Abstract
Studies on various forms of synaptic plasticity have demonstrated a link between mRNA translation, learning and memory. Like memory, synaptic plasticity includes an early phase which depends on modification of pre-existing proteins, and a late phase that requires transcription and synthesis of new proteins1,2. Activation of post-synaptic targets appears to trigger the transcription of plasticity-related genes. The new mRNAs are either translated in the soma or transported to synapses before translation. GCN2, a key protein kinase, regulates the initiation of translation. We now report a unique feature of hippocampal slices from GCN2 -/- mice: in CA1, a single 100 Hz train induces a strong and sustained long-term potentiation (late-LTP or L-LTP), which is transcription and translation dependent. In contrast, stimulation that elicits late-LTP in wild type slices, such as four 100 Hz trains or forskolin, fails to evoke L-LTP in GCN2 -/- slices. This aberrant synaptic plasticity is mirrored in the behavior of GCN2 -/- mice in the Morris water maze: after weak training, their spatial memory is enhanced, but it is impaired after more intense training. Activated GCN2 stimulates mRNA translation of ATF4, a CREB antagonist. Accordingly, in the hippocampus of GCN2 -/- mice, the expression of ATF4 is reduced and CREB activity is increased. Our study provides genetic, physiological, behavioral and molecular evidence that GCN2 regulates synaptic plasticity, as well as learning and memory through modulation of the ATF4/CREB pathway.
Translation of eukaryotic mRNAs is primarily regulated at the level of initiation3. Binding of the initiator tRNA, Met-tRNAiMet, to the 40S subunit is facilitated by the initiation factor 2 (eIF2) which forms a ternary complex with GTP and Met-tRNAiMet. Although phosphorylation of the α subunit of eIF2 can inhibit general translation4,5, it stimulates the mRNA translation of the transcriptional modulator ATF46, which inhibits synaptic plasticity and behavioral learning in diverse phyla7-10. In view of the need for translation for modulation of synaptic activity and strong evidence that eIF2α phosphorylation controls translation of ATF4 mRNA6,11,12, eIF2α kinase(s) may regulate synaptic plasticity. Because GCN2 is the most evolutionarily conserved eIF2α kinase and GCN2 mRNA is enriched in the brain of flies13 and mammals (as well as in liver)14,15 (see supplementary Fig. 1), we explored the role of GCN2 in synaptic plasticity and behavioral learning.
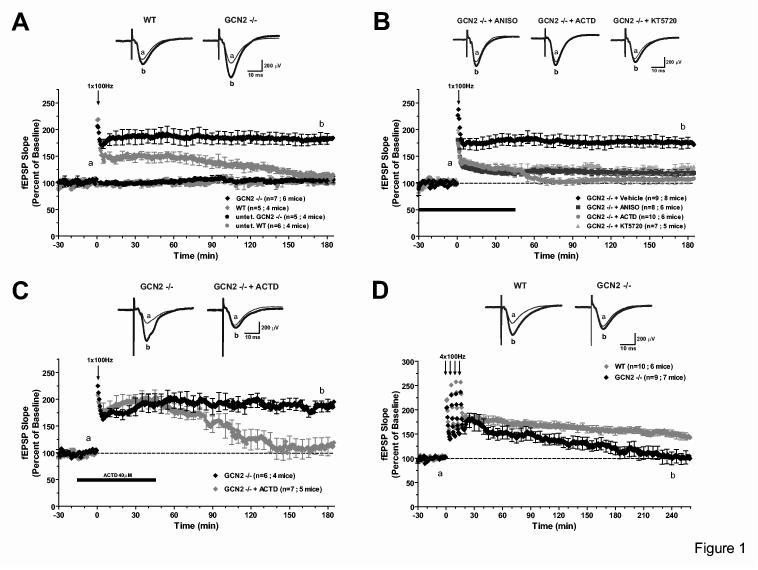
Figure 1.
