Abstract
Experiments were carried out to test the hypothesis that, in the absence of vision, position sense at the human forearm is generated by the combined input from muscle spindles in elbow flexor muscles and signals of central origin giving rise to a sense of effort. In a forearm position-matching task, to remove a possible contribution from the sense of effort, the reference arm was held supported at the test angle. Subjects were less accurate in matching elbow position of the supported forearm than when it was unsupported. Adding a 2 kg weight to the unsupported reference arm led subjects to make matching errors consistent with an increase in the effort signal. Evidence of a contribution from muscle spindles was provided by showing that the direction of position matching errors could be systematically altered by flexion or extension conditioning of the reference arm before its placement at the test angle. Such changes in errors with conditioning could be shown to be present when the reference arm was supported, unsupported, or unsupported and weighted. It is concluded that both peripheral signals from muscle spindles and signals of central origin, associated with the motor command required to maintain arm position against the force of gravity, can provide information about forearm position.
The question of whether we have a ‘muscular sense’ has fascinated neuroscientists since the 19th century. Von Helmholtz (1867) put forward the theory of ‘sensation of innervation’ whereby sensations apparently arising from within the muscles actually took origin somewhere in the brain, in association with the motor commands. That view was not shared by Sherrington (1900) who believed that muscle sensations were the result of activity of afferents located within the muscles. Sherrington's view persists in various forms to the present day.
Throughout much of the 20th century, it was believed that the main group of receptors responsible for kinaesthesia, the sense of limb position and movement, were slowly adapting joint receptors (Skoglund, 1973). It was the experiments of Goodwin et al. (1972) on the sensory effects of muscle vibration that provided the first evidence for a role of muscle spindles in conscious sensation. Today we believe that in the absence of visual information, the primary endings of muscle spindles are responsible for the sense of position and movement of our limbs, and that secondary endings of spindles contribute to the sense of position while tendon organs provide us with a sense of tension. At some, particularly the more distal joints, additional information is provided by skin and joint afferents. For a review, see Gandevia (1996).
However, there remain some uncertainties. Thus, it was reported by several groups that forearm position sense was rather poor when the forearms were passively positioned (Goodwin et al. 1972; Gregory et al. 1988). Subjects became more accurate if they placed the forearms themselves and held them, unsupported (Paillard & Brouchon, 1968). It suggested that a level of muscle contraction improved positional acuity. As it was known that the fusimotor neurones to muscle spindles were coactivated during voluntary contractions (Vallbo, 1974), one explanation for the improved performance was that the increased spindle activity during the contraction provided more precise positional information.
Our own experiments in this area were concerned with the consequences for proprioception of periods of intense exercise. We demonstrated in a forearm force-matching task, that significant matching errors arose when elbow flexors of one arm had been fatigued (Weerakkody et al. 2003). The simplest explanation, supported by the observations of others (Carson et al. 2002), was that subjects were matching the effort required to achieve a given force, not forces themselves. If muscles were fatigued so that more effort was required to achieve a given level of force, this led to force-matching errors between the fatigued and unfatigued arms.
More recently, we have shown that fatigue from exercise can lead to significant errors in a forearm position matching task (Walsh et al. 2004; Allen & Proske, 2005). When one arm was fatigued, it matched the angle set by the unfatigued arm by adopting a more vertical position, where the same effort would be required to maintain its position. The observations suggested that if muscle spindles contributed to position sense, an additional cue was provided by the amount of effort required to maintain the position.
In the experiments described here we have tried to examine more closely how the signals from muscle spindles and from the sense of effort might contribute to position sense at the forearm.
Methods
A feature of these experiments was their simplicity. The experimental design was arranged to bring out the main points that we wanted to make. A whole series of more detailed experiments could have been carried out, but these might have clouded the issue and they have been left for the future.
A total of 15 subjects (6 males and 9 females) participated in the four experiments. Four subjects participated in all four experiments, 10 subjects participated in three, 11 in two, and four in one, giving a total of 11, 11, 10 and 8 subjects, respectively, for each experiment. Subjects gave their informed, written consent prior to undertaking the experiments, which were all approved by the Monash University Committee for Human Experimentation, and ethical aspects of the experiments conformed with the Declaration of Helsinki.
Each subject attended several sessions. A series of control trials was carried out to help familiarize subjects with the equipment and procedures. In the unsupported position sense measurements, subjects were required to achieve an accuracy of < 3 deg of error and a standard deviation of < 3.5 deg. In the event, three of the 18 subjects tested were excluded from the experiments, because they were unable to achieve the required matching accuracy.
