculogram.
Figure 1.
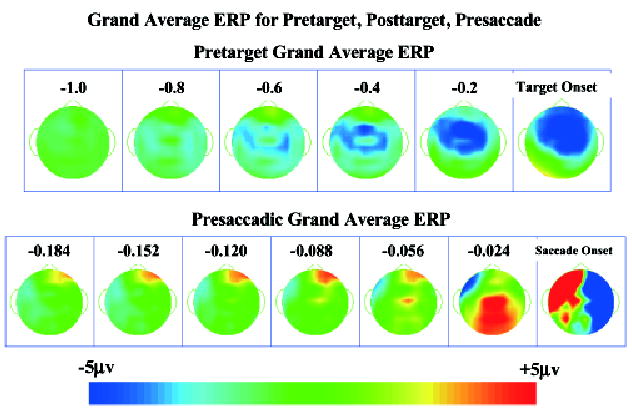
Topographical scalp potential maps for the grand average ERP in the pretarget period and immediately preceding the saccade, collapsed across conditions. The pretarget map is plotted as the average for 200-ms intervals from 1 s until target onset. The presaccade map is plotted as 32-ms averages from 182 ms preceding the saccade through 40 ms following saccade onset. For all topographical maps, the stimulus-locked segments are positioned so that the target appeared on the left side and the response-locked segments are positioned so that the eye movement went toward the left side.
Figure 2.
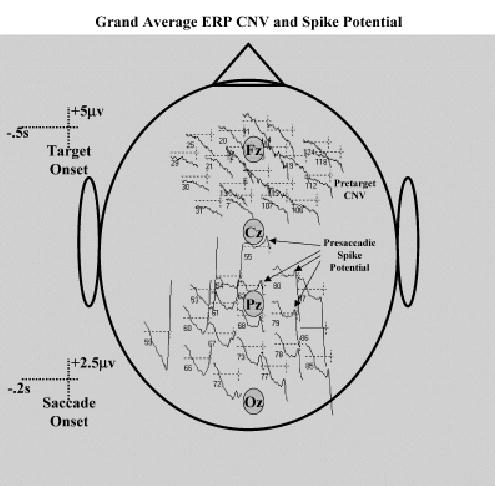
Grand average ERP for representative electrodes. The frontal-central electrodes contain data from the pretarget period and represent the negative pretarget potential (CNV). The parietal-occipital electrodes contain data from the presaccade period and represent the spike potential. The approximate location of the 10–20 midline electrodes are shown.
Principal Components Analysis: Eigenvectors
The ERP were analyzed with principal components analysis. Figure 3 shows topographical maps of the average eigenvector weights from the clusters for these PCs. The first row shows the PCs from the stimulus-locked ERP. The first PC (far left) was always the one with the largest eigenvalue and probably represents overall variance in the ERP segments. The third PC represents negative activity in the frontal-central electrodes, and the fourth PC represents positive activity in the parietal electrodes. A fifth PC (Figure 3, bottom half) represents positive scalp activity in the occipital area contralateral to the target location. The second row of Figure 3 shows PCs from the response-locked ERP. Four PCs were nearly identical to those from the stimulus-locked ERP analysis (four PCs in Figure 3, second row). Two PCs in the presaccadic segments represent activity in the lateral occipital area (fourth row). These two PCs likely correspond to the single contralateral occipital PC in the target onset segments, and are on opposite sides due to the switching of lateral leads for the eye movements for the presaccadic data (i.e., right occipital activity represents a left target and a prosaccade eye movement to the left, left occipital activity represents a right target and an antisaccade eye movement to the left). The last two PCs in Figure 3 represent left and right frontal activity and do not correspond to any PC in the target onset segments. The five clusters of the stimulus-locked PCs represent 402 of the 1,000 PCs (20 PC × 50 participants) coming from the analysis, and the eigenvalues representing these PCs account for 45% of the total variance in the ERP. The eight clusters of the response-locked ERP segments represent 594 of the 1,000 PCs coming from the analysis, and the eigenvalues representing these PCs account for 57% of the total variance in the ERP. The rest of the PCs did not cluster together well or had idiosyncratic patterns of loading weights. These unclustered PCs also were examined in relation to experimental events (i.e., activations analyzed in next section) and no systematic relation between the PCs’ activations and the experiment events was found.
Figure 3.
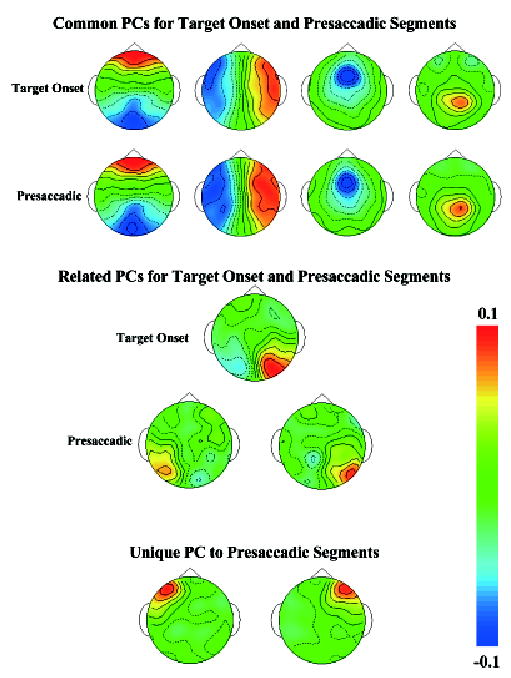
Topographical scalp potential maps for the average of the PC clusters (collapsed across conditions). The top two panels show clusters that were nearly identical in the stimulus-locked and response-locked EEG segments. The bottom panels show clusters that were unique in these periods. The eigenvector weights of the PC are plotted.
Component Activation and Projection
The activations (PC scores) were calculated from the PC eigenvectors in 25-ms bins for each trial. The activation represents the amount of activity in the ERP recording in the spatial axis defined by the PC eigenvector. The activation defines the time course of the ERP component represented by the spatial PC and may be evaluated in relation to the experimental procedures. The projections of the PCs into the electrode recording space were done. The projections represent the activity of those PCs that were used in the electrode recording space and uses units identical to the raw data. The projections of the PC into the electrode recording space were calculated for PCs with statistically significant relations to experimental events. The activations were analyzed for the PC clusters, whereas the projections were calculated for all PCs occurring in that interval. The intervals before target onset, immediately subsequent to target onset, and immediately preceding saccade onset were analyzed.
Pretarget
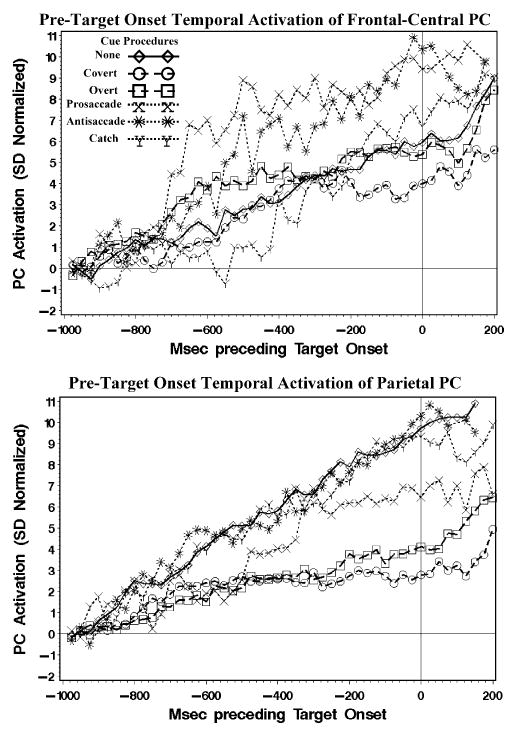
The activations from −1 s to the onset of the target were first analyzed. The cue procedures before the target onset included no cue, a peripheral cue, a central cue, or one of three cues indicating a prosaccade, antisaccade, or catch trial. These were used as experimental factors in the design. The activations were analyzed with a Cue Procedure (6: no, peripheral, central, prosaccade, antisaccade, catch) × Intervals (40: 25-ms intervals) design, using repeated-measures ANOVA.4 Three of the clusters showed no significant main effects or interactions. These include the cluster with bilateral frontal-occipital activity, the cluster with right–left activity, and the cluster with contralateral occipital activity (Figure 3). The cluster with negative bilateral-frontal-central activity (Figure 3) showed a main effect of the intervals factor, F(39,1638, ɛ = .3051) = 6.55, p <.001. Figure 4 (top figure) shows the activation for this PC for the six procedures. The activation increased linearly over the pretarget interval, that is, steadily increasing negative ERP over the frontal-central scalp area (Figure 1). This increase primarily occurred in the pretarget period, whereas the activation was stable in the 200 ms following the target onset. The steadily increasing negative potential did not differ significantly for the six cueing procedures. However, the cues for prosaccade or antisaccade eye movements in the movement cueing procedure did result in a significantly higher activation of the PC immediately preceding target onset (i.e., Figure 4, top figure, 0 ms is target onset).
