Abstract
Expression of N-methyl-d-aspartate (NMDA) receptor-dependent long-term potentiation (LTP) in the CA1 region of the hippocampus can be divided into an early (1–2 h), protein synthesis-independent phase and a late (>4 h), protein synthesis-dependent phase. In this study we have addressed whether the de novo protein synthesis required for the expression of late-LTP can be sustained solely from the translation of mRNAs located in the dendrites of CA1 pyramidal neurones. Our results show that late-LTP, lasting at least 5 h, can be maintained in hippocampal slices where the dendrites located in stratum radiatum have been isolated from their cell bodies by a microsurgical cut. The magnitude of the potentiation of the slope of field EPSPs in these ‘isolated’ slices was similar to that recorded in ‘intact’ slices. Incubation of the slices with the mRNA translation inhibitor cycloheximide or the mammalian target of rapamycin (mTOR) inhibitor rapamycin blocked late-LTP in both ‘intact’ and ‘isolated’ slice preparations. In contrast, incubation of slices with the transcription inhibitor, actinomycin D, resulted in a reduction of sustained potentiation, at 4 h, in ‘intact’ slices while in ‘isolated’ slices the magnitude of potentiation was similar to that seen in untreated slices. These results indicate that late-LTP can be induced and maintained in ‘isolated’ dendritic preparations via translation of pre-existing mRNAs.
N-methyl-d-aspartate (NMDA) receptor-dependent long-term potentiation (LTP) in the CA1 region of the mammalian hippocampus is the most widely studied and best characterized electrophysiological model of learning and memory. This form of LTP is typically induced by high frequency stimulation (HFS) of the Schaeffer collateral/commissural input to synapses located in stratum radiatum of the CA1 region of the hippocampus. Two forms of LTP have been described following HFS of these inputs – an early-phase LTP that lasts for up to 2 h and is not blocked by the inhibition of de novo protein synthesis but requires modification of pre-existing proteins and a longer phase, late-LTP, which is critically dependent on new protein synthesis at the time of LTP induction (for reviews see Kelleher et al. 2004b; Lynch, 2004).
One of the characteristics of NMDA receptor-dependent plasticity is the potential to induce synapse-specific changes. While it is generally accepted that protein synthesis is needed for long-lasting forms of synaptic plasticity (late-LTP), a global upregulation of protein synthesis via new transcription and transport is difficult to reconcile with this idea. The ‘tagging hypothesis’ has been proposed as one way of solving this specificity problem (see Frey & Morris, 1997). Alternatively, activity-dependent local protein synthesis in dendrites could allow synapse specificity to be retained (Steward & Fass, 1983; Steward & Schuman, 2001) This latter idea has gained support due to observations that numerous mRNAs have been identified in neuronal dendrites, in addition to translational machinery (Crino & Eberwine, 1996; Tian et al. 1999; for reviews see Jiang & Schuman, 2002; Steward & Schuman, 2003). Furthermore, LTP-inducing stimuli result in the translocation of ribosomes to active sites (Ostroff et al. 2002). In addition, both brain-derived neurotrophic factor (BDNF)-induced LTP and dihydroxyphenylglycine (DHPG)-sensitive long-term depression (LTD) have been shown to exist in the absence of an intact cell soma–dendrite connection (Kang & Schuman, 1996; Huber et al. 2000), arguing that at least some forms of plasticity rely on local translation of the pre-existing pool of mRNAs.
A requirement for local protein synthesis in NMDA receptor-dependent LTP has remained less clear. Early work concerning the role of local protein synthesis and LTP suggested that late-LTP could not be established in slices where dendrites and cell bodies had been physically separated (Frey et al. 1989). However, accumulating cellular and molecular evidence suggests that activity-dependent dendritic translation occurs, and is probably involved in synaptic plasticity mechanisms. For example, alpha calcium/calmodulin-dependent kinase II (αCAMKII) mRNA has been shown to associate with polysomes following chemical stimulation in synaptosomes (Bagni et al. 2000), and polyadenylation and translation of αCAMKII mRNA has been observed following visual experience in vivo (Wu et al. 1998). Alongside this, recent studies have shown the activity-dependent translation and trafficking of overexpressed AMPA receptor subunits in cultured dendrites that have been isolated from their cell bodies (Ju et al. 2004). Nonetheless, a clear physiological demonstration that local protein synthesis is involved in the maintenance of late-LTP remains to be shown. In this study we show that the induction and maintenance of protein synthesis-dependent late-LTP, lasting >5 h, can occur in isolated dendrites of hippocampal CA1 pyramidal neurones. We provide evidence to suggest that this late-LTP is dependent on translation and not transcription.
Methods
Preparation of slices
Male Wistar rats (6–8 weeks old) were decapitated under halothane anaesthesia (in accordance with current UK Home Office procedures), their brains removed rapidly and placed in ice-cold (2–4°C) recording solution (mm: NaCl 120, KCl 2.5, MgSO4 1.3, CaCl2 2.5, NaH2PO4 1, NaHCO3 26 and glucose 11), gassed with 95% O2 and 5% CO2. The hippocampus was isolated and transverse slices (400 μm thick) were prepared using a tissue slicer (Stoelting, IL, USA). Slices were incubated in recording solution for 1 h before being transferred to an interface humidified chamber (FST, Canada) where they were perfused, at a flow rate of 3 ml min−1 with continuously gassed recording solution. All experiments were carried out at 33°C.