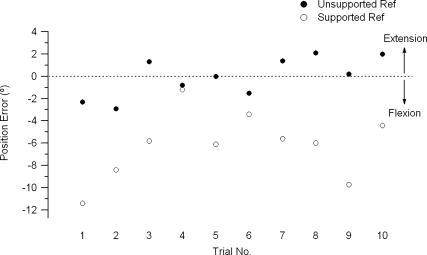
In these experiments we chose to use a single test angle (∼ 45 deg, depending on the accuracy of placement by the experimenter). In practice, angles in the range of 40–50 deg were achieved, and these were matched by the subject. In previous experiments of this kind, we had selected three different test angles (Walsh et al. 2004). However, for the kinds of measurements we were making, we found a single test angle adequate. In none of the trials was there any evidence of learning during successive matches (see, for example, Fig. 2).
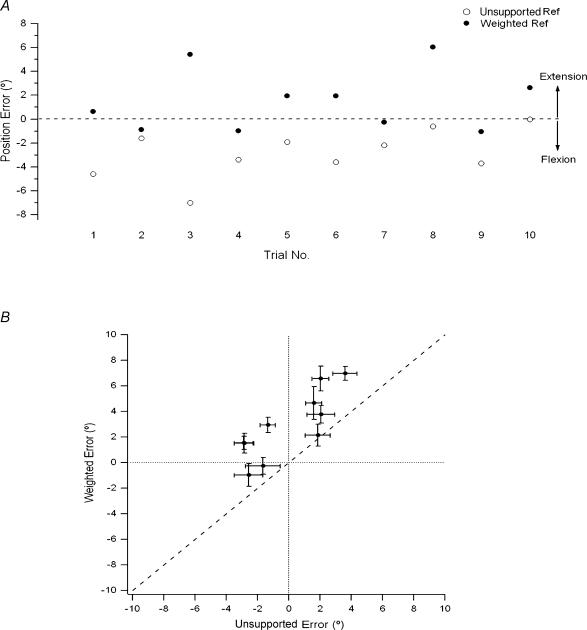
Figure 2. Elbow position matching trials.
Position errors in degrees, i.e. difference between the reference arm, sitting at 45 deg (dotted line), and the indicator arm, for 10 successive trials, for one subject. Errors were calculated as the difference between reference angle and indicator angle. Positive errors are in the direction of extension relative to the test angle and negative errors in the direction of flexion. Filled symbols, reference arm unsupported; open symbols, reference arm supported.
Position sense
The measurement procedure used was the same as that described in Walsh et al. (2004). The subject had both forearms strapped to lightweight paddles, with the paddle hinges arranged to be coincident with the elbow joint. The height of the apparatus was adjusted so that when the forearms were strapped to the paddles, the upper arms were at ∼ 45 deg. Potentiometers attached to the paddle hinges provided an analog signal proportional to the elbow joint angle. Position signals were acquired at 40 Hz using MacLab 4/s running Chart software (ADInstruments, Castle Hill, NSW, Australia) on a Macintosh computer. Resolution of elbow angles was 0.2 deg with this system.
Experiment 1: supported versus unsupported arm
In the first experiment, the blindfolded subject was instructed to slowly move their reference arm from the horizontal position (0 deg), in the direction of flexion, until they were told to stop. At that point, arm position was ∼ 45 deg from the horizontal. They were then asked to match it, by similar placement of their indicator arm. The angles adopted by the two arms were noted. After each match forearms were returned to the horizontal position, ready for the next match. A total of 10 trials was carried out for each subject.
This was followed by another series of 10 trials in which the reference arm was supported. For this task, subjects were instructed to keep their reference arm relaxed throughout the procedure. The experimenter moved the reference arm to the 45 deg position and then placed it on a support. To ensure that subjects complied with the instructions and kept their reference elbow flexors relaxed, they were provided with audio feedback of electromyographic (EMG) activity recorded from the surface of biceps brachii. This was done using Ag–AgCl electrodes with an adhesive base and solid gel contact point (3M Health Care, London, Ontario, Canada). The EMG signals were amplified using a BIO Amp (AD Instruments, Castle Hill, NSW, Australia) and fed through a speaker. Once the relaxed reference arm was on the support, the subject was asked to match its position by active placement of their indicator arm.
Experiment 2: conditioning the supported arm
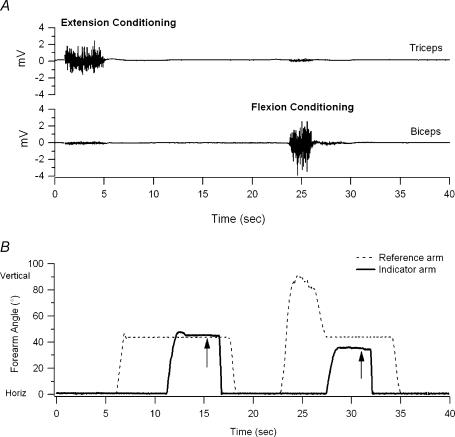
In the second experiment, the arm was placed on the support as before, but after elbow muscles had first been conditioned. In each of 10 pairs of matching trials we alternated between two forms of conditioning (Gregory et al. 1988). In elbow flexion conditioning, the elbow was flexed and subjects were asked to contract biceps, that is, apply a flexion force. For extension conditioning, subjects were instructed to place their arm on the table and to push downwards in the direction of extension, ensuring a contraction of triceps brachii (Fig. 1). The important consideration for these kinds of conditioning procedures was to make sure that the muscle which had been shortened in the conditioning position was contracted. The reason was to remove any slack in the intrafusal fibres of the spindles in the short muscle, thereby preventing a fall in spindle stretch sensitivity (Proske et al. 1993). Here we had to keep in mind that signals could arise from both elbow flexors and extensors (Inglis & Frank, 1990). In practice, subjects invariably carried out conditioning co-contractions of elbow muscles, which, from a conditioning point of view, was satisfactory.
