Abstract
Endothelium-dependent dilatation (EDD) is impaired with ageing in sedentary, but not in regularly exercising adults. We tested the hypotheses that differences in tetrahydrobiopterin (BH4) bioactivity are key mechanisms explaining the impairment in EDD with sedentary ageing, and the maintenance of EDD with ageing in regularly exercising adults. Brachial artery flow-mediated dilatation (FMD), normalized for local shear stress, was measured after acute oral placebo or BH4 in young sedentary (YS) (n = 10; 22 ± 1 years, mean ± s.e.m.), older sedentary (OS) (n = 9; 62 ± 2), and older habitually aerobically trained (OT) (n = 12; 66 ± 1) healthy men. At baseline, FMD was ∼50% lower in OS versus YS (1.12 ± 0.09 versus 0.57 ± 0.09 (Δmm (dyn cm−2)) × 10−2, P < 0.001; 1 dyn = 10−5 N), but was preserved in OT (0.93 ± 0.08 (Δmm (dyn cm−2)) × 10−2). BH4 administration improved FMD by ∼45% in OS (1.00 ± 0.10 (Δmm (dyn cm−2)) × 10−2, P < 0.01 versus baseline), but did not affect FMD in YS or OT. Endothelium-independent dilatation neither differed between groups at baseline nor changed with BH4 administration. These results suggest that BH4 bioactivity may be a key mechanism involved in the impairment of conduit artery EDD with sedentary ageing, and the EDD-preserving effect of habitual exercise.
Ageing is a major risk factor for the development of cardiovascular diseases (CVD) (Lakatta & Levy, 2003). A central feature of the age-associated increase in CVD risk is a reduction in vascular endothelium-dependent dilatation (EDD) (Celermajer et al. 1994; Taddei et al. 1995). As such, determining the mechanisms by which ageing impairs EDD and establishing strategies for the prevention of this adverse effect have important clinical implications.
The decrease in EDD with ageing in sedentary adults is mediated by oxidative stress (Taddei et al. 2000; Eskurza et al. 2004b), whereas EDD is preserved with ageing in the absence of oxidative stress in habitually exercising adults (Taddei et al. 2000; Eskurza et al. 2004b). However, the mechanisms by which the presence and absence of oxidative stress modulates EDD with ageing in sedentary and physically active humans, respectively, are unknown.
Oxidative stress can suppress EDD by oxidizing tetrahydrobiopterin (BH4), an essential cofactor for endothelial nitric oxide synthase (eNOS), thus reducing BH4 bioactivity (Milstien & Katusic, 1999; Cosentino et al. 2001; Laursen et al. 2001; Landmesser et al. 2003). Reduced BH4 bioactivity leads to the ‘uncoupling’ of eNOS (Pou et al. 1992; Vasquez-Vivar et al. 1998, 2002), resulting in greater formation of superoxide anions than NO and impaired EDD (Vasquez-Vivar et al. 1998; Cosentino & Luscher, 1999; Cosentino et al. 2001; Laursen et al. 2001; Landmesser et al. 2003). BH4 supplementation improves EDD in patients with clinical cardiovascular disorders (Stroes et al. 1997; Maier et al. 2000; Setoguchi et al. 2002). However, the role of BH4 in the modulation of EDD with sedentary and physically active ageing in healthy adults is unknown.
In the present study, we tested the hypothesis that differences in BH4 bioactivity are a key mechanism explaining the impairment in endothelium-independent dilatation (EDD) with sedentary ageing and the maintenance of EDD with ageing in regularly exercising adults. To do so, we determined the effect of acute BH4 supplementation (single oral dose, 10 mg kg−1) on brachial artery flow-mediated dilatation (FMD), a clinically important measure of conduit artery EDD, in groups of young and older sedentary and aerobic exercise-trained healthy men. Because group differences in brachial artery FMD are influenced by corresponding differences in the dilatory stimulus (i.e. local shear stress or blood flow) (Mitchell et al. 2004), brachial artery FMD was normalized for local shear stress.
Methods
Subjects
A total of 31 healthy men were studied: 10 young (aged 19–26 years) and 21 older (aged 55–74 years). During the previous 2 years, the older men were either sedentary (no regular physical activity) (n = 9) or habitually exercising (vigorous aerobic-endurance exercise, more than three sessions per week) (n = 12). Subjects were normotensive (blood pressure, <140/90), nonsmokers, nonobese, and free of CVD, as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG (older men only). Subjects were excluded if they were classified as having the metabolic syndrome according to the criteria established by the National Cholesterol Education Program Adult Treatment Panel III expert panel or a modification of the original (World Health Organization 2001; Laaksonen et al. 2002; Lakka et al. 2002). Candidates who had used antioxidants (e.g. vitamins C and E) within 6 weeks of study or were taking other medications were excluded. Subjects gave their written informed consent to participate. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder.