In order to separate the dendrites of CA1 pyramidal neurones from their cell bodies, a small cut was made immediately below the cell body layer. Such slices were allowed to recover for a further hour before any attempts were made to record field potentials. We refer to slices in which this cut was made as ‘isolated’ slices to distinguish them from ‘intact’ slices in which no cut was made. Isolation of the dendrites from cell bodies was confirmed by visual inspection and subsequent cresyl violet staining, as well as by the inability to induce population spikes in the field potential recordings when high stimulus intensities were applied.
Electrophysiological recording of field potentials
Field excitatory postsynaptic potentials (fEPSPs) were recorded via an electrode placed in the stratum radiatum. Recording electrodes were made from thick-walled borosilicate glass and had resistances of ∼2 MΩ. Two tungsten bipolar electrodes were placed either side of the recording electrode in order to evoke fEPSPs in two independent pathways. The S1 pathway was used to apply the tetanus stimulation to evoke LTP, while the S2 pathway acted as a control. Constant current stimuli were used to evoke fEPSPs whose slopes were approximately one-third of the magnitude of those evoked by a maximal stimulus. In ‘intact’ slices these ranged between 50 and 100 μA, while in ‘isolated’ slices higher intensities were required and were typically between 100 and 150 μA. S1 and S2 pathways were stimulated, alternately, at a frequency of 0.033 Hz. Larger stimulus artefacts were evident in recordings of fEPSPs in ‘isolated’ slices when compared to recordings from ‘intact’ preparations. This prevented the clear visualization of fibre volleys in such recordings. However in control experiments, application of tetrodotoxin (500 nm) abolished all synaptic activity while leaving the large stimulus artefact unchanged. Two pulses, 50 ms apart, were applied in order to measure paired-pulse facilitation (PPF) ratios before and after the induction of LTP. fEPSPs were amplified via an EXT-102F amplifier (NPI, Tamm, Germany). The LTP Program (Anderson & Collingridge, 2001) was used to display fEPSPs and allow the monitoring of online measurements of the slopes of fEPSPs. Subsequent off-line analysis was carried out to provide an accurate measure of these slopes. Prior to evoking LTP in either ‘intact’ or ‘isolated’ slices, stable baselines were recorded for at least 1 h. LTP was induced by the application of three high-frequency trains of stimuli 5 min apart, each comprising of a single 1 s 100 Hz tetanus. The assignment of S1 and S2 pathways, relative to the position of the recording electrode, was changed regularly to ensure that there was no bias in attempting to induce LTP in only one particular recording configuration. Several criteria had to be satisfied in order for an experiment to be included. Baseline recordings of fEPSPs from both S1 and S2 pathways had to be stable for at least 50 min prior to the application of the tetanic pulses, and any slice which showed a >10% change in the slope of the fEPSP in either pathway was discarded from further analysis. In addition, following the application of the tetanic pulses to the S1 pathway, the slope of fEPSPs in the control (S2) pathway was not allowed to drift, by more than 15% in any 60 min recording period. Finally, LTP was deemed to have been established if the slopes of fEPSPs in the S1 pathway were at least 150% of baseline values (at 60 min post-tetanus). Approximately 70% of all recordings satisfied our criteria for acceptance, and the main reason for rejection of an experiment was drift in the control (S2) pathway following the application of the tetanus.
In our recordings of late-LTP in non-drug-treated slices we monitored fEPSPs for at least 5 h following the application of the tetanus. The inhibitors, actinomycin D, rapamycin and cycloheximide (Sigma) were dissolved in dimethylsulfoxide (DMSO), the final volume of which did not exceed 1%. This concentration of DMSO did not affect the duration or magnitude of late-LTP. The ionotropic glutamate receptor antagonists d-2-amino-5-phosphonopentanoic acid (d-AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were purchased from Tocris Biosciences (Avonmouth, UK).
Cresyl violet staining and 35S-methionine labelling of hippocampal slices
After recording, hippocampal slices were submerged overnight in fixative (2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 m sodium phosphate buffer (PB), pH 7.4). 50 μm transverse sections were then cut on a freezing microtome (Vibratome, Lancer) and selected sections were mounted onto glass slides and incubated in a 0.1% solution of cresyl violet (Sigma). Sections were then dehydrated through a graded series of ethanol before being immersed in xylene and coverslipped with DPX. To assess the efficacy of the inhibitors, slices were labelled with 35S-methionine at 15 μCi ml−1 in the absence or presence of rapamycin or cycloheximide. Inhibitors were applied while the slices were held in a modified holding chamber and bathed in the standard, oxygenated recording solution. After 2 h, slices were homogenized in a deoxycholate buffer (1% sodium deoxycholate, 50 mm Tris pH 9, 50 mm NaF, 20 μm ZnCl2, 1 mm Na2VO4, 0.5 mm phenylmethylsulphonylfluoride, 2 μg ml−1 aprotinin and 2 μg ml−1 leupeptin). Homogenates were incubated, on ice, for 1 h and then centrifuged at 20 000 r.p.m. for 1 h. Protein concentration was determined using BCA assay (Pierce, Rockford, USA), and 50 μg of the resulting sample subjected to reducing SDS-PAGE. The gel was dried and exposed on a Fuji imaging plate (Fuji, Japan). The plate was then scanned using a Typhoon variable mode imager (Amersham Pharmacia). The scan of the autoradiograph was analysed using Scion Image (Scion Corporation).
Statistical analysis
Results are presented as mean ± standard error of the mean (s.e.m.) and statistical comparison between data sets was assessed by ANOVA.