Unusual properties of LTP induced in slices from GCN2 -/- mice. A) One train (100 Hz for 1s) of high-frequency stimulation (HFS) elicited E-LTP in WT slices but produced a robust and L-LTP in GCN2 -/- slices. B) Sustained LTP in GCN2 -/- slices is reduced by anisomycin (ANISO, 40μM), actinomycin-D (ACTD, 40μM) or the PKA inhibitor KT5720 (1μM). C) The enhanced LTP in GCN2 -/- slices is reduced by actinomycin-D, at later time points (>90 min) when applied 15 min prior to and 45 min post-tetanus. D) L-LTP induced by four 100 Hz trains at 5 min intervals is stable in WT slices but not in GCN2 -/- slices.
The GCN2 gene was inactivated by homologous recombination in ES cells (supplementary Fig. 1A and text). Hippocampal immunohistochemistry and in situ histohybridization show that GCN2, normally expressed mainly in CA1 and CA3 as well as in dentate gyrus, is undetectable in brain slices from GCN2 -/- mice (supplementary Figs. 1 and 2).
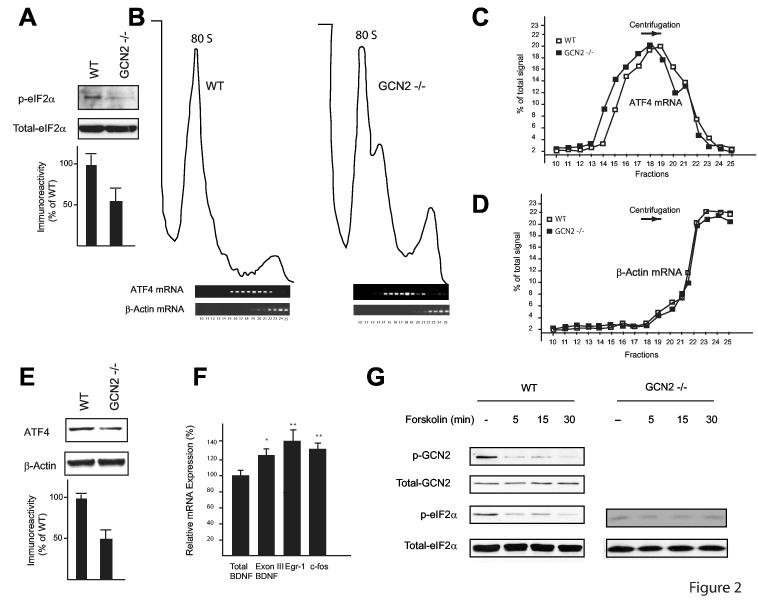
Figure 2.
ATF4 mRNA translation is downregulated in GCN2 -/- mice. A) Western blots performed on hippocampal extracts show that eIF2α phosphorylation is reduced in GCN2 -/- (n=3) as compared to WT mice (n=3). B) In polysome profiles from hippocampal extracts, ATF4 mRNA is in lighter fractions in GCN2 -/- (left panel) than in WT (right panel) controls as determined by RT-PCR analysis. C) Quantification of the band intensities in each fraction from ATF4 mRNA, panel B. D) for data in B, band intensities are quantified for each fraction of β-actin mRNA. E) In pooled hippocampal extracts, expression of ATF4 is reduced in GCN2 -/- mice. F) Real-time RT-PCR analysis reveals increased expression of CREB-dependent genes in hippocampal extracts from GCN2 -/- vs. WT mice (for both, n=5); mRNA expression is given as % of controls; *p < 0.05, **p < 0.01. G) Forskolin decreases GCN2 and eIF2α phosphorylation. In immunoblots of homogenates of CA1 region (from slices frozen immediately after tetanic stimulation), phosphorylated GCN2 (top) and eIF2α (middle panel) are decreased five minutes after forskolin application.
