Abstract
Two experiments examined how different frustration contexts affect the instrumental and emotional responses of 4- to 5-month-old infants. Three different frustrating contexts were investigated: loss of stimulation (extinction), reduction in contingent stimulation (partial reinforcement), and loss of stimulus control (noncontingency). In both experiments, changes in arm activity and facial expressions of anger and sadness coded according to the Maximally Discriminative Facial Movement Coding System (MAX) were the measures of frustration. Both experiments showed that (a) arm responses increased when the contingent stimulus was lost or reduced but decreased when control of the stimulus was lost under noncontingency, (b) MAX-coded anger, but not MAX-coded sad or blends of anger and sad, was associated with frustration, and (c) the pattern of anger and arm responses varied with the frustration context. When contingent stimulation was lost or reduced, both anger and arm responses increased, but when expected control was lost under noncontingency, arm responses decreased while anger increased.
Infants’ responses to distinctive eliciting contexts have a long history in the assessment of emotions during the 1st year of life. For example, tickling, having the infant taste lemon juice in contrast to sweet and bitter solutions, sudden violations such as that produced by a jack-in-the-box, the entry of a masked stranger, arm restraint, or removal of a teething biscuit have all been used to elicit facial expressions in young infants (Bendersky, Sullivan, Alessandri, & Lewis, 1995; Braungart-Reiker & Stifter, 1996; Charlesworth, 1969; Fox & Davidson, 1986; Granchrow, Steiner, & Daher, 1983; Kochanska, Coy, Tjebkes, & Husarek, 1998; Rosenstein & Oster, 1988; Scarr & Salapatek, 1970; Skarkin, 1977; Sroufe & Wunsch, 1972; Steiner, 1979; Stenberg, Campos, & Emde, 1983). These situations represent only a sample of the various contexts that have been devised. Studying facial expressions and behavior in varied contexts (a) can demonstrate whether there are relatively unique sets of facial expressions for a given context and whether the same expression serves different functions across contexts and (b) allows some confidence about the “signal value” of infant facial expressions (Bennett, Bendersky, & Lewis, 2002; Lewis & Michalson, 1983). Examination of facial expressions across a variety of well-controlled and systematically varied contexts should also help to clarify issues related to infant facial expressions and their organization early in development.
The literature on infant facial expression has many problems (Lewis & Michalson, 1983). First is the issue of emotional expression as an index of emotional state. Others are the relation between emotional expressions and experiences, the connection between emotional state and experience, and finally, the relation between specific elicitors and facial expressions. Differential emotions theory (DET; Izard & Ackerman, 2000; Izard et al., 1995) suggests a direct one-to-one correspondence between facial expressions and emotional states as well as a direct connection between expressions and experiences. The theory also proposes that certain elicitors are linked to specific expressions.
Lewis and Michalson (1983) argued that expressions, states, and experiences undergo developmental change and that the connections or disconnections among them arise as a function of social development and learning. These authors, as well as those who take a functional approach to emotion (e.g., Campos, Campos, & Barrett, 1989), theorized that a one-to-one correspondence between emotional state, expression, and emotional experience is unlikely. Lewis and Michalson specifically proposed that the relation of states to facial expressions varies with the age and/or temperament of the infant and with the particular emotion. Learning in context and experience are seen as critical in determining which emotional behaviors and expressions are observed, their correspondence to states, and how each changes over time. Camras (1992, 1994; Camras, Malatesta, & Izard, 1991) addressed a portion of this problem, namely, the relation between specific elicitors and specific facial expressions. Using a dynamical systems theory (DST) perspective, Camras also proposed that expressions, like other forms of motor behavior, are “softly assembled,” such that no single facial expression ever characterizes a specific emotion. DST is a complex theory offering many hypotheses about the organization of facial expression components, most of which have yet to be tested empirically. It predicts, for example, that movements of various regions of the face might not initially cohere as “whole face” expressions. Instead, expressive components may become gradually coordinated, emerging as stable, recognizable expressions in a given context. This stable “attractor” facial pattern emerges when a critical variable, termed a “control parameter,” changes and “leads to corresponding changes . . . in the collective behavior of the system,” creating rapid, discontinuous appearances in a given motor pattern (Thelen & Ulrich, 1991, p. 11). In short, DST hypothesizes that the expressions observed depend on the interaction between the central nervous system, coordinated motor components, and environmental circumstances.
Because extant data challenge DET’s assumptions of expression specificity and because there is as yet no conclusive evidence of a one-to-one correspondence between infant expressions and underlying, distinctive emotional states or experiences, in this article we treat expressions in their own right, independent of any assumption about the quality of an underlying state or experience. We are concerned here primarily with the coherence between behavior and expression in context. Whether and when particular facial expressions occur, an analysis of the contexts in which they occur, and how contextual variation affects them are important for an understanding of the organization and signal value of infant expressions in and of themselves. It is in context that the meaning of expressions is discerned and responded to by others (Lewis & Michalson, 1983).
In this article, we focus primarily on frustration contexts for several reasons. First, such contexts have been widely studied in human development. Second, an existing theoretical model proposes that multiple negative responses always occur in initial response to a given frustration context (Berkowitz, 1989). Accounting for multiple expressions within a given context is a problem for DET, which allows for multiple expressions, blends, and intersubject variability within a context but does not propose mechanisms (apart from constitutional differences) for how or why varied expressions might arise. Third, frustration contexts can be defined empirically and manipulated experimentally in order to study the consequences of that variation on facial expressions, behavioral actions, and the relation between them.
Frustration contexts are those in which the expected access to goals or rewards is violated in some way. Such violations usually result in anger (Averil, 1982; Ben-Zur & Bernitz, 1991; de Rivera, 1991; Eisenberg, Fabes, & Nyman, 1991). In young infants, the violation of expected reward also has been linked with the appearance of anger expressions. The withdrawal of an expected reward during a brief extinction period has been shown to frustrate infants 2 to 8 months of age (Alessandri, Sullivan, & Lewis, 1990; Lewis, Alessandri, & Sullivan, 1990; Sullivan, Lewis, & Alessandri, 1992); that is, increases in a formerly contingent arm response and in anger expressions occur when an expected pleasant event fails to appear. Anger is the predominant expression, although low levels of the sad expression also can be observed. Infants apparently attempt to regain the contingent event by increasing their arm activity and show facial anger when they cannot. Although this expectancy violation hypothesis accounts for the observed behavior, it does not reveal what aspects of the violation lead to both increased arm responses and facial displays of anger. What contextual change causes the infant’s frustration response when a contingency is lost? Is it the loss of the expected event itself or the loss of control over its occurrence?
Lewis and colleagues (Lewis et al., 1990; Lewis & Goldberg, 1969), as well as Gunnar (1980), have argued that the critical aspect of a violated contingency is not the loss of the pleasant, rewarding event itself. Rather, it is the disruption of the infant’s expected control over the contingent stimulus. Almost any change in a contingency context might conceivably produce anger expressions. However, if perceived control is critical in triggering negative emotion, then loss of control alone should produce greater anger expressions than should other frustration contexts.
A second question is whether varying the frustration context affects infants’ display of other negative expressions. Other work (e.g., Camras et al., 1991; Oster, Hegley, & Nagel, 1992) has shown that many negative expressions co-occur when infants become upset or distressed. Blended expressions of anger and sadness are likely as well. The quality and pattern of these other negative expressions might also be affected by context. Some frustration contexts might be more likely to promote sad expressions or alter the relative distribution of sad and anger expressions, for example. Alternatively, because negative expressions are thought to be less well differentiated in young infants, both anger and sad expressions may show similar patterns within and across frustration contexts.
To address these issues, we designed two experiments to explore how the type of expectancy violation affects instrumental and expressive behavior. We examined three potentially frustrating contexts following contingency training: extinction, partial contingency, and noncontingency. Each of these contexts violates some aspect of the contingency learning context. As defined by Berkowitz (1989), potentially frustrating conditions must create either a reduction or a loss of expected reward in order to elicit negative affect. Each of the three contexts studied represents such loss or reduction. Moreover, because each also varies how the contingency is violated, each also represents a qualitatively different frustration context, as described below.
Contingency is a context in which a response consistently produces a particular outcome and that outcome only follows that response. Infants’ learning of the relation between the response and the outcome is indexed by a sustained increase in responding above baseline levels. When there is consistent exposure to a contingency context, infants presumably learn two aspects of the situation (Bower, 1997; Symons & Moran, 1994; Watson, 1979, 1985, 1997). As shown in Table 1, they learn to expect (a) that a particular action produces an outcome (i.e., pulling activity by the arm produces pleasant slides and music) and (b) that this outcome co-occurs only with that action. These two expectancies define the infant’s experience during the learning or “acquisition” phase. Several different frustration contexts can be contrasted with contingency learning and with each other by manipulating these two expectancies.
