Abstract
We sought to determine whether decreased neuromuscular use in the form of hindlimb unweighting (HU) would affect the properties of innervating motoneurones. Hindlimb weight-bearing was removed in rats for a period of 2 weeks via hindlimb suspension by the tail. Following this the electrophysiological properties of tibial motoneurones were recorded under anaesthesia in situ. After HU, motoneurones had significantly (P < 0.05) elevated rheobase currents, lower antidromic spike amplitudes, lower afterhyperpolarization (AHP) amplitudes, faster membrane time constants, lower cell capacitances, and depolarized spike thresholds. Frequency–current (f–I) relationships were shifted significantly to the right (i.e. more current required to obtain a given firing frequency), although there was no change in f–I slopes. ‘Slow’ motoneurones (AHP half-decay times, > 20 ms) were unchanged in proportions in HU compared to weight-bearing rats. Slow motoneurones had significantly lower minimum firing frequencies and minimum currents necessary for rhythmic firing than ‘fast’ motoneurones in weight-bearing rats; these differences were lost in HU rats, where slow motoneurones resembled fast motoneurones in these properties. In a five-compartment motoneurone model with ion conductances incorporated to resemble firing behaviour in vivo, most of the changes in passive and rhythmic firing properties could be reproduced by reducing sodium conductance by 25% and 15% in the initial segment and soma, respectively, or by increasing potassium conductance by 55% and 42%, respectively. This supports previous conclusions that changes in chronic neuromuscular activity, either an increase or decrease, may result in physiological adaptations in motoneurones due to chronic changes in ion conductances.
The rat model of hindlimb unloading (HU), also known as hindlimb suspension, has proven to be a valuable ground-based model of the weightlessness that occurs during space flight (Morey-Holton & Globus, 2002). Despite the wealth of information from studies using HU on changes that occur in muscle following the removal of weight-bearing, very little is known about changes in the electrophysiological properties of the innervating motoneurones. Reported changes in voluntary recruitment and locomotor patterns following space flight and HU are consistent with possible changes in the excitability of motoneurones (Layne et al. 1997; Canu & Falempin, 1997, 1998; Anderson et al. 1999; Edgerton et al. 2000; Recktenwald et al. 2000; Hodgson et al. 2000; Canu et al. 2001). Changes have been reported in hindlimb Hoffman reflex gain following 3 weeks of HU; however, it is unknown to what extent motoneurone excitability versus synaptic efficacy were involved in this adaptation (Anderson et al. 1999). During various models of decreased neuromuscular activity, including joint immobilization, bed rest, ‘dry’ water immersion and space flight, the ability to maximally activate muscles voluntarily is compromised, and there is EMG evidence that motor control is significantly altered (Duchateau & Hainaut, 1990; Duchateau, 1995; Ploutz-Snyder et al. 1995; Berg et al. 1997; Zanette et al. 1997; Correia, 1998; Koryak, 1998). However, direct evidence of changes in motoneurone properties under these conditions is lacking.
There is some evidence that motoneurones are influenced by decreased usage. Thus, decreased weight-bearing results in decreased succinic dehydrogenase activity in a subpopulation of lumbar motoneurones in rat (Ishihara et al. 1997), and an attenuation of dendritic development of motoneurones in growing rats raised in a weightless environment (Inglis et al. 2000). Sciatic nerves of rats subjected to HU show an elevation in choline acetyltransferase (Gupta et al. 1985). HU also results in a reduction in GABA-immunoreactive cells and terminals in the hindlimb representation of the rat somatosensory cortex (D'Amelio et al. 1996).