Experimental procedures
All procedures were performed in the University of Colorado at Boulder General Clinical Research Center. After completion of screening procedures, two main experimental sessions (i.e. placebo baseline and BH4 treatment) were conducted within 2 days of each other. Prior to the main sessions, subjects fasted for 10–12 h and abstained from any physical exercise on the previous day. During the experimental sessions, subjects were in the supine position and instrumented with an intravenous catheter in one arm for acquisition of blood.
Measurements
Brachial artery FMD and EDD
Brachial artery FMD was assessed noninvasively as described originally by Celermajer et al. (1992) and more recently by our laboratory (Eskurza et al. 2004b). Brachial artery baseline and peak (post-ischaemia) diameters were analysed off line with an automatic wall-tracking system (Vascular Analysis Tools, 4.0, Medical Imaging Analysis, LLC, IA, USA). The same investigator (I.E.) performed all analyses blinded to the group assignment of the subject and the experimental condition. Velocities and diameters were analysed on separate days. Baseline and peak mean blood velocities were measured as previously described (Eskurza et al. 2004b). The reliability of mean blood velocities obtained for nine subjects measured on two separate days (r= 0.97, P < 0.001) was similar to that reported in a recent population study (Mitchell et al. 2004).
Shear stress (SS) (dyn cm−2; 1 dyn = 10−5 N) averaged over the whole entire cycle was calculated using the formula SS = 8 ×μ×V/D, where μ, V and D represent viscosity (dyn × (s cm−2)), velocity (cm s−1) and diameter (cm), respectively, as described recently (Mitchell et al. 2004). To calculate baseline SS (SSbase), baseline V and D were used. Because brachial artery diameter is lower during the end of the occlusion period than before occlusion (Levenson et al. 2001), the peak velocity and corresponding diameter obtained during the last 15–20 s of occlusion was used to calculate peak SS (SSpeak).
Because the conventional expression of FMD as percentage change from baseline is highly dependent on baseline brachial artery diameter (i.e. the same absolute change in diameter results in a greater percentage change the smaller the baseline diameter), to normalize FMD for local SS we used the following formula: normalized FMD = (peak D− baseline D)/SSpeak. SSpeak was used to normalize FMD rather than the area under the SS curve (AUC) for two main reasons: (1) in our laboratory we are currently unable to determine the AUC of SS, and (2) the normalization of FMD with peak shear rate (i.e. an estimate of SS commonly used when viscosity in not measured) versus AUC of shear rate provides comparable efficacy for eliminating the effect of baseline diameter FMD among individuals (Pyke et al. 2004).
Brachial artery endothelium-independent dilatation was measured with sublingual nitroglycerin, as previously described (Eskurza et al. 2004b).
Arterial blood pressure
Resting blood pressure was measured over the brachial artery using a semi-automated device (Dynamap XL; Johnson and Johnson).
Plasma marker of oxidative stress
Plasma samples were analysed for oxidized low-density lipoproteins (ALPCO Diagnostics, Windham, NH, USA).
Body composition
Total body fat mass and fat-free mass (FFM) were measured by dual-energy X-ray absorptiometry (DXA-GE; Lunar corporation, Madison, WI, USA; software version 5.60.003), and waist and hip circumferences and body mass index (BMI) by anthropometry (Van Pelt et al. 1998).
Maximal oxygen consumption V̇O2 max
V̇O2 maxwas measured during graded treadmill exercise test using open-circuit spirometry, as previously described (DeSouza et al. 2000).
Blood viscosity
Whole-blood viscosity was measured at shear rates of 0.3–60 r.p.m. (2.5 × 10−6 to 6.0 × 10−5g) at 37°C using a viscometer (model DV-I+; Brookfield Engineering Laboratories, Inc., Staughton, MA, USA), as previously described by our laboratory (Dinenno et al. 2001). Blood viscosity values at rates of 60 r.p.m. were used to calculate wall SS (see above).
Dietary analyses
Macronutrient and antioxidant (vitamins A, C and E) intake were quantified using the food-frequency questionnaire (NHANES III food-intake database).
