Abstract
The substantia nigra pars reticulata (SNR) represents an endogenous seizure suppressing system, which may be targeted to develop treatments for generalized or multifocal epilepsies. This review summarizes the region-, age-, and sex-specific features of the SNR-based seizure-controlling network.
The substantia nigra pars reticulata (SNR), a midbrain structure that is a well-recognized component of motor control systems, also plays a critical role in the modulation of seizures (1, 2). The SNR is the larger part of the substantia nigra proper, and contains mainly GABAergic neurons with high spontaneous firing rates. These neurons receive inputs from striatum by two distinct pathways (3). The striato–nigral direct pathway exerts GABA-mediated inhibitory effects on SNR neurons. The indirect striato-pallido-subthalamic-nigral pathway provides excitatory glutamatergic inputs from the subthalamic nucleus. During normal behavioral conditions, the indirect glutamatergic input seems to have an insignificant role in the regulation of SNR neurons (4), which may not be the case during seizures. Furthermore, a series of experimental studies strongly suggest that the role of the SNR in the control of seizures is different in male and female rats and also changes as a function of age.
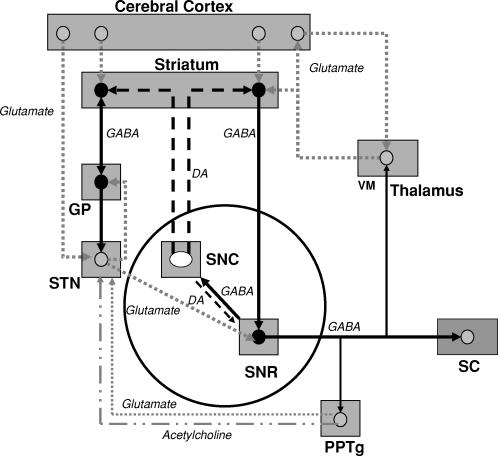
The SNR plays an important transmitting role by directing information either back to the striatum or to the output structures of the basal ganglia (i.e., the thalamus, superior colliculus, and brainstem, via the pedunculopontine tegmental nucleus). The striato-nigro-striatal loop serves as an inhibitory feedback control for the activity of the SNR neurons. GABA release at the striato–nigral terminals or exogenous application of GABA decreases the firing rate of the SNR GABAergic neurons (5, 6). Either event results in a decrease of GABA release in the output structures, leading to their disinhibition (see Figure 1).
FIGURE 1.
Simplified diagram of the afferent and efferent connectivity of the SNR obtained from studies in adult male rats. The activity of each efferent pathway can be influenced by the location of the SNR GABAergic neurons in SNRanterior or SNRposterior as well as by age and sex (29). Disinhibition occurs following GABA application in the SNR, which supresses nigral GABAergic neuron activity, leading to increased neuronal firing in neurons of the output structures, such as the ventromedial thalamus or superior colliculus (5).
Role of the SNR in Seizure Control
The involvement of the SNR in seizures was recognized from metabolic mapping studies using 14C-2-deoxyglucose (2DG) in adult rats. Among brain structures, the increase in glucose uptake in the SNR during different types of seizures is especially striking. While the patterns of 2DG uptake vary in different seizure models, the SNR is always activated, especially during generalized seizures (7–10). A recent study showed that distinct regions of the SNR are activated sequentially during the evolution of a generalized seizure. The posterior part of the SNR (SNRposterior) becomes involved just before the expression of the seizure, while the anterior part (SNRanterior) is activated during the seizure (10).