Figure 1. The muscle conditioning procedure.
A, triceps and biceps EMG. B, elbow angle. Subjects were asked to extend their reference arm and contract triceps (extension conditioning), then to relax while the experimenter moved the passive arm to the test position where it was placed on a support. Subjects were asked to match the reference position (dashed trace, B) with their indicator arm (continuous trace). They were to declare when they believed they had achieved an accurate match (arrow). The conditioning procedure was then repeated with the arm flexed and a conditioning contraction carried out in biceps (flexion conditioning). This was followed by another position matching trial.
Experiment 3: unsupported versus weighted arm
The third experiment involved the same arrangement as the first, except that unsupported position matching was used and compared with and without a 2 kg weight. The weight was strapped to the paddle of the reference arm, 33 cm from the point of rotation and represented a torque of 6 Nm with the forearm at 45 deg.
Experiment 4: conditioning the unsupported versus the weighted arm
In the fourth experiment, matching of the unsupported reference arm was done after muscle conditioning, and then repeated with the reference arm weighted with 2 kg (as above).
Statistical analysis
Position matching errors were calculated as:
angle (reference arm) − angle (indicator arm),
where horizontal forearm angle = 0 deg and vertical forearm angle = 90 deg. Data were analysed using the software Igor Pro v.4 (Wavemetrics, Lake Oswego, OR, USA) running on an Apple iMac computer. Statistical Analysis used Data Desk 5.0 (Data Description, Ithaca, NY, USA). Analysis used a one-way ANOVA. with repeated measures to test for differences in position error between the paired conditions of supported and unsupported reference arm, extension and flexion conditioning of the reference arm (for unsupported, supported, and weighted reference) and for the unsupported and weighted reference arm. To test for variability in matching errors for the unsupported versus the supported arm, and for the unsupported versus the weighted arm, a repeated-measures ANOVA was performed using the standard deviation values of each group. Significance was accorded a P-value < 0.05. Results in the text are given as mean ± standard error of the mean (s.e.m.).
Results
A total of four experiments was carried out. The working hypothesis was that forearm position sense, when the forearms are held unsupported, in front of the body, is the result of peripheral cues coming from muscle spindles as well as centrally generated cues associated with the motor command. The motor activity was necessary to allow the arm to be held unsupported against the force of gravity. It was postulated to give rise to a sense of effort. No such effort signal would be present when the arm was supported.
Experiment 1: supported versus unsupported arm
A total of 11 subjects successfully carried out this experiment. In terms of the above hypothesis, placing the forearm on a support and ensuring that elbow flexors remained relaxed should eliminate any effort signal.
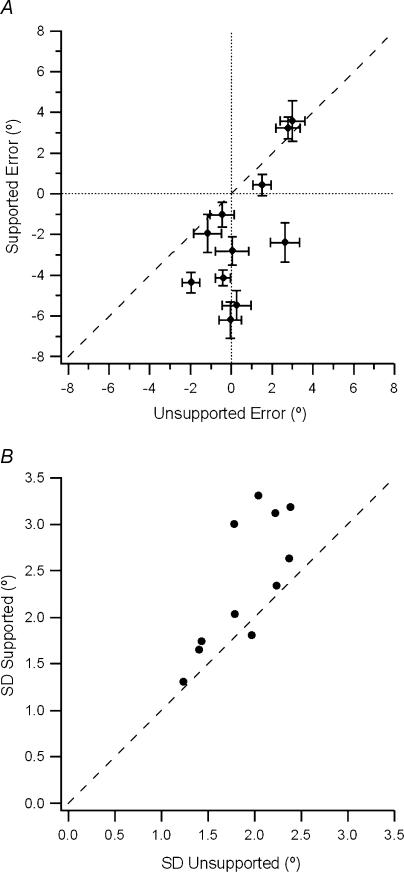
The result of this experiment for one subject is shown in Fig. 2. When the reference arm was supported, it can be seen that the subject was much more erratic in their matching performance (s.d. was ± 3.0 deg for the supported arm compared to an s.d. of ± 1.8 deg for the unsupported arm). In addition, the subject tended to match the unsupported reference arm with position errors that lay in a more extended direction than for the supported arm. For the 11 subjects such differences were significant (ANOVA, F1,10= 11.7, P = 0.01, Fig. 3A). The scatter of values, expressed as the standard deviation of position matching errors, was significantly greater for the supported arm when compared with the unsupported arm (ANOVA, F1,10= 9.7, P = 0.01, Fig. 3B).