Results
Properties of fEPSPs recorded in ‘isolated’ hippocampal slice preparations
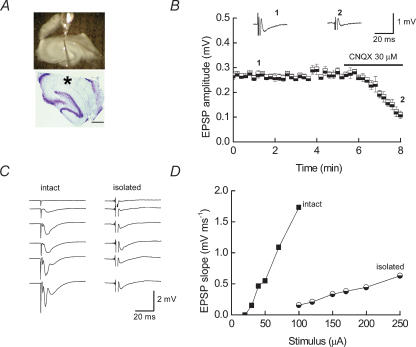
Figure 1A shows an example of a typical ‘isolated’ dendritic slice preparation clearly showing that the stratum pyramidale and stratum oriens are physically isolated from the dendrites of the CA1 pyramidal neurones located in stratum radiatum. The upper panel is a view from the electrophysiological recording setup with the two stimulating electrodes placed to either side of the glass recording electrode. The lower panel shows an example of a cresyl violet-stained hippocampal slice with the cell body layer completely ablated. The asterisk indicates where the recording electrode was placed in this preparation. fEPSPs mediated by α-amino-3-hydroxy-5-methyl isoxazole propionic acid (AMPA) receptors could be evoked in such ‘isolated’ preparations as assessed by blockade with CNQX (30 μm) (Fig. 1B). To verify complete separation of cell bodies from their dendrites, we assessed whether the ‘isolated’ slices generated cell-body-mediated population spikes at higher stimulus intensities. Input–output relationships for fEPSPs illustrate that population spikes were only obtained from ‘intact’ slices at the high stimulus intensities and not from ‘isolated slices (Fig. 1C, compare the lower two traces in the left-hand panel to those on the right). The latency from the stimulus artefact and the fEPSP is similar for both slice preparations. Figure 1D compares the stimulus intensities required to evoke fEPSPs in each type of preparation. In agreement with previous studies (Kang & Schuman, 1996), where fEPSPs have been recorded from ‘isolated’ dendritic preparations, we observed that higher stimulus intensities were required to evoke fEPSPs and the fEPSPs were of lower amplitude when compared to those recorded in ‘intact’ slices. It is likely that the increased stimulus intensities required to induce a field response are in part due to incomplete sealing of cut dendrites resulting in a need for larger synaptic depolarizations to produce the field potentials.
Figure 1. Properties of fEPSPs recorded from ‘isolated’ dendritic hippocampal slice preparations.
A, upper panel, view of an ‘isolated’ hippocampal slice preparation. The separation of the cell body layer from the dendritic layer is clearly visible. The glass recording electrode is placed in the stratum radiatum immediately below the area which has been cut away from the stratum pyramidale and stratum oriens. Two stimulating electrodes are placed to either side of the recording electrode in order to activate independent inputs. The lower panel shows a representative cresyl violet-stained slice. The cell bodies of neurones located in the dentate gyrus and CA3 regions are visible. Although the somata of some CA1 neurones are still present in the slice, there is no evidence of any CA1 pyramidal cell bodies in the region, as indicated by the asterisk, where the recording electrode was placed. Scale bar 500 μm. B, plot of the amplitudes of fEPSPs recorded in ‘isolated’ slice preparations shows that they are blocked by the AMPA receptor antagonist, CNQX (30 μm). Representative fEPSPs recorded at the times indicated are shown above the plot. C, examples of fEPSPs recorded in ‘intact’ (left-hand panel) and ‘isolated’ slices (right-hand panel) in response to increasing stimulus intensities (ranges 20–100 μA for ‘intact’; 100–250 μA for ‘isolated’). Evidence of population spikes is seen only in recordings from the ‘intact’ slice. D, plot of fEPSP slope against stimulus intensity for data shown in C.
Late-LTP can be elicited in ‘isolated’ hippocampal CA1 dendrites
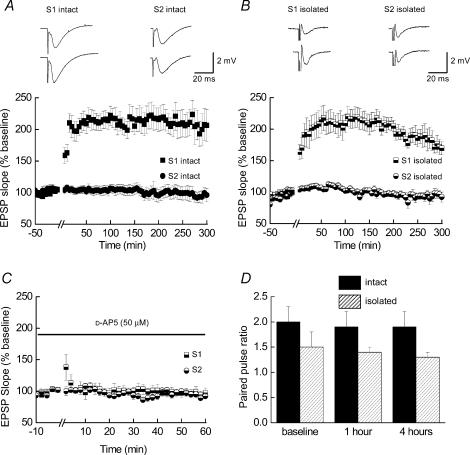
We first determined whether it was possible to induce long-lasting LTP in ‘isolated’ hippocampal slices. Pooled data from ‘intact’ (Fig. 2A) and ‘isolated’ (Fig. 2B) slice preparations (n = 10 for each) demonstrates that it is possible to induce and maintain late-LTP, for at least 5 h, in slices in which the dendrites have been isolated from their cell bodies. The magnitude of the potentiation 5 h following the application of the tetanus stimulation (S1 pathway, see Methods) was similar in both ‘intact’ and ‘isolated’ slices (206 ± 26% and 173 ± 10% of baseline ‘control’ values, respectively). As can be seen, the non-tetanized (S2) pathways did not show any significant overall change throughout the duration of these experiments. The mean slope values at 5 h were 97 ± 11% and 92 ± 8% of pretetanus values in ‘intact’ and ‘isolated’ slices, respectively. Representative fEPSPs recorded from each of the two pathways during the pretetanus baseline, 1 h and 5 h after the tetanus are also illustrated above each of the plots. Although the illustrations of late-LTP in Fig. 2A and B are shown to 5 h post-tetanus we did record late-LTP in several ‘intact’ slices for at least 8 h, and in one case 17 h, while in ‘isolated’ slices several recordings of late-LTP were made until 7 h post-tetanus without the indication of a decline in the slope of potentiated fEPSPs.