Pharmacological studies using focal drug applications have contributed greatly to the understanding of how the SNR regulates the seizures. Most of these studies examined the role of the SNRanterior. Treatments that decrease the activity of the SNRanterior GABAergic neurons lead to attenuation of seizures. The original studies showed that precisely localized bilateral microinfusions of GABAA receptor agonists, such as muscimol, bilaterally into the SNR produce anticonvulsant effects (1). In contrast, localized SNR microinfusions of the GABAA receptor antagonist bicuculline have proconvulsant effects (11). In addition, suppression of the SNR glutamatergic input from the subthalamic nucleus by local microinfusions of glutamate receptor antagonists (e.g., AP7, an N-methyl-d-aspartate [NMDA] receptor antagonist) into the SNR decreases the firing rate of SNR neurons and, thus, induces anticonvulsant effects (12, 13). The fact that seizure activity is sensitive to manipulations of both GABA and glutamate receptor systems within the SNR suggests that, unlike during normal behavioral conditions (4), the nigral glutamatergic inputs are actively involved during seizures. These results, together with data from lesion studies (14), demonstrate that the anticonvulsant effects are associated with decrements in the activity of the SNRanterior GABAergic neurons leading to disinhibition of the output structures (5): the superior colliculus and the pedunculopontine tegmental nucleus (3). The disinhibition is essential for the anticonvulsant effect. Accordingly, bicuculline infusions in the superior colliculus or pedunculopontine nucleus are anticonvulsant but localized muscimol infusions or lesions are proconvulsant (15–18).
Region-Specific Regulation of Seizures by the SNR in Adult Male Rats
The two SNR regions can be further distinguished on the basis of the differences in their local features (e.g., cytoarchitectonics, the complement and subunit composition of receptors, electrophysiologic responses) as well as differences in the activation of the output target (19–22). For example, there are regional differences in the distribution and expression of the two major GABAA receptor subunits, the α1 and γ2 subunits, within the SNR, as determined by in situ hybridization (23). The density of hybridization grains per cell for both GABAA receptor subunits is significantly higher in the SNRanterior compared with the SNRposterior. Because the functional properties of GABAA receptors depend on their subunit composition (24), the GABAA receptors within the two regions may be functionally distinct. An immunohistochemistry study also showed that the GABA content per neuron is higher in the SNRanterior than in the SNRposterior (25). In addition, the neuronal-specific potassium chloride cotransporter 2 (KCC2), which maintains lower intracellular Cl−, and thus is critical to the presence of hyperpolarizing GABA responses (26), is expressed in higher levels in the neurons of the SNRanterior than in the SNRposterior (27). The regional differences between the SNRanterior and SNRposterior are not confined to the GABAergic system. Pharmacological studies showed similar SNRanterior–posterior differences in the response to infusions of AP7 (13), while a binding study shows that NMDA and metabotropic glutamate receptor density is higher in the SNRposterior than in the SNRanterior (28).
The two SNR regions also utilize GABA-sensitive output networks, as revealed by the 2DG mapping technique (19, 29). The previously mentioned 2DG study revealed that during the preclonic state of flurothyl generalized myoclonic seizures, the SNRposterior is selectively involved, suggesting that it may act as a “gateway” for seizure propagation (10). As the seizure evolves, the SNRanterior becomes involved, now exhibiting increased glucose uptake. The involvement of the SNRanterior is associated with a decrease in glucose uptake in superior colliculus and striatum. As the 2DG uptake reflects changes at the presynaptic terminals (30, 31), the SNRanterior seizure-controlling network may include increased GABA release from the striato–nigral projection (reflected by increased 2DG uptake in the SNRanterior), which has inhibitory effects on the SNR GABAergic neuron firing. These events result in decreased GABA release in the superior colliculus and, thus, disinhibition within this SNR output structure, initiating a process to terminate the ongoing seizure. This finding is supported by the results of the pharmacological studies, which demonstrate that exogenous administration of GABAA agonists in the SNRanterior augments the efficacy of the SNR-based endogenous anticonvulsant system (32–34).
The postclonic seizure state is associated with generalized brain hypometabolism that has specific decreases in 2DG uptake in the pedunculopontine tegmental nucleus, increased 2DG uptake in the subthalamic nucleus, and no changes in the SNR (10). The general hypometabolism reflects exhaustion in energy stores within neurons, especially in structures highly active during the seizure activity, as in the SNR. The lack of energy is associated with activation of inwardly rectifying ATP-sensitive potassium (KATP) channels (35). These channels powerfully regulate neuronal activity during states of metabolic distress. A decrease in ATP/ADP ratio results in channel opening, which leads to hyperpolarization of the neurons. The SNR contains a very high density of the KATP channels with subunit composition SUR1/Kir6.2 that are especially sensitive to energy deficits (36, 37). Thus, during seizures, the opening of the KATP channels that is due to the energy depletion (or metabolic stress) causes hyperpolarization of the nigral GABAergic neurons, leading to decreases in their firing rates. These events are reflected as decreased 2DG uptake in the terminals of the neurons in the pedunculopontine tegmental nucleus. The net result is disinhibition of neurons in the pedunculopontine tegmental nucleus, which produces an increase in cholinergic and glutamatergic drive on the subthalamic nucleus, an important ascending projection of the pedunculopontine tegmental nucleus (38). This sequence of events is depicted as increases 2DG uptake in the subthalamic nucleus, another structure implicated in seizure control (10).