We have demonstrated previously (Beaumont & Gardiner, 2002, 2003) that increased chronic activity in the form of daily voluntary or forced treadmill training has demonstrable effects on several biophysical properties of tibial motoneurones which would influence motoneurone excitability and firing characteristics. Thus, motoneurones do indeed ‘detect’ increased chronic activation, and adapt to it. In a previous report, we (Cormery et al. 2000) also demonstrated some effects on tibial motoneurones resulting from 4 weeks of hindlimb paralysis induced by chronic superfusion of the sciatic nerve with tetrodotoxin. However, this latter model is difficult to interpret, because muscle may be paralysed while the soma continues to be activated by intact spinal circuitry. Similarly, we (Beaumont et al. 2004) and others (Hochman & McCrea, 1994a, b) have shown biophysical changes in motoneurones distal to a spinal cord lesion, which are, for the most part, in a direction opposite to those seen with increased activity. However, these latter adaptations may be due to more than merely an absence of weight-bearing.
Our purpose was therefore to determine whether the absence of weight-bearing, as seen in the model of HU in the rat, would have measurable effects on the electrophysiological properties of motoneurones. This is an especially interesting issue given that motoneurones that innervate fast and slow muscle fibres also exhibit ‘fast’ and ‘slow’ properties (afterhyperpolarizations, input resistance and rheobase current), and that muscle fibres tend to change towards fast type with HU. Thus, a change in motoneurone properties towards fast characteristics after HU might reinforce the existence of molecular mechanisms that allow such matching of motoneurone–muscle fibre properties to occur under normal circumstances.
Methods
Treatment of animals
Female rats weighing 200–225 g were obtained from Charles River (St Constant, Québec, Canada), and initially housed in groups of three in plastic cages situated in an environmentally controlled room maintained at 23°C and kept on a 12 h light–12 h dark cycle. Rats were randomly assigned to a hindlimb unweighted (HU, n = 24) or weight-beariing (WB, n = 24) group within 7 days of arrival. The rats were provided water and food ad libitum throughout the experiment. Animals belonging to the HU group were suspended in a head-tilt position at an angle of approximately 30 deg from the horizontal plane via a non-invasive apparatus affixed to the proximal end of the tail as described by Morey-Holton & Globus (2002). Briefly, the animal's tail was washed, dried and wrapped in breathable adhesive tape with a paper clip attached to the end. The paper clip acted as a hook by which the animal could be secured onto an elevated swivel system built into the top of the cage. The hindlimbs were prevented from touching any supportive surfaces of the cage while the forelimbs maintained full contact with the cage floor allowing free movement and access to food and water. Daily inspection of the animals' tail was performed, to check for discolouration or lesions. Body mass was evaluated every 48 h as an indication of tolerance to the suspension condition. Any animal demonstrating signs of distress or intolerance was immediately excluded from the experimental protocol. Rats in the WB group did not have their tails prepared as in the HU group (thus not constituting true ‘controls’ for the HU rats), and were not manipulated during the course of the experiment. All procedures were approved by the animal ethics committee of the Université de Montréal and were in accordance with the guidelines of the Canadian Council of Animal Care.
Measurement of motoneurone properties
For the terminal experiment, WB rats were taken from their cages and anaesthetized with ketamine/xylazine (80/10 mg kg−1, i.p.). HU rats were anaesthetized while still suspended. The terminal experiment proceeded as described previously (Beaumont & Gardiner, 2002, 2003). Briefly, the anaesthetized rat was surgically prepared for impalement of spinal motoneurones following an incision to allow stimulation of the tibial nerve of the left hindlimb, and a laminectomy was performed from T12 to S1. Anaesthesia was maintained by constant infusion via a jugular vein catheter of a solution containing ketamine/xylazine (8/1 mg h−1), in a physiological saline solution which also contained plasma expander (Ficoll 70, Amersham Pharmacia, Uppsala, Sweden), delivered at a rate of 40 mg h−1. The rat also received an intraperitoneal injection (2 ml) of saline containing 100 mg dextrose and 0.05 mg kg−1 atropine. Depth of anaesthesia was verified frequently using the ear pinch and eye blink reflexes. Rectal temperature was monitored and maintained near 37°C using a heating blanket. The head, thoracic and lumbar vertebrae, hips and left foot were immobilized with clamps, and the open leg and back incisions were filled with mineral oil. The intubated rat was ventilated with pure oxygen-enriched room air, at a volume of approximately 2 ml, and a rate of approximately 80–120 strokes min−1. The dura mater covering the spinal cord was incised, and the large dorsal roots representing afferents from the left hindlimb were cut and reflected over the right side of the cord. An opening was made in the pia just lateral to the entry zone of these roots into the cord, in preparation for introduction of the glass microelectrode. Immediately before beginning the search for motoneurones, a pneumothorax was performed by making a 5-mm incision between ribs t5 and t6, on the left side of the thorax.