A lower L-LTP threshold in GCN2 -/- mice
There were no gross morphological changes in the hippocampus or other regions of the brain of GCN2 -/- mice (supplementary Fig. 3) and basal synaptic transmission in CA1 was unaltered as indicated by the following: 1) relation of fEPSPs to stimulus intensity, 2) size of fiber volley, 3) paired-pulse facilitation (PPF) and 4) peak response to tetanic stimulation (supplementary Fig. 4 and text). Normally, a single high-frequency tetanus (100 Hz for 1 s) elicits in the Schaffer collateral/commissural pathway a transient form of long-term potentiation known as early-LTP (E-LTP) which decays in 2-3 h and does not require RNA or protein synthesis1. In slices from GCN2 -/- mice, a single tetanus induces a robust and sustained late-LTP (Fig. 1A; at 180 min, p < 0.001) and the initial potentiation is greater than in slices from wild type (WT) mice (Fig. 1A; at 15 min, p < 0.05). This increase was synapse specific since a control input that received only test stimulation remained stable for the entire experimental session (supplementary Fig. 5A). Thus, GCN2 affects the duration of LTP and its initial amplitude.
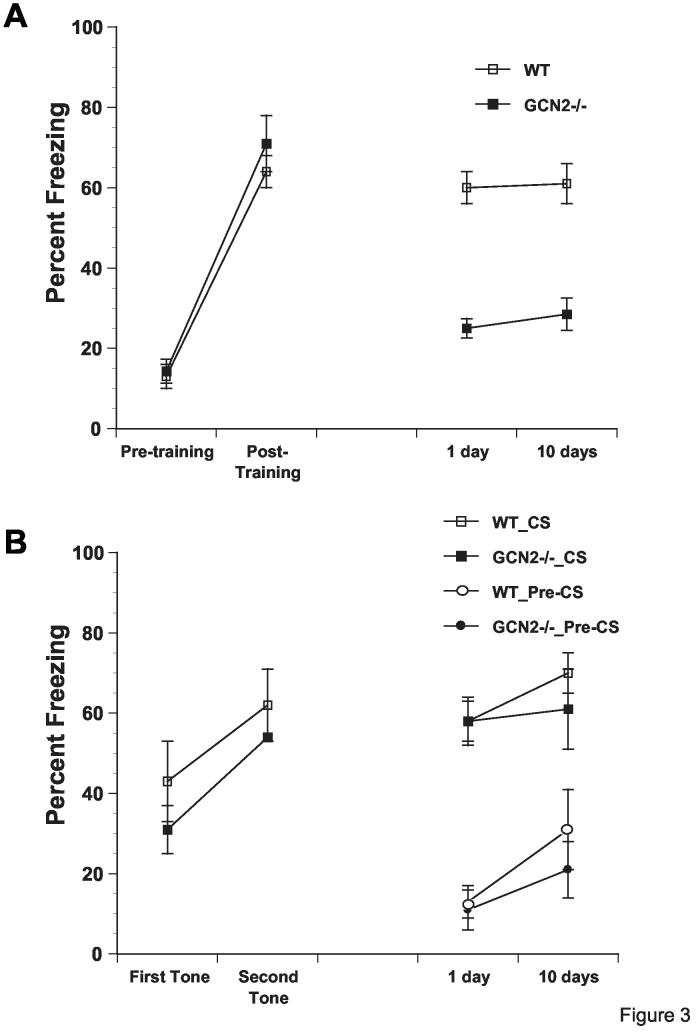
Figure 3.
GCN2 -/- mice are impaired in contextual but not auditory fear conditioning. A) Though acquisition of contextual freezing (with two pairings) is similar in GCN2 -/- (filled squares, n=10) and WT (open squares, n=12) mice, contextual freezing, measured during 2 min period before first shock (pre-shocks) and 1 min period after last shock (post-shocks), is impaired in GCN2 -/- mice 1 and 10 days after acquisition. B) Auditory fear conditioning is intact in freezing to tone over two pairings and in long term memory tests at 1 and 10 days later in GCN2 -/- mice. Labels indicate genotype and whether freezing was to tone (CS) or during 2 min before tone (pre-CS) (Means ± SEM).