Region-Specific Regulation of Seizures by the SNR in Adult Female Rats
Less information is available on the role of the SNR during seizures in female rats. Interestingly, a recent kindling study showed that in females, muscimol infusions in the SNRanterior have less powerful anticonvulsant effects (39) than had been reported in male rats (40). This finding may explain the observation that SNR lesions in females have no effect on kindling-induced seizures (41). In vivo single-unit recordings show that neuronal activity is increased in the SNRposterior but not in the SNRanterior following amygdala kindling (42), suggesting that two functionally distinct regions also exist in females. The role of the SNR may be more prominent in generalized seizures. Muscimol infusions in the SNRanterior have anticonvulsant effects but no effect in the SNRposterior in flurothyl-induced generalized seizures (21). Indeed, the SNRanterior has higher expression of the α1 and γ2 subunits of GABAA receptor and of KCC2 mRNA as well as higher GABA levels per neuron, compared with the SNRposterior (25, 27). Each SNR region also utilizes distinct networks following unilateral localized muscimol infusions (29). However, the most striking finding is that in contrast to male rats, in females, there is no proconvulsant muscimol-sensitive region (21).
Age-Specific Effects of the SNR in Seizure Control
Developmental studies show that the effectiveness of the SNR-based seizure-suppressing system is different in immature rats compared with adult rats. In contrast to adult male rats, in 2-week-old male pups, activation of the GABAA receptors by muscimol has proconvulsant effects (43), and there is only one functional region at this age (19, 21). In addition, the SNR GABAB receptor system seems to play a more important role in developing male rats than in adult male rats. While in adult male rats infusions of the GABAB receptor agonist baclofen or the receptor antagonist CGP 35348 have no effect on seizure threshold, in 2-week-old rats baclofen infusions have anticonvulsant effects, and CGP 35348 infusions are proconvulsant (44, 45). Other developmental differences between developing male and adult male rats include lower expression of α1 and γ2 subunits of GABAA receptors (23), lower GABA content per neuron (25), and lower expression of KCC2 (27) in the male rat pups. Developmental studies in female rats also reveal that functionality of the SNR-based seizure-suppressing system changes with age. As in 2-week-old male rats, muscimol infusions in the SNR of 2-week-old female rats do not distinguish between SNRanterior and SNRposterior. But in contrast to males, these infusions in females do not alter the seizure threshold (21).
Sex-Specific Effects of the SNR in Seizure Control Early in Life
In both male and female rats, the switch to the mature SNR with two distinct regions occurs around puberty (21). The timing corresponds to dramatic hormonal changes and may not be coincidental, indicating that the maturation of the SNR may be under the influence of sex hormones (21). Recent studies suggest that the SNR is equipped with both estrogen and androgen receptors, which are expressed at birth (46). During development, circulating sex hormones have organizing effects, leading to permanent differences between males and females (47). This fact explains the sex-specific SNR effects in seizure control, such as the previously cited difference in the effects of muscimol infusions into the SNR of 2-week-old male rats (proconvulsant) and female rats (no effect) (21). The crucial factor in the formation of the sex differences in the SNR is the presence of testosterone or its metabolites (estrogen and dihydrotestosterone), especially during early postnatal development. In male rats, depletion of testosterone by orchiectomy immediately after birth leads to the female SNR phenotype while, in females, postnatal administration of testosterone leads to the male SNR phenotype (21). The male–female differences in seizure control seem to be associated with sex-specific differences in the GABAergic system within the SNR as well as in connectivity patterns (29). In the SNR of a male rat, the GABA content per neuron and GABAA receptor α1 subunit mRNA expression is lower compared to females at the same age (25). The male SNR neurons express lower levels of the neuronal-specific KCC2 mRNA than females, which may explain the depolarizing responses to bath application of muscimol or synaptically released GABA, while female neurons respond by membrane hyperpolarization of the SNR GABAergic neurons (27, 48). In addition, in 2-week-old rats, acute sex hormone administration regulates the expression of KCC2 in the SNR, further suggesting that the hormonal surges during development also may be responsible for the sex-differences in the modulation of seizures by the SNR-based system (49).