Thin-walled glass microelectodes (o.d., 1.0 mm) were pulled, and filled with 2 m potassium citrate. Electrode impedances ranged from 2 to 10 MΩ. The tip of the electrode was positioned at a hole in the pia, and was lowered into the cord in steps of 10 μm. The tibial nerve was stimulated with a bipolar silver microelectrode at a frequency of 1 s−1, and the microelectrode driven into the cord while monitoring the field potential. Evidence of successful impalement of an α-motoneurone was a sudden increase in potential to at least −55 mV, and an antidromic action potential with a positive overshoot and a reproducible latency of less than 2.5 ms from the stimulation artifact.
When recording had stabilized for at least 2 min, resting membrane potential was recorded. Subsequently, the following were recorded: antidromic action potential (average of 10), rheobase current (50-ms square-wave current amplitude resulting in spikes 50% of the time), action potential in response to a 1-ms current pulse of supramaximal intensity (average of 40), and antidromic spikes, with and without superimposition of a 150-ms depolarizing current of 1 nA, a 150-ms hyperpolarization of 1 nA (average of 100) and a series (100) of 1-nA hyperpolarizing pulses lasting 50 ms, for measurement of membrane time constant. Cells were then challenged with a series of increasing and then decreasing amplitudes of depolarizing current injections lasting 500 ms every 2 s. Current was increased in steps until blocking occurred before the end of the 500-ms period, after which current steps of decreasing intensity were administered (see Fig. 1). During recording, resting membrane potential and time were frequently recorded. At the end of these measurements, the microelectrode was backed out of the cell in 5-μm steps, and the voltage outside the cell was recorded. Typically, experiments yielded from four to seven motoneurones with complete complements of data. At the end of the experiment, the rat was killed by an overdose of pentobarbital, and the muscles were removed and weighed.
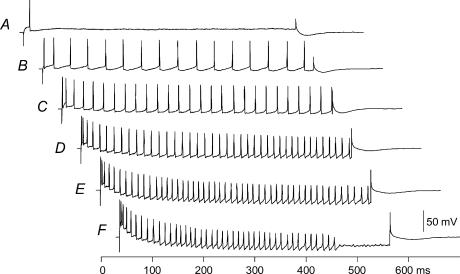
Figure 1. f–I relationship for a typical motoneurone.
Cell generates a single spike at a current intensity of 6.5 nA (A), and began firing during the entire 500-ms injection period at an intensity of 7.6 nA (B). Frequencies increase with increasing current intensity (C–E). At a current intensity of 20.7 nA (F), spiking is blocked before the end of the 500-ms current injection period. Minimum current for steady-state firing and minimum steady-state firing frequency (from last three interspike intervals) were measured for this cell from record B, and maximum steady-state firing frequency (from last three interspike intervals) from record E.
From these recordings we determined antidromic spike height and timecourse, amplitude and half-decay time of the afterhyperpolarization (AHP) following an action potential evoked by a 1-ms current pulse, cell input resistance using the spike-height method (Frank & Fuortes, 1956), and membrane time constant, length constant and total cell capacitance using the curve-peeling method (Ito & Oshima, 1965). AHP half-decay time was used to separate slow from fast motoneurones, as we have done previously (Beaumont & Gardiner, 2002, 2003). Spike threshold was determined by subtracting the rheobasic spike amplitude from the amplitude of the spike evoked by a short current pulse, and adding this value to the resting membrane potential.