Figure 2. Late-LTP can be induced and maintained in ‘isolated’ hippocampal slice preparations.
A and B, pooled data (n = 10 for both) showing the time-course of LTP induced in ‘intact’ (A) and isolated (B) hippocampal slices. In both types of slice preparation, LTP can be maintained for at least 5 h following tetanic stimulation of the Schaeffer collateral/commissural pathway (S1). Non-tetanized (S2) pathways show no significant increase in fEPSP slopes during these recordings. Examples of fEPSPs recorded as a result of the stimulation of S1 and S2 pathways in both ‘intact’ and ‘isolated’ slice preparations are shown above each graph. C, LTP in ‘isolated’ slices is blocked when tetanic stimulation is carried out in the presence of the NMDA receptor antagonist, d-AP5 (n = 3). A small amount of post-tetanic potentiation is visible in the S1 pathway. D, plot of paired-pulse facilitation ratios recorded prior to and 1 h and 4 h after the application of the tetanus for LTP recorded in ‘intact’ (filled bars) and ‘isolated’ (shaded bars) slice preparations (n = 4 for both). Although PPF ratios are higher in ‘intact’ slices, when compared to ‘isolated’ slices there is no significant difference in these ratios within each preparation at the time points indicated.
Tetanus-induced LTP in the CA1 region of the hippocampus is NMDA receptor dependent (Collingridge et al. 1983). We confirmed that the LTP-inducing protocol we used in ‘isolated’ slices was also dependent on NMDA receptor activation. Figure 2C shows pooled data from ‘isolated’slices (n = 3) where the tetanus was applied in the presence of d-AP5 (50 μm). Only a small amount of post-tetanic potentiation is present in these recordings, confirming that the separation of dendrites from cell bodies does not change this fundamental property of LTP. In addition, a common feature of NMDA receptor-dependent LTP in the CA1 region is the observation that PPF ratios do not change following LTP expression (see for example Manabe et al. 1993). We measured PPF ratios in a subset of slices (n = 4) and for ‘intact’ slices PPF ratios for pretetanus and 1 h and 4 h post-tetanus were 2.0 ± 0.3, 1.9 ± 0.3 and 1.9 ± 0.3, respectively. The corresponding values for ‘isolated’ slices were 1.5 ± 0.3, 1.4 ± 0.1 and 1.3 ± 0.1. Although PPF ratios were significantly greater in ‘intact’ slices compared to ‘isolated’ slice preparations (P < 0.05), in neither case were the values of PPF recorded 1 h and 4 h after tetanic stimulation significantly different from PPF values recorded during the baseline period with each group (P = 0.9, ‘intact’; P = 0.6, ‘isolated’; ANOVA; Fig. 2D).
Thus this initial characterization of late-LTP demonstrates that isolating the dendrites of CA1 pyramidal neurones does not prevent the ability to induce a long-lasting potentiation of synaptic transmission. Furthermore, the late-LTP observed shares several of the fundamental properties of late-LTP seen in conventional recordings, and indicates that ‘isolated’ slice preparations are a viable system to investigate mechanisms underlying long-lasting changes in synaptic efficacy.
Late-LTP in ‘isolated’ slices is blocked by inhibitors of translation
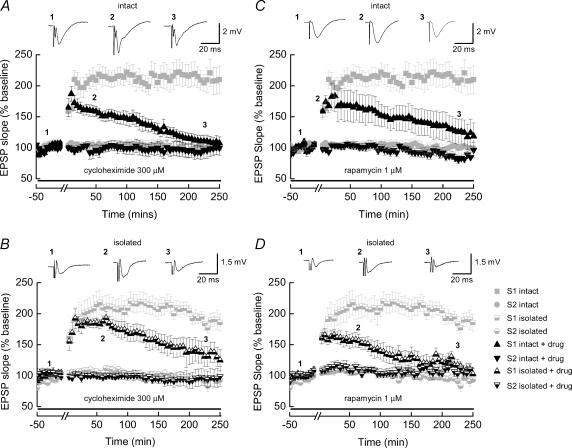
Late-LTP is, by definition, LTP that is dependent upon new protein synthesis. In order to establish when this form of LTP was occurring in our preparations, we examined the profile of LTP stability in ‘intact’ slices exposed to the general translation inhibitor, cycloheximide. Addition of cycloheximide (300 μm, Fig. 3A) did not effect the initial potentiation of fEPSPs (n = 8), and indeed the magnitude of the potentiation was similar to that observed in untreated ‘control’ slices. However following the initial potentiation of fEPSPs, a decay to near-baseline levels was observed and there was no evidence of sustained, late-LTP occurring in any of the slices examined. Thus, 4 h following the application of the tetanus there was no significant potentiation of fEPSPs when compared to the pretetanus baseline values. This result establishes that, in our system, ‘late-LTP’ can be defined as LTP lasting more than 4 h. As the ‘isolated’ slices lacked cell bodies, as determined both visually and electrophysiologically (see Fig. 1), ‘isolated’ slice late-LTP was presumably being maintained via the local translation of pre-existing dendritic mRNAs. To confirm this, we also exposed ‘isolated’ slices to cycloheximide (n = 7, Fig. 3B). Similar to the ‘intact’ slices, we found that late-LTP was not sustained, and decayed to near baseline within 4 h, with a similar decay profile to that seen in the ‘intact’ slices. Taken together our results support the idea that late-LTP can be maintained purely due to the local translation of pre-existing dendritic mRNAs.