Figure 4.
The activations of the PCs with the negative potential found in the frontal-central scalp electrodes and the positive potential found in the parietal leads. The PCs were from the 1,200-ms stimulus-locked EEG segments, and the activations came from 25-ms segments.
The PC cluster with the positive parietal activity (Figure 3) was analyzed with the procedures and intervals factors in an ANOVA. There was a significant main effect of intervals, F(39,1911, ɛ = .1050) = 10.89, p <.001. The procedure main effect and the interaction between procedure and intervals were not significant. However, there was a significant interaction between the linear polynomial trend of the intervals effect and the procedures, F(5,1755, ɛ = .1050) = 24.47, p <.001. Figure 4 (bottom figure) shows the activation of this PC cluster for the six procedures. The increase in activation over the interval was a steady increase similar to the negative PC cluster (Figure 4, top figure). Post hoc tests showed that this linear trend was larger for the no cue and the three movement cues than it was for the two cue conditions (Figure 4, bottom figure). That is, when the cue (peripheral or central) indicated the location in which the target would appear, the parietal activity was not as large when there was no cue or the cue was uninformative for spatial location.
In summary, there was a large increase in the negative bilateral frontal-central activity preceding the onset of the target. This negative potential did not differ for the six cueing procedures. A similiar increase in the positive parietal potential was smaller for the procedures in which a cue indicated the position of the upcoming target than when no cue, or a cue that was uninformative for spatial location, occurred.
Posttarget
The activations occurring at the onset of the target and continuing for several milliseconds were analyzed. The targets for the cue procedures had information about the eye movement, so the procedures were defined as the no cue, peripheral cue, central cue, and movement cue, and eye movement type was defined as prosaccade, antisaccade, or catch trials. These were used in experimental factors and analyzed with a Cue Procedure (4) × Movement Type (3) × Intervals design, using repeated-measures ANOVA. Five of the eight clusters found in the response-locked segments showed no statistically significant main effects or interactions. The lateralized occipital clusters (Figure 3) did show statistically significant main effects of the cueing procedure within the first 200 ms of target onset. Given the complicated analyses concerning side of target for these two clusters, the analogous cluster from the stimulus-locked segments (−1 s before target to 200 ms after target) with positive occipital activity contralateral to the target was analyzed (Figure 3). There was a main effect of intervals on the activation for this PC, F(7,217, ɛ = .9903) = 4.46, p = .002, a significant interaction between the cueing procedure and the intervals factors, F(21,126, ɛ = .9903) = 1.67, p = .046, but no effects involving the eye movement type. Figure 5 (top panel) shows the activation for this PC component. The peripheral cueing procedure shows activation beginning at 75 ms following target onset, peaking at about 100–125 ms, followed by a return to baseline levels. The central cueing procedure showed an increase beginning later (about 100 or 125 ms) with a clear peak later (175 ms) than that for the peripheral cue, with a possible earlier peak in the same time frame as the peripheral cue. There was no significant activation of this PC for the no-cue and the movement-cue procedures.
Figure 5.
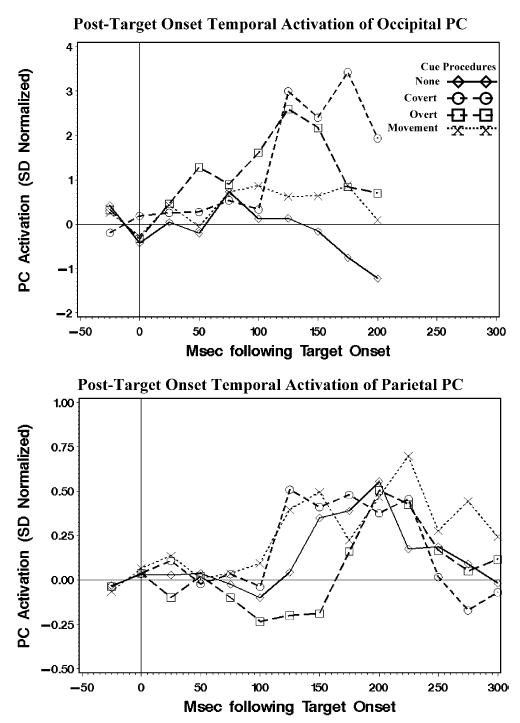
The activations in the posttarget period of the PCs with the occipital activity contralateral to the target location and the positive parietal activity following target onset.
The other PC cluster that showed significant activation related to the target onset was the positive parietal activity (Figure 3). This was analyzed with a Procedure × Eye Movement Type × Intervals ANOVA for 300 ms following target onset. The intervals effect was not statistically significant (p = .084), however, the linear, quadratic, and cubic polynomial trends were statistically significant (all ps <.001). Figure 5 (bottom panel) shows the activation for the parietal PC cluster separately for the four cue procedures. The activation occurred as a curvilinear change with a peak about 200 ms following target onset. There were no other main effects or interactions affecting this cluster.
A summary of the PC activations for the posttarget data is that there was a significant change in the activation of the lateralized occipital clusters only for the two cueing procedures, and the parietal cluster was activated for all procedures. Figure 6 shows the projections of the PC into the electrode recording space. The four cue procedures show the parietal activity about 200 ms following target onset. The “early” and “late” occipital activity is seen in the peripheral and central cueing conditions, respectively. Notice in this figure that the early occipital activity in the peripheral cueing procedure is more central-posterior, specifically over the lateral occipital scalp area (10–20 electrodes O1 and O2). The later occipital activity in the central cueing procedure is more lateral and anterior than the early activity, perhaps occurring over lateral parietal leads (10–20 electrodes P3 or P4) or posterior temporal electrodes (10–20 electrodes T7 or T8), and may temporally overlap the parietal activity (i.e., 175 ms).
Figure 6.
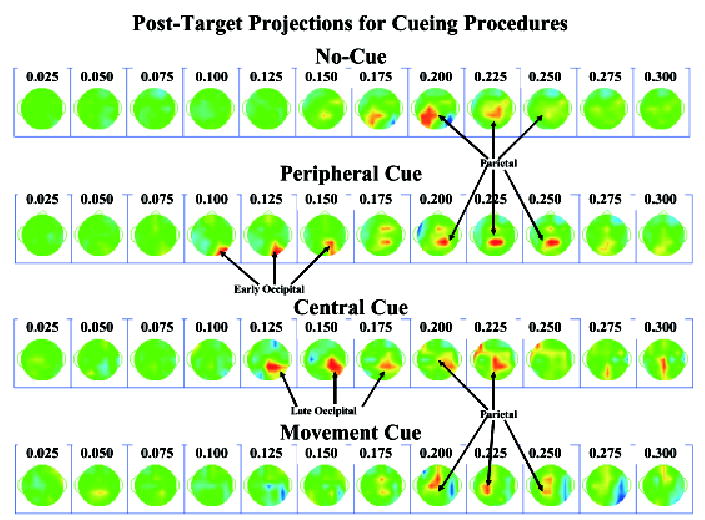
Topographical scalp potential maps for the projections in the posttarget interval, separately for the four cueing procedures. The peripheral cue procedure resulted in an “early P1,” the spatial cue procedure resulted in a “late P1,” and all four procedures had significant parietal activity.