Study design
We conducted a double-blind crossover study in which subjects were randomly assigned to take either placebo or BH4 (single dose of 10 mg (kg body weight)−1) pills (Schirks Laboratories, Switzwerland). The selection of this single dose was based on the facts that: (1) it increases plasma biopterin levels by ∼50-fold (Fiege et al. 2004), (2) improvements in brachial artery FMD are observed with as little as a fourfold increase in BH4 above normal baseline concentrations (Ueda et al. 2000), and (3) commercially available BH4 is used therapeutically to treat special forms of phenylketonuria at a dose of 2–10 mg per kg of body weight, and a single dose of 10 mg (kg body weight)−1) decreases symptoms in these conditions (Niederwieser et al. 1982; Kure et al. 1999). Two days were allowed between the phases of the crossover study in order to ensure an adequate washout period, as previously described (Fiege et al. 2004). The randomization of the treatments was performed by the General Clinical Research Center pharmacist who was not involved in data acquisition or analysis. To ensure compliance with the treatments, subjects were contacted by phone the night prior to the experimental session. With the unavoidable exception of knowing the age group (young versus older) of the subjects during data collection, the investigator (I.E.) who performed the data collection and analysis was blinded to the group and treatment condition. Measurements were obtained ∼3 h after ingestion when the dose of BH4 used reaches its maximal plasma concentration (Fiege et al. 2004).
Statistical analyses
Statistical analyses were performed with SPSS statistical package (version 11.0; SPSS, Chicago, IL, USA). Differences in subject characteristics across the three groups were determined by one-way ANOVA. To determine the effects of placebo versus BH4 administration on all outcome measures, repeated measures ANOVA was used. In the case of significant F values, post hoc analyses were performed using the Bonferroni correction to identify significant differences among mean values. To examine relations between variables of interest, bivariate relations were performed with Pearson product–moment correlations.
Results
Subject characteristics
Values are shown in Tables 1 and 2. The groups did not differ in body mass, total cholesterol, low-density lipoprotein-cholesterol (LDL-C), triglycerides, glucose, blood viscosity, haematocrit and systolic blood pressure. BMI, total body fat, waist and hip circumferences, waist-to-hip ratio (WHR) and plasma insulin concentration were greater, and V̇O2 max was lower, in the older sedentary men compared with the other two groups (P < 0.05). High-density lipoprotein-cholesterol (HDL-C) was greater, and resting heart rate was lower, in the older exercising men than in the other two groups (P < 0.05). Although well within the normal range, diastolic blood pressure was higher in both older groups compared with the young controls (P < 0.05). Plasma concentrations of oxidized LDL were higher in the older compared with the young sedentary men (60.5 ± 6.5 versus 45.8 ± 5.8 U l−1, P = 0.05), but were not different in the young sedentary and older exercising (52.1 ± 4.8 U l−1) men. Groups did not differ in dietary intake of major antioxidants such as vitamins A, E and C (data not shown).
Table 1.
Subject characteristics
| Young sedentary | Older sedentary | Older trained | |
|---|---|---|---|
| Age (years) | 22 ± 1 | 62 ± 2* | 66 ± 1* |
| Body mass (kg) | 73 ± 3 | 79 ± 3 | 70 ± 2 |
| Body fat (%) | 15.4 ± 2.2 | 27.3 ± 2.6* | 16.9 ± 1.1† |
| BMI (kg m−2) | 23.3 ± 0.9 | 25.6 ± 1.0 | 22.8 ± 0.6† |
| Waist circumference (cm) | 78.8 ± 2.4 | 94.8 ± 3.1* | 82.8 ± 1.8† |
| Hip circumference (cm) | 93.0 ± 1.7 | 99.9 ± 1.9* | 93.4 ± 1.4† |
| WHR | 0.84 ± 0.01 | 0.95 ± 0.02* | 0.89 ± 0.02† |
| Total cholesterol (mmol l−1) | 4.5 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.2 |
| LDL cholesterol (mmol l−1) | 2.7 ± 0.2 | 3.3 ± 0.2 | 3.0 ± 0.2 |
| HDL cholesterol (mmol l−1) | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1*† |
| Triglycerides (mmol l−1) | 1.2 ± 0.2 | 1.1 ± 0.1 | 0.86 ± 0.1 |
| Plasma insulin (μIU ml−1) | 3.7 ± 0.5 | 6.3 ± 1.0* | 3.3 ± 0.5† |
| Plasma glucose (mmol l−1) | 4.9 ± 0.1 | 5.3 ± 0.2 | 4.8 ± 0.1 |
| V̇O2 max | 48.0 ± 1.3 | 29.1 ± 2.1* | 41.3 ± 1.2*† |
| BV (dyn cm−2) | 0.036 ± 0.001 | 0.033 ± 0.002 | 0.036 ± 0.003 |
| Haematocrit (%) | 45.0 ± 0.8 | 44.4 ± 1.1 | 43.8 ± 0.7 |
Data are means ± s.e.m. BMI, body mass index; WHR, waist-to-hip ratio; V̇O2 max, maximal oxygen consumption; BV, blood viscosity (1 dyn = 10−5 N).