χ2 analysis was used to determine the effect of HU on the proportions of fast and slow motoneurones in the sample. Data were analysed using two-factor analysis of variance on the factors of treatment (WB versus HU) and motoneurone ‘type’ (fast versus slow). Where significant interaction effect emerged, Tukey's post hoc test was used to determine significance of differences among individual means.
Results
Muscle and body weight responses
The 2-week period of HU evoked the classical significant decrease (25%) in soleus muscle wet weight (WB, 145 ± 5 g; HU, 109 ± 6 g, means ± 1 s.d.) (Roy et al. 1987; Talmadge et al. 1996), with a non-significant loss in body weight of 2.7%.
Passive and threshold motoneurone properties
We recorded tibial motoneurone properties from 23 WB rats (154 motoneurones) and 15 HU rats (75 motoneurones). Experiments in which between four and 11 motoneurone recordings were taken were included in the data set. This latter criterion eliminated one of the original 24 WB rats, and six of the HU rats (less than four motoneurones). Three HU rats were eliminated as a result of detaching themselves from the HU apparatus (two) or damage to the tail (one).
The effects of the 2-week period of HU are summarized in Table 1. As expected, fast and slow motoneurones, distinguished as in previous reports using 20-ms AHP half-decay time, demonstrated significant differences in rheobase (slow < fast) and input resistance (slow > fast). Slow motoneurones also demonstrated a slightly larger antidromic spike (by about 3 mV) compared to fast motoneurones. Although there was a tendency for the proportion of fast motoneurones to be larger in the HU sample (68%) compared to the WB sample (60%), this difference was not significant (P = 0.3) on χ2 analysis.
Table 1.
Motoneurone passive and threshold properties
| Weight-bearing | Unweighted | ||||
|---|---|---|---|---|---|
| Property | Fast | Slow | Fast | Slow | Difference |
| Rheobase (nA) | 8.0 ± 4.7 (88) | 6.0 ± 3.8 (60) | 10.6 ± 4.3 (52) | 6.4 ± 4.2 (23) | B |
| Input resistance (MΩ) | 1.7 ± 1.5 (55) | 2.7 ± 1.4 (51) | 1.7 ± 1.3 (45) | 2.6 ± 1.3 (20) | A |
| Spike amplitude (mV) | 77 ± 12.3 (91) | 84 ± 12.1 (58) | 73 ± 12.0 (51) | 74 ± 11.5 (24) | B |
| Rheobasic spike amplitude (mV) | 58 ± 7.5 (89) | 64 ± 7.7 (60) | 52 ± 7.8 (51) | 55 ± 7.2 (19) | B |
| RMP (mV) | −61 ± 7.4 (87) | −63 ± 7.5 (57) | −59 ± 7.5 (48) | −63 ± 7.7 (24) | |
| AHP half-decay time (ms) | 14.4 ± 3.8 (93) | 28.6 ± 3.9 (61) | 14.6 ± 4.3 (52) | 27.0 ± 3.8 (24) | A |
| AHP amplitude (mV) | 1.5 ± 0.9 (80) | 2.9 ± 0.7 (57) | 1.3 ± 0.7 (52) | 2.0 ± 1.0 (24) | B |
| Spike threshold (mV) | −43 ± 9.1 (83) | −42 ± 8.8 (55) | −38 ± 9.3 (46) | −45 ± 8.9 (19) | C |
| Time constant (ms) | 4.5 ± 1.3 (53) | 5.4 ± 1.9 (46) | 4.0 ± 0.9 (47) | 4.4 ± 0.9 (22) | B |
| Cell capacitance (nF) | 3.5 ± 1.8 (30) | 2.8 ± 1.8(34) | 2.8 ± 1.5 (38) | 2.1 ± 1.2 (20) | B |
Data are presented as means ± 1 s.d. Two-way ANOVA was used for analysis. In right-hand column, A indicates type (slow versus fast) of significant main effect only (P < 0.01) and B indicates both group (weight-bearing versus unweighted) and type (fast versus slow) significant main effects (P < 0.02), with difference due to group effect shown in bold, and C indicates a significant interaction effect (P < 0.02), with mean which is different from all others shown in bold.