Figure 3. Late-LTP in both ‘intact’ and ‘isolated’ hippocampal slices is blocked by inhibitors of mRNA translation.
A and C, pooled data obtained from ‘intact’ slices where LTP was induced in the presence of the mRNA translation inhibitors cycloheximide (A, n = 8) or rapamycin (C, n = 5). In each case these inhibitors did not affect the initial size of the potentiation obtained but prevented the establishment of stable late-LTP. Thus, 4 h after the application of the tetanic stimulation the slope values for the fEPSPs have returned to control (pretetanus) levels. B and D, pooled data obtained from ‘isolated’ hippocampal slices treated with cycloheximide (B, n = 7) or rapamycin (D, n = 9). As is the case for ‘intact’ slices, late-LTP was blocked under these recording conditions. Representative fEPSPs recorded from stimulation of the S1 pathway and taken from the time points are shown above each of the plots. For comparison, in each of the plots shown, the magnitudes and time-courses of late-LTP obtained in control slices (and shown in Fig, 2) are indicated by the light grey symbols.
We assessed next the ability of rapamycin to block late-LTP in these preparations. Rapamycin is a selective inhibitor of the mammalian target of rapamycin (mTOR), a protein involved in regulating both the translation initiation step and generation of translation elongation factors; and the mTOR pathway has previously been shown to play a role in hippocampal late-LTP (Tang et al. 2002; Cammalleri et al. 2003). Results with rapamycin were more pronounced than those seen with cycloheximide application. Addition of rapamycin (1 μm) to either ‘intact (Fig. 3C, n = 5) or ‘isolated’ (Figs 3D, n = 9) slices did not affect the initial potentiation of fEPSPs, but LTP again decayed back to baseline within 4 h following the application of the tetanus. These results further strengthen the conclusion that ‘isolated’ slice late-LTP utilizes similar mechanisms to that seen with ‘intact’ slice late-LTP, and that late-LTP involves the regulation of local protein synthesis through upregulation of translational signalling pathways. Control non-tetanized (S2) pathways were not affected by these inhibitors, indicating that regular synaptic transmission, over the time period examined here, is not dependent on protein synthesis.
Late-LTP in ‘isolated’ slices is not blocked by inhibitors of mRNA transcription
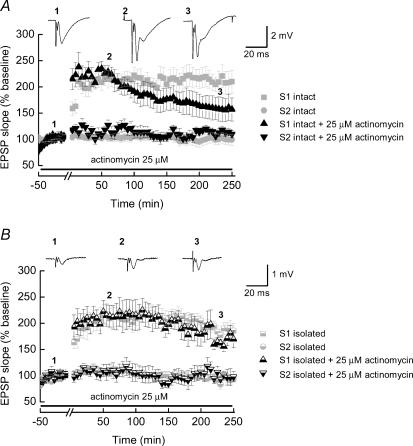
While our results thus far argue that ‘isolated’ slice late-LTP is identical to ‘intact’ slice LTP, previous results have suggested that late-LTP actually requires new gene transcription, not just new translation of pre-existing mRNAs (Frey et al. 1996). Transcription-dependent protein synthesis occurs, by definition, in the cell soma where the nucleus resides. Two possibilities could explain these conflicting results. First, it is possible that the requirement for transcription actually reflects a non-cell-autonomous action (i.e. gene expression from other support cells). Alternatively, late-LTP might in fact normally occur via both transcription and translation, and in the absence of nuclear availability, translation alone might be able to still sustain this process. To address these two possibilities, we tested the effect of incubating slices in the transcription inhibitor, actinomycin D (25 μm) prior to and during tetanic stimulation, to determine whether we could observe a differential effect of this drug in ‘intact’ and ‘isolated’ slice preparations. Figure 4 shows pooled data from ‘intact’ (Fig. 4A, n = 8) and ‘isolated’ (Fig. 4B, n = 6) slices where the profiles of ‘control’ late-LTP in each sort of slice are shown in grey to aid comparison. In both data sets the magnitude of LTP observed in the first hour following the tetanus is not significantly different from that seen in ‘control’ recordings (‘intact’= 219 ± 14%; ‘isolated’= 214 ± 21%). Following this initial potentiation, LTP in ‘intact’ slices is decremental, such that at 4 h post-tetanus the level of potentiation (156 ± 22%) while still elevated compared to the pretetanus baseline is reduced when compared to untreated ‘control’ slices. This failure to establish stable late-LTP, in ‘intact’ slices, by inhibiting transcription with actinomycin D is consistent with previously published data (Frey et al. 1996). In contrast, actinomycin D had no effect on the magnitude or profile of late-LTP in ‘isolated’ slices. As shown in Fig. 4B the potentiation mirrors that seen in untreated slices with the mean slope of the fEPSP, at 4 h, being 171 ± 14% of the pretetanus value. These results both confirm that our ‘isolated’ slices are indeed free from somatic input, and also suggest that local protein synthesis is sufficient to maintain late-LTP. However, it seems likely that ‘normal’ late-LTP utilizes a combination of transcription and local translation. One possibility is that transcription-dependent processes are normally involved in more general neural maintenance, while local translation serves as a mechanism by which synapse specificity can be achieved.
Figure 4. Differential effect of mRNA transcription inhibitors on late-LTP in ‘intact’ and ‘isolated’ hippocampal slices.