Presaccadic
The activations occurring up to 350 ms before the saccade onset were analyzed. The cueing procedures were the no cue, peripheral cue, central cue, and movement cue, and eye movement type was defined as prosaccade or antisaccade. These were used in experimental factors and analyzed with a Cue Procedure (4) × Movement Type (2) × Intervals design, using repeated-measures ANOVA. Five of the eight clusters did not show statistically significant main effects or interactions when the ERP segments were averaged from the onset of the saccade and prior to its onset (“presaccadic ERP”).
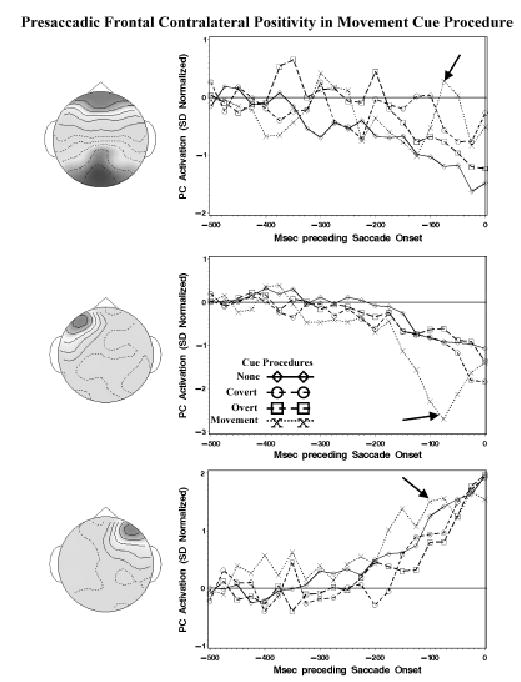
The three PC clusters that showed significant presaccadic activation were the bilateral frontal-occipital cluster and the two lateral frontal clusters (Figure 3). The summary of these effects is that during the movement cue condition, there was a significant peaked activation, whereas these PCs showed only the late “spike potential” activation for the no-cue, peripheral-cue, and central-cue conditions. There was a significant intervals effect for these three PC clusters; bilateral frontal-central, F(13,520, ɛ = .5784) = 1.89, p = .070; ipsilateral (to eye movement) frontal, F(13,468, ɛ = .5564) = 7.19, p <.001; contralateral frontal, F(13,520, ɛ = .5375) = 5.64, p <.001. The linear polynomial trend affected the activations of these three clusters, and the quadratic polynomial trend also was significant for the contralateral frontal cluster. Although the omnibus intervals by cue procedure effect was not significant, the linear polynomial intervals effect interacted with the cue procedure for the bilateral frontal-occipital cluster, F(3,273, ɛ = .5784) = 8.83, p = .003, and for the ipsilateral frontal cluster, F(3,260, ɛ = .5564) = 4.78, p = .030. Figure 7 shows the activation pattern for these three clusters. For the no-cue, peripheral-cue, and central-cue procedures, there was a strong linear activation of the PC immediately prior to the saccade onset for the three PC clusters (“spike potential”; positive for the contralateral frontal component and negative for the other components). For the movement-cue procedure, there was a clear curvilinear component that peaked about 75 ms prior to saccade onset. This activation would show up in the ERP signal as a large positive component over the frontal area contralateral to the eye movement and a negative component over the frontal area ipsilateral to the eye movement.
Figure 7.
The activations in the presaccadic period of the PCs with frontal-occipital activity, activity ipsilateral to the eye movement, and activity contralateral to the eye movement. The arrow points to an apparent ERP component occurring about 75 ms prior to saccade onset primarily in the movement cue procedure. Immediately preceding saccade onset, there was a large activation of all three PCs probably corresponding to the spike potential seen in the grand average ERP (Figures 1 and 2).
The type of eye movement also affected this presaccadic frontal ERP component. For the bilateral frontal-occipital PC cluster, there was a significant interaction between the type of eye movement and the intervals factors, F(13,507, ɛ = .5784) = 2.53, p = .015. For the contralateral frontal activity, the quadratic intervals trend interacted with the eye movement type, F(1,481, ɛ = .5375) = 4.77, p = .009, as well as the cubic intervals trend, F(1,481, ɛ = .5375) = 4.18, p = .016. The plots of the activations separate for the eye movement types showed that the activation at the time of the curvilinear peak, primarily in the movement-cue procedure, was larger for the antisaccade eye movements than for the prosaccade eye movements. This was also slightly larger for the other three conditions, though the prosaccade–antisaccade difference in the other three cueing procedures was not as large in the movement-cue condition.
The PCs were used to project the activations into the electrode recording space averaged from the saccade backwards in time for 150 ms, using the clusters for the response-locked ERP segments. Figure 8 shows these for the prosaccades and antisaccades, and for the movement cue and the other three cues. A positive potential shift over the parietal area occurred beginning 50 to 75 ms prior to saccade onset. The spike potential is obvious in all four series, with an onset about 25 ms prior to saccade onset, a peak at the 25 ms or onset temporal frame, and distributed from the anterior-central to the posterior-occipital leads. The presaccadic positive potential in the frontal scalp area peaked at about 75 ms before saccade onset (cf. activation in Figure 7), occurring primarily over the contralateral frontal scalp areas, was strongest in the antisaccade eye movements on the movement cue trials, at an intermediate level in the prosaccade eye movements on the movement-cue trials, at a small level for antisaccade eye movements in the non-movement-cue conditions, and not apparent in the prosaccade eye movements on the non-movement-cue conditions. The contralateral positive potential was accompanied on the movement-cue procedure with an ipsilateral negative potential (cf. activation in Figure 7).
Figure 8.
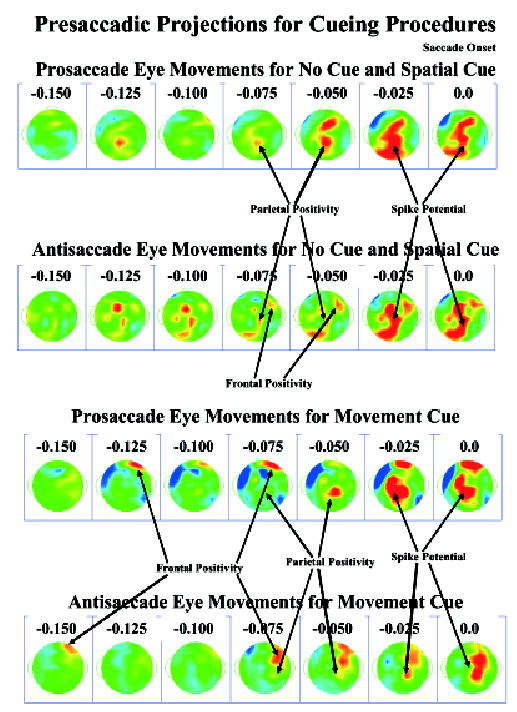
Topographical scalp potential maps for the projections in the presaccadic interval, separately for the movement cue procedure and the other procedures. The positive presaccadic activity occurring about 75 ms before saccade onset corresponds to the ERP component found in the activations (Figure 7). The spike potential occurs in the last two panels, but was maximal in the grand average ERP about 8 to 12 ms prior to saccade onset (Figure 2).
Summary
The results of the activations will be briefly summarized. The pretarget activity occurred primarily as a frontal-central negative pretarget potential for the cue procedures, and a positive parietal potential for the no-cue and movement-cue procedures (Figures 1 and 4). The frontal-central negative activity was similar to the “Premotor Negative” potential identified in eye movement research, or the contingent negative variation. There was significant activation in the occipital area contralateral to the target location for the two cueing procedures (Figures 5 and 6). This activation appeared to be earlier in the peripheral than in the central cueing procedure and in a different location for the two procedures. There was a significant parietal activation that was identical for the cueing procedures (Figure 5). The occipital activation appears to be similar to the P1 enhancement found in spatial cueing paradigms. The presaccadic activation included a clear activation centered over the parietal cortex and broadly including central and anterior-central scalp leads (Figure 8). The presaccadic frontal positive potential occurring about 75 ms prior to saccade onset occurred most strongly in the movement conditions (Figure 7), and more strongly for antisaccade eye movements than for prosaccade eye movements (Figure 8).