Table 1.
Definitions of Contingency and Frustration Contexts
| Context | Operational definition | Real-world significance |
|---|---|---|
| Contingency | (a) Outcome consistently occurs.
(b) Outcome occurs only with arm movements. |
It responds to me. |
| Frustration | ||
| Extinction | (a) Arm responses no longer work but I don’t know why.
(b) Outcome never occurs. |
It stopped. |
| Partial | (a) Outcome occurs consistently one third of the time.
(b) Outcome always follows an arm pull but not other times. |
It’s not responding as much. |
| Noncontingency | (a) Outcome no longer follows arm responses.
(b) Outcome always occurs independent of arm responses. |
It’s not responding to me. |
When infants subsequently receive an extinction, no stimulation occurs. In this context, the expected outcome is completely lost and cannot be regained. When extinction is introduced, infants perceive that pulling does not result in the outcome and that the outcome will never occur. This process, just like initial learning, takes time. Once the uncertainty about the outcome’s occurrence is resolved, responding stops, or is “extinguished.” Extinction procedures in most infant learning studies are sufficiently brief so that full cessation of the response is rarely reached. Responding usually remains above baseline during these brief extinction periods, and this behavior serves as a useful index of immediate memory (Rovee-Collier, 1987). In some cases, responding actually may show an “extinction burst” such that responses increase at first. With prolonged or repeated extinction contexts, responding will decrease (Miller, 1996; Tarabulsy, Tessier, & Kappas, 1996). In other words, extinction is a context that violates an expected outcome, but it does not immediately violate the expected control of that outcome.
When infants experience partial contingency, control of the outcome is only reduced, not lost. The response that controls the outcome remains unchanged, but the expected outcome is obtainable at a lesser rate. For example, in a partial contingency (or partial reinforcement schedule), a contingent outcome might occur once for every third arm response, but not at other times. Thus, the expectancy about the stimulus dependence of arm responses is less strongly violated in this context than in extinction.
A full noncontingency experience provides yet another potential frustration context. In this case, the contingent event still occurs but is now independent of the infant’s activity. During noncontingency, control of the outcome is lost, but not the outcome itself, as happens in extinction. The independence of the stimulus from the arm response confirms that the stimulus is no longer under the infant’s control.
Little is known about the emotional responses of infants to noncontingency and partial contingency. Although noncontingency has been studied in infancy and has been associated with negative emotional behavior (DeCasper & Carstens, 1981; Dunham & Dunham, 1990; Fagen & Rovee, 1976; Gunnar,1980; Hains & Muir, 1996), partial contingency has received relatively little attention in the infant literature, probably because it is unlikely to lead to rapid learning (Weir, Toland, & King, 1998). However, because it represents a reduction in reward, partial contingency is a potential frustration condition—one that stresses the infant’s perceived control of the stimulus (Amsel, 1962; Scull, 1973).
We proposed that each of these three frustration contexts would differentially affect infants’ behavioral responses and facial expressions. Because earlier work (Lewis et al., 1990; Lewis, Sullivan, Ramsay, & Alessandri, 1992; Sullivan et al., 1992) demonstrated that arm responses, as well as angry expressions, increased during a 2-min extinction, arm responses, facial expressions, and the relation between expressions and behavior are the main focus of the present analyses. On the basis of previous findings with the extinction context, we expected anger to be the predominant emotion and the one most sensitive to contextual variation. We proposed three alternative hypotheses accounting for variation in arm responses and anger across the three contexts:
If loss of control underlies the frustration response in learning contexts, the greatest anger and arm response should occur in response to noncontingency because of the sudden complete loss of control. Partial contingency and extinction represent a reduction in the expected rate of the stimulus and the loss of the stimulus, respectively, and should be less frustrating.
If loss of the stimulus alone underlies the frustration response, then extinction should produce the most frustration and noncontingency the least. Extinction, the most severe loss of stimulation, should be the most frustrating because the expected outcome never occurs and there are no cues on how to reinstate it. Partial contingency, or a reduction in stimulation, should produce much milder frustration by comparison. In noncontingency, because the outcome is still available and continues at the same level as during training, there should be no frustration.
If only expectancy violation underlies frustration, changing the contingency in any way should be sufficient to produce the frustration response, and the specific context should not matter. All contexts should be equally frustrating because all thwart access to an expected and presumably rewarding outcome. In this case, frustration can be understood as a generic response to any unexpected context change.
In Experiment 1, anger and sad expressions (coded with the Maximally Discriminative Facial Movement Coding System [MAX, Revised; Izard, 1995]) and pulling in response to the three frustration contexts were examined after 4 min of contingency training. In Experiment 2, a longer training period was used in order to assess the stability of the findings in 4½- to 5½-month-old infants. We expected that the same frustration responses would occur as long as infants learned the contingency, regardless of the learning time. We also examined differences in MAX-specified anger, sadness, and anger/sadness blends more closely in Experiment 2. In addition, surprise/interest expressions were examined to determine the degree to which the various frustration conditions led to disengagement from the context.
Experiment 1
Method
Participants
Sixty-eight full-term, healthy infants born in a university-affiliated hospital participated in the study. The mean age of the subjects was 21 weeks (± 2 weeks). Equal numbers of male and female infants were seen. Infants were randomly assigned to one of three frustration groups (extinction, n = 24; partial contingency, n = 19; and noncontingency, n = 25). The groups did not differ in age or gender distribution.
The infants were predominantly White and of European ancestry, but the sample included African American (8%), Hispanic American (8%), and Asian American (5%) infants.1 Mothers were approached on the maternity ward shortly after delivery and asked if they could be contacted subsequently for participation in the study. Approximately 60% of all eligible births were sampled, with a high percentage of mothers agreeing to subsequent contact.
Procedure
The contingency procedure has been described in detail elsewhere (Bendersky et al., 1995; Lewis et al., 1990; Lewis, Sullivan, & Brooks-Gunn, 1985). Infants were seated in an infant seat, facing a rear-projection screen. A colored slide of a happy baby, accompanied by music lasting 3 s, appeared during the procedure consistent with experimental phase and group assignment. A video camera, mounted below the screen, recorded infants’ facial expressions during the entire procedure. Infants wore an elastic bracelet attached to a ribbon. Pulling on the ribbon closed a switch, recording responses on the laboratory computer. The computer also controlled the timing and delivery of the slide and music for the various phases and conditions as follows:
All infants received 2 min of baseline training and 4 min of contingency training in which pulling with the right arm resulted in slides and music. Ribbon tension was set so that hand-to-mouth movement was insufficient to activate the outcome. The frustration period immediately followed the initial contingency training and lasted 2 min. Thereafter, there was a reinstatement of the initial contingency for all participants. Thus, infants were treated exactly the same during baseline and contingency learning. During the frustration phase, however, groups differed in how the frustration context was accomplished.
During frustration, the relation between the contingent event and arm responses was disrupted in one of three ways. The slide and music either did not occur (extinction), occurred at a reduced rate of one onset for every third arm response (FR3 [FR = fixed reinforcement] or 33% partial contingency), or occurred at the expected rate but independent of arm activity (noncontingency), as described in Table 1. The rate of noncontingent presentation was determined by the infant’s response rate in the final 2 min of the contingency phase.
Response criterion
To ensure that individual infants had actually increased their arm responses and remained above baseline during the contingency phase, we applied a minimum response criterion to each infant’s learning data before examining facial and behavioral responses to frustration. This criterion was used because we wished to assess emotion related to violation of expectancy. If negative emotion depends on the violation of a learned expectancy, then demonstrating a minimal level of contingent response during the learning phase is critical. Not all infants who are exposed briefly to contingent stimulation will exceed baseline levels of responding, nor will any increase in responding that is due to sensitization or arousal unrelated to contingency learning be sustained. Mean learning data obscure such within-session response variability during learning (McSweeny, Hinson, & Cannon, 1996). Infants who exceeded the criterion were considered to have responded to the contingency appropriately; infants who were below the criterion were considered to have not responded appropriately. Because the latter infants were unlikely to have learned to expect anything, they should not be frustrated by a change in the occurrence of an event. These infants were retained as an arousal control group indexing changes in emotion over time in the absence of demonstrable learning. These infants actually make the test of expressions in context more stringent, because the analysis tests whether expressions in the frustration contexts are greater than any negative expressions that occur when infants simply remain in the procedure for the same amount of time in the absence of a perceived contingency.