A, pooled data obtained from ‘intact’ slices (n = 8) where LTP was induced in the presence of the mRNA transcription inhibitor actinomycin D. The LTP obtained in the presence of actinomycin D did not show a stable profile. B, late-LTP in ‘isolated’ slice preparations (n = 6) in the presence of actinomycin D shows a similar time-course and magnitude to late-LTP obtained in ‘isolated’ slices in non-drug-treated preparations. Again, for ease of comparison, in each of the plots shown, the magnitudes and time-courses of late-LTP obtained in control slices (and shown in Fig 2) are indicated by the light grey symbols.
Reduction in 35S-methionine incorporation in hippocampal slices treated with mRNA translation inhibitors
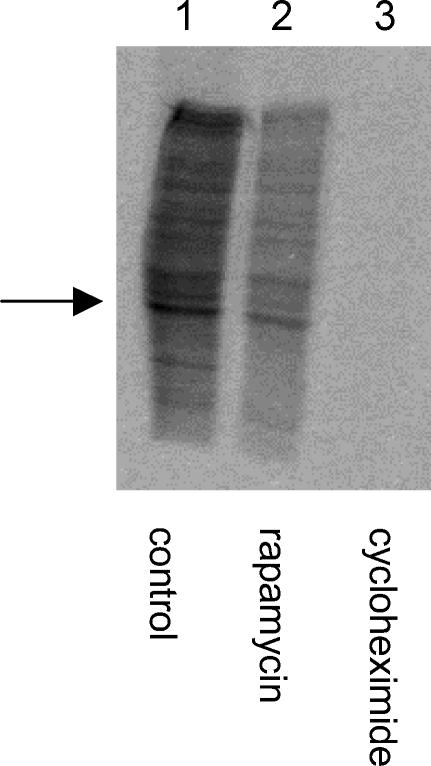
To assess independently the effects of the mRNA translation inhibitors, we compared the incorporation of 35S-methionine into newly synthesized proteins in control slices with those treated with rapamycin or cycloheximide. Figure 5 shows an autoradiograph of a SDS-PAGE gel that highlights the reduction in 35S-methionine incorporation seen in the presence of either rapamycin or cycloheximide. In this example, the band indicated by the arrow was analysed using densitometry. The mTOR inhibitor, rapamycin, reduced 35S-methionine incorporation by 67%, while the general mRNA translation inhibitor reduced 35S-methionine incorporation by 94%. These levels of inhibition with rapamycin and cycloheximide are similar to those reported previously by other groups who have used specific and general mRNA translation inhibitors (see Cracco et al. 2005 and references therein).
Figure 5. mRNA translation inhibitors reduce the incorporation of 35S-methionine in hippocampal slices.
Autoradiograph of a SDS-PAGE gel showing the levels of 35S-methionine incorporation into untreated hippocampal slices (lane 1) and slices treated with rapamycin (1 μm, lane 2) or cycloheximide (300 μm, Lane 3). Clear reductions in these levels are seen in lanes 2 and 3.
Discussion
Late-LTP and the locus of protein synthesis
The two main findings of this study are that (1) late-LTP can be induced and maintained in hippocampal slices in which there is physical isolation of dendrites from their cell bodies; and (2) that this late-LTP requires translation and not transcription. The interpretation that late-LTP can be established in ‘isolated’ slice preparations, where there is no influence of somatic protein synthesis, is dependent critically on the fact that we had successfully isolated, in the area from which recordings were made, dendrites of pyramidal neurones from their cell bodies. Cresyl violet staining of ‘isolated’ slice preparations showing the complete removal of the CA1 region from the recording area and the fact that we did not observe population spikes at high stimulus intensities argues against the idea that there is a subpopulation of ‘displaced’ pyramidal neurones whose cell bodies are located in the stratum radiatum. Thus, based on visual inspection, biochemical staining and electrophysiological characterization, our data demonstrate, for the first time, that ‘isolated’ slice preparations are capable of sustaining protein synthesis-dependent LTP that lasts for at least 5 h.
Our findings complement and extend recent results reported by three groups which have implicated a role for dendritic translation of mRNAs in LTP (Bradshaw et al. 2003; Cracco et al. 2005; Tsokas et al. 2005). In the first of these, local perfusion of emetine (an mRNA translation inhibitor) was shown to reduce late-LTP. However, this study was carried out using conventional ‘intact’ slices, and the possibility remains that the action of the translation inhibitor was not confined to the dendritic layer. In the study by Cracco et al. (2005), an intermediate (1 h) duration LTP, in an isolated dendritic preparation, was shown to be sensitive to rapamycin. The third study by Tsokas et al. (2005) showed that LTP-inducing stimuli in ‘isolated’ dendritic hippocampal slice preparations could enhance the expression of elongation factor 1A (eEF1A), an effect that could be blocked by rapamycin. Our present study extends these findings by demonstrating that it is possible to maintain late-LTP in ‘isolated’ dendritic slice preparations for several hours, and that this potentiation is blocked by inhibitors of mRNA translation. Our findings are, however, in contrast to those of Frey et al. (1989) who reported that LTP lasting up to 3 h could be observed in isolated dendrites but that this decayed rapidly to pretetanus levels. The effects of mRNA translation inhibitors were not examined on this shorter-lasting LTP. Therefore it is unclear whether the difference between these two studies is simply one of duration of the potentiation or whether our LTP-inducing protocol results in alternative expression mechanisms.