Cortical Source Analysis
The cortical source analysis of the PC clusters was done. The “Equivalent Current Dipole” (ECD) analysis of the EMSE computer program was used. The first step was an ECD analysis of the individual PC loading weights. This was done to get an approximate location for the cortical sources that generated the spatial PCs found in the individual topographic loadings.
For the stimulus-locked ERP data, I focused on the negative bilateral frontal-central, the positive parietal, and the contra-lateral occipital clusters (Figure 3). Table 2 contains information about the average coordinates of the ECD locations for these clusters, and Figure 9 shows coronal and axial MR slices with the ECD sources for each participant PC plotted for the pretarget PC clusters. For the negative bilateral frontal-central PC cluster, the participant PCs resulted in ECDs in the orbital-frontal gyrus (Brodmann area 11), the middle and superior frontal lobes (Brodmann area 9), and the superior frontal lobe (Brodmann area 6). The orbital-frontal gyrus ECD occurred on the side ipsilateral to the upcoming target, whereas the other two locations had bilateral representation. Figure 9 shows the three sources for the negative scalp activity and the distribution of individual participant ECDs. For the positive parietal activity, the sources were located in the superior parietal lobe (Brodmann area 7). There appeared to be a distinct separation between sources located on the medial cortical surface (precuneate gyrus) and sources located on the lateral portions of the superior parietal lobe.
Table 2.
Equivalent Current Dipole Locations for the PC Clusters for Stimulus-Locked ERP Segments
| N | Saggitala | Coronal | Axial | Mag. SDb | Brodmann area | Cortical area |
|---|---|---|---|---|---|---|
| Negative bilateral frontal-central | ||||||
| 19 | −7.8 | 55.9 | −11.4 | 18.8 | 11 | Orbital-frontal gyrus |
| 13 | ±10.0 | 46.1 | 34.3 | 12.4 | 9 | Middle and superior frontal gyrus |
| 34 | ±12.2 | 21.4 | 50.9 | 15.7 | 6 | Superior frontal gyrus |
| Positive parietal | ||||||
| 20 | ±6.8 | −53.3 | 55.4 | 16.0 | 7 | Precuneate gyrus, medial superior parietal lobe |
| 46 | ±27.3 | −57.9 | 50.1 | 15.1 | 7 | Lateral superior parietal lobe |
| Contralateral occipital | ||||||
| 7 | 13.3 | −93.0 | −0.4 | 11.5 | 17 | Calcarine fissure |
| 23 | 20.6 | −88.5 | 23.6 | 10.7 | 19 | Superior occipital lobe |
| 9 | 27.7 | −61.3 | 38.3 | 19.5 | 7 | Lateral superior parietal lobe |
The ECDs occurring in bilateral locations are indicated with a ± in front of the saggital coordinate, and the average saggital location was calculated as the absolute value of the saggital coordinate. The ERP data was organized in the saggital plane so that the target occurred on the left side (saggital coordinate is negative). The scale of the coordinates is in millimeters and the coordinates are given in Talairach coordinates (Talairach & Tournoux, 1988).
The magnitude of the SD, calculated as the length of a vector from the ECD location to the SD, indicates the approximate radius of a sphere for locations within 1 SD of the centroid. For bilateral locations, the magnitude of the SD is around the absolute value of the saggital coordinates.
Figure 9.
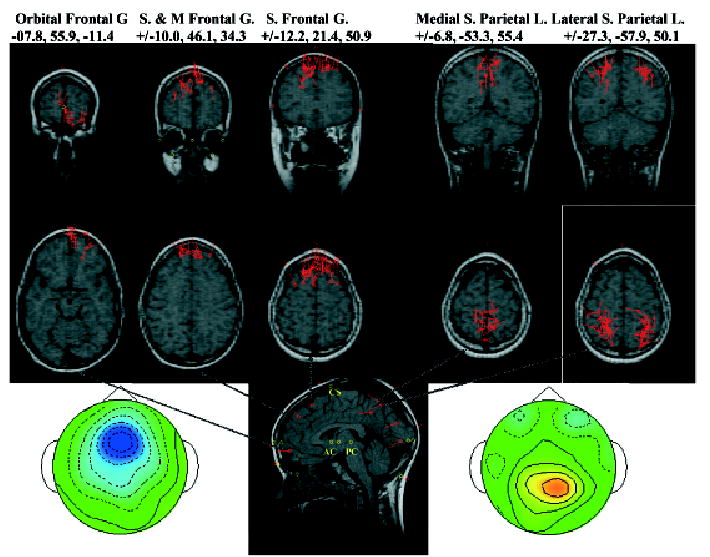
Equivalent current dipole locations for the pretarget PC clusters. Each location on the MRI recording represents a PC from one individual. The three MRIs on the left came from the negative frontal-central activity and the two on the right from the positive parietal activity. More information about the locations may be found in Table 2.
The sources for the occipital PC cluster appeared in three distinct locations. Most of the ECD sources for this cluster were located in the lateral portion of the superior occipital lobe (Brodmann area 19). A small number were located in the calcarine fissure (Brodmann area 17) and in the lateral superior parietal lobe (Brodmann area 7). These latter sources, though clustered on the basis of scalp topography with the occipital lobe sources, were in the same area as the lateral superior parietal lobe dipole sources that clustered with the positive parietal activity (Figure 3). The average coordinates for the dipole sources of the occipital PC cluster are shown in Table 2 and the average coronal-axial locations may be seen on the saggital slice in Figure 9.
The negative bilateral frontal-central PC cluster and positive parietal PC cluster had similar ECD locations for the response-locked ERP segments as they did for the stimulus-locked ERP segments (see Table 2). For the response-locked ERP segments, I focused on the bilateral frontal-occipital cluster, and the two lateral frontal clusters (Figure 3). Table 3 contains information about the average coordinates of the ECD locations. Figure 10 shows coronal and axial MR slices with the ECD sources for each participant PC plotted for the bilateral frontal-occipital cluster, and the two lateral frontal clusters. The bilateral frontal-central PC cluster had ECDs in the orbital-frontal gyrus (Brodmann area 11), similar to that found with the bilateral negative frontal-central PC cluster (cf. Tables 2 and 3; cf. Figures 9 and 10).
Table 3.
Equivalent Current Dipole Locations for the PC Clusters for Response-Locked ERP Segmentsa
| N | Saggitalb | Coronal | Axial | Mag. SDc | Brodmann area | Cortical area |
|---|---|---|---|---|---|---|
| Frontal-positive and occipital-negative | ||||||
| 41 | ±6.6 | 60.4 | −13.0 | 17.4 | 11 | Orbital-frontal gyrus |
| Ipsilateral frontal | ||||||
| 9 | −15.6 | 72.8 | 18.9 | 8.7 | 10 | Anterior & medial superior frontal gyrus |
| 14 | −37.5 | 54.8 | 2.8 | 8.4 | 10 | Anterior & lateral superior frontal gyrus |
| 12 | −44.2 | 27.6 | 37.2 | 15.1 | 6 & 8 | Lateral middle frontal gyrus |
| Contralateral frontal | ||||||
| 18 | 26.6 | 66.9 | 0.4 | 10.6 | 10 | Anterior superior frontal gyrus |
| 12 | 33.7 | 57.7 | 1.4 | 12.9 | 10 | Anterior middle frontal gyrus |
| 11 | 19.4 | 40.4 | 50.0 | 10.3 | 8 | Superior frontal gyrus |
| 9 | 36.2 | 34.1 | 44.1 | 12.6 | 8 | Middle frontal gyrus |
A negative bilateral frontal-central PCA cluster, and a positive parietal PCA cluster, had similar ECD locations as the stimulus-locked ERP segments (Table 2).