The criterion was sustaining an average pulling rate of 15% or more above the average baseline response for the entire 4-min learning phase (baseline × 1.15). The minimum response criterion was derived from continuous learning curves for 4- to 6-month-olds in this procedure (Lewis et al., 1985) and was confirmed in pilot data. The pilot data showed that infants who responded at this level were likely to remain consistently above baseline throughout the learning phase and to exceed the criterion for 3 out of 4 min. Thus, the criterion ensured that infants’ responding was consistently above the baseline level during the contingency period. Because the criterion assessed minimum response levels, maximum (peak) and final learning levels of infants in this paradigm were typically greater. Infants who met the minimum criterion were also likely to meet a commonly accepted learning criterion of contingency mastery, that is, 1.5 × baseline for 2 consecutive minutes (Hartshorn et al., 1998).
Frustration measures
The two aspects of the frustration response assessed were changes in arm response and changes in facial expression. Both were examined because the different frustration conditions might have affected the relation between arm responses and expressions. For example, both arm responses and anger expressions were expected to increase during extinction.
The first measure of frustration was the change in mean arm response observed between the frustration and learning periods. Arm responses were averaged over the 4-min learning and the 2-min frustration periods to yield a rate of pulling per minute. The average arm response rate during the frustration period was subtracted from the average arm response rate during the contingency learning period to yield a frustration score.
The second measure of frustration was the frequency per minute of anger and sad facial expressions. The duration of expressions was not scored because there was no reason to expect differences in their duration by frustration context. Facial movements of anger and sadness were coded second by second from videotapes with the MAX coding system (Izard, 1995). Expressions were coded only when the infant’s head was within 45° of forward facing. Raters were blind to the hypotheses of the experiment and scored the tapes without knowing which frustration group was being coded. Only anger and sadness occurred with sufficient frequency during the frustration period to permit analysis. Blends of anger and sadness were not scored in this study. As in previously reported work, expressions of disgust, contempt, shame, and physical pain occurred with such low frequency that analysis of them was precluded (Lewis et al., 1990). Consequently, the occurrence of MAX-specified anger (25–33–54/55/0) and sadness (23–33–56/0) constituted the measures of negative emotion. Because the study was designed to elicit frustration, few positive emotion expressions were anticipated. A dearth of positive emotions during extinction, with the exception of interest, was consistent with findings from previous work, as were the levels of anger and sadness expressions (Lewis et al., 1990).
Reliability
Coders trained with MAX on slides and previously coded videotapes from earlier studies until they reached an intercoder agreement of 80% or better and demonstrated better than chance agreement (kappa) on the expressions. Interrater agreement for scoring MAX anger and sad codes was checked with a random sampling of 25% of the tapes. Interclass correlations for single, randomly assigned raters were used to index the proportion of coder variance attributable to actual behavioral variance (Shrout, 1995). This method was used because the frequency of particular expressions was of interest. Intercoder agreement on expressions met or exceeded training-level agreement on study tapes: .94 for MAX anger and .93 for MAX sad.
Results
We present the learning data first to identify those infants who responded appropriately to the contingency in terms of rates of pulling and to establish that the number of subjects who met the minimum criterion did not differ by frustration context assignment. Because frustration was predicated on having learned the contingency, we needed to observe that learning occurred and occurred similarly across infants in each of the frustration contexts.
Arm activity during learning
Overall, there was a 64% increase in arm responses above baseline during learning with no differences by frustration group, as expected. When the minimum criterion was applied to the response data of individuals, 37% of the sample exceeded the minimum response criterion during the learning phase; that is, they exceeded baseline by an average of at least 15% during the 4-min contingency period. The number of subjects who exceeded criterion also was similar in each frustration group: extinction, 10; partial contingency, 7; and noncontingency, 8. There were 13, 15, and 14 nonlearners in the extinction, partial contingency, and noncontingency frustration groups, respectively. On average, infants meeting the criterion achieved a response rate that was more than three times their base rate (M = 3.03, SD = 1.09) at the end of the contingency phase. In contrast, nonlearners were not significantly above baseline in the final 2 min of learning (M = 0.78, SD = 0.23).
The criterion was neither too easy nor too difficult and compared favorably with other commonly used indices of contingency mastery (Hartshorn et al., 1998). For example, all of the subjects meeting the current criterion sustained response increments at least 25% above baseline for at least 2 consecutive minutes during the contingency phase, and 90% were at least 50% above baseline in any 2 consecutive minutes. In contrast, only 1 nonlearner had 2 consecutive minutes in which responding exceeded 25% of baseline, and these occurred in the first 2 min; this was followed by below-baseline responding in the final 2 contingency minutes, suggesting habituation. Maximum response rates attained also compare favorably with data previously reported by Alessandri et al. (1990). Thus, the criterion discriminated infants who exhibited increases in responding over 4 min of contingency learning from infants who remained in the session for an equivalent amount of time without stable increases. Those infants not meeting the minimum response criterion served as a control for changes in emotion over time in the procedure during the frustration phase. Infants who did not learn the contingency between their response and the outcome logically cannot be frustrated by the removal or alteration of this relation. The negative expressions of learners and nonlearners should differ because the eliciting context is different.
To concretely illustrate the difference between the learners and nonlearners, Figure 1 shows the arm response data averaged in consecutive 2-min learning blocks for both learners (left panel) and nonlearners (right panel) broken down by frustration group. Two-minute averages were used for the phase comparisons because there were no significant minute-to-minute changes during learning. The figure shows that learners remained above baseline in the second 2-min block, whereas nonlearners did not, F(1, 62) = 39.09, p < .01.
Figure 1.
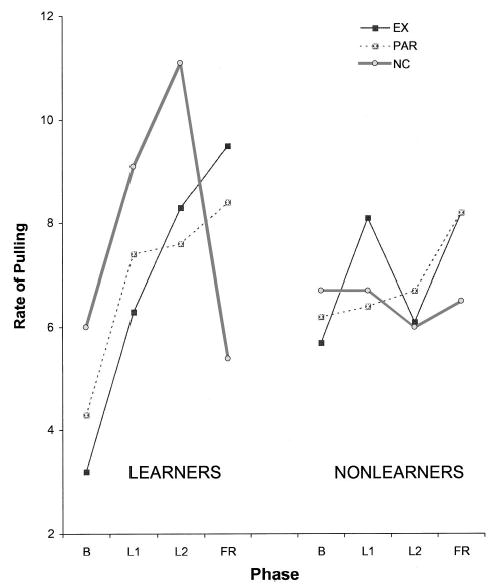
Arm responses over learning time in learners and nonlearners. Learners had a mean response at least 15% above baseline over 4 min. EX = extinction; PAR = partial contingency; NC = noncontingency; B = baseline; L1 = first 2-min learning block; L2 = second 2-min learning block; FR = frustration.
Most important, learners showed no differences by frustration condition in their response increments during the learning phase (extinction, M = 3.27, SD = 2.31; partial, M = 2.50, SD = 1.50; noncontingency, M = 3.23, SD = 1.70). We are therefore confident that among learners, there was no difference in learning at the end of the contingency phase of the experiment.
Arm activity in response to frustration
Figure 1 also shows the change in average arm response during frustration for the learners and the controls. Averages are reported because there was no change over the 2 min of frustration, as is typical in this procedure (Alessandri et al., 1990; Sullivan et al., 1992). Among the learners, the extinction and partial contingency groups increased their pulling above the learning level, whereas the non-contingent group decreased their pulling to below baseline levels. Among the controls, all three groups showed little change as a function of frustration. The change in pulling shown during frustration was subjected to a Context (3) × Criterion Group (2) analysis of variance (ANOVA), and the two-way interaction was confirmed, F(2, 62) = 3.0, p < .05. Follow-up one-way ANOVAs within each group indicated that pulling differed by context only among the learners, F(2, 21) = 6.50, p < .01.
Among the learners, the extinction context showed the expected increase in response rate (M = 2.15, SE = 1.1), an increase similar to that reported in earlier work for 4- to 6-month-olds (M = 2.80; Alessandri et al., 1990; Sullivan et al., 1992). In contrast, infants in the partial contingency context showed little change in pulling response (M = 1.14, SE = 1.1), and those in the noncontingent context showed a sharp decrease (M = −4.72, SE = 2.1). Planned contrasts indicated that infants in the noncontingent context decreased pulling significantly relative to those exposed to the other two contexts (p < .05), but the extinction and partial contingency contexts were not significantly different from one another.