As mentioned in the Introduction, local protein synthesis has been proposed as an alternative mechanism to the ‘synaptic tagging hypothesis’ (Frey & Morris, 1997) to explain the requirement of de novo protein synthesis while retaining synapse specificity. The results reported here do not address the issue of synaptic tagging and furthermore, as has been pointed out, these two mechanisms are not necessarily mutually exclusive (for a review see Wang & Tiedge, 2004). While it has been demonstrated that ‘synaptic tags’ can be set under conditions where protein synthesis is blocked (Frey & Morris, 1997), the nature of these elements and indeed the ‘plasticity factors’ with which they interact remain to be identified. While the latter may indeed be proteins it is possible that they could be mRNAs (Fonseca et al. 2004; Kelleher et al. 2004b).
The differential effect of the transcription inhibitor, actinomycin D, on the inhibition of late-LTP in ‘intact’ and ‘isolated’ slice preparations indicates that the role of transcription, when it occurs in LTP, is cell-autonomous. If transcription-dependent LTP was due to the block of synthesis of proteins in glia, interneurones or surrounding (intact) pyramidal neurones, then it might have been expected that late-LTP in ‘isolated’ slices would have been reduced in the presence of actinomycin D. There is a reduction in the magnitude of late-LTP seen in ‘intact’ slices treated with actinomycin compared to control recordings. However, the level of potentiation observed was still significantly greater than pretetanus (baseline) values. These data provide evidence for an adequate pool of pre-existing mRNAs in dendrites, unaffected by actinomycin treatment, which can maintain late-LTP for at least 4 h.
Very few studies have examined directly the properties of LTP in isolated hippocampal dendrites. The data reported here provide evidence that protein synthesis from pre-existing mRNAs is sufficient for the maintenance of late-LTP. Our experiments cannot exclude the possibility that the protein synthesis required for late-LTP is derived from presynaptic terminals, glial cells or interneurones. However, for the following reasons we consider these possibilities to be unlikely. We observed no change in PPF ratios following LTP induction (see Manabe et al. 1993). More detailed studies addressing the possibility that increased presynaptic glutamate release contributes to LTP have failed to demonstrate changes in glutamate transporter currents as a result of LTP induction, specifically at CA3–CA1 synapses (Diamond et al. 1998; Luscher et al. 1998). Moreover, several recent studies have provided evidence for hippocampal dendritic translation of mRNAs, suggesting that the postsynaptic neurone is the locus for this de novo protein synthesis. Synaptosome preparations have been shown to contain mRNAs that are translated upon stimulation (Bagni et al. 2000), while cultured neuronal preparations, free from glia and interneurones, have been shown to undergo activity-dependent local dendritic translation of recombinant receptors (Ju et al. 2004). Furthermore, a recent report has implicated a role for mTOR-dependent synthesis of the eEF1A in the dendrites of CA1 pyramidal neurones following induction of LTP (Tsokas et al. 2005). In addition, evidence from in-vivo studies has shown that the expression of eEF1A in hippocampal pyramidal cell dendrites is increased following alterations in synaptic strength (Huang et al. 2005). Thus our data, together with these other recent reports, are consistent with a postsynaptic (dendritic) locus for the required protein synthesis in late-LTP.
Local translation and synaptic plasticity
The aim of this study was not to identify specific regulatory mRNA translation pathways or particular mRNAs that may be translated and whose products are components of late-LTP expression mechanisms. Nonetheless our results are consistent with data obtained recently from studies where more biochemical and molecular genetic approaches have been adopted (Kelleher et al. 2004a; Tsokas et al. 2005).
Using drug-based inhibitor studies, both our work, and that of others has suggested that NMDA-dependent LTP results in ‘non-specific’ regulation of translation by generally increasing the activity of the translation initiation machinery. The application of rapamycin prevents the phosphorylation of the translational regulator 4E-BP1 by mTOR. In its unphosphorylated state, 4E-BP1 remains bound to the translation initiation factor, euykaryotic initiation factor 4E (eIF4E), and the initiation of translation is inhibited. Our results, and those of others, support a role for this mTOR-signalling pathway in regulating the local synthesis of proteins during late-LTP (Tang et al. 2002; Cammalleri et al. 2003). In this respect the effect of rapamycin overlaps with reports that the extracellular regulated kinase (ERK) signalling pathway is also involved in the transcription-independent, translation-dependent phase of late-LTP (Kelleher et al. 2004a), as ERK signalling also results in increased activity of eIF4E (Banko et al. 2004).
In addition to these more general mechanisms leading to upregulation of translation at synapses, mRNA-specific mechanisms also appear to be activated whereby specific subsets of mRNAs are translated. Numerous studies of mRNAs during development have shown that elements within individual mRNAs can control when and where they are translated (for a review see Wilkie et al. 2003). Similar mechanisms have been shown to occur in neurones. For example mRNA for αCaMKII is translated in an NMDA receptor-dependent manner following visual experience (Wu et al. 1998), and can be delivered to synaptic sites following high-frequency burst stimulation in vivo (Havik et al. 2003). In addition, activity-dependent overexpression of recombinant AMPA receptor subunits can occur in isolated dendrites in culture (Ju et al. 2004). Furthermore, it is clear that many mRNAs coding for proteins implicated in the expression and maintenance of LTP in mammals or long-term facilitation in Aplysia are localized to dendrites/processes (Moccia et al. 2003; for a review see Steward & Schuman, 2003). It is to be anticipated that specific regulatory elements within these mRNAs may be the targets of processes initiated by activity-dependent alterations in synaptic strength. In addition to being potential mediators of alterations in synaptic strength in dendrites, regulation of mRNA translation and local protein synthesis have also been suggested to play a role in axons where they have been shown to contribute to growth cone guidance (Campbell & Holt, 2001), axonal regeneration (Verma et al. 2005), axonal repair following injury (Willis et al. 2005) and synaptic growth (Menon et al. 2004).