See notes a and b in Table 2. The ERP data was organized in the saggital plane so that the eye movement was toward the left side (saggital coordinate is negative).
Figure 10.
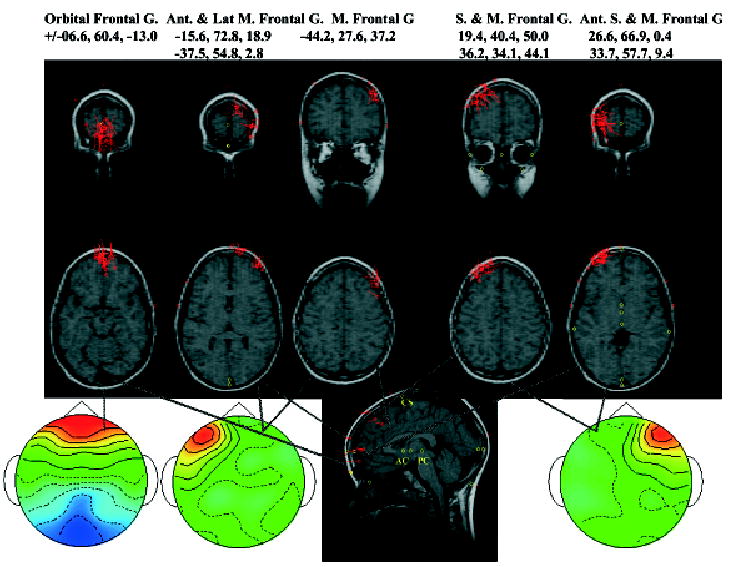
Equivalent current dipole locations for the presaccadic PC clusters. Each location on the MRI recording represents a PC from one individual. The MRI on the left came from the PC with the frontal-occipital activity, the next two came from the PC with activity in the frontal scalp region ipsilateral to the saccade, and the two on the right from the PC with activity in the frontal scalp region contralateral to the saccade. More information about the locations may be found in Table 3.
The ipsilateral frontal PC cluster had three distinct areas in which ECDs occurred. There were ECDs in the anterior and medial portion of the superior frontal gyrus (Brodmann area 10) and in the anterior and lateral portion of this same gyrus (Brodmann area 10). The ECDs for the ipsilateral frontal cluster also appeared in the lateral portion of the middle frontal gyrus (Brodmann areas 6 and 8). The contralateral frontal PC cluster had two distinct areas in which ECDs occurred, related to two Brodmann areas. First, there was a cluster of ECDs that began in the anterior pole of the superior frontal gyrus and swept posterior-lateral through the anterior pole of the middle frontal gyrus. The mean ECD was done separately in Table 3 for the superior and middle frontal gyri, but these appear to be a continuous range of ECDs (Figure 10). Second, there was a cluster of ECDs, located in Broadmann area 8, in the superior and middle frontal gyri. The mean ECD location was done separately for Table 3 for the superior and middle frontal gyri, but these appear in Figure 10 to be continuous across this area.
Discussion
Presaccadic ERP
There were several types of ERP activity found in this study that replicated findings from other studies. This study found a slowly increasing positive component over parietal leads (Becker et al., 1973; Csibra et al., 1997; Kurtzberg & Vaughan, 1980, 1982; Moster & Goldberg, 1990) and a spike potential (Balaban & Weinstein, 1985; Becker et al., 1973; Csibra et al., 1997; Kurtzberg & Vaughan, 1980, 1982; Weinstein et al., 1991) temporally closer to the eye movement than the negative ERP potential. For example, the increase in the parietal PC cluster immediately prior to saccade onset was likely a combination of the presaccadic positive potential and the spike potential. The presaccadic positive potential may be seen in the grand average ERP topography beginning about 80 to 100 ms prior to saccade onset (Figure 1) and in the PC projections beginning about 50 to 75 ms prior to saccade onset (Figure 8). As with other studies of prosaccade and antisaccade eye movements (Evdokimidis et al., 1996; Everling et al., 1997; Everling, Spantekow, et al., 1998), no difference was found in the activation of the parietal PC cluster for this presaccadic activity. I also found in this study increased activity in the occipital scalp area that was enhanced when a cue signaled which location the target would occur (Figures 5 and 6). This enhancement is likely a “P1 validity effect” (Hillyard, Mangun, Woldroff, & Luck, 1995). The present study further showed an “early P1” for the peripheral cue procedure and a “late P1” for the cue procedure (cf. Martinez et al., 1999).
The methods of the current study suggest the negative potential shift in the presaccadic ERP is a “contingent negative variation” rather than a “premotor negativity” (PMN). Several studies of the presaccadic ERP have reported a negative shift in EEG that begins up to 1 s prior to saccade onset and has its maximum values over the vertex (Becker et al., 1973; Klein et al., 2000; Klosterman et al., 1994; Kurtzberg & Vaughn, 1980 Kurtzberg & Vaughn, 1982; Moster & Goldberg, 1990). This is often labeled the premotor negativity. The PMN is hypothesized to be similar to movement-related potentials that occur prior to the making a voluntary hand or leg motor movement. A similar PMN has been reported in studies of antisaccade eye movements (Brickett et al., 1984; Evdokimidis et al., 1996; Everling et al., 1997; Everling, Spantekow, et al., 1998). However, in these studies and in the current study the saccadic eye movements are made in response to a target rather than being strictly participant-initiated eye movements. Thus, they might be considered to be more similar to the slowly shifting negative potential found in a S1–S2 task known as the “contingent negative variation” (CNV; Walter, Cooper, Aldridge, MacCallum, & Winter, 1964). The topographical distribution (bilateral, vertex, and frontal) of the ERP (Figure 1), the PC topography (Figure 3), and its temporal characteristics (Figures 1 and 4), suggest this negative shift was more like the CNV than negative movement-related activity. Also, in the current study, this negative potential occurred equally to targets for which an eye movement would occur and to the catch trials. On the movement-cued catch trials, the participant knew in advance that no eye movement would be necessary. The CNVcan occur in the absence of overt motor movements, whereas premotor negativity is closely associated with overt motor movements (Fabiani, Gratton, & Coles, 2000).
One difference found between studies of antisaccade presaccadic ERP change is the effect (or lack of effect) of the antisaccade eye movements on this negative potential shift. The studies using a mixed-trials design, in which prosaccade and antisaccade trials were given in a single block and in which the target indicates the eye movement direction, have reported that this negative presaccadic potential does not differ between prosaccade and antisaccade trials (Evdokimidis et al., 1996). This is not totally unexpected, as the time course of this negative potential (up to 1 s prior to saccade onset) and the latency of the saccade following target onset (~450 ms) imply that the negative potential occurs prior to the time of the target so that the participant does not yet know which eye movement will occur. Alternatively, blocked designs in which the prosaccade and antisaccade trials were presented in different blocks, report that this negative potential was larger for the antisaccade trial block (Everling et al., 1997). Similarly, in at least one study in which a warning stimulus indicated the type of movement (prosaccade or antisaccade) and an imperative stimulus indicated the type of movement, the difference in this negative presaccadic potential was shown (Klein et al., 2000). One study using cued gap trials to elicit antisaccade errors (i.e., eye movements toward the antisaccade target) found that correct antisaccade trials were preceded by a larger negative potential shift than error antisaccade trials (Everling, Spantekow, et al., 1998). In two studies showing ERP averages timed at stimulus onset, this negative potential clearly peaks at target stimulus onset rather than saccade onset (Figure 3 in Evdokimidis et al., 1996; Figure 4 in Everling, Spantekow, et al., 1998).