P < 0.05 versus young;
P < 0.05 versus older sedentary.
Table 2.
Systemic haemodynamics and brachial artery parameters after placebo and BH4 supplementation
| Young sedentary | Older sedentary | Older endurance-trained | ||||
|---|---|---|---|---|---|---|
| Placebo | BH4 | Placebo | BH4 | Placebo | BH4 | |
| Systolic BP (mmHg) | 110 ± 3 | 111 ± 4 | 111 ± 4 | 113 ± 5 | 107 ± 3 | 108 ± 4 |
| Diastolic BP (mmHg) | 58 ± 2 | 59 ± 2 | 69 ± 1* | 69 ± 2* | 66 ± 2* | 67 ± 2* |
| Heart rate (beats min−1) | 52 ± 3 | 50 ± 3 | 58 ± 3 | 55 ± 2 | 46 ± 2*† | 46 ± 2*† |
| Baseline D (mm) | 4.13 ± 0.11 | 4.10 ± 0.11 | 4.36 ± 0.16 | 4.33 ± 0.16 | 3.99 ± 0.14 | 3.96 ± 0.14 |
| Baseline MBV (cm s−1) | 5.5 ± 0.5 | 5.3 ± 0.5 | 5.7 ± 0.3 | 5.4 ± 0.4 | 4.8 ± 0.3 | 4.9 ± 0.3 |
| Peak MBV (cm s−1) | 42.9 ± 2.5 | 43.8 ± 1.4 | 44.6 ± 3.4 | 40.9 ± 2.3 | 37.2 ± 2.1† | 36.2 ± 2.2* |
| SSbase (dyn cm−2) | 3.8 ± 0.3 | 3.5 ± 0.2 | 3.9 ± 0.3 | 3.7 ± 0.3 | 3.3 ± 0.3 | 3.5 ± 0.5 |
| SSpeak (dyn cm−2) | 28.6 ± 1.7 | 29.2 ± 1.1 | 29.0 ± 3.2 | 26.2 ± 1.8 | 26.7 ± 2.3 | 24.6 ± 1.7 |
| FMD (Δmm) | 0.32 ± 0.02 | 0.31 ± 0.02 | 0.16 ± 0.03* | 0.26 ± 0.02‡ | 0.23 ± 0.01† | 0.24 ± 0.02 |
| FMD (%) | 7.7 ± 0.5 | 7.6 ± 0.6 | 3.9 ± 0.7* | 6.0 ± 0.7‡ | 5.9 ± 0.5† | 6.3 ± 0.6 |
Data are means ± s.e.m. BH4, tetrahydrobiopterin. BP, blood pressure; D, diameter; MBV, mean blood velocity, FMD, flow-mediated dilatation; SSbase, baseline shear stress (1 dyn = 10−5 N); SSpeak, peak shear stress.
P < 0.05 versus young sedentary;
P < 0.05 versus older sedentary;
P < 0.05 versus placebo in the same group.
Brachial artery response to BH4
Baseline brachial artery diameter (i.e. placebo condition) did not differ among groups and was not affected by BH4 administration (Table 2).