Negative expressions
The means and standard errors for MAX-specified anger and sad expressions during the frustration phase for learners and nonlearners are shown in Table 2. Anger and sad expressions could not be examined by ANOVA because the sample was small and the data were not normally distributed. Mann-Whitney U tests were used instead to examine the frequency of MAX-coded anger and sad expressions by frustration context. Since little or no negative emotion occurred during the initial learning phase (i.e., anger and sad expressions were practically at zero level), difference scores were not calculated because they would be equivalent to the amount observed during frustration (see also Lewis et al., 1990).
Table 2.
Means (and Standard Errors) of Negative Expressions During Three Types of Frustration
| Frustration context
|
|||
|---|---|---|---|
| Expression | Extinction | Noncontingent | Partial |
| Anger | |||
| Learners | 4.31 (1.9)a | 8.89 (2.7)b | 2.00 (1.1)a |
| Nonlearners | 1.29 (1.8)c | 1.63 (0.54)c | 4.10 (0.98)c |
| Sadness | |||
| Learners | 0.47 (0.22)d,e | 2.09 (0.69)e | 0.96 (0.57)d |
| Nonlearners | 0.54 (0.25)d | 0.95 (0.55)d | 1.18 (0.76)d |
Note. Scores within rows or columns with different subscripts are significantly different from one another by Mann–Whitney U test (two-tailed probability, corrected for ties).
Anger
The data show that the learners in the extinction group increased their anger expressions, a result replicating previous work. Learners and controls showed different levels of anger expression (U = 9.0, p < .05). Among the learners, infants in the noncontingent context showed more anger expressions than those in the partial and extinction contexts (Us = 8.0 or more, ps < .05). The levels of anger expressions in the partial and extinction contexts were similar. Lewis and colleagues (Lewis et al., 1990; Sullivan et al., 1992) reported a mean level of anger expressions equivalent to the mean level of the learners observed here. Among controls, there were no differences by frustration context, as expected.
Sad expressions
There also were more sad expressions among learners than among controls (U = 20.0, p < .05). Among learners, the only significant difference was observed between the extinction and noncontingent contexts. Infants in the noncontingent context showed more sad expressions (U = 13.5, p < .05).
Discussion
The results of Experiment 1 were consistent with the hypothesis that how infants are frustrated affects their responding. Differences observed in the frustration responses (instrumental pulling and negative facial expressions) were specific to having learned the contingency because the control groups failed to show frustration responses. The findings of Lewis and colleagues with respect to extinction were replicated (Alessandri et al., 1990; Lewis et al., 1990; Sullivan et al., 1992). A distinctive pattern of responses emerged in response to noncontingency. When control was violated during noncontingency, there was more total negative expression than in either the extinction or the partial contingency contexts. More important, noncontingency following contingency resulted in less arm response. Negative emotion and decreased instrumental responding in infants exposed to noncontingency have been described by others (DeCasper & Carstens, 1981; Seligman, 1975), but our results suggest that both the loss of control (noncontingency) and the loss of outcome (extinction) led primarily to anger expressions. Shifting infants to partial contingency after 4 min of contingency resulted in only slightly more anger expressions and little change in either pulling or sad expressions. Because noncontingency resulted in the highest levels of anger and sad expressions, loss of control appeared to elicit the greatest frustration response.
A limitation of the data was the small percentage of subjects who met the minimum criterion of sustained responding during the 4-min learning phase. In order to allow more infants to meet the criterion, we replicated the experiment using a longer learning phase to allow infants more time to reach criterion. In addition, we coded a wider range of emotion expressions during the frustration period. In addition to anger and sadness, we examined blends of anger and sadness because these commonly occur in situations in which discrete anger and sadness expressions are observed (Camras, 1992; Matias & Cohen, 1993). We also examined surprise. Although the validity of MAX-coded surprise has been challenged (Camras, Lambrecht, & Michel, 1996; Michel, Camras, & Sullivan, 1992), this expression is consistent with attention to the contingent outcome in our procedure and was scored separately as “surprise/interest,” as opposed to other MAX-coded interest forms. There were no specific hypotheses regarding variation in interest as a function of frustration condition. We hypothesized only that interest would decline during frustration as infants became angry. Enjoyment was not scored because this expression occurs at very low frequency during initial contingency learning (Lewis, Sullivan, & Michalson, 1984; Sullivan, Rovee-Collier, & Tynes, 1979).
Experiment 2
Method
Participants
Seventy-seven full-term infants (40 boys and 37 girls) were recruited for study and randomly assigned to the three frustration groups. The mean age of the infants was 17 weeks (± 2 weeks). The ethnic diversity of the sample was similar to that in the first study (11% African American, 4% Hispanic ancestry, 7% Asian American, and 3% mixed ancestry). Eight additional infants were excluded from the study. Four were untestable (i.e., cried continuously during baseline), and their mothers were unable to reschedule. Four more were excluded for the following reasons: crying continuously for more than 60 s when the contingency began (2), poor quality video (1), or equipment malfunction (1). Application of the learning criterion to the data identified 47 infants, or 61% of the sample, as learners, a larger number than in Experiment 1.
Procedure
The procedure differed from that of Experiment 1 only in that the contingency learning phase lasted 6 min instead of 4 min. So that it corresponded with the longer training period, the criterion was adjusted to require a minimum increment of 15% across all 6 min. This still required that the average response remain above baseline throughout the learning phase despite the additional learning time. The learners were distributed as follows: extinction, 14; partial, 14; and noncontingency, 19. The non-learner/controls were distributed as follows: extinction, 10; partial, 11; and noncontingency, 9.
Frustration measures
Both arm responses and facial expressions were again scored. In Experiment 2, anger, sadness, as well as blends of anger and sadness (25–33–56 or 23–33–54 or 55) were scored.2 MAX-coded surprise/interest expressions were scored when the infant’s head and eyes were level and facing directly forward, as recommended by Camras et al. (1996). MAX surprise/interest was scored as the combination of MAX codes 20–30, and either 50 or 0 in the mouth area. Other forms of MAX interest, including MAX upper face codes 20–30 with any of the other various MAX mouth codes designating interest, including code 51, were excluded. Examples of all the facial expressions scored appear in Figure 2. The arm response rate was calculated over 2 min, as in Experiment 1. The average frequency of anger expressions per minute was computed across the final learning blocks (4 min) and the 2-min frustration phase.
Figure 2.
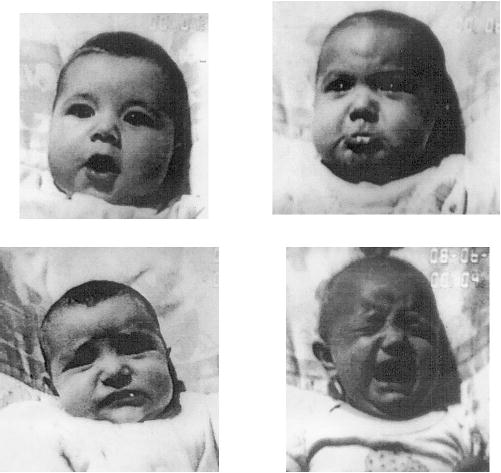
Examples of MAX-coded (Maximally Discriminative Facial Movement Coding System [Izard, 1995]) facial expressions taken from videotape: surprise/interest (top left), anger/sad blend (top right), sad (bottom left), and anger (bottom right).
Reliability
Emotion expressions were coded by a different set of coders than in Experiment 1; however, identical training and preliminary reliability assessment were provided in order to ensure equivalence in coding methods. Initial reliability was established at the level of .85 interobserver agreement or better, with kappas for the individual expressions ranging from .69 for surprise/interest to .93 for sadness. All kappas indicated above-chance agreement. Reliability was monitored subsequently by having the two coders score 25% of the tapes. Reliability was uniformly high for all expressions. Interrater reliability coefficients using interclass correlations for single raters (Shrout, 1995) were as follows: anger, .94; anger/sad blends, .85; all other anger blends, .85; sad, .95; and surprise/interest, .87.
Results
Arm activity during learning
Analyses of the infants’ responding during the learning phase established that there were no differences in arm responses by either gender or frustration context during the learning phase. However, over all subjects, there was a significant increase in pulling from the baseline response in the first learning block, F(1, 77) = 29.30, p < .04. Arm responses were more than twice the baseline level at the end of 6 min of training (M = 2.03, SD = 2.68).
As in Experiment 1, the number of infants meeting criterion compared well with commonly used contingency mastery criteria. We found that 90% of infants responded at least 50% above baseline for at least 2 consecutive minutes (Hartshorn et al., 1998), and all exceeded 25% of baseline in the final 2 min. Only 2 infants who did not meet the criterion met the Hartshorn et al. (1998) criterion (50% above baseline in Minutes 2–3 or Minutes 3– 4, respectively), but they responded below baseline during 3 of the 4 remaining minutes. Thus, the minimum criterion again provided a valid method for discriminating among infants who consistently maintained response rates above their baseline rates throughout the session. Those who did not meet the criterion were again considered controls for context-specific expressions during frustration.