Figure 3. Position matching errors for the supported versus the unsupported arm.
A, position errors for supported and unsupported reference arms. The sizes of the errors, in degrees, with the reference arm supported are plotted against the sizes of the errors for the unsupported arm for 11 subjects. Each symbol is the mean (± s.e.m.) of 10 trials for one subject. Positive errors refer to the indicator arm being more extended than reference arm; negative errors are in the direction of flexion. The dotted line indicates zero error, the dashed line, line of equality. Notice that when the arm was supported, errors tended to lie in a direction more flexed than the target, while when the arm was unsupported, errors were distributed more evenly about the target position. B, variability of position errors. For each of the 11 subjects, the standard deviation of position errors, when the reference arm was supported, is plotted against the standard deviation of errors for the unsupported reference arm. Dashed line, line of equality.
Experiment 2: muscle conditioning
Our interpretation of the above experiment was that subjects were more erratic in matching the position of a supported arm because the positional cue arising from the sense of effort had been eliminated. It meant that the remaining positional cue, according to our hypothesis, was that coming primarily from muscle spindles. Could we provide evidence that spindles were indeed contributing to forearm position sense?
The maintained discharge rate of muscle spindles at a given muscle length can be manipulated by muscle conditioning (Proske et al. 1993). We have previously demonstrated such changes in spindle resting activity in an animal model, and that similar conditioning can lead to systematic changes in forearm position sense in human subjects (Gregory et al. 1988). Here we have repeated and extended that result.
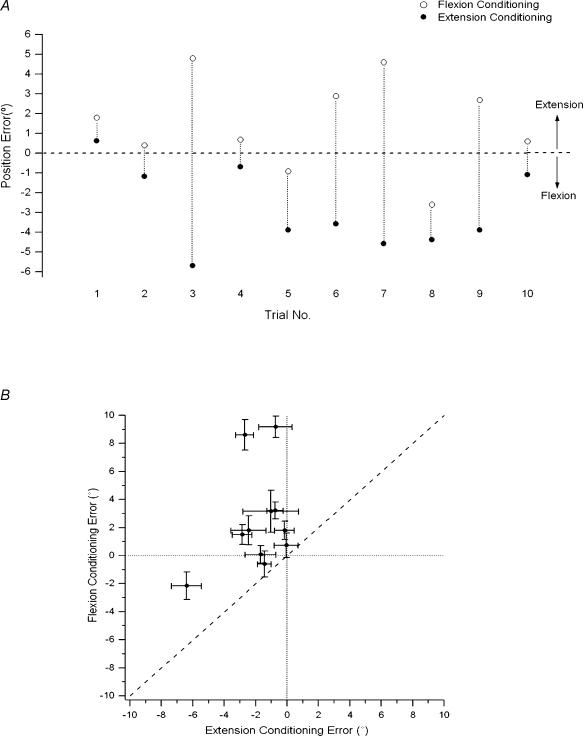
Eleven subjects participated in this experiment. Following the conditioning procedures (see Methods) subjects showed systematic differences in position matching errors. The indicator arm adopted a more extended position after flexion conditioning, compared with that after extension conditioning. Data for one subject are displayed in Fig. 4A. Mean errors are displayed in Fig. 4B; the mean difference (± s.e.m.) for the group was 4.3 ± 1.1 deg. A repeated-measures ANOVA showed that the differences in errors for the two forms of conditioning were significant (F1,10= 17.6, P = 0.002). Notice that when the arm was supported, position matching accuracy was poor for both forms of conditioning, but the relative position adopted in the match was always dependent on the form of conditioning that preceded it.
Figure 4. Effect of muscle conditioning on supported matching errors.
A, position errors after muscle conditioning of reference arm. Position matching in degrees, for 10 pairs of trials for one subject after flexion conditioning (open symbols) and extension conditioning (filled symbols) of the supported reference arm. Each flexion conditioning trial was followed by an extension conditioning trial. Each pair of trials has been identified by the dotted line joining them. Dashed line, zero error B, distribution of conditioning-dependent errors. Mean position errors (± s.e.m.) in degrees after flexion conditioning, plotted against errors after extension conditioning of the supported reference arm for the 11 subjects. Notice errors lie mainly in the upper left-hand quadrant; that is, positive errors (direction of extension) after flexion conditioning, and negative errors (direction of flexion) after extension conditioning. Dashed line, line of equality, dotted lines, zero error.
Experiment 3: unsupported versus weighted arm
In the previous experiment, after removing any effort-related positional cue by supporting the arm, the dependence of position sense on a muscle spindle signal had been tested by conditioning the muscle. In this experiment, the aim was to alter the effort signal by increasing the weight of the arm. The working hypothesis was that the extra weight would increase the effort required to maintain position of the reference arm, and therefore would lead to positional errors.