The HU condition resulted in an increase in rheobase (by about 16%, P = 0.021) in both fast and slow motoneurones. This increase occurred in spite of no effect of HU on cell input resistance (Table 1). HU also resulted in a significant decrease in mean cell capacitance and membrane time constant, which affected both slow and fast motoneurones.
An additional effect of HU on motoneurones was a reduction of the amplitude of the spike measured at rheobase during a 50-ms current injection (‘rheobasic spike’, Table 1). Such a reduction in rheobasic spikes with unchanged antidromic spike amplitudes would normally indicate a more depolarized spike threshold. In fact, HU resulted in depolarization of the spike threshold in fast, but not slow, motoneurones.
Rhythmic firing properties
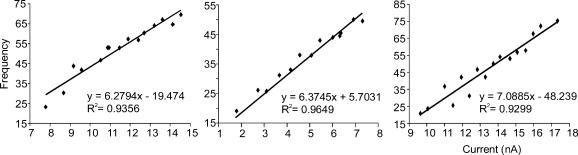
A subset (n = 62) of the motoneurones shown in Table 1 was injected with current steps lasting 500 ms in order to measure their rhythmic firing responses (Fig. 1). Examples of steady-state frequency–current (f–I) slopes for three of these motoneurones are shown in Fig. 2, and rhythmic firing properties are summarized in Figs 3, 4, 5.
Figure 2. Three examples of f–I relationships for steady-state firing.
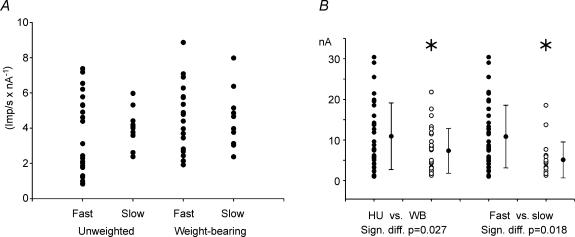
Figure 3. Effects of HU on F-I slopes and minimum currents for steady-state firing.
A, slopes of steady-state firing. No differences were found in comparing fast versus slow motoneurones, or unweighted versus weight-bearing conditions. B, significant main effects of minimum current required for steady-state firing. Motoneurones of hindlimb unweighted (HU; •) rats had significantly higher minimum currents than weight-bearing (WB) rats (○, P = 0.027), and fast (•) had higher currents than slow (○) motoneurones (P = 0.018).
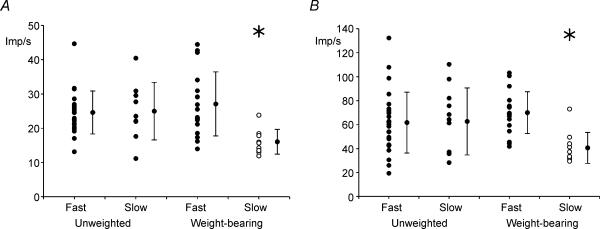
Figure 4. Effects of HU on minimum and maximum steady-state firing frequencies.
Significant interaction effects were found for minimum (A, P = 0.009) and maximum (B, P = 0.014) steady-state firing frequencies. For both measures, slow motoneurones in weight-bearing rats had significantly lower steady-state firing frequencies than all other groups; thus, slow motoneurones in unweighted rats had steady-state firing frequency ranges similar to fast motoneurones.
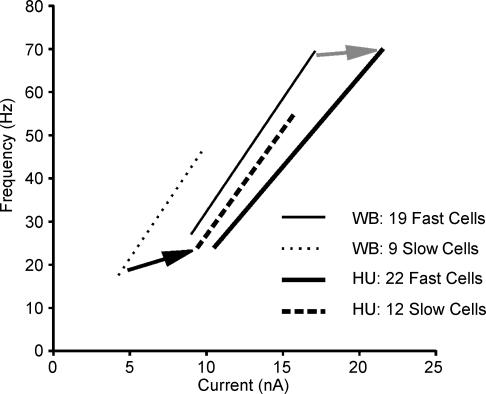
Figure 5. Synopsis of changes occurring in the f–I relationships of fast and slow motoneurones as a result of HU.