Figure 3 shows arm responses for learners and controls (non-learners) averaged over successive 2-min contingency learning blocks so that the data can be compared with the data from Experiment 1. Learners showed the expected change over the three contingency learning blocks, but controls did not. Rates of pulling per minute were comparable to those of Experiment 1 during the second learning block and were sustained at or above this level during the additional 2 min of Experiment 2.
Figure 3.
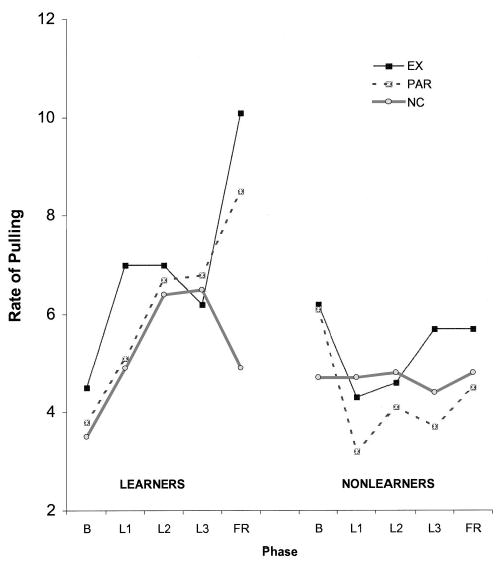
Arm responses averaged over 2-min blocks during learning and frustration among learners and nonlearners. Learners had a mean response 15% above baseline over 6 min. EX = extinction; PAR = partial contingency; NC = noncontingency; B = baseline; L1 = first 2-min learning block; L2 = second 2-min learning block; L3 = third 2-min learning block; FR = frustration.
The average increments in arm responses attained by learners and controls during contingency learning differed, with learners again tripling their response on average (M = 3.03, SD = 1.97) and controls responding below baseline as in Experiment 1 (M = 0.76, SD = 0.27). We concluded that the contingency was detected by a majority of the infants, that the learners and non-learner/controls differed significantly on every learning measure considered, and that arm rates did not differ significantly among the randomly assigned frustration contexts at the end of learning.
Arm activity in response to frustration
Figure 3 also shows the arm response during frustration by learning group and frustration condition. There were main effects of phase, F(1, 71) = 13.64, p < .01, and criterion group, F(1, 71) = 19.96, p < .01, on the rate of pulling. Infants exposed to extinction and the partial contingency increased arm responses during the frustration phase, whereas infants exposed to noncontingency decreased their pulling. For the controls, there was little change during the frustration phase and no condition differences, as expected. Learners exposed to extinction, partial contingency, and noncontingency during the frustration phase responded significantly above baseline (for all ts, p < .03), whereas none of the controls did. Thus, changes in responding that were due to the change in context occurred only in subjects who learned, which replicates the results of Experiment 1. That is, prior learning was necessary to observe a response when the frustration contexts were introduced.
To follow up, we conducted separate Phase × Condition multivariate analyses of variance (MANOVAs) for learners and controls. For learners, a significant Phase × Context effect was obtained, as expected, F(2, 47) = 4.77, p < .01. In the extinction and partial contingency contexts, infants pulled at similar rates during the frustration phase, but infants in the noncontingent context showed a decrease in pulling relative to infants in the extinction context. In contrast, controls showed no significant change over phase and no differences by context.
To confirm that the change in pulling during frustration differed by frustration context, we examined the change in arm response of learners during frustration (frustration block minus the final 2 min of contingency) in a one-way ANOVA with planned contrasts. There was no difference in the change in arm response rate observed between infants in the partial and extinction contexts. Infants exposed to extinction and partial contingency increased pulling significantly more than did infants exposed to noncontingency: t(44) = 2.17, p < .04, and t(44) = 1.85, p < .08, respectively.
Expression differences
To establish whether expressions changed with the shift from the learning phase to the frustration phase, and whether phase changes interacted with the frustration context and/or learning status, we conducted a MANOVA with repeated measures over expressions (MAX anger, MAX sad, anger/sad blends, and MAX surprise/interest) and phase (2) with between-subjects factors of frustration context (3) and criterion group (2). We observed significant differences in expression, F(3, 72) = 7.48, p < .01. Overall, MAX anger was more frequently expressed than was either MAX sad or anger/sad blends, F(2, 70) = 29.61, p < .01, and was observed more frequently than were these two expressions combined, F(1, 55) = 55.02, p < .01.
We also observed an Expression × Phase interaction, F(3, 72) = 3.63, p < .02, Wilks’s criteria. Within-subjects average Fs also indicated a significant Expression × Learning Criterion interaction, Greenhouse–Geiser F(3, 222) = 4.45, p < .02. Table 3 shows the means for MAX anger, MAX sad, anger/sad blends, and surprise/interest over the final 2 min of learning and the frustration phase for learners and nonlearners (controls), collapsed over the frustration context. MAX anger was greater among the learners than among the nonlearners, F(1, 71) = 5.43, p < .02, but none of the other negative expressions differed between learners and controls.
Table 3.
Phase Changes in Expressions in Response to Different Frustration Contexts
| Phase
|
|||
|---|---|---|---|
| Expression | MAX coding | Learning | Frustration |
| Anger | 25–33–54/55/0 | ||
| Learners | 2.45 (2.18)a | 4.22 (4.12)b | |
| Nonlearners | 1.70 (2.49)a | 2.18 (2.55)a | |
| Anger/sad blends | 25–33–56/23–33–54/55 | ||
| Learners | 0.81 (1.09) | 0.81 (1.22) | |
| Nonlearners | 0.39 (0.56) | 0.56 (1.54) | |
| Sad | 23–33–56/0 | ||
| Learners | 0.19 (0.65) | 0.30 (0.54) | |
| Nonlearners | 0.15 (0.41) | 0.39 (1.07) | |
| Surprise/interest | 20–30–50/0 | ||
| Learners | 1.40 (1.42)c | 0.78 (1.35)d | |
| Nonlearners | 1.90 (2.00)c | 0.98 (1.40)d | |
Note. Means (standard deviations appear in parentheses) within each set of rows or columns with different subscripts are significantly different at p < .05 or better using planned contrasts. The overall Phase × Expression interaction was significant by multivariate analysis of variance. For learners, n = 47; for nonlearners, n = 30. MAX = Maximally Discriminative Facial Movement Coding System (Izard, 1995).
Phase changes in negative expressions
Phase changes in the negative expressions also were significant in the overall MANOVA, F(1, 71) = 11.25, p < .01, and were greater among learners than controls, F(1, 71) = 3.12, p < .05. Table 3 shows that anger expressions increased the most during the frustration phase, whereas sad and anger/sad expressions did not. Anger expressions showed a greater linear increase from learning to frustration compared with the other negative expressions, which remained at low, relatively stable levels, F(1, 71) = 8.89, p < .01. Learners also displayed more negative expressions during frustration than did all the other groups, F(2, 71) = 3.12, p < .05. A significant Expression × Phase × Criterion Group effect confirmed that the phase change for anger expressions was greater among learners than among the nonlearner/controls, F(1, 72) = 6.02, p < .02.
Negative expressions during frustration
Table 4 shows all of the expressions by frustration context during the frustration phase for the learners and nonlearner/controls. To examine the mean differences in detail, we examined the negative expressions and surprise/interest separately by MANOVA, followed by planned contrasts. All mean comparisons used separate variance estimates when appropriate. As confirmed by the MANOVA, overall, learners and nonlearner/controls differed on anger expressions during frustration, but not on the other negative expressions. There were significant differences between learners and nonlearners exposed to noncontingency ( p < .05) but not between learners and non-learners exposed to extinction or partial contingency.
Table 4.