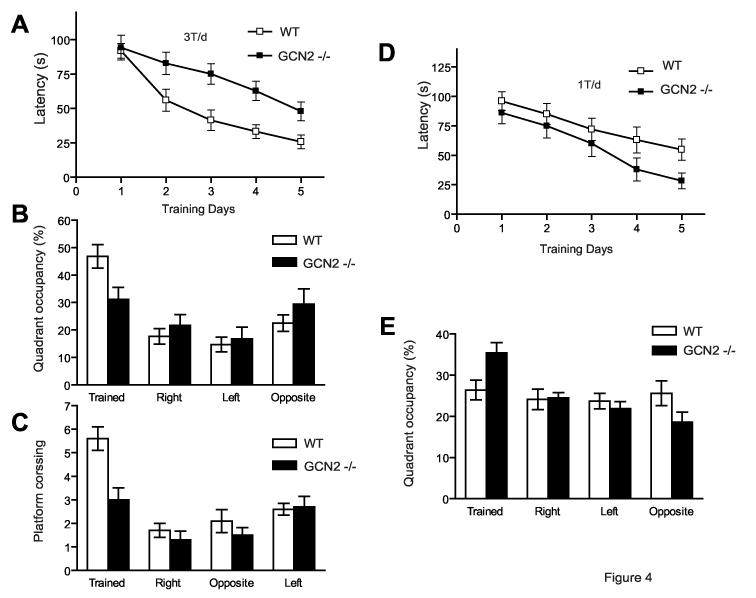
Figure 4.
Long-term spatial memory of GCN2 -/- mice is enhanced after weak training but impaired after more intense training (in the Morris water maze) A) Escape latencies in hidden platform tests (3 trial per day), plotted as function of training days (WT, open squares, n = 16 and GCN2 -/-, filled squares, n = 15), are shorter for WT than GCN2 -/- mice. B) After completion of training, WT mice show preferential quadrant occupancy. C) WT mice crossed the previous site where the platform was located more times than GCN2 -/- mice (p < 0.001). D) When locating hidden platform (1 trial per day), escape latencies were consistently shorter for GCN2 -/- mice than for WT (for both, n=15). E) In occupancy test, GCN2 -/- mice spent more time in the trained quadrant (Means ± SEM).
Like the L–LTP normally elicited by four tetanic trains, the L-LTP induced by a single tetanus in slices from GCN2 -/- mice depends on PKA (Fig. 1B; at 180 min, p < 0.01), new mRNA (Fig. 1B; at 180 min, p < 0.01) and protein synthesis (Fig. 1B; 180 min, p < 0.001) and is resistant to depotentiation (supplementary Fig. 5C). As expected, E-LTP elicited in slices from WT mice by a single tetanus was not affected by inhibiting these pathways and could be depotentiated (supplementary Figs. 5B and 5C). Anisomycin (a translation inhibitor) and actinomycin-D (a transcription inhibitor) not only prevented the persistence of LTP in slices from GCN2-/- mice, but also caused an immediate decrease of the early phase of LTP (Fig. 1B). Similar to our results, the effects of anisomycin on L-LTP in the Schaffer collateral pathway often show an immediate decrement in the magnitude of potentiation, indicating that protein synthesis-dependent processes are required early following L-LTP induction16,17. The early effect of actinomycin-D suggests that the increased amplitude of initial potentiation is due to the translation of immediate-early-genes whose mRNAs are quickly turned over. Indeed, when actinomycin-D is applied 15 min (instead of 30 min) before the onset of tetanization to minimize the effects of steady state levels of rapidly turning over mRNAs, the drug did not have an immediate effect (Fig. 1C, at 60 min, p > 0.05). Instead, there was a delayed decrease in LTP consistent with the lack of induction of new mRNAs necessary for the maintenance of LTP (Fig. 1C, at 180 min, p < 0.01).