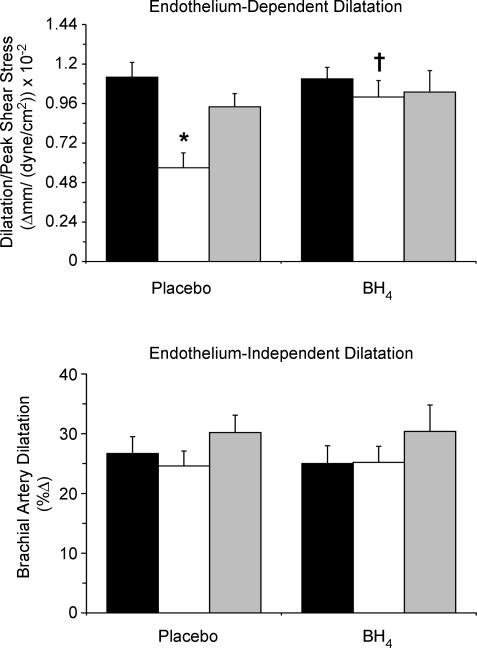
Brachial artery normalized FMD at baseline (after placebo) and in response to BH4 is shown in Fig. 1. At baseline, FMD was ∼50% lower in the older sedentary men compared with the young controls (P < 0.001), but was not different in the older exercising and young men. Brachial artery FMD was increased by ∼45% after BH4 administration in the older sedentary men (P < 0.01 versus placebo baseline), but was not affected by BH4 in the other two groups. There were no significant group differences in brachial artery FMD after BH4 administration (all P > 0.05).
Figure 1.
Brachial artery flow-mediated dilatation (FMD) (top panel) and endothelium-independent dilatation (bottom panel) in young sedentary (black bars) and older sedentary (white bars) and regularly exercising (grey bars) healthy men after acute oral placebo (baseline conditions) or tetrahydrobiopterin (BH4) supplementation. FMD values are normalized for the stimulus. Mean ± s.e.m. values are shown. *P < 0.001 versus young and older exercising men. †P < 0.01 versus placebo condition.
There were no group differences in brachial artery dilatation in response to sublingual nitroglycerin at baseline, and the responses to nitroglycerin were not affected by BH4 in any of the groups (Table 2).
Other brachial artery and haemodynamic information is presented in Table 2. Systolic and diastolic blood pressures, heart rate, baseline mean blood velocity, SSbase and SSpeak were similar in response to placebo and BH4 administration. Whole-blood viscosity and haematocrit did not change with BH4 administration in any of the groups (data not shown).
In the pooled sample, the improvement in normalized FMD in response to BH4 was inversely related to V̇O2 max (r=−0.46; P = 0.01) and normalized FMD at baseline (r=−0.61; P < 0.001), and was positively related to plasma insulin concentration (r= 0.37; P = 0.04).
Discussion
This study provides new insight into the potential mechanisms mediating the impairment in peripheral conduit artery EDD with sedentary ageing, and the maintenance of EDD with physically active ageing in humans. Specifically, the main findings of this study are that acute administration of BH4 improved brachial FMD in older sedentary men, but did not affect FMD in young sedentary or habitually exercising older men. These results suggest that: (1) reduced BH4 bioactivity may contribute to the suppression of brachial artery FMD with sedentary human ageing; and (2) maintained BH4 bioactivity may be involved in the preserved FMD with ageing seen in habitually exercising men.
Role of BH4 on EDD with sedentary ageing
In the present study, baseline brachial artery FMD (placebo control condition) was ∼50% lower in older compared with young healthy men, whereas dilatation in response to sublingual nitroglycerin, a NO donor, was not different. Consistent with previous findings (Celermajer et al. 1994; Eskurza et al. 2004b), these results indicate an age-related impairment in peripheral conduit artery EDD in the absence of any reduction in endothelium-independent dilatation in sedentary healthy adults.
We (Eskurza et al. 2004b) and others (Taddei et al. 2000) previously demonstrated that supraphysiological concentrations of ascorbic acid, a potent antioxidant, restore the age-related loss of EDD in older sedentary adults, suggesting that oxidative stress is the key mechanism involved. This is in agreement with the observation that the aortas of older rats and mice have increased vascular production of reactive oxygen species (ROS), which is associated with reductions in NO bioavailability and EDD (van der Loo et al. 2000; Blackwell et al. 2004). In the present study, we also found that plasma concentrations of oxidized LDL, a relatively insensitive systemic marker of oxidative stress, were higher in the older compared with the young sedentary men, as reported previously (Mosinger, 1997; Eskurza et al. 2004a).
We hypothesized that an important mechanism by which oxidative stress could impair EDD with sedentary ageing is by influencing the biological activity of BH4, the essential cofactor of eNOS for NO synthesis (Pollock et al. 1991). BH4 is one of the most potent endogenous reducing agents. Therefore, vascular oxidative stress, specifically peroxynitrate, rapidly oxidizes BH4, with subsequent generation of dihydrobiopterin (BH2), its oxidized and inactive form (Milstien & Katusic, 1999; Laursen et al. 2001). The experimental observation that the aortas of older rats show increased production of peroxynitrate (van der Loo et al. 2000) was also consistent with the idea of increased BH4 oxidation with ageing.