Ten subjects participated in this experiment. The result for one subject of adding a 2 kg weight to the reference paddle is shown in Fig. 5A. Subjects were found to systematically adopt a more extended position with their indicator arm, when the reference arm was weighted (Fig. 5B). Positional errors were significantly different between the weighted arm and unweighted arm (repeated-measures ANOVA, F1,9= 35.0, P < 0.001). Accompanying the positional errors, there was no significant increase in standard deviation of the errors.
Figure 5. Position matching errors for the unsupported versus the weighted arm.
A, position errors for the weighted reference arm. Data for one subject. The reference arm was unsupported, bearing its own weight (open circles), or with an additional 2 kg load fixed to the reference paddle (filled circles). For the weighted reference arm, errors made by the indicator arm were in the direction of extension relative to the errors for the unsupported arm. Dotted line, zero error. B, mean position errors after weighting the reference arm. Position errors (± s.e.m.), in degrees, for each of the 10 subjects when matching the weighted reference arm plotted against matching errors for the unsupported reference arm. After weighting, errors were positive (direction of extension). Dashed line, line of equality, dotted lines, zero error.
Experiment 4: conditioning unsupported versus conditioning weighted arm
Our previous work had shown that differences observed in the responses of muscle spindles following muscle conditioning could be reduced by interposing an extrafusal contraction after the conditioning (Gregory et al. 1986). Here we have shown conditioning-dependent errors in position sense when the reference arm was supported (Fig. 4). Now we wanted to see whether such errors persisted when a low level of contraction was present from holding the arm unsupported. Furthermore, such a contraction-history dependence should become progressively less as the level of muscle activity increased. Therefore, weighting the arm should further reduce any differences after extension or flexion conditioning.
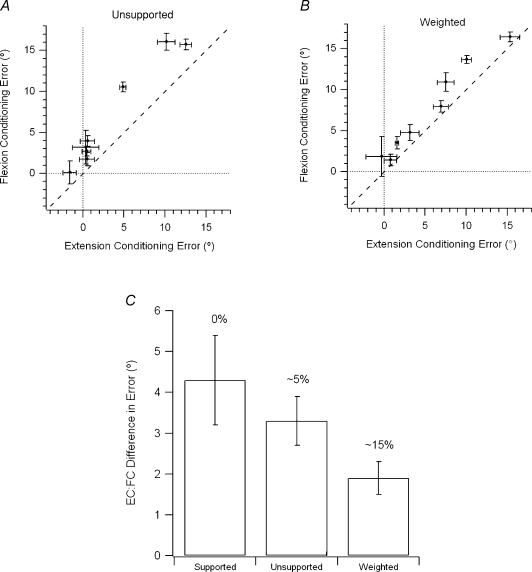
In the event, conditioning errors persisted in position matching of the unsupported arm (Fig. 6A). Flexion conditioning led to position errors that were significantly different when compared to extension conditioning (repeated-measures ANOVA, F1,7= 30.7, P < 0.001). Furthermore, the difference in position errors between the two conditioning procedures remained significant when the arm was weighted. The average size of this difference became less for the unsupported and weighted arm. So the mean difference in errors fell from 4.3 ± 1.1 deg (supported arm) to 3.3 ± 0.6 deg (unsupported arm) to 1.9 ± 0.4 deg (weighted arm), see Fig. 6C. However, while the differences in position error due to conditioning were significant for the unsupported versus the weighted arm (Scheffe post hoc, P = 0.03) they were not for the supported arm versus the other two. This was likely to be due to the large standard deviation for the supported condition.
Figure 6. Effects of muscle conditioning compared for the unsupported and weighted arms.
A, conditioning-dependent errors for the unsupported arm. Differences in errors, in degrees (mean ± s.e.m.), for the two forms of conditioning for eight subjects carrying out 10 trials each in a position matching task where the unsupported reference arm was conditioned while flexed, or while extended. Positive errors the in direction of extension from the target, negative errors in the direction of flexion. Dashed line, line of equality, dotted lines, zero error. B, conditioning-dependent errors for the weighted arm. Differences in errors in degrees (mean ± s.e.m.), for the two forms of conditioning, for each of eight subjects after flexion or extension conditioning of the unsupported reference arm, carrying a 2 kg load. Directions of errors as above. Dashed line, line of equality. C, differences after muscle conditioning. Histogram of mean differences (± s.e.m.) in position matching errors, in degrees, after the two forms of conditioning for the task where the reference arm was supported, where it was unsupported, and where it was weighted (2 kg). The values above each column indicate the approximate percentages of MVC required to support the arm under each condition. EC, extension conditioning. FC, flexion conditioning.