Slopes were not different between fast (continuous lines) and slow (dashed/dotted lines) motoneurones, and HU had no effect on slope. HU resulted in a shift of f–I relationship to the right (increased minimum current necessary for rhythmic firing), with the relationship for slow motoneurones also shifted upwards (higher minimum and maximum frequencies).
Slopes ranged from < 1 to 9 impulses s−1 nA−1. No significant differences were noted in mean f–I slopes between fast and slow motoneurones, or between WB and HU conditions (Fig. 3A).
As would be expected from the results in Table 1, the minimum current for steady-state firing (defined as the minimum current evoking firing during 500 ms) was significantly (P = 0.018) different between fast and slow motoneurones (slow < fast, Fig. 3B). The HU condition resulted in a significant increase (P = 0.027) in this threshold current. This effect was similar in fast and slow motoneurones, as evidenced by a lack of significant interaction term in the two-way ANOVA. This was reminiscent of the increased rheobase current that occurred in both fast and slow motoneurones following HU (Table 1). The minimum current for steady-state firing was 50% higher than rheobase current, consistent with previous reports (Granit et al. 1963).
Minimum frequency during steady-state firing (defined as the lowest frequency evoked at the end of a 500-ms current injection) was also altered in HU motoneurones. However, in this case, the effect was seen only for the slow motoneurones. For example, in WB rats, slow motoneurones had a significantly (P < 0.001) lower minimum steady-state firing frequency than fast motoneurones; this difference was eliminated following HU (difference between fast and slow, P = 0.9). Consistent with this, slow motoneurones in HU rats had significantly (P = 0.013) higher minimum firing frequencies than slow motoneurones in WB rats (Fig. 4A).
As with minimum steady-state firing, maximum steady-state firing was influenced by HU, such that slow motoneurones, which had values significantly (P = 0.002) lower than fast motoneurones in WB rats, were not different from fast motoneurones following HU (P = 0.9, Fig. 4B).
Figure 5 combines and summarizes information concerning minimum and maximum steady-state firing frequencies, minimum current thresholds for steady-state firing and f–I slopes. Although slopes were not significantly influenced by HU (Fig. 3A), the relationships were shifted to the right, indicating loss in excitability. This rightward shift in the f–I relationship for both fast and slow motoneurones is supported by the increase in the minimum currents required for rhythmic firing, combined with the lack of influence on the average slope. The movement of the slow motoneurone f–I relationship in an upward direction is evidenced by increases in minimum and maximum steady-state firing frequencies. Thus, all motoneurones became somewhat less excitable, and slow motoneurones became more like fast motoneurones in firing properties, with no effects of HU on motoneurone ‘gain’.
Modelling
We made use of the five-compartment model presented by Dai et al. (2002) for slow and fast motoneurones, to attempt to determine possible ionic mechanisms that might be involved in these chronic motoneurone adaptations. Model fast and slow motoneurones were of the properties described in Table 1 of their report. Reducing the sodium conductance at the initial segment by 25% and in the soma by 15% in the model resulted in motoneurones with increased rheobase, unaltered input resistance and AHP duration, reduced antidromic spike amplitudes, slight changes in AHP amplitude, slightly depolarized spike threshold and a rightward shift of the f–I slope (Table 2); results that resemble in many respects the measured changes (Table 1). Similar results were seen by increasing delayed rectifier potassium conductance in the initial segment by 55% and in the soma by 42%.
Table 2.