According to these observations, GCN2 deletion leads to an enhanced response to a single tetanus, resulting in L-LTP instead of E-LTP. Does GCN2 deletion also affect the L-LTP normally induced by repeated tetani? To address this question, we examined L-LTP induced in CA1 by two different protocols: tetanic stimulation with four trains at 100 Hz, and forskolin, an activator of PKA18. As expected, in slices from WT mice, four trains elicited L-LTP that persisted for at least 4 hr. By contrast, in slices from GCN2 -/- mice, the LTP decayed to baseline within 3 hr (Fig. 1D, at 240 min, p < 0.01). In slices from WT mice, forskolin elicited the usual L-LTP whereas in GCN2-/- slices the L-LTP was not sustained (supplementary Fig. 5D). The GCN2 deletion specifically affected LTP because long-term depression (LTD), which is induced by low frequency stimulation or by incubation with an agonist of group I mGluRs, DHPG19, was unaltered in GCN2 -/- slices (supplementary Fig. 6 and text).
ATF4 is reduced and CREB activity is increased in GCN2 -/- mice.
GCN2 activation can inhibit translation initiation by eIF2α phosphorylation, but paradoxically, stimulates ATF4 mRNA translation6. We therefore measured eIF2α phosphorylation in hippocampal extracts from WT and GCN2 -/- mice and found that it is reduced (50% ± 19%) in GCN2 -/- mice (Fig. 2A). Consistent with this finding, ATF4 mRNA was shifted to the lighter polysome fractions of hippocampal extracts from GCN2 -/- mice (Figs. 2B and 2C). In agreement with a weak basal translation of ATF4 mRNA, ATF4 protein was correspondingly reduced (49% ± 11%; Fig. 2E). By contrast, β-actin mRNA sedimented predominantly in the heavy polysome fractions as would be expected for an efficiently translated mRNA (Figs. 2B and 2D). Thus, GCN2 deletion leads to a decrease in translation of ATF4 mRNA in the hippocampus. In accordance with the inhibition of CREB by ATF4, decreased translation of ATF4 mRNA in GCN2 -/- mice was associated with enhanced CREB function: expression of immediate-early-genes regulated by CREB (BDNF, cFos, Egr-1) was 25-35% greater in GCN2 -/- hippocampal extracts (Fig. 2F).
To further investigate how synaptic plasticity affects GCN2, we examined the effects of forskolin or 4 trains at 100 Hz (both induce L-LTP and stimulate CRE-mediated gene expression)20 on GCN2 and eIF2α phosphorylation. Both protocols reduced GCN2 and eIF2α phosphorylation in WT but not in GCN2 -/- slices (Fig. 2G and supplementary Fig. 7A). However, E-LTP elicited by a single train was not associated with a reduction in GCN2 and eIF2α phosphorylation (supplementary Fig. 7B). Thus, GCN2 activity is regulated by two forms of strong stimulation that elicit L-LTP, but not by a weaker stimulation that only induces E-LTP.
Defective hippocampus-dependent memory of GCN2 -/- mice
The effects of GCN2 deletion on long-term learning and memory were first studied in a fear conditioning paradigm. Fear conditioning by 2 tone-shock pairings has two components. One is contextual fear conditioning, which associates the training context and the footshock and requires both the hippocampus and the amygdala. The second, which associates the tone and the footshock, requires the amygdala but not the hippocampus21. When tested 1 and 10 days after training, GCN2 -/- mice showed a deficit in contextual fear conditioning (Fig. 3A, p < 0.05 and supplemental text). By contrast, auditory fear conditioning (tested in a different chamber) was intact (Fig. 3B, p > 0.05 and supplemental text).