The present results extend previous findings by demonstrating that BH4 administration restores the age-associated loss of brachial artery FMD in sedentary healthy men. BH4 did not affect the dilatory response to sublingual nitroglycerin. Therefore, these findings suggest that reduced BH4 bioactivity may be a key mechanism involved in the impairment of peripheral conduit artery EDD with sedentary ageing. Our results are consistent with earlier experimental observations that showed that acute BH4 supplementation improved or restored EDD in groups at risk of or with clinical CVD, i.e. states characterized by vascular oxidative stress (Stroes et al. 1997; Heitzer et al. 2000; Maier et al. 2000; Ueda et al. 2000; Setoguchi et al. 2002).
The exact mechanism by which BH4 contributes to reduced brachial artery FMD with sedentary ageing in humans cannot be discerned from the present results. It is possible that conduit artery EDD may become impaired with ageing in part via increased endothelial cell production of ROS with consequent increased BH4 oxidation and increased BH2 relative to BH4. Indeed, the BH4/BH2 ratio determines superoxide versus NO generation from eNOS (Vasquez-Vivar et al. 2002). As such, a reduction in the active form of the cofactor (BH4) would result in decreased BH4 biological activity and, thus, the uncoupling (dysfunction) of eNOS, thereby generating superoxide instead of NO (Pou et al. 1992; Vasquez-Vivar et al. 1998, 2002). However, in mice, BH4 and BH2 concentrations in aorta, although tending to be lower in older animals, are not significantly different than in young mice, and the BH4/BH2 ratio is similar (Blackwell et al. 2004). Moreover, guanosine 5′-triphosphate cyclohydrolase I (GTPC I), the rate-limiting enzyme in BH4 synthesis, is not different in the young and older mice (Blackwell et al. 2004). There are no published data on vascular BH4 or BH2 concentrations or GTPC I enzyme activity with ageing in humans. The findings in mice with ageing (Blackwell et al. 2004) differ from observations in experimental models of diabetes and low-renin hypertension in which reductions in vascular endothelial BH4 production and GTPC I activity appear to play an important role in mediating associated endothelial dysfunction (Zheng et al. 2003; Meininger et al. 2004). Interestingly, stimulation of BH4 synthesis restores vascular endothelial function in some animal models of hypercholesterolaemia despite elevated baseline levels of BH4 (d'Uscio et al. 2003; Alp et al. 2004).
In vitro, half-maximal and maximal NO synthesis, estimated from l-citrulline production, occurs in the micromolar range of BH4 concentrations (0.1 and 1 μm, respectively) (Chen et al. 1995). However, much smaller plasma BH4 concentrations (in the nanomolar range) achieved after a single oral dose of 2 mg (kg body weight)−1 of sapropterin hydrochloride, a BH4 precursor, improve brachial FMD in young adult smokers (Ueda et al. 2000). The discrepancy between in vitro and in vivo results, including the present findings, may have at least two explanations. First, it is possible that some eNOS is activated at much lower BH4 concentrations than those reported in in vitro preparations. Second, BH4 increases the affinity of eNOS for l-arginine (Klatt et al. 1994). Thus, it is possible that relatively low BH4 concentrations could produce an amount of NO greater than expected based on the BH4 concentration alone.
In the present study, we did not specifically inhibit NO bioactivity and therefore cannot exclude the possibility that other endothelium-dependent dilatory mechanisms (e.g. endothelium-derived hyperpolarizing factor or prostacyclin) were involved in the BH4-related improvement of FMD seen in the older sedentary men. However, conduit artery FMD is primarily mediated by endothelial-derived NO (Lieberman et al. 1996; Mullen et al. 2001). Moreover, to the best of our knowledge, BH4 is a cofactor only for eNOS synthesis of NO and not for any other endothelium-dependent dilator.
Finally, we wish to emphasize that formation of ROS from other vascular sources, such as fibroblasts, smooth muscle cells and inflammatory cells, may also act to reduce the bioavailability of NO (Ushio-Fukai et al. 1996; Griendling et al. 2000), and NO production could be impaired with ageing via other mechanisms including reductions in eNOS expression, although this is unclear (Barton et al. 1997; van der Loo et al. 2000).