Conclusion
Recent advances in technology offer possibilities for new treatments of epileptic disorders. One promising procedure may be deep brain high-frequency stimulation (50). However, for a successful use of such a treatment in epilepsy, identification of structures controlling the general seizure activity and multifocal epilepsies is essential. The SNR seems to be one of the promising regions as a target for treatments involving high-frequency stimulation (51) or focal drug delivery, which potentially could replace systemic antiepileptic therapy. Understanding the region-, sex-, and age-specific features of the SNR seizure-controlling network is important for developing precisely targeted therapies that would take into account the maturational state and gender-related factors.
Acknowledgments
Supported in part by grant NS-20253 from NINDS. SLM is a Martin A. and Emily L. Fisher Fellow in Neurology and Neuroscience.
References
- 1.Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by g-aminobutyric acid. Science. 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- 2.Velíšková J, Claudio OI, Galanopoulou AS, Kyrozis A, Lado FA, Ravizza T, Velíšek L, Moshé SL. Developmental aspects of the basal ganglia and therapeutic perspectives. Epileptic Disord. 2002;4(suppl 3):S73–S82. [PubMed] [Google Scholar]
- 3.Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windels F, Kiyatkin EA. GABA, not glutamate, controls the activity of substantia nigra reticulata neurons in awake, unrestrained rats. J Neurosci. 2004;24:6751–6754. doi: 10.1523/JNEUROSCI.1528-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigra influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res. 1985;334:227–233. doi: 10.1016/0006-8993(85)90214-8. [DOI] [PubMed] [Google Scholar]
- 6.Niemi-Junkola UJ, Westby MGW. Spatial variation in the effects of inactivation of substantia nigra on neuronal activity in rat superior colliculus. Neurosci Lett. 1998;241:175–179. doi: 10.1016/s0304-3940(97)00956-7. [DOI] [PubMed] [Google Scholar]
- 7.Albala BJ, Moshé SL, Okada R. Kainic-acid-induced seizures: a developmental study. Dev Brain Res. 1984;13:139–148. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 9.Nehlig A, Vergnes M, Marescaux C, Boyet S. Mapping of cerebral energy metabolism in rats with genetic generalized nonconvulsive epilepsy. J Neural Transm. 1992;35:141–153. doi: 10.1007/978-3-7091-9206-1_10. [DOI] [PubMed] [Google Scholar]
- 10.Velíšková J, Miller AM, Nunes ML, Brown LL. Regional neural activity within the substantia nigra during peri-ictal flurothyl generalized seizure stages. Neurobiol Dis. 2005;20:752–759. doi: 10.1016/j.nbd.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperber EF, Wurpel JND, Zhao DY, Moshé SL. Evidence for the involvement of nigral GABAA receptors in seizures of adult rats. Brain Res. 1989;480:378–382. doi: 10.1016/0006-8993(89)90211-4. [DOI] [PubMed] [Google Scholar]
- 12.Turski L, Cavalheiro E, Turski W, Meldrum B. Excitatory neurotransmission within substantia nigra pars reticulata regulates threshold for seizures produced by pilocarpine in rats: effects of intranigral 2-amino-7-phosphonoheptanoate and N-methyl-d-aspartate. Neuroscience. 1986;18:61–77. doi: 10.1016/0306-4522(86)90179-x. [DOI] [PubMed] [Google Scholar]
- 13.Velíšková J, Liptáková S, Hussain S. The effects of N-methyl-d-aspartate antagonist 2-amino-7-phosphonoheptanoic acid microinfusions into the adult male rat substantia nigra pars reticulata are site-specific. Neurosci Lett. 2001;316:108–110. doi: 10.1016/s0304-3940(01)02379-5. [DOI] [PubMed] [Google Scholar]
- 14.Garant D, Gale K. Lesions of substantia nigra protect against experimentally induced seizures. Brain Res. 1983;273:156–161. doi: 10.1016/0006-8993(83)91105-8. [DOI] [PubMed] [Google Scholar]