Means (and Standard Deviations) of Emotions Expressed per Minute in Response to Different Forms of Frustration in 5-Month-Old Infants
| Frustration context
|
||||
|---|---|---|---|---|
| Expression | MAX coding | Extinction | Noncontingent | Partial |
| Anger | 25–33–54/55/0 | |||
| Learners | 3.89 (4.26) | 4.21 (3.75)* | 4.53 (4.23)† | |
| Nonlearners | 2.60 (1.90) | 1.88 (1.88)* | 2.02 (2.91)† | |
| Anger/sad blends | 25–33–56/23–33–54/55 | |||
| Learners | 0.52 (0.89) | 0.63 (0.67) | 1.28 (1.81) | |
| Nonlearners | 0.78 (2.52) | 0.66 (0.79) | 0.25 (0.72) | |
| Sad | 23–33–56/0 | |||
| Learners | 0.30 (0.47) | 0.31 (0.66) | 0.29 (0.53) | |
| Nonlearners | 0.90 (1.20) | 0.22 (0.39) | 0.04 (0.14) | |
| Surprise/interest | 20–30–50/0 | |||
| Learners | 1.20 (1.46) | 0.30 (0.82) | 0.89 (1.10) | |
| Nonlearners | 1.10 (1.36) | 1.40 (1.05) | 0.45 (0.53) | |
Note. Learners (n = 47) and nonlearners (n = 30) differed significantly overall by multivariate analysis of variance ( p < .05). The two-way interaction was not significant. MAX = Maximally Discriminative Facial Movement Coding System (Izard, 1995).
Indicates pairs that differ at p < .08 (two-tailed).
Indicates pairs that differ at p < .05 (two-tailed).
As in Experiment 1, the mean level of anger expression in response to noncontingency (M = 4.21) was greater than that in response to extinction (M = 3.89) among the learners, but not significantly so. There were no significant differences in MAX sad expressions or anger/sad blends by frustration context and no interactions.
Surprise/interest
MAX surprise/interest showed a linear decline from the learning phase to the frustration phase, F(1, 72) = 17.90, p < .01. The decline was similar in both learners and controls. There were no effects of frustration context or any interactions.
General Discussion
Darwin (1872/1965) and others (e.g., Berkowitz 1989) have advanced the view that anger expressions and other vocal and bodily responses are the action patterns that occur when access to a goal is thwarted. Facial expressions of anger and increased instrumental responding occur when an expected event is disrupted, and this pattern occurs in animals as well as in children and adults (Amsel, 1962; Amsel & Roussel, 1952). The present experiments as well as earlier work (Lewis et al., 1990; Sullivan et al.,1992) have shown that when expected consequences are altered, increases in motor responses in human infants are accompanied by predominantly angry facial expressions. Moreover, the type of expectancy violation affects both instrumental motor action and emotion expressions somewhat differently. Anger expressions are elicited by any of the three types of contingency disruption (extinction, partial contingency, and noncontingency). As long as learning occurs, alteration in the contingency produces, for the most part, anger expressions. However, changes in the instrumental action of pulling depend on the specific form of contingency violation that defines the frustration context.
The decrease in pulling when infants receive a shift from contingency to noncontingent outcomes suggests that infants quickly perceive that their arm responses no longer have an effect. This is due most likely to the strong contrast between contingent and noncontingent events, that is, between being able to control the event and losing control of it. The loss of control has consistently been shown to have important effects on the relation of negative emotion to instrumental responding. Decreased instrumental responding and elevated negative expressions, observed in both of the present experiments during exposure to noncontingency, are consistent with previously reported results (DeCasper & Carstens, 1981; Dunham & Dunham, 1990; Fagen & Rovee, 1976; Hains & Muir, 1996). In most of the earlier work, however, negative emotions were indexed exclusively by crying. Our results, by including facial expressions, extend these findings and, more important, show a dissociation between facial expressions and instrumental pulling when noncontingent outcomes follow contingent ones. The dissociation of motor action and facial expressions when noncontingency was introduced was found in both experiments and was striking in comparison to the other two frustration contexts.
That there are, in general, more anger expressions in response to noncontingency than in response to extinction may be due to a hydraulic model of emotion expressions. If arm activity and anger expressions are both aspects of the anger response, a decrease in one response may lead to a corresponding increase in the other. There is some evidence for this process. For example, in the stranger approach situation, infants show more wary facial expressions when they are restricted to their infant seats than when they can freely move about the room (Lewis & Rosenblum, 1974). When infants are restricted motorically, the signal value of moving away from the stranger is negated, and so an increase in facial expression becomes more important. Infants who are allowed to move away from the stranger show less facial expression in this situation. The overall amount of negative arousal is relatively constant in both contexts but can be expressed differentially through the various responses. In the present case, when negative emotion was not expressed as arm activity, infants in the noncontingent group in both experiments showed more anger expressions. In contrast, infants who experienced the extinction context showed more arm behavior but less anger expression.
The results of these experiments also show the importance of contingency as a source of motivation and as a source of expectancies about the world. The change in the infants’ behavior when a contingent context shifts to a noncontingent one is similar to the change in infant behavior observed in the enface paradigm when maternal behavior shifts from contingent to noncontingent interaction (Bigelow, Maclean, & MacDonald, 1996). In both cases, the infant shows negative emotional behavior in response to this change, although anger faces have seldom been studied in the enface paradigm. It seems clear from a variety of studies that contingent control is an important contextual variable that affects the infant from the early weeks on.
Exactly how specific facial expression patterns become associated with specific contexts is still unresolved, and the present study was not designed to address this issue directly. Both DET and DST may find support in certain aspects of these data, but neither theory is fully supported. It is clear that the particular frustration contexts represented in these experiments elicit a particular set of facial expressions. For Darwin (1872/1965), as for DET, action patterns are thought to be innately connected to particular contexts. These data support a connection between expression and context, because frustrating events, such as extinction or noncontingency, do not produce positive facial expressions, like joy, but only negative ones.
How then might a connection arise between these contexts of expectancy violation and the negative expressions they elicit? Either, as Darwin (1872/1965) proposed, there are innate action patterns associated with a particular adaptation— overcoming an obstacle— or there is an association between action patterns and context that is learned early. In either case, by 4–5 months, violations of contingent expectations are already associated with negative facial expressions. It is clear from this study, as well as from other work on frustration, that only negative emotions are expressed when a goal is blocked. The data here do not resolve whether DET or DST offers a better account because infants seen 4–5 months after birth may have already had early experiences that serve to organize expressions, as proposed by DST. Observation of facial expressions in neonates in a contingency violation paradigm might resolve this question.
A related question pertains to how negative expressions are patterned. DET and DST differ with respect to this question because DET predicts that a particular facial configuration emerges as a discrete and fully formed display, whereas DST predicts that a particular facial configuration will come to predominate only as a critical value of one or more “control parameters” is reached (Camras, 1992; Thelen & Ulrich, 1991). There was no evidence in the present experiments to suggest that the pattern of expressions changed over time except in response to the shift in context from contingency to frustration. Although the data do not appear to support the DST view, to fairly test whether DST accounts for emergent anger expressions in response to frustration, a longitudinal study of change in the relative amounts of anger, sad, and anger/sad blended expressions during contingency learning and frustration is needed. How the distribution of these expressions changes in context over time and with repeated experience is an important area for study and is critical to assessing how well DST accounts for facial expression patterns. The data from both experiments do show that the quality of the prior contingency perception had an organizing effect on expressions because the negative expressions during frustration were less strongly differentiated among those who had not met the learning criterion than among those who had. Thus, the nature and quality of the experience that infants bring to the frustration context are important influences on their expressions, a finding with which most emotion theorists can be satisfied.
The findings on facial expression also have a bearing on the question of the type of negative expression that is elicited by the frustration. On the whole, the data are consistent with the infant expression literature in showing that sad expressions and anger/sad blends co-occur to some degree with anger, weakly supporting DST (Camras, 1992; Matias & Cohen, 1993). However, sad expressions and anger/sad blends do not increase in parallel with anger expressions when a frustration is introduced, as might be expected of loosely differentiated negative expressions. Because only 3% of the negative expressions in the present experiments were sadness alone, sadness was not a significant part of the frustration response, a finding that replicates past work on emotional responses to extinction (Lewis et al., 1990; Sullivan et al., 1992). Sadness and anger/sad blends occurred at similar levels in all three forms of contingency violation in Experiment 2. These findings suggest that anger expressions are already the predominant response to this context by 5 months.
The arm response data indicated that learning occurred but that not all subjects learned and that, except in the noncontingent group, increases in arm responses occurred when there was a change in the contingency. There were a few minor differences in arm responses between the experiments. These might have been due to the 2 min of additional contingency learning time in Experiment 2. For example, the partial contingency group increased responding during the frustration, as expected, in Experiment 2, whereas in Experiment 1, arm responses under partial contingency were not significantly different from those during initial learning. The additional 2 min of exposure to the contingency in Experiment 2 may have helped make the shift to partial contingency more salient.3 The increase in arm responses observed in Experiment 2 is the expected direct consequence of the higher response requirement of partial reinforcement (Reese, 1976). When partial contingency had the expected effect on arm responses, anger also increased as expected.