Finally, recent studies have shown that pharmacologically induced changes in synaptic strength, as measured electrophysiologically, can result from translation of dendritically localized mRNAs. BDNF-induced potentiation fEPSPs in hippocampal slices can occur in preparations where dendrites and cell bodies are physically isolated (Kang & Schuman, 1996; though see Frerking et al. 1998), and metabotropic glutamate receptor-mediated LTD can also be established in ‘isolated’ dendritic slice preparations (Huber et al. 2000). In this latter example, it is interesting to note that the reduction in synaptic efficacy induced by the metabotropic receptor agonist DHPG involves eIF4E, since it can be prevented when slices are incubated with the mRNA cap analogue, m7GpppG.
Conclusion
This study has demonstrated that it is possible to induce NMDA receptor-dependent, protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal neurones. Our results support the emerging consensus that the translation of pre-existing mRNAs is an important and physiologically relevant mechanism for synapse-specific alterations and neuronal adaptation. Whether the pool of mRNAs available for translation limits the extent and duration of the LTP that can be maintained, and how ultimately translation of these mRNAs is integrated with somatic protein synthesis remains to be determined.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (15/C19143 to KSD and DJAW). K.S.D. was a recipient of a Royal Society of Edinburgh Personal Research Fellowship. We thank Gordon Arbuthnott and Ann Wright for assistance with cresyl violet staining and Colin O'Carroll for advice and assistance with preliminary experiments. We thank Roger Nicoll for providing constructive and critical comments on the manuscript and our colleagues in the Cognition, Learning and Synaptic Plasticity Group at the University of Edinburgh for helpful discussions during the course of this project.
References
- Anderson WW, Collingridge GL. The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Meth. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Bagni C, Mannucci L, Dotti CG, Amaldi F. Chemical stimulation of synaptosomes modulates alpha-Ca2+/calmodulin-dependent protein kinase II mRNA association to polysomes. J Neurosci. 2000;20:RC76. doi: 10.1523/JNEUROSCI.20-10-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw KD, Emptage NJ, Bliss TV. A role for dendritic protein synthesis in hippocampal late LTP. Eur J Neurosci. 2003;18:3150–3152. doi: 10.1111/j.1460-9568.2003.03054.x. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci U S A. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus. 2005;15:551–556. doi: 10.1002/hipo.20078. [DOI] [PubMed] [Google Scholar]
- Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Bergles DE, Jahr CE. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron. 1998;21:425–433. doi: 10.1016/s0896-6273(00)80551-6. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Nargel UV, Morris RG, Bonhoeffer T. Competing for memory: hippocampal LTP under regimes of reduced protein synthesis. Neuron. 2004;44:1011–1020. doi: 10.1016/j.neuron.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Frey U, Frey S, Schollmeier F, Krug M. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol. 1996;490((3)):703–711. doi: 10.1113/jphysiol.1996.sp021179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Krug M, Brodemann R, Reymann K, Matthies H. Long-term potentiation induced in dendrites separated from rat's CA1 pyramidal somata does not establish a late phase. Neurosci Lett. 1989;97:135–139. doi: 10.1016/0304-3940(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Havik B, Rokke H, Bardsen K, Davanger S, Bramham CR. Bursts of high-frequency stimulation trigger rapid delivery of pre-existing alpha-CaMKII mRNA to synapses: a mechanism in dendritic protein synthesis during long-term potentiation in adult awake rats. Eur J Neurosci. 2003;17:2679–2689. doi: 10.1046/j.1460-9568.2003.02712.x. [DOI] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1 is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25:7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Jiang C, Schuman EM. Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem Sci. 2002;27:506–513. doi: 10.1016/s0968-0004(02)02190-4. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004a;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004b;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron. 1998;21:435–441. doi: 10.1016/s0896-6273(00)80552-8. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Menon KP, Sanyal S, Habara Y, Sanchez R, Wharton RP, Ramaswami M, Zinn K. The translational repressor Pumilio regulates presynaptic morphology and controls postsynaptic accumulation of translation factor eIF-4E. Neuron. 2004;44:663–676. doi: 10.1016/j.neuron.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya EEY, Kalachikov S, Spahn CM, Frank J, Kandel ER, Barad M, Martin KC. An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci. 2003;23:9409–9417. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff L, Fiala J, Allwardt B, Harris K. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Fass B. Polyribosomes associated with dendritic spines in the denervated dentate gyrus: evidence for local regulation of protein synthesis during reinnervation. Prog Brain Res. 1983;58:131–136. doi: 10.1016/S0079-6123(08)60013-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian QB, Nakayama K, Okano A, Suzuki T. Identification of mRNAs localizing in the postsynaptic region. Brain Res Mol Brain Res. 1999;72:147–157. doi: 10.1016/s0169-328x(99)00214-4. [DOI] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tiedge H. Translational control at the synapse. Neuroscientist. 2004;10:456–466. doi: 10.1177/1073858404265866. [DOI] [PubMed] [Google Scholar]
- Wilkie GS, Dickson KS, Gray NK. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem Sci. 2003;28:182–188. doi: 10.1016/S0968-0004(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Wells D, Tay J, Mendis D, Abbott MA, Barnitt A, Quinlan E, Heynen A, Fallon JR, Richter JD. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21:1129–1139. doi: 10.1016/s0896-6273(00)80630-3. [DOI] [PubMed] [Google Scholar]