Next, hippocampus-dependent spatial memory was tested in the Morris water maze22. In the course of training (three times per day, at 30 min intervals) the performance of both groups improved (Fig. 4A, p < 0.001), but WT mice learned faster than did GCN2 -/- mice (Fig. 4A; at 5 days, p < 0.01). In probe tests performed after the end of training, the platform was removed and the mice were allowed to search for 60s (Fig. 4B). Unlike WT mice (Fig. 4B, p < 0.001), GCN2 -/- mice showed no preference for the training quadrant (Fig. 4B, p > 0.05) and fewer platform crossings (Fig. 4C, p < 0.001). Vision and locomotor functions were equally efficient in WT and GCN2 -/- mice, judging by swimming speed (p > 0.05) and latency of escape to a visible platform (p > 0.05). Thus, GCN2 deletion is associated with a specific impairment of hippocampus-dependent learning and memory.
Hippocampus-dependent spatial memory of GCN2 -/- mice is enhanced after weak training.
Because a single tetanus elicits L-LTP in slices from GCN2 -/- mice (Fig. 1A), we reasoned that mnemonic processes may be enhanced during weaker conditioning. Indeed, when mice were trained only once (vs. three times) per day, Tukey's test showed that on day 5, escape latencies were shorter for GCN2 -/- than WT mice (Fig. 4D; at 5 days, p < 0.02). Enhanced spatial learning by GCN2 -/- mice was also evident in the probe tests that were conducted three days following the end of training (Fig. 4E). According to repeated measures ANOVA, the GCN2 -/- mice spent significantly more time in the target quadrant (“Trained” in Fig. 4E) as compared to WT mice (p < 0.001). Thus, in agreement with the findings on LTP, memory is enhanced after weak training.
Discussion
The major finding of this study is that a decrease in threshold for L-LTP in CA1 (in slices) is associated with an improved spatial memory of weak conditioning in GCN2 -/- mice. A switch from short- to long-term plasticity8,10,23,24 is generally associated with enhanced gene expression. Indeed, CREB-dependent gene expression is increased in GCN2 -/- mice. Thus, GCN2 could affect long-lasting changes in plasticity by modulating CREB-activity. The dependence of the early phase of LTP in GCN2 -/- mice on transcription and translation may be due to translation of mRNAs coding for CRE-dependent immediate-early-genes (which are up-regulated at the basal state). Because they turn over rapidly25,26, these mRNAs are likely to be down-regulated during the 30 min of preincubation with actinomycin-D, whereas in WT slices they are scarce in the basal state but are induced by repeated tetani. Therefore, L-LTP in WT slices requires a stronger stimulation and is inhibited only at later times. Thus, two mechanisms underlie L-LTP in GCN2 -/- slices: first, translation of pre-existing transcripts immediately increases LTP, and second, increased transcription of specific mRNAs generates persistent L-LTP.
How does GCN2 affect synaptic plasticity and learning? One possible model is based on the translational regulation of ATF4 mRNA via GCN2-mediated phosphorylation of eIF2α. A pivotal point is that ATF4 represses neuronal CREB activity1,7,8,10. Thus, under basal conditions, when GCN2 and eIF2α are phosphorylated and ATF4 levels are high, CREB-dependent transcription, synaptic plasticity and learning are repressed. By reducing GCN2 and eIF2α phosphorylation, LTP-inducing stimulation would remove this inhibition of synaptic plasticity and memory formation. In this manner, GCN2 regulates the switch from short- to long-term memory.