With regard to differences in emotion between the two experiments, both are consistent in showing that anger increases in response to both extinction and noncontingency. Infants exposed to noncontingency tended to express more anger in both experiments, although not significantly more in Experiment 2. A significant change in sad expressions in this group also was not replicated in Experiment 2. The mean amounts of both anger and sad expressions in response to noncontingency were greater in Experiment 1 than in Experiment 2, but in Experiment 1, the sample size was smaller and expression levels during learning were lesser. Thus, these minor differences might be due to any number of factors and cannot be attributed exclusively to the 2-min difference in contingency exposure, and firm conclusions about the source of these minor differences are not possible. Nonetheless, the findings of an increase in anger expressions in response to violation of contingency and of a difference in the relation between instrumental and expressive behavior in response to noncontingency were consistent across the experiments.
The clearest finding to emerge from these experiments is that by 5 months of age, expectancy violation is a frustration context associated predominantly with anger expressions. Although a few behaviorists still reject the expectancy construct (cf. Gewirtz & Pelaez-Nogueras, 1992, 1993), others have found it useful in accounting for the representation of contingency learning in the brain and have argued that it does not imply a sophisticated or conscious level of cognitive processing or means– ends knowledge (Bower, 1997; Fagen, 1993; Lewis, 1999; Tarabulsy et al., 1996). Recent evidence on the neural basis of motor learning supports this view. The learning of some skills requires awareness of the goal of movement for learning to occur, but other aspects of self-awareness are not necessary (Willingham, 1999). Our experiments demonstrate that expectancy violation paradigms can also be an important tool for understanding early expressive and motivational development. This methodology provides a way to operationally define contextual change as well as to control for the prior experience of the child, because both factors are likely to influence the nature of the expressions shown. In addition, we have shown that it is possible to manipulate the form of expectancy violation in order to explore the variability of negative expressions across contexts and to determine whether negative expressions are specific to those variations. If we accept the premise that specific negative expressions are only partially or loosely differentiated in the early months of life, then a method to identify how and under what circumstances negative expressions are or become differentiated is sorely needed.
The weight of the evidence in these experiments suggests that anger expressions increase when there is any disruption of a learned expectancy, but further questions remain about how the form and pattern of negative expressions change with both age and experience. Examination of negative expressions in specific contexts and following repeated expectancy violation offers the prospect of determining how negative expressions are organized and how particular expression systems emerge. For example, repeated exposure to noncontingency has been associated with failure to develop generalized expectancies (Lewis & Goldberg, 1969) and with depressed affect (Seligman, 1975). Repeated noncontingency experiences might eventually come to elicit more sad expressions than anger expressions. Individual differences in this process are likely, but the advantage of an expectancy violation procedure is that one can observe directly whether and how experience affects the regulation and organization of negative expressions. However, in young infants, it is clear that both facial expressions and instrumental behavior are affected by what has changed in relation to infants’ prior experience.
Acknowledgments
We thank the following individuals for assistance with data collection and coding: Samantha Levine and Charles Morris (Study 1) and Dan Hitchcock and Rebecca Fraser (Study 2).
Footnotes
This research was supported by National Science Foundation Grant BNS-921319 and National Institute of Mental Health Grant HD35138.
No differences by ethnicity or gender were expected, and none were revealed in preliminary analyses.
Rules for classifying MAX expressions into discrete and blended expression categories are well described (Izard, 1995). Blends of anger and sadness have been the most frequently observed blended expression in our past work. Of the two possible combinations, the blend 25–33–56 appears more commonly, but individual differences appear with some infants showing the sad/anger combination (23–33–54). Because the meaning of these blends is still unclear and their frequency is low, treating them as a single category of expression is currently the most reasonable approach. The incidence of other expressions blended with anger or sadness was so low that it was not possible to analyze them as separate categories. Very high arousal did not occur in these experiments because infants cried only briefly, if at all, and because maximum arousal as indexed by the presence of MAX pain eye components was not observed.
The minor and nonsignificant age difference between experiments (21 weeks vs. 17 weeks) is unlikely to account for the difference in response to the partial contingency. In none of our previous work using this procedure did we find a difference in the rate of learning between 4 and 5 months of age. The mean rates of response of 4- and 6-month-olds were not significantly different, nor were the numbers of 4- and 6-month-old infants meeting the response criterion (Alessandri et al., 1990; Sullivan et al., 1992).
References
- Alessandri SM, Sullivan MW, Lewis M. Violation of expectancy and frustration in early infancy. Developmental Psychology. 1990;26:738–744. [Google Scholar]
- Amsel A. Frustrative nonreward in partial reinforcement and discrimination learning. Psychological Review. 1962;69:306–328. doi: 10.1037/h0046200. [DOI] [PubMed] [Google Scholar]
- Amsel A, Roussel J. Motivational properties of frustration. Journal of Experimental Psychology. 1952;43:363–368. doi: 10.1037/h0059393. [DOI] [PubMed] [Google Scholar]
- Averil, J. R. (1982). Anger and aggression: An essay on emotion. New York: Springer-Verlag.
- Bendersky, M., Sullivan, M. W., Alessandri, S., & Lewis, M. (1995). Measuring the effects of prenatal cocaine exposure. In M. Lewis & M. Bendersky (Eds.), Mothers, babies, and cocaine: The role of toxins in development (pp. 163–178). Mahwah, NJ: Erlbaum.
- Bennett D, Bendersky M, Lewis M. Facial expressivity at four months: A context by expression analysis. Infancy. 2002;3:97–114. doi: 10.1207/S15327078IN0301_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zur H, Bernitz S. What makes people angry: The dimensions of anger-evoking events. Journal of Research in Personality. 1991;25:1–22. [Google Scholar]
- Berkowitz L. Frustration-aggression hypothesis: Examination and reformulation. Psychological Bulletin. 1989;106:59–73. doi: 10.1037/0033-2909.106.1.59. [DOI] [PubMed] [Google Scholar]
- Bigelow AE, Maclean BK, MacDonald D. Infants’ response to live and replay interactions with self and mother. Merrill-Palmer Quarterly. 1996;42:596– 611. [Google Scholar]
- Bower TGR. Contingencies, logic and learning. The Behavior Analyst. 1997;20:141–148. doi: 10.1007/BF03392771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braungart-Reiker JM, Stifter CA. Infants’ responses to frustrating situations: Continuity and change in reactivity and regulation. Child Development. 1996;67:1767–1779. [PubMed] [Google Scholar]
- Campos J, Campos R, Barrett K. Emergent themes in the study of emotional development. Developmental Psychology. 1989;25:394–402. [Google Scholar]
- Camras L. Expressive development and basic emotions. Cognition and Emotion. 1992;6:269–283. [Google Scholar]
- Camras, L. (1994). Two aspects of emotional development: Expression and elicitation. In P. Ekman & R. J. Davidson (Eds.), The nature of emotion: Fundamental questions (pp. 347–351). New York: Oxford University Press.
- Camras L, Lambrecht L, Michel GF. Infant “surprise” expressions as coordinative motor structures. Journal of Nonverbal Behavior. 1996;20(3):183–195. [Google Scholar]
- Camras, L., Malatesta, C., & Izard, C. (1991). The development of facial expressions in infancy. In R. S. Feldman & B. Rimé (Eds.), Fundamentals of nonverbal behavior (pp. 73–105). New York: Cambridge University Press.
- Charlesworth, W. R. (1969). The role of surprise in cognitive development. In D. Elkind & J. H. Flavell (Eds.), Studies in cognitive development: Essays in honor of Jean Piaget (pp. 257–314). London: Oxford University Press.
- Darwin, C. (1965). The expression of the emotions in man and animals Chicago: University of Illinois Press. (Original work published 1872)
- DeCasper AJ, Carstens AA. Contingencies of stimulation: Effects on learning and motivation. Infant Behavior and Development. 1981;4:19–35. [Google Scholar]
- de Rivera, J. (1991). The structure and dynamics of emotion. In D. Ozer, J. M. Healy Jr., & A. J. Stewart (Vol. Eds.), Perspectives in personality: Vol. 3. Self and emotion: Approaches to understanding lives (pp. 191–212). London: Jessica Kingsley.