Preservation of FMD with age in habitually exercising men
Consistent with our recent findings (Eskurza et al. 2004b), in the present study, brachial artery FMD was preserved in older men who habitually exercise. Similar observations have been made for EDD in forearm resistance vessels (DeSouza et al. 2000; Taddei et al. 2000). In the present study, BH4 supplementation had no effect on brachial artery FMD in either young sedentary or older exercising men. Consistent with this, the difference (improvement) in brachial artery FMD between the placebo and BH4 treatment conditions was inversely related to baseline V̇O2 max, i.e. maximal aerobic exercise capacity. Taken together, these findings support the idea that the beneficial effects of habitual exercise/high aerobic fitness on EDD with ageing may be associated with the maintenance BH4 bioactivity.
The mechanisms by which regular aerobic exercise preserves BH4 bioactivity and EDD with ageing are unknown, but they are likely to involve, at least in part, the absence of oxidative stress.
Aerobic (endurance) exercise training is associated with an upregulation of important enzymatic antioxidants, as well as reduced production of superoxide anions (Sen, 1995; Fukai et al. 2000; Rush et al. 2000). Furthermore, the maintenance of resistance vessel and conduit artery EDD with ageing in physically active adults is associated with a lack of improvement in EDD in response to ascorbic acid administration (Taddei et al. 2000; Eskurza et al. 2004b), suggesting an absence of baseline oxidative stress in contrast to their sedentary peers. The present findings suggest that the maintenance of BH4 bioactivity may be a novel mechanism by which regular aerobic exercise preserves EDD with ageing. One possibility is that regular exercise may prevent the development of oxidative stress with ageing, thus reducing the oxidation of BH4, which maintains normal eNOS function and production of NO.
Limitations
This study has at least four important limitations. First, acute BH4 supplementation may have improved EDD in the older sedentary men in part via its non-specific antioxidant effects. Supraphysiological concentrations of BH4 scavenge ROS, including superoxide anions, in vitro (Hyun et al. 1995; Kojima et al. 1995). However, based on electron paramagnetic resonance kinetic analysis, superoxide scanvenging by BH4 is not a major reaction in vivo (Vasquez-Vivar et al. 2001). More importantly, the ‘antioxidant’ effect of BH4 was due to its ability to decrease superoxide formation and increase NO synthesis from eNOS (Vasquez-Vivar et al. 2002).
Second, we attempted, but were unable to measure plasma BH4 concentrations. As such, we cannot rule out the possibility of group differences at baseline or in response to BH4 supplementation. To our knowledge, only one previous study has reported plasma BH4 concentrations at baseline and after experimental stimulation of BH4 in humans (chronic smokers) (Ueda et al. 2000). However, the single dose used in the present study increases plasma biopterin levels by ∼50-fold (Fiege et al. 2004) and improvements in brachial artery FMD are observed with as little as a fourfold increase in BH4 above normal baseline concentrations (Ueda et al. 2000), consistent with the fact that a significant improvement in brachial FMD was observed with BH4 in the older sedentary subjects in the present study. Moreover, plasma concentrations do not necessarily reflect group differences in intra-endothelial BH4 concentrations. In patients with coronary artery disease, baseline plasma concentrations of BH4 are high (Tatzber et al. 1991; Schumacher et al. 1997), but acute supplementation of BH4 nevertheless restores EDD (Maier et al. 2000).
Third, conduit artery EDD and resistance vessel EDD do not necessary correlate (Eskurza et al. 2001). Therefore, our findings cannot be extended to smaller vessel function. However, intra-arterial administration of BH4 improves EDD of resistance vessels in patients with CVD (Stroes et al. 1997; Heitzer et al. 2000; Setoguchi et al. 2002), suggesting that reduced BH4 bioavailability may contribute to endothelial dysfunction in both conduit and resistance vessels.
Finally, in the present study a single high dose of BH4 was used because the experimental aim was to gain mechanistic insight into the role of BH4 in the tonic modulatory influences of age and habitual exercise on baseline EDD. We recognize that based on the present results and those of others (Stroes et al. 1997; Heitzer et al. 2000; Maier et al. 2000; Ueda et al. 2000; Setoguchi et al. 2002), it may now be of clinical interest to examine the potential therapeutic benefits of sustained lower-dose administration of BH4 in patients at risk of CVD or with existing clinical disorders.
Conclusions
The results from the present investigation suggest for the first time that reduced BH4 bioactivity may be a key mechanism involved in the impairment of peripheral conduit artery EDD with sedentary ageing. Our findings also provide the first experimental evidence supporting the hypothesis that preservation of conduit artery EDD with physically active ageing may be associated with the maintenance of BH4 bioactivity.