Discussion
We have provided evidence from four experiments in support of the view that when we place our arms in front of us, in their working space, in the absence of vision, we are able to determine their location by signals of both peripheral and central origin. The signal of central origin, postulated to give rise to a sense of effort, can be removed by asking subjects to match forearm position while the reference arm is supported. It leads subjects to demonstrate an increase in uncertainty about the position of their arm in space (Figs 2 and 3).
This result is not new. Goodwin et al. (1972) commented on the fact that when the experimenter held the subject's reference arm, their ability to match its position was rather poor with errors of 12–15 deg (see also McCloskey 1973; Horch et al. 1975; Gregory et al. 1988). Similar comments were made by Paillard & Brouchon (1968), who added that subjects' ability to match position was improved when they were able to actively move their arm. The result is also consistent with the findings of Gooey et al. (2000), who found that when the reference arm was counterweighted, to allow it to move without effort, the standard deviation of position errors significantly increased.
It could be argued that the smaller position errors with the unsupported arm were the result of a larger spindle signal, as some spindles became activated by the fusimotor system during the voluntary contraction required to maintain arm position. If so, weighting the arm should not have introduced new position errors and positional acuity should have further improved as more spindles became coactivated. Such trends were not observed (Fig. 5B).
The way we carried out the matching task here was much as others had done, by beginning each trial with both arms extended, placed on the table in front of the subject (Goodwin et al. 1972). For the supported matching, the relaxed reference arm was placed by the experimenter on the support at the requisite angle. For the unsupported match, the subject moved their reference arm themselves. Carrying out the experiment in this way resulted in errors which were approximately evenly distributed about the test angle when the reference arm was unsupported, but the errors were biased in the direction of flexion when the arm was supported (Fig. 3A). Our interpretation of the direction of errors is that passive placement of the arm carried out in this way corresponded to extension conditioning as we used it in the next experiment, but without any accompanying conditioning contraction.
Muscle spindles
The proposal that, for a supported arm, the remnant position signal was coming from muscle spindles was based on the observation that the distribution of the errors with a supported arm could be manipulated by muscle conditioning (Fig. 4). This is against a background of evidence in support of a peripheral signal provided by muscle vibration experiments (Goodwin et al. 1972; Roll et al. 1989).
Why does muscle conditioning affect position matching errors? In the simplest view, muscle spindles are able to provide a position signal because they are stretch receptors. As the muscle lengthens, the spindle discharge increases, in direct proportion to the length of the muscle. The monotonic relationship between maintained spindle firing rate and muscle length could be used by the central nervous system to derive information about the length of the muscle and therefore the position of the limb. However, all of this presupposes that the spindles remain passive, that is, there is no fusimotor activity. Furthermore, even the responses of passive spindles are not invariant and are subject to confounding influences from the thixotropic properties of their intrafusal fibres (Proske et al. 1993).
When a muscle is contracted voluntarily, there is coactivation of skeletomotor and fusimotor neurones (Vallbo, 1974). The fusimotor activity sets the resting length of the intrafusal fibres following the contraction. If the contraction is carried out at a long muscle length, once the muscle has relaxed, the intrafusal fibres at rest remain long. If the passive muscle is then shortened, such long intrafusal fibres fall slack. They fall slack rather than shorten because their resting stiffness has been raised by the presence of stable cross-bridges between myofilaments in sarcomeres (Proske & Morgan, 1999). The presence of intrafusal slack reduces the strain on the sensory endings of the spindle, and lowers levels of resting discharge. Alternatively, if the voluntary contraction is carried out at a short muscle length, intrafusal length will be short and no slack will be present. There will be a greater strain on the sensory endings as the muscle lengthens, resulting in a higher level of resting discharge. Such conditioning-dependent changes in spindle resting activity have been previously seen in the responses of single identified muscle spindles in an animal model, and they have been associated with systematic changes in errors in a forearm position matching task in human subjects (Gregory et al. 1988). The errors observed in the present study are consistent with these findings (Fig. 4).
An important requirement for the generation of muscle conditioning-dependent changes in spindle resting activity is that the spindles remain passive. If, for example, resting discharge was low because slack had been introduced in intrafusal fibres, a subsequent period of fusimotor activity would lead to take-up of the slack and so raise the level of resting discharge (Gregory et al. 1986, 1991). There would therefore no longer be a difference in resting activity from the two forms of conditioning.
As we saw conditioning-dependent changes in position errors, this suggests that the position signal was coming from passive spindles. Interestingly, conditioning-dependent errors were present both when the reference arm was supported (Fig. 6A), and when it was held unsupported by the subject, although differences in errors were smaller for the unsupported arm (Fig. 6B). Presumably the level of voluntary activity associated with holding the unsupported arm was sufficiently low, to leave a number of spindles passive, allowing the generation of conditioning-dependent errors. Indeed, when the arm was loaded conditioning-dependent errors were still present, although differences were smaller again (Fig. 6C). This was presumably because now only a small number of spindles in the population remained passive.