The current study provides a direct comparison between trials when the cue preceding the target either provided information about the type of eye movement (movement-cue procedure) or did not (no-cue or spatial-cue procedure). The negative presaccadic potential was related to target onset and not to saccade onset (Evdokimidis et al., 1996; Everling, Spantekow, et al., 1998). This potential did not differ on movement-cued antisaccade and movement-cued prosaccade trials and was not significantly different from non-cued or spatial-cued trials, except perhaps at the period immediately preceding target onset on the movement cue procedure (Figure 4; cf. Klein et al., 2000). This suggests that studies with blocked trials may induce a response set on antisaccade blocks similar to the movement-cue procedure blocks in this study. These findings imply that the negative potential shift is more closely associated with a CNV-type expectancy shift related to target onset and target evaluation rather than to the processes differing for the neural control of prosaccades and antisaccades. A study directly comparing this negative potential in blocked trials, uncued mixed trials, and movement-cued mixed trials would help to clarify this issue.
The present study is the first to report the presaccadic ERP activity occurring over the frontal cortex. This activity was positive in the frontal scalp leads contralateral to the upcoming saccade and negative in frontal leads ipsilateral to the saccade. This presaccadic ERP activity was found in the overall grand average ERP topographical map (Figure 1), the activation for three PCs from the response-locked EEG segments (Figure 7), and the PC projections (Figure 8). This presaccadic ERP activity has the characteristics of a “classic ERP component,” that is, specific scalp topography, temporal morphology, and relation to experimental variables (Donchin, Ritter, & McCallum, 1978; Fabiani et al., 2000; Spencer et al., 1999). This component was at its maximum when an antisaccade eye movement was cued in advance of the target presentation (i.e., Figure 8). However, it also occurred on the movement-cued prosaccade trials, and to a lesser degree, on antisaccade trials for the no-cue and spatial-cue procedures (Figure 8). I suggest that this ERP component is involved in the planning of the targeted eye movement. Its occurrence in movement-cued prosaccade and movement-cued antisaccade trials implies that it is not reflecting the inhibition of reflexive eye movement toward the target but must be involved in the computation of the saccade to the targeted field. Its occurrence on the non-movement-cued antisaccade trials suggests that such movement planning is heightened on antisaccade trials in the absence of specific movement cueing, whereas prosaccade trials without movement cueing may use reflexive subcortical eye movement control processes.
The contralateral frontal ERP component has not been reported upon in prior studies of prosaccade and antisaccade eye movements (Evdokimidis et al., 1996; Everling et al., 1997; Everling, Spantekow et al., 1998). There are several possible reasons for this. First, this component is emphasized most clearly in the eye movements occurring in the movement-cue procedures. These procedures provide a mixed-trials block design with the movement cue indicating the type of eye movement on a trial-by-trial basis. Prior studies either used a no-cue procedure (Evdokimidis et al., 1996) or a blocked trials design (Everling et al., 1997; Everling, Spantekow, et al., 1998). If the movement cue procedure enhances the need for targeted movement planning, then this ERP component would not be found in those studies. Second, the prior studies used a low-density EEG recording system and the lateral frontal area was covered by three electrodes (e.g., Fp2, F4, F8). This component in this study in the grand average ERP topographical maps (Figure 1) or the PC projections (Figure 8) occurred primarily in electrodes anterior and/or lateral to these electrodes. The EGI sensor net electrodes in this area were numbers 2, 3, 9, and 123, which roughly correspond to locations near AF2, AF4, and F6 of the 10–20 system (Luu & Ferree, 2000). Finally, it is possible that this ERP component may be seen in ERP tracings and topographical plots from those studies. For example, Figure 2A in Evdokimidis et al. (1996) has a temporal topographical map of the antisaccade data (Note: Figure 2A is mislabeled as prosaccade, but should be antisaccade—see text description). There was in that figure at about 75 ms a brief positive section in the frontal contralateral map (~+2 μV). Similarly, the average ERP tracings in Figure 1 of Everling et al. (1996) show a small peak in the presaccadic positive potential immediately preceding the spike potential in F4 and F8, whereas the positive presaccadic potential in the parietal (and other posterior) leads was a simple steep decline without a peak. Although these are not definitive evidence for such a component in these studies, it is possible that such a component did occur, was smaller because of the cueing conditions, was masked by the large parietal positive activity, or was not detected because of the lower density of electrode placements.
This presaccadic potential has some characteristics of the retinal potential accompaning the saccade (e.g., Figure 1, bottom half, far right panel; cf. with Figure 8). That is, there is a large negative potential near one retina and a large positive potential near the other one. In this case, the presaccadic potential has the opposite polarity of the eye movement. I believe for several reasons that this potential is cortical in origin rather than due to preparatory eye movement activity. First, activity seems to be localized around electrodes posterior to the electrode nearest the eye that picks up the “electrooculogram.” Thus this activity is localized behind where the EOG produces muscle activity. Second, I have examined individual trial data, and this presaccadic potential may be seen in the electrodes on scalp areas immediately above the cortex and when it is absent in the EOG electrodes. Thus, it does not represent a small eye movement in the direction opposite the final saccade, such as an incorrect prosaccade in the antisaccade trial. Finally, source analyses of the saccadic potential accompaning the postsaccadic ERP (not reported in this article) localize the electrical sources of the eye movement in a location where one would expect the eye muscle to be located and not in cortical locations found for the presaccadic activity.
Cortical Source of Presaccadic Activity in Antisaccade Task
One goal of this study was to relate the temporal morphology found with the presaccadic ERP to the localization of cortical sources possible in PET (and fMRI) neuroimaging. There have been several neuroimaging studies of eye movement and the antisaccade task. Neural activity has been localized in these studies to just about every cortical area known to be involved in eye movement control in primates (i.e., FEF, SEF, superior parietal lobe, DPC [areas 9 and 46], anterior medial PFC [areas 8 and 9], ventromedial PFC [area 10]; Everling & Fischer, 1998). These studies have used the block-presentation design, so that neural activity potentially is affected by block-level response set processes, and temporal relations to specific aspects of the procedure (stimulus-locked, response-locked) cannot be determined. The temporal aspect of the ERP data and the activations of the PCs may be useful in identifying the time course of the cognitive processes and associating the cognitive processes with neural areas.
Scalp-recorded ERP activity was related to neural activity in four intervals: prior to target onset, in response to the target, the contralateral presaccadic ERP, and the spike potential. First, it appears from this study that many of the areas that have been active in the PET/fMRI neuroimaging studies were related to target onset or in response to the target rather than in relation to the eye movements. For example, in this study, the large negative potential shift (CNV) was related to the onset of the target signaling the type of eye movement that was to occur. The cortical sources for this potential shift occurred in Brodmann areas 6, 9, and 11. The location in this study of the activity in area 6 would be closest to the SEF (SMA) and area 9 would be closest to the DPC. Neuroimaging studies using PET have found these two areas to have more activity on the antisaccade trial blocks than in the prosaccade trial blocks (e.g., O’Driscoll et al., 1995; Sweeney et al., 1996; also see review by Everling & Fischer, 1998). I argued previously that this scalp ERP activity was related to target onset and that the blocked-trials design is necessary to show a prosaccade–antisaccade difference in this activity in the ERP (cf. this study, Everling et al., 1997, and Evdokimidis et al., 1996). This suggests that the SEF and DPC areas were closely related to the response set processes manipulated in the blocked-trial design and not to specific processes of eye movement control. The positive parietal PC and occipital PC also were closely tied to target onset. These areas have been found to be active in fMRI and PET studies of the antisaccade task (O’Driscoll et al., 1995; Sweeney et al., 1996).