- 15.Dean P, Gale K. Anticonvulsant action of GABA receptor blockade in the nigrotectal target region. Brain Res. 1989;477:391–395. doi: 10.1016/0006-8993(89)91434-0. [DOI] [PubMed] [Google Scholar]
- 16.Redgrave P, Simkins M, Overton P, Dean P. Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy—I. Mapping of dorsal midbrain with bicuculline. Neuroscience. 1992;46:379–390. doi: 10.1016/0306-4522(92)90059-b. [DOI] [PubMed] [Google Scholar]
- 17.Okada R, Nagishi N, Nagaya H. The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol induced seizures. Brain Res. 1989;480:383–387. doi: 10.1016/0006-8993(89)90212-6. [DOI] [PubMed] [Google Scholar]
- 18.Depaulis A, Liu Z, Vergnes M, Marescaux C, Micheletti G, Warter JM. Suppression of spontaneous generalized non-convulsive seizures in the rat by microinjection of GABA antagonists into the superior colliculus. Epilepsy Res. 1990;5:192–198. doi: 10.1016/0920-1211(90)90038-w. [DOI] [PubMed] [Google Scholar]
- 19.Moshé SL, Brown LL, Kubová H, Velíšková J, Zukin RS, Sperber EF. Maturation and segregation of brain networks that modify seizures. Brain Res. 1994;665:141–146. doi: 10.1016/0006-8993(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 20.Thompson K, Anantharam V, Behrstock S, Bongarzone E, Campagnoni A, Tobin AJ. Conditionally immortalized cell lines, engineered to produce and release GABA, modulate the development of behavioral seizures. Exp Neurol. 2000;161:481–489. doi: 10.1006/exnr.1999.7305. [DOI] [PubMed] [Google Scholar]
- 21.Velíšková J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- 22.Velíšková J, Velíšek L, Nunes ML, Moshé SL. Developmental regulation of regional functionality of substantia nigra GABAA receptors involved in seizures. Eur J Pharmacol. 1996;309:167–173. doi: 10.1016/0014-2999(96)00341-x. [DOI] [PubMed] [Google Scholar]
- 23.Velíšková J, Kubová H, Friedman LK, Wu R, Sperber EF, Zukin RS, Moshé SL. The expression of GABAA receptor subunits in the substantia nigra is developmentally regulated and region-specific. Ital J Neurol Sci. 1998;19:205–210. doi: 10.1007/BF02427602. [DOI] [PubMed] [Google Scholar]
- 24.Levitan ES, Schofield PR, Burt DR, Rhee LM, Wisden W, Köhler M, Fujita N, Rogriguez H, Stephenson FA, Darlison MG, Barnard EA, Seeburg PH. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988;335:76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- 25.Ravizza T, Friedman LK, Moshé SL, Velíšková J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 26.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 27.Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp Neurol. 2003;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 28.Hedberg TG, Velíšková J, Sperber EF, Nunes ML, Moshé SL. Age-related differences in NMDA/metabotropic glutamate receptor binding in rat substantia nigra. Int J Dev Neurosci. 2003;21:95–103. doi: 10.1016/s0736-5748(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 29.Velíšek L, Velíšková J, Ravizza T, Giorgi FS, Moshé SL. Circling behavior and [14C]2-deoxyglucose mapping in rats: possible implications for autistic repetitive behaviors. Neurobiol Dis. 2005;18:346–355. doi: 10.1016/j.nbd.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 31.Ackermann RF, Finch DM, Babb TL, Engel J., Jr Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depaulis A, Vergnes M, Liu Z, Kempf E, Marescaux C. Involvement of the nigral output pathways in the inhibitory control of the substantia nigra over generalized non-convulsive seizures in the rat. Neuroscience. 1990;39:339–349. doi: 10.1016/0306-4522(90)90272-6. [DOI] [PubMed] [Google Scholar]