- Dunham P, Dunham F. Effects of mother–infant social interaction on infants’ subsequent contingency task performance. Child Development. 1990;61:785–793. [PubMed] [Google Scholar]
- Eisenberg N, Fabes R, Nyman M. The relations of children’s emotionality and regulation to children’s anger-related reactions. Child Development. 1991;65:109–128. [PubMed] [Google Scholar]
- Fagen JW. Reinforcement is not enough: Learned expectancies and infant behavior. American Psychologist. 1993;47:1153–1155. doi: 10.1037//0003-066x.48.11.1153. [DOI] [PubMed] [Google Scholar]
- Fagen JW, Rovee CK. Effects of quantitative shifts in a visual reinforcer on the instrumental response of infants. Journal of Experimental Child Psychology. 1976;21:346–360. doi: 10.1016/0022-0965(76)90048-5. [DOI] [PubMed] [Google Scholar]
- Fox N, Davidson R. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia. 1986;24:417– 422. doi: 10.1016/0028-3932(86)90028-x. [DOI] [PubMed] [Google Scholar]
- Gewirtz JL, Pelaez-Nogueras M. B. F. Skinner’s legacy to human infant behavior and development. American Psychologist. 1992;47:1411–1422. doi: 10.1037//0003-066x.47.11.1411. [DOI] [PubMed] [Google Scholar]
- Gewirtz JL, Pelaez-Nogueras M. “Expectancy”: Sleight-of-hand mentalism, not mechanism or process”. American Psychologist. 1993;47:1156–1157. [Google Scholar]
- Granchrow JR, Steiner JE, Daher M. Neonatal facial expressions in response to different qualities and intensities of gustatory stimulation. Infant Behavior & Development. 1983;6:153–157. [Google Scholar]
- Gunnar M. Control warning signs and distress in infancy. Developmental Psychology. 1980;16:153–157. [Google Scholar]
- Hains SMJ, Muir DW. Effects of stimulus contingency in infant–adult interactions. Infant Behavior & Development. 1996;19:49– 61. [Google Scholar]
- Hartshorn K, Rovee-Collier C, Gerhardstein P, Bhatt RS, Wondoloski TL, Klein P, et al. The ontogeny of long-term memory over the first year-and-a-half of life. Developmental Psychology. 1998;32:69–89. [PubMed] [Google Scholar]
- Izard, C. E. (1995). The maximally discriminative facial movement coding system (MAX) (Rev. ed.). Newark: University of Delaware.
- Izard, C. E., & Ackerman, B. P. (2000). Motivational, organizational, and regulatory functions of discrete emotions. In M. Lewis & J. M. Haviland-Jones (Eds.), Handbook of emotions (2nd ed., pp. 203–264). New York: Guilford Press.
- Izard CE, Fantauzzo CA, Castle JM, Haynes OM, Rayias MF, Putnam PH. The ontogeny and significance of infants’ facial expressions in the first 9 months of life. Developmental Psychology. 1995;31:997–1013. [Google Scholar]
- Kochanska G, Coy K, Tjebkes R, Husarek S. Individual differences in emotionality in infancy. Child Development. 1998;64:375–390. [PubMed] [Google Scholar]
- Lewis, M. (1999). Social cognition and the self. In P. Rochat (Ed.), Early social cognition (pp. 81–98). Mahwah, NJ: Erlbaum.
- Lewis M, Alessandri SM, Sullivan MW. Violation of expectancy, loss of control, and anger in young infants. Developmental Psychology. 1990;26:745–751. [Google Scholar]
- Lewis M, Goldberg S. Perceptual cognitive development in infancy: A generalized expectancy model as a function of mother–infant interaction. Merrill-Palmer Quarterly. 1969;3:307–316. [Google Scholar]
- Lewis, M., & Michalson, L. (1983). Children’s emotions and moods New York: Plenum Press.
- Lewis, M., & Rosenblum, L. (1974). Introduction. In M. Lewis & L. Rosenblum (Eds.), The origins of fear: The origins of behavior (Vol. 2, pp. 1–10). New York: Wiley.
- Lewis M, Sullivan MW, Brooks-Gunn J. Emotional behavior during the learning of a contingency in early infancy. British Journal of Developmental Psychology. 1985;3:307–316. [Google Scholar]
- Lewis, M., Sullivan, M. W., & Michalson, L. (1984). The cognitive emotional fugue. In C. E. Izard, J. Kagan, & R. B. Zajonc (Eds.), Emotions, cognition, and behavior (pp. 264–288). Cambridge, England: Cambridge University Press.
- Lewis M, Sullivan MW, Ramsay D, Alessandri SM. Individual differences in anger and sad expressions during extinction: Antecedents and consequences. Infant Behavior & Development. 1992;15:443–452. [Google Scholar]
- Matias R, Cohen JF. Are Max-specified infant facial expressions during face-to-face interaction consistent with differential emotions theory? Developmental Psychology. 1993;29:524–531. [Google Scholar]
- McSweeny FK, Hinson JM, Cannon CB. Sensitization-habituation may occur during operant learning. Psychological Bulletin. 1996;120:256–271. [Google Scholar]
- Michel GF, Camras L, Sullivan J. Infant interest expressions as coordinative motor structures. Infant Behavior & Development. 1992;15:347–358. [Google Scholar]
- Miller, K. (1996). Principles of everyday behavior analysis Pacific Grove, CA: Brooks Cole.
- Oster H, Hegley D, Nagel L. Adult judgments and fine-grained analysis of infant facial expressions: Testing the validity of a priori coding formulas. Developmental Psychology. 1992;28:1115–1131. [Google Scholar]
- Reese, H. W. (1976). Basic learning processes in childhood New York: Holt, Rinehart & Winston.
- Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Development. 1988;59:1555–1568. [PubMed] [Google Scholar]
- Rovee-Collier, C. K. (1987). Learning and memory in infancy. In J. D. Osofsky (Ed.), Handbook of infant development (pp. 98–133). New York: Wiley.
- Scarr S, Salapatek P. Patterns of fear development during infancy. Merrill-Palmer Quarterly. 1970;16:53–90. [Google Scholar]
- Scull JW. The Amsel frustration effect. Psychological Review. 1973;79:352–361. doi: 10.1037/h0034430. [DOI] [PubMed] [Google Scholar]
- Seligman, M. E. P. (1975). Helplessness: On depression, development, and death San Francisco: Freeman.
- Shrout, P. E. (1995). Measuring degree of consensus in personality judgments. In P. E. Shrout & S. T. Fiske (Eds.), Personality research, methods, and theory. Hillsdale, NJ: Erlbaum.
- Skarkin K. Cognitive and contextual determinants of stranger fear in six- and eleven-month-old infants. Child Development. 1977;48:537–544. [PubMed] [Google Scholar]
- Sroufe LA, Wunsch JP. The development of laughter in the first year of life. Child Development. 1972;43:1326–1344. [PubMed] [Google Scholar]
- Steiner JE. Human facial expressions in response to taste and smell stimulation. Advances in Child Development and Behavior. 1979;13:178–184. doi: 10.1016/s0065-2407(08)60349-3. [DOI] [PubMed] [Google Scholar]
- Stenberg CR, Campos J, Emde R. The facial expression of anger in seven-month-old infants. Child Development. 1983;54:178–184. doi: 10.1111/j.1467-8624.1983.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MW, Lewis M, Alessandri SM. Cross-age stability in emotional expressions during learning and extinction. Developmental Psychology. 1992;28:58– 63. [Google Scholar]
- Sullivan MW, Rovee-Collier CK, Tynes DM. A conditioning analysis of infant memory. Child Development. 1979;58:152–162. [PubMed] [Google Scholar]
- Symons DK, Moran G. Using social signals to describe the contingency environment of early mother–infant interaction. Infant Behavior & Development. 1994;17:209–214. [Google Scholar]
- Tarabulsy GM, Tessier R, Kappas A. Contingency detection and the contingent organization of behavior in interactions: Implications for socioemotional development in infancy. Psychological Bulletin. 1996;120:25–41. doi: 10.1037/0033-2909.120.1.25. [DOI] [PubMed] [Google Scholar]
- Thelen, E., & Ulrich, B. (1991). Hidden skills. Monographs of the Society for Research in Child Development, 56(1, Serial No. 223). [PubMed]
- Watson, J. (1979). Perception of contingency as a determinant of social responsiveness. In E. Thoman (Ed.), Origins of infants’ social responsiveness (pp. 33– 64). Hillsdale, NJ: Erlbaum.
- Watson, J. S. (1985). Contingency perception of early social development. In T. Field & N. Fox (Eds.), Comparative perspectives on the development of memory (pp. 159–179). Hillsdale, NJ: Erlbaum.
- Watson JS. Contingency and its two indices within conditional probability analysis. The Behavior Analyst. 1997;20:129–140. doi: 10.1007/BF03392770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, C., Toland, C., & King, R. A. (1998, April). Demonstrations of continuous or partial reinforcement on performance of 6- and 12-month olds Paper presented at the International Conference on Infant Studies, Atlanta, GA.
- Willingham DB. The neural basis of motor skill. Current Directions in Psychological Science. 1999;8:178–182. [Google Scholar]