Acknowledgments
This study was supported by NIH awards AG13038, AG06537 and RR00051.
References
- Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Chen PF, Tsai AL, Wu KK. Cysteine 99 of endothelial nitric oxide synthase (NOS-III) is critical for tetrahydrobiopterin-dependent NOS-III stability and activity. Biochem Biophys Res Commun. 1995;215:1119–1129. doi: 10.1006/bbrc.1995.2579. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res. 1999;43:274–278. doi: 10.1016/s0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004a;286:H1528–1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004b;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol. 2001;88:1067–1069. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, Thony B, Blau N. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab. 2004;81:45–51. doi: 10.1016/j.ymgme.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- Hyun J, Komori Y, Chaudhuri G, Ignarro LJ, Fukuto JM. The protective effect of tetrahydrobiopterin on the nitric oxide-mediated inhibition of purified nitric oxide synthase. Biochem Biophys Res Commun. 1995;206:380–386. doi: 10.1006/bbrc.1995.1052. [DOI] [PubMed] [Google Scholar]
- Klatt P, Schmid M, Leopold E, Schmidt K, Werner ER, Mayer B. The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem. 1994;269:13861–13866. [PubMed] [Google Scholar]
- Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K. Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res. 1995;23:419–430. doi: 10.3109/10715769509065263. [DOI] [PubMed] [Google Scholar]
- Kure S, Hou DC, Ohura T, Iwamoto H, Suzuki S, Sugiyama N, Sakamoto O, Fujii K, Matsubara Y, Narisawa K. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr. 1999;135:375–378. doi: 10.1016/s0022-3476(99)70138-1. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol. 2001;38:1668–1674. doi: 10.1016/s0735-1097(01)01604-7. [DOI] [PubMed] [Google Scholar]
- Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, Wood MK, Wade LA, Wu G. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J. 2004;18:1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Mosinger BJ. Human low-density lipoproteins: oxidative modification and its relation to age, gender, menopausal status and cholesterol concentrations. Eur J Clin Chem Clin Biochem. 1997;35:207–214. doi: 10.1515/cclm.1997.35.3.207. [DOI] [PubMed] [Google Scholar]
- Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- Niederwieser A, Curtius HC, Wang M, Leupold D. Atypical phenylketonuria with defective biopterin metabolism. Monotherapy with tetrahydrobiopterin or sepiapterin, screening and study of biosynthesis in man. Eur J Pediatr. 1982;138:110–112. doi: 10.1007/BF00441135. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol. 2000;279:H2068–2076. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Halwachs G, Tatzber F, Fruhwald FM, Zweiker R, Watzinger N, Eber B, Wilders-Truschnig M, Esterbauer H, Klein W. Increased neopterin in patients with chronic and acute coronary syndromes. J Am Coll Cardiol. 1997;30:703–707. doi: 10.1016/s0735-1097(97)00172-1. [DOI] [PubMed] [Google Scholar]
- Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- Setoguchi S, Hirooka Y, Eshima K, Shimokawa H, Takeshita A. Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J Cardiovasc Pharmacol. 2002;39:363–368. doi: 10.1097/00005344-200203000-00007. [DOI] [PubMed] [Google Scholar]
- Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tatzber F, Rabl H, Koriska K, Erhart U, Puhl H, Waeg G, Krebs A, Esterbauer H. Elevated serum neopterin levels in atherosclerosis. Atherosclerosis. 1991;89:203–208. doi: 10.1016/0021-9150(91)90061-7. [DOI] [PubMed] [Google Scholar]
- Ueda S, Matsuoka H, Miyazaki H, Usui M, Okuda S, Imaizumi T. Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol. 2000;35:71–75. doi: 10.1016/s0735-1097(99)00523-9. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin-II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Davy KP, Stevenson ET, Wilson TM, Jones PP, Desouza CA, Seals DR. Smaller differences in total and regional adiposity with age in women who regularly perform endurance exercise. Am J Physiol Endocrinol Metab. 1998;275:E626–634. doi: 10.1152/ajpendo.1998.275.4.E626. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Whitsett J, Martasek P, Hogg N, Kalyanaraman B. Reaction of tetrahydrobiopterin with superoxide: EPR-kinetic analysis and characterization of the pteridine radical. Free Radic Biol Med. 2001;31:975–985. doi: 10.1016/s0891-5849(01)00680-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adults Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation. 2003;108:1238–1245. doi: 10.1161/01.CIR.0000089082.40285.C3. [DOI] [PubMed] [Google Scholar]