The activity that was closely related to saccade onset in this study was the presaccadic ERP occurring about 50 to 80 ms prior to saccade onset and located primarily in the ERP in scalp leads contralateral to the saccade. This ERP component was larger on antisaccade than on prosaccade trials, but also was relatively large on the movement-cued trials of either movement type (either prosaccade or antisaccade). The primary cortical sources for this component were located in the anterior portions of the PFC (i.e., areas 10, anterior, medial, and lateral portions of superior frontal gyrus, Table 3; area 11, orbital-frontal gyrus, Table 3; also see Figure 10), and Brodmann area 8 (Table 3, Figure 10). Some of these locations are similiar to those found in PET and fMRI studies. For example, Sweeney et al. (1996) reported neural activity in the anterior medial PFC near the border of Brodmann areas 8 and 9, and in a ventromedial region in area 10 near the frontal pole. I suggested in the prior section of the discussion that this ERP component reflects neural activity involved in the planning of the targeted eye movement. Its occurrence in these anterior portions of the PFC closely resembles neuropsychological work. Damage to prefrontal regions such as the ventrolateral frontal cortex that does not include damage to the FEF (Pierrot-Deseilligny, Rivaud, Gaymard, & Agid, 1991; Rivaud, Muri, Gaymard, Vermersch, & Pierrot-Deseilligny, 1994; Walker, Husain, Hodgson, Harrison, & Kennard, 1998) increases error rates in the antisaccade task. If eye movement planning occurs in these areas as I suggest, then damage to these areas leads to errors in the antisaccade task when cortical preparation is necessary to overcome the reflexive saccade toward the target. This is consistent with the view that the cortical areas that suppress the prosaccade reflexive eye movement, initiate the antisaccade eye movement, or plan the eye movement are in prefrontal cortex. The DPC, SEF, FEF, and parietal cortex may affect central cue disengagement (saccade latency), target evaluation, and be significantly affected by response set processes.
There was a surprising absence of activity in the present analysis in the areas of the cortex representing the FEF or the SEF. The FEF in humans is located near the junction of the superior frontal sulcus and the precentral sulcus (Paus, 1996). The FEF (or SEF) have been found to be active prior to eye movements in single-unit recording studies (e.g., Funahashi et al., 1993; Schlag-Rey et al., 1997), PET and fMRI studies (O’Driscoll et al., 1995; Sweeney et al., 1996), and damage to these areas results in eye movement deficits (Pierrot-Deseilligny et al., 1991; Rivaud et al., 1994; Walker et al., 1998). In the current study, the closest activity was found in Brodmann area 6 (Table 3, Figure 9), the superior frontal gyrus, which is anterior to the the human FEF location. There are several possible reasons for this discrepancy. First, activity from the FEF may be obscured in the scalp-recorded EEG. This may occur because it is located near the areas that generate the large potential shift of the CNV in the EEG. It is possible that the electrical activity is obscured in the EEG segments because of the presence of this CNVactivity. Second, some aspect of the principal components analysis may overlook activity in this region. The PCs coming from this analysis represent topographically organized co-occurrence of activity in the EEG segments. If this activity was not independent from the CNV, it is possible that the PC thought to represent the CNV obscured a PC representing the FEF or SEF activity. Finally, the clustering procedure may have ignored this activity because it was similar to one of the other clusters, or these PCs were not clustered together because they were dissimilar enough across individuals to not be recognized in the clustering algorithm. This could be tested by adopting a procedure that generated a priori scalp topographies from FEF-located ECDs and then fitting the empirical PC weights into categories defined by the a priori topographies. Given these potential difficulties with the analyses in the current article, it is premature to conclude that scalp-recorded ERP activity cannot be used to identify the FEF or SEF as cortical sources in these eye movement tasks.
Footnotes
The choice of 100 kΩ as the maximum impedance value was based on the high input impedance of the EGI amplifiers. These amplifiers have an input impedance of about 200 MΩ compared with traditional EEG amplifier impedances of about 10 MΩ. Given the recommendation of interelectrode impedances being at least 1% of amplifier input impedance (e.g., 10 kΩ for 10 MΩ amplifier; Picton et al., 2000), 100 kΩ is appropriate for this amplifier. Ferree, Luu, Russell, and Tucker (2001) estimate that for this amplifier system a 50-kΩ preparation would lead to a maximum 0.025% signal loss, and therefore the current levels should lead to no more than a 0.050% signal loss. They found no discernible signal loss with electrode preparations at about 40 kΩ.
Principal components analysis of ERP activity often is followed by a rotation of the obtained PC vectors (e.g., “varimax” rotation; Chapman & McCrary, 1996; Spencer et al., 1999). However, the rationale for such rotations is not specifically germane to ERP components and was not done in this study. The variances of the first 20 of 128 eigenvalues of the PC analysis were examined. The percent of variance accounted for by the first 20 eigenvalues ranged across individuals from 75 to 97% (M = 87.79%, SD = 0.056). The last 5 of the first 20 eigenvectors (16 to 20) accounted for about 3% of the total variance, and the 20th eigenvector accounted for less than 1% of the total variance for each participant.
The ANOVAs for the analyses were done with a general linear models approach using nonorthogonal design because of the unequal number of eye movements across factors, and because of the different numbers of eye movements across subjects (see Hocking, 1985; Searle, 1971, 1987). The sums of squares (hypothesis and error) for the nested effects in the design were estimated using “subjects” as a class and nesting repeated measures (e.g., session) within this class variable. The “PROC GLM” of SAS was used for the computations. The saccade latency also was tested with log-transformed values, and with the mean of the median latency per subject, with similar results. In all analyses, the Scheffe method was used to control for inflation of testwise error rate. The error mean squares for each post hoc comparison was obtained from the error term for the omnibus interaction for that post hoc evaluation. The significance of the post hoc tests was p <.05 for all tests and these individual probabilities were not reported in the text.
The ANOVAs involving the intervals effects first tested the effects due to the omnibus test. If the intervals effect was significant, the linear, quadratic, and cubic trends for the intervals effect and the interaction of intervals and the experimental factors were tested. However, these trends were tested against the error term for the total intervals effects to provide a conservative test of the intervals effects. The intervals effects were adjusted by the Huynh–Feldt ɛ adjustment to the degrees of freedom to control for inflated error rates with psychophysiological measures (Huynh & Feldt, 1970; Jennings & Wood, 1976; Keselman & Keselman, 1988; Pivik et al., 1993).
This research was supported by grants from the National Institute of Child Health and Human Development, #R01-HD18942, and a Major Research Instrumentation Award, #BCS-9977198, from the National Science Foundation. I wish to acknowledge Michael Stevens and William Campbell for their aid in testing participants, data editing, and analysis, and Jeffrey Schatz and Jennifer Vendemia for help on principal component and cortical source analysis.
References
- Achim A, Richer F, Saint-Hilaire J. Methods for separating temporally overlapping sources of neuroelectric data. Brain Topography. 1988;1:22–28. doi: 10.1007/BF01129336. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Weinstein JM. The human presaccadic spike potential: Influences of a visual target, saccade direction, electrode laterality and instructions to perform saccades. Brain Research. 1985;347:49–57. doi: 10.1016/0006-8993(85)90888-1. [DOI] [PubMed] [Google Scholar]
- Becker W, Hoehne O, Iwase K, Kornhuber HH. Cerebral and ocular muscle potentials preceding voluntary eye movements in man. Electroencephalography and Clinical Neurophysiology Supplement. 1973;33:99–104. [PubMed] [Google Scholar]
- Brickett PA, Weinberg H, Davis CM. Cerebral potentials preceding visually triggered saccades. Annuals of the New York Academy of Science. 1984;424:429–433. doi: 10.1111/j.1749-6632.1984.tb23563.x. [DOI] [PubMed] [Google Scholar]
- Csibra G, Johnson MH, Tucker LA. Attention and oculomotor control: A high-density ERP study of the gap effect. Neuropsychologia. 1997;35:855–865. doi: 10.1016/s0028-3932(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Donchin, E., Ritter, W., & McCallum, W. C. (1978). Cognitive psychophysiology: The endogenous components of the ERP. In E. Callaway, P. Tueting, & S. H. Koslow (Eds.), Brain event-related potentials in man New York: Academic Press.