The torque exerted by elbow flexors holding an arm at 45 deg is ∼ 5% of maximum voluntary contraction (MVC). Weighting the arm increases this value to ∼ 15%. In other words, our observations suggest that during a voluntary contraction approaching one fifth of maximum, some spindles continue to remain passive (Fig. 6C). This conclusion is consistent with the observation of Edin & Vallbo (1990) that 75% of spindles were coactivated at 10% MVC. Extrapolation from our data suggests that by 30% MVC all spindles would have become fusimotor-activated.
The sense of effort
The most important evidence in support of a sense of effort contributing to position sense is that after fatigue from exercise, subjects make position matching errors (Walsh et al. 2004). There is no evidence of fatigue altering the responsiveness of muscle receptors. In an animal model, fatigue and damage from a period of severe eccentric exercise did not disturb responsiveness of muscle spindles (Gregory et al. 2004), or tendon organs (Gregory et al. 2002). Supporting evidence for the sense of effort hypothesis provided by the present study is that increasing the load on the reference arm led subjects to produce significant matching errors in the direction of elbow extension (Figs 5 and 6). The simplest interpretation of this result is that with the extra load it required more effort to support the arm. If subjects were matching arm position by comparing efforts, the indicator arm would be placed in a more horizontal position, where a larger vector of the force of gravity would be acting on the arm (Walsh et al. 2004; Fig. 6).
The experiments on muscle vibration (Goodwin et al. 1972) have implicated muscle spindles both in the sense of limb position and the sense of limb movement. Indeed the authors emphasized that 100 Hz vibration produced predominantly an illusion of movement. We have recently explored the possibility that the sense of effort contributed to movement sensation (Allen & Proske, 2005). It was found that fatiguing elbow flexors by 30% MVC led to significant position matching errors but subjects were still able to accurately carry out a movement tracking task. It was concluded that the sense of effort did not contribute to movement sensation and that this sense was generated entirely by signals from muscle spindles. Vibration illusions are known to be present, if somewhat slower, up to relatively high voluntary contraction forces (McCloskey, 1973), suggesting that spindles are able to provide movement information even when they are under fusimotor control.
Wider implications
An interesting conclusion from this work is that the peripheral position signal is likely to be coming from passive muscle spindles. A long-standing problem with muscle spindles as position sensors has been the fact that they provide potentially ambiguous signals, depending on whether the activity is generated by muscle stretch or fusimotor activity. McCloskey and colleagues (McCloskey, 1981; McCloskey et al. 1983) got around this problem by postulating, as had first been proposed by von Holst & Mittelstaedt (1950) for invertebrate animals, that a central subtraction process took place. In particular, all of the afferent activity generated by fusimotor impulses (reafference) was subtracted from the total spindle signal to give access to consciousness for only that component of the response which was due to the environmental stimulus (exafference), in this case muscle stretch. The fact that muscle conditioning is able to manifest itself as position errors points to passive spindles as responsible for these errors. Any exafferent spindle signal derived from a central subtraction process should not show muscle history effects. That is because the fusimotor activity would eliminate such effects. It leads to the prediction that as the arm is progressively loaded, muscle conditioning dependence of position errors would become progressively less (Fig. 6C). At the same time, fidelity of the position signal from spindles should remain essentially unchanged, provided the coactivated spindles, after undergoing the subtraction process, were able to provide a position signal comparable to that for passive spindles. If a subtraction process does not take place and the fusimotor-activated spindles were not contributing to position sense, the decrease in conditioning dependence should be accompanied by a loss of positional acuity, as fewer spindles would be available to contribute to a position signal.
At face value, the lack of an increase in the scatter of values for position errors with the weighted arm (Fig. 5B) supports the idea of an ongoing central subtraction process. However, interpretation of our data is not so straightforward. Weighting the reference arm, according to our hypothesis, increased the effort signal, a conclusion supported by significant position errors in the direction of extension (Fig. 5). So increasing the weight of the arm could have reduced the available spindle signal, while increasing the effort signal, providing subjects with confidence about the position of their arm, even though they were making errors. On balance, the evidence provided by our experiments on active position matching does not support the existence of a position sense derived only from spindle signals. Such a conclusion removes the need to postulate a central subtraction process for fusimotor-activated spindles. Our current working hypothesis is that as soon as spindles are activated through the fusimotor system, they no longer contribute to position sense. At the same time, the effort signal generated by the motor command provides additional positional information.
Presumably whenever we carry out movements against the force of gravity, we are provided with effort cues. It would account for the difficulties encountered by astronauts in outer space carrying out motor tasks in the absence of vision (Young et al. 1993). A similar situation would presumably pertain to scuba divers. Furthermore if fatigue alters the effort signal and effort contributes to position sense, it means that exercising to fatigue is accompanied by proprioceptive disturbances (Walsh et al. 2004). This is an issue of importance for competing athletes. In addition, it has been suggested that strain injuries in leg muscles may arise from inappropriate placement of the leg during fatigue (Orchard, 2002). Perhaps leg placement becomes less precise as a result of the disturbed proprioception in the face of fatigue. Such propositions should be able to be put to the test, experimentally.