We documented a correlation between L-LTP and spatial memory. In accordance with the low threshold for L-LTP and its suppression after strong stimulation, the spatial memory of GCN2-/- mice depended on the intensity of training: it was impaired by strong training and enhanced by weaker training. A likely explanation is that strong stimulation (behavioral or by four trains in slices) potentiates an inhibitory pathway that is facilitated in GCN2 -/- mice. The nature of this mechanism will be an important target of future studies and may involve changes in regulation of gene expression and/or synaptic translation. Our results suggest that neurons have not only a threshold for activation of gene expression, but a second threshold where too much gene expression blocks synaptic plasticity. Shutting off plasticity could be important under conditions of excessive activity such as seizures. Our results provide novel genetic evidence that translational control by GCN2 is critical for synaptic plasticity, learning and memory. In addition, they raise the intriguing idea that memory formation is regulated via translational control of transcription.
Methods
Generation of Transgenic Mice by GCN2.KO4 targeting
GCN2 was deleted by a targeting vector constructed from PCR fragments amplified from cloned 129SvEv genomic DNA (see supplemental Methods). Chimeric mice derived from GCN2.KO4ex/+ ES cells were prepared by blastocyst injection and the mutant allele transmitted through the germline to isogenic 129SvEv mice which were bred to homozygosity. GCN2 -/- mice were phenotypically normal as compared to their wild-type littermates and were obtained in a Mendelian ratio.
In situ hybridization histochemistry (ISH).
Mouse sense and antisense cRNA probes coding for exon 12 of GCN2 were labeled with [35S]UTP and [35S]CTP (1,250 Ci/mmol; Amersham) and ISH was performed as previously reported27.
Immunoprecipitation, Immunohistochemistry and Western-Blotting
Antibodies against the C-terminal portion (kinase domain) of mouse GCN2 kinase (C-term) and ATF4 have been described6. The antibody against the N-terminal portion (amino acids 1 to 363, N-term) of human GCN2 was produced as a GST-fusion protein in BL-21, and purified on glutathione-Sepharose (APB). Immunoblotting and immunohistochemistry were as reported6,28. Anti- phospho-eIF2α, total eIF2α and β-actin antibodies were purchased from Cell Signaling and Technology Laboratories.
Electrophysiology
After decapitation of WT (GCN2 +/+) or transgenic (GCN2 -/-) age-matched littermates (6-12 weeks old), 400 μm hippocampal slices were cut with a vibratome and kept submerged at 27-28°C. Slices were perfused (at 1-2 ml/min) with oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing: 124 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1.3 mM MgSO4, 2.5 mM CaCl2, 26 mM NaHCO3 and 10 mM glucose. Bipolar tungsten electrodes were placed in CA1 stratum radiatum to stimulate Schaffer collateral and commissural fibers, and extracellular field EPSPs (fEPSPs) were recorded from stratum radiatum with a glass microelectrode (2-3 MΩ, filled with 2 M NaCl). Stimulus (0.1 ms duration) was adjusted to evoke 35-40% maximal fEPSPs at 0.033 Hz. LTP was induced with 1 or 4 trains (1 s) at 100 Hz delivered 5 min apart. For LTD experiments, 1Hz stimulation was applied for 15 min. Forskolin (50 μM, Sigma) or DHPG (50 μM, Tocris) was added to the bath after at least 30 min of stable recording. Anisomycin (40 μM, Calbiochem), actinomycin-D (40 μM, Calbiochem) or KT5720 (1 μM, Biomol) was applied 30 min, or as indicated otherwise, before tetanic stimulation. Statistical analysis utilized t-tests and two-way ANOVA. All data are presented as mean ± SEM and “n” indicates the number of slices. The experimenter was blind to the mouse genotype.
Fear conditioning.
The experimenter (blind to mouse genotype) compared GCN2-/- and WT littermates (males, 2–4 months old). Training consisted of two pairings of a tone (2800 Hz, 85 db, 30 s) with a co-terminating foot-shock (0.7 mA, 2 s). The first tone started 120 s after animals were placed in the conditioning chamber, where they remained for an additional minute after the second pairing, and were then returned to their home cage. Mice were tested 1 and 10 days later for freezing in response to training context in a counterbalanced manner (Supplementary text).
Morris water maze task.