- Evdokimidis I, Constantinidis TS, Gourtzelidis P, Liakopoulos D, Papageorgiou C. Changes of presaccadic cortical activity when performing horizontal, visually guided saccades. Electroencephalography and Clinical Neurophysiology. 1997;102:256–260. doi: 10.1016/s0013-4694(96)96583-2. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Liakopoulos D, Constantinidis TS, Papageorgiou C. Cortical potentials with antisaccades. Electroencephalography and Clinical Neurophysiology. 1996;98:377–384. doi: 10.1016/0013-4694(96)94699-4. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Liakopoulos D, Papageorgiou C. Cortical potentials preceding centrifugal and centripetal self-paced horizontal saccades. Electroencephalography and Clinical Neurophysiology. 1991;79:503–505. doi: 10.1016/0013-4694(91)90169-5. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Mergner T, Lucking CH. Dependence of presaccadic cortical potentials on the type of saccadic eye movement. Electroencephalography and Clinical Neurophysiology. 1992;83:179–191. doi: 10.1016/0013-4694(92)90143-6. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. Journal of Neurophysiology. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: A review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Flohr H. Cortical potentials preceding pro- and antisaccades in man. Electroencephalography and Clinical Neurophysiology. 1997;102:356–362. doi: 10.1016/s0013-4694(96)96569-4. [DOI] [PubMed] [Google Scholar]
- Everling S, Spantekow A, Krappmann P, Flohr H. Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Experimental Brain Research. 1998;118:27–34. doi: 10.1007/s002210050252. [DOI] [PubMed] [Google Scholar]
- Fabiani, M., Gratton, G., & Coles, M. G. H. (2000). Event-related brain potentials: Methods, theory, and applications. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 53–84). New York: Cambridge.
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Journal of Clinical Neurophysiology. 2001;112–113:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Characteristics of “anti” saccades in man. Experimental Brain Research. 1992;89:415–424. doi: 10.1007/BF00228257. [DOI] [PubMed] [Google Scholar]
- Fox PT, Fox JM, Raichle ME, Burde RM. The role of cerebral cortex in the generation of voluntary saccades: A positron emission tomographic study. Journal of Neurophysiology. 1985;54:348–369. doi: 10.1152/jn.1985.54.2.348. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–758. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Hallet PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hillyard, S. A., Mangun, G. R., Woldroff, M. G., & Luck, S. J. (1995). Neural systems mediating selective attention. In M. S. Gazzaniga (Ed.), Cognitive neurosciences (pp. 665–682). Cambridge, MA: MIT Press.
- Hocking, R. R. (1985). The analysis of linear models Monterey, CA: Brooks/Cole Publishers.
- Huizenga HM, Molenaar PCM. Estimating and testing the sources of evoked potentials in the brain. Multivariate Behavioral Research. 1994;29:237–262. doi: 10.1207/s15327906mbr2903_3. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Conditions under which the mean square ratios in repeated measurements designs have exact F distributions. Journal of the American Statistical Association. 1970;65:1582–1589. [Google Scholar]
- Jennings JR, Wood CC. The ɛ -adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Keselman JC. Comparing repeated measures means in factorial designs. Psychophysiology. 1988;25:612–618. doi: 10.1111/j.1469-8986.1988.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Klein C, Heinks T, Andresen B, Berg P, Moritz S. Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biological Psychiatry. 2000;47:978–990. doi: 10.1016/s0006-3223(00)00234-1. [DOI] [PubMed] [Google Scholar]
- Klostermann W, Kompf D, Heide W, Verleger R, Wauschkuhn B, Seyfert T. The presaccadic cortical negativity prior to self-paced saccades and without visual guidance. Electroencephalography and Clinical Neurophysiology. 1994;91:219–228. doi: 10.1016/0013-4694(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Kurtzberg, D., & Vaughan, H. G. (1980). Differential topography of human eye movement potentials preceding visually triggered and self-initiated saccades. In H. H. Kornhuber & L. Deecke (Eds.), Motivation, motor and sensory processes of the brain (pp. 203–208). Amsterdam: Elsevier Science Publishers. [DOI] [PubMed]
- Kurtzberg D, Vaughan HG. Topographic analysis of human cortical potentials preceding self-initiated and visually triggered saccades. Brain Research. 1982;243:1–9. doi: 10.1016/0006-8993(82)91115-5. [DOI] [PubMed] [Google Scholar]
- Luu, P., & Ferree, T. (2000). Determination of the Geodesic Sensor Nets’ electrode positions and their 10-10 international equivalents (Technical note Eugene, OR: Electrical Geodesics, Inc.
- Maier J, Dagnelie H, Spekreijse H, van Dijk B. Principal components analysis for source localization of VEPs in man. Vision Research. 1987;27:165–177. doi: 10.1016/0042-6989(87)90179-9. [DOI] [PubMed] [Google Scholar]
- Makeig S, Bell AH, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. Advances in Neural Information Processing Systems. 1996;8:145–151. [Google Scholar]
- Makeig S, Jung TP, Bell AH, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proceedings of the National Academy of Sciences, USA. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Harato H. Detection of rapid phases of eye movements using third-order derivatives. Japanese Journal of Ergonomics. 1983;19:147–153. [Google Scholar]
- Matsuoka K, Ueda Y. Frequency characteristics of the smooth pursuit component in tracking eye movements. Ergonomics. 1986;29:197–214. doi: 10.1080/00140138608968260. [DOI] [PubMed] [Google Scholar]
- Mosher JC, Lewis PS, Leahy RM. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Transactions on Biomedical Engineering. 1992;39:541–557. doi: 10.1109/10.141192. [DOI] [PubMed] [Google Scholar]
- Moster ML, Goldberg G. Topography of scalp potentials preceding self-initiated saccades. Neurology. 1990;40:644–648. doi: 10.1212/wnl.40.4.644. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysee SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proceedings of the National Academy of Sciences, USA. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: A selective review. Neuropsychologia. 1996;24:474–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Scalp current density mapping: Value and estimation from brain data. IEEE Transactions on Biomedical Engineering. 1987;34:283–288. doi: 10.1109/tbme.1987.326089. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Cortical source analysis of ERP of individual participants in psychophysiological experiments. Psychophysiology. 2002;39(Suppl):S69. [Google Scholar]
- Richards JE, Hunter SK. Peripheral stimulus localization by infants with eye and head movements during visual attention. Vision Research. 1997;37:3021–3035. doi: 10.1016/s0042-6989(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Experimental Brain Research. 1994;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Scherg, M. (1990). Fundamentals of dipole source potential analysis. In F. Grandon, M. Hoke, & G. L. Romani (Eds.), Auditory evoked magnetic fields and potentials (Vol. 6, pp. 40–69). Basel, Switzerland: Karger.
- Scherg M. Functional imaging and localization of electromagnetic brain activity. Brain Topography. 1992;5:103–111. doi: 10.1007/BF01129037. [DOI] [PubMed] [Google Scholar]
- Scherg, M., & Picton, T. W. (1991). Separation and identification of event-related potential components by brain electrical source analysis. In C. H. M. Brunia, G. Mulder, & M. N. Verbaten (Eds.), Event-related brain research (pp. 24–37). Amsterdam: Elsevier Science Publishers. [PubMed]
- Schlag-Rey M, Amador N, Sanchez H, Schalg J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Searle, S. R. (1971). Linear models New York: John Wiley & Sons.
- Searle, S. R. (1987). Linear models for unbalanced data New York: John Wiley & Sons.
- Sharma, S. (1996). Applied multivariate techniques New York: John Wiley & Sons.
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atals of the human brain New York: Thieme Medical Publishers.
- Thickbroom GW, Mastaglia FL. Presaccadic “spike” potential: Investigation of topography and source. Brain Research. 1985;339:271–280. doi: 10.1016/0006-8993(85)90092-7. [DOI] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: The geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Liotti M, Potts GF, Russell GS, Posner MI. Spatiotemporal analysis of brain electrical fields. Human Brain Mapping. 1994;1:134–152. [Google Scholar]
- Walker R, Husain M, Hodgson TL, Harrison J, Kennard C. Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia. 1998;36:1141–1159. doi: 10.1016/s0028-3932(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: An electrical sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Weinstein JM, Balaban CD, VerHoeve JN. Directional tuning of the human presaccadic spike potential. Brain Research. 1991;543:243–250. doi: 10.1016/0006-8993(91)90034-s. [DOI] [PubMed] [Google Scholar]