Acknowledgments
This work was carried out with financial support from the National Health and Medical Research Council of Australia.
References
- Allen TJ, Proske U. Effect of muscle fatigue on the sense of limb position and movement. Exp Brain Res. 2005 doi: 10.1007/s00221-005-0174-z. (in press) [DOI] [PubMed] [Google Scholar]
- Carson RG, Riek S, Shahbazpour N. Central and peripheral mediation of human force sensation following eccentric or concentric contractions. J Physiol. 2002;539:913–925. doi: 10.1113/jphysiol.2001.013385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol. 1990;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Kinaesthesia: Roles for afferent signals and motor commands. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 128–172. [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Gooey K, Bradfield O, Talbot J, Morgan DL, Proske U. Effects of body orientation, load and vibration on sensing position and movement at the human elbow joint. Exp Brain Res. 2000;133:340–348. doi: 10.1007/s002210000380. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Brockett CL, Morgan DL, Whitehead NP, Proske U. Effect of eccentric muscle contractions on Golgi tendon organ responses to passive and active tension in the cat. J Physiol. 2002;538:209–218. doi: 10.1113/jphysiol.2001.012785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the response of cat muscle spindles. J Neurophysiol. 1986;56:451–461. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol. 1988;59:1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Two kinds of resting discharge in cat muscle spindles. J Neurophysiol. 1991;66:602–612. doi: 10.1152/jn.1991.66.2.602. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Responses of muscle spindles following a series of eccentric contractions. Exp Brain Res. 2004;157:234–240. doi: 10.1007/s00221-004-1838-9. [DOI] [PubMed] [Google Scholar]
- Horch KW, Clark FJ, Burgess PR. Awareness of knee joint angle under static conditions. J Neurophysiol. 1975;38:1436–1447. doi: 10.1152/jn.1975.38.6.1436. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Frank JS. The effect of agonist/antagonist muscle vibration on human position sense. Exp Brain Res. 1990;81:573–580. doi: 10.1007/BF02423506. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Differences between the senses of movement and position shown by the effects of loading and vibration of muscles in man. Brain Res. 1973;63:119–131. doi: 10.1016/0006-8993(73)90521-0. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Corollary discharges: motor commands and perception. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda: American Physiological Society; 1981. pp. 1415–1447. [Google Scholar]
- McCloskey DI, Gandevia S, Potter EK, Colebatch JG. Muscle sense and effort: motor commands and judgements about muscular contractions. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 151–167. [PubMed] [Google Scholar]
- Orchard J. Biomechanics of muscle strain injury. NZ J Sports Medicine. 2002;30:92–98. [Google Scholar]
- Paillard J, Brouchon M. Active and passsive movements in the calibration of position sense. In: Freeman SJ, editor. The Neuropsychology of Spatially Oriented Behaviour. Homewood, USA: Dorsey Press; 1968. pp. 37–55. [Google Scholar]
- Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell Motil. 1999;20:433–442. doi: 10.1023/a:1005573625675. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot-Ciscar E. Alterations of proprioceptive messages induced by leg tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The muscular sense. In: Schafer EA, editor. Textbook of Physiology. Edinburgh: Pentland; 1900. pp. 1002–102. [Google Scholar]
- Skoglund S. Joint receptors and kinaesthetics. In: Iggo A, editor. Handbook of Sensory Physiology. New York: Springer-Verlag; 1973. pp. 111–136. [Google Scholar]
- Vallbo A. Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiol Scand. 1974;90:319–336. doi: 10.1111/j.1748-1716.1974.tb05594.x. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. Optical Society of America. Vol. 3. Wisconsin: Menasha; 1867. Treatise on physiological optics. 1925.(Translation by J.P.C. Southall of 3rd German edition) [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprinzip (Wechselwirkungen zwischen Zentralnervensystem und Peripherie) Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Walsh LD, Hesse CW, Morgan DL, Proske U. Human forearm position sense after fatigue of elbow flexor muscles. J Physiol. 2004;558:705–715. doi: 10.1113/jphysiol.2004.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Percival P, Morgan DL, Gregory JE, Proske U. Matching different levels of isometric torque in elbow flexor muscles after eccentric exercise. Exp Brain Res. 2003;149:141–150. doi: 10.1007/s00221-002-1341-0. [DOI] [PubMed] [Google Scholar]
- Young LR, Oman CM, Merfeld C, Watt D, Roy S, DeLuca C, Balkwill D, Christie J, Groleau N, Jackson DK. Spatial orientation and posture during and following weightlessness: human experiments in Spacelab Life Sciences 1. J Vest Res. 1993;3:231–239. [PubMed] [Google Scholar]