The pool was 100 cm in diameter and the water was rendered opaque by adding white tempera. Water temperature was kept at 20°C. The platform was 4.5 cm in diameter. Mice were trained three times per day (3T/d) at intervals of 30 min, or once per day (1T/d) over five consecutive days. In each trial the mouse swam until it found the platform, or after 120 s was guided to the platform; the mouse was then placed on the platform for 10 s before being picked up. At the end of the testing period, a probe trial (60s) was done. Statistical analysis was based on univariate and multivariate ANOVA and between-group comparisons by Tukey's Test.
Polysome profile analysis and RT-PCR
Hippocampal slices were washed twice with cold PBS + 100 μg/ml cycloheximide, suspended in lysis buffer, homogenized with 15 strokes (7-ml Wheaton Dounce) on ice, and then centrifuged for 2 min at 14,000g. Gradients were prepared and analyzed as described29. For detection of ATF4 and β-Actin mRNAs, RNA from individual fractions was amplified in one-tube RT-PCR reactions which were optimized to detect the exponential phase on the amplification curve.
Quantitative RT-PCR
The one-step RT-PCR LightCycler RNA Master SYBR Green kit (Roche, Canada) was used to quantify CRE-dependent gene expression, as recommended by the manufacturer. Primers, RT-PCR conditions and normalization procedures were as described30.
Supplementary Material
Acknowledgements
We thank Eric Kandel, Kresimir Krnjević, Kobi Rosenblum, Emmanuel Landau, Cristina Alberini, Yael Mamane and Tamar Lubell for comments on the manuscript, Yuhong Zhang, Rivka Jungreis and Annie Sylvestre for assisting in the production and maintenance of the GCN2 -/- mice and Colin Lister for excellent assistance. This work was supported by grants from the Canadian Institute of Health Research (CIHR) and Howard Hughes Medical Institute (HHMI) to N.S, a CIHR Group Grant to J-C.L and W.S; a CIHR grant to N. Seidah; a NIH and DK47119 to D. R; a CIHR grant to A.C.C. N.S. is a CIHR and Distinguished Scientist and a HHMI International scholar. M.C-M is supported by a CIHR post-doctoral fellowship.
References
- 1.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 2.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 3.Mathews MB, Sonenberg N, Hershey JWB. In: Translational Control of Gene Expression. Sonenberg N, Hershey JWB, Merrick WC, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 1–33. [Google Scholar]
- 4.Hinnebusch AG. In: Translational Control of Gene Expression. Sonenberg N, Hershey JWB, Merrick WC, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 185–244. [Google Scholar]
- 5.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 6.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 7.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch D, et al. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–92. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 9.Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–41. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 10.Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–69. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 11.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 13.Santoyo J, Alcalde J, Mendez R, Pulido D, de Haro C. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2alpha (eIF-2alpha) kinase from Drosophila melanogaster. Homology To yeast GCN2 protein kinase. J Biol Chem. 1997;272:12544–50. doi: 10.1074/jbc.272.19.12544. [DOI] [PubMed] [Google Scholar]
- 14.Berlanga JJ, Santoyo J, De Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem. 1999;265:754–62. doi: 10.1046/j.1432-1327.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 15.Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–6. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 17.Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–79. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 18.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–50. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–32. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 20.Impey S, et al. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–82. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 21.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 23.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 24.Malleret G, et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–86. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen CY, Shyu AB. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol Cell Biol. 1994;14:8471–82. doi: 10.1128/mcb.14.12.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shyu AB, Greenberg ME, Belasco JG. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- 27.Seidah NG, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–33. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapointe V, et al. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. The Journal of Physiology. 2004;555:125–35. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan J, Khan SN, Harvey I, Merrick W, Pelletier J. Eukaryotic protein synthesis inhibitors identified by comparison of cytotoxicity profiles. Rna. 2004;10:528–43. doi: 10.1261/rna.5200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saura CA, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.