- 33.Redgrave P, Marrow L, Dean P. Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy—II. Pathways from substantia nigra pars lateralis and adjacent peripeduncular area to the dorsal midbrain. Neuroscience. 1992;46:391–406. doi: 10.1016/0306-4522(92)90060-f. [DOI] [PubMed] [Google Scholar]
- 34.Garant DS, Gale K. Substantia nigra-mediated anticonvulsant actions: role of nigral output pathways. Exp Neurol. 1987;97:143–159. doi: 10.1016/0014-4886(87)90289-5. [DOI] [PubMed] [Google Scholar]
- 35.Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 36.Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 37.Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. Embo J. 1999;18:833–846. doi: 10.1093/emboj/18.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247(suppl 5):V1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- 39.Gernert M, Löscher W. Lack of robust anticonvulsant effects of muscimol microinfusions in the anterior substantia nigra of kindled rats. Eur J Pharmacol. 2001;432:35–41. doi: 10.1016/s0014-2999(01)01458-3. [DOI] [PubMed] [Google Scholar]
- 40.McNamara JO, Galloway MT, Rigsbee LC, Shin C. Evidence implicating substantia nigra in regulation of kindled seizure threshold. J Neurosci. 1984;4:2410–2417. doi: 10.1523/JNEUROSCI.04-09-02410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahnschaffe U, Löscher W. Selective bilateral destruction of substantia nigra has no effect on kindled seizures induced from stimulation of amygdala or piriform cortex in rats. Neurosci Lett. 1990;113:205–210. doi: 10.1016/0304-3940(90)90304-r. [DOI] [PubMed] [Google Scholar]
- 42.Gernert M, Fedrowitz M, Wlaz P, Löscher W. Subregional changes in discharge rate, pattern, and drug sensitivity of putative GABAergic nigral neurons in the kindling model of epilepsy. Eur J Neurosci. 2004;20:2377–2386. doi: 10.1111/j.1460-9568.2004.03699.x. [DOI] [PubMed] [Google Scholar]
- 43.Sperber EF, Wong BY, Wurpel JN, Moshé SL. Nigral infusions of muscimol or bicuculline facilitate seizures in developing rats. Brain Res. 1987;465:243–250. doi: 10.1016/0165-3806(87)90245-8. [DOI] [PubMed] [Google Scholar]
- 44.Sperber EF, Wurpel JND, Moshé SL. Evidence for the involvement of nigral GABAB receptors in seizures in rat pups. Dev Brain Res. 1989;47:143–146. doi: 10.1016/0165-3806(89)90117-x. [DOI] [PubMed] [Google Scholar]
- 45.Velíšková J, Garant DS, Xu S-G, Moshé SL. Further evidence of involvement of substantia nigra GABAB receptors in seizure suppression in developing rats. Dev Brain Res. 1994;79:297–300. doi: 10.1016/0165-3806(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 46.Ravizza T, Galanopoulou AS, Velíšková J, Moshé SL. Sex differences in androgen and estrogen receptor expression in rat substantia nigra during development: an immunohistochemical study. Neuroscience. 2002;115:685–696. doi: 10.1016/s0306-4522(02)00491-8. [DOI] [PubMed] [Google Scholar]
- 47.McEwen BS. Permanence of brain sex differences and structural plasticity of the adult brain. Proc Natl Acad Sci U S A. 1999;96:7128–7130. doi: 10.1073/pnas.96.13.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyrozis A, Chudomel O, Moshé SL, Galanopoulou AS. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci Lett. doi: 10.1016/j.neulet.2005.12.018. 2006 Mar 13 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184:1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- 50.Loddenkemper T, Pan A, Neme S, Baker KB, Rezai AR, Dinner DS, Montgomery EB, Jr, Luders HO. Deep brain stimulation in epilepsy. J Clin Neurophysiol. 2001;18:514–532. doi: 10.1097/00004691-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Velíšek L, Velíšková J, Moshé SL. Electrical stimulation of substantia nigra pars reticulata is anticonvulsant in adult and young male rats. Exp Neurol. 2002;173:145–152. doi: 10.1006/exnr.2001.7830. [DOI] [PubMed] [Google Scholar]