Effects of altering ion conductances on motoneurone properties in a five-compartment motoneurone model of Dai et al. (2002)
| Condition 1 | Condition 2 | ||||
|---|---|---|---|---|---|
| Property | Slow Mn | Fast Mn | Slow Mn | Fast Mn | Agreement with data |
| Rheobase (nA) | +3 | +3 | +3 | +3 | A |
| Input resistance (MΩ) | 0 | 0 | 0 | 0 | A |
| Spike amplitude (mV) | −14.1 | −7.1 | −3.2 | −2.6 | B |
| AHP width (ms) | 0 | 0 | 0 | reduced | B |
| AHP amplitude (mV) | −1.8 | +0.4 | −1.4 | −0.4 | B |
| Spike threshold (mV) | +3.2 | +4.0 | +2.2 | +2.4 | A |
| f–I relationship shift | right | right | right | right | A |
Condition 1, reducing initial segment sodium conductance (gNa) by 25% and soma gNa by 15%. Condition 2, increasing initial segment delayed rectifier potassium conductance (gKdr) by 55% and soma gKdr by 42%. In right-hand column, A indicates very strong agreement, B indicates somewhat in agreement (see Table 1).
Discussion
In this paper we demonstrate for the first time that elimination of weight-bearing for 2 weeks has consequences on the passive, threshold and rhythmic firing properties of motoneurones innervating the muscles subjected to decreased use, in this case, primarily the ankle extensors which are innervated by the tibial nerve. Some of these effects are common, while some are specific, to motoneurone types (fast versus slow). In general, it appears that motoneurones become less excitable during reduced weight-bearing, and slow motoneurones change in some properties towards those of fast motoneurones.
The changes noted here may be caused by a decreased weight-bearing, or may be due to a decrease in activation of the motoneurone. For example, integrated EMG recorded over 24 h from hindlimb muscles is significantly reduced for a few days following the initiation of reduced weight-bearing, after which it returns to near-control values (Alford et al. 1987). Thus, motoneurones appear to experience only a transient decrease in aggregate activity which lasts only a few days, before resuming original daily activation levels. This finding might suggest that muscle changes resulting from reduced weight-bearing, independent from a reduction in motoneuronal activity, are the source of the motoneurone changes. However, a subsequent report using a more detailed analysis of EMG shows significant reductions and changes in patterns of hindlimb EMG of HU rats (Blewett & Elder, 1993), which might suggest that a reduction or change in pattern in motoneurone activation alone could be the root of the adaptations noted here.
The previous literature on hindlimb suspension suggests that, after 4 weeks, there are considerable changes in myosin heavy chains towards the fast phenotype, especially in soleus muscle (Roy et al. 1987; Fitts et al. 1989; Talmadge et al. 1996). The finding that many soleus motor units become heterogeneous in fibre type composition (Picquet et al. 2000) would suggest that muscle fibre changes most probably precede motoneurone changes, which would in turn suggest that substances produced by altered muscle fibres are influential in bringing about motoneuronal changes, via a retrograde influence. Reasonably strong evidence of the effects of muscle substances on motoneurone properties exists in the literature (Czeh et al. 1978; Foehring et al. 1987; Gonzalez & Collins, 1997). An attractive hypothesis is that an inactivity substance (or lack of an activity substance) produced by unweighted muscles exerts an influence on the synthesis and incorporation of ion conductance channels via a retrograde mechanism. Of course, a change in conductance could come about via modulation of existing ion conductances, as seems to occur during fictive locomotion (Krawitz et al. 2001). However, these latter alterations are normally short-lived, as compared to the longer-lasting (at least 48 h) adaptations that we have witnessed.
A decrease in afferent information as a result of the hindlimb unweighting condition may also play a role in some of the changes seen in motoneurone properties. For example, surgical deafferentation in the cat results in a decreased motoneuronal excitability and decreased membrane time constant (Gustafsson et al. 1982), similar to our results.
Many of the significant changes in motoneuronal passive properties that we noted with decreased weightbearing were opposite to those seen following chronically increased neuromuscular activity (spike threshold, spike amplitude, AHP amplitude, rheobase) (Beaumont & Gardiner, 2002, 2003). Changes seen in spike threshold (more depolarized) and f–I relationships (less excitable) in the current study were similar in direction to changes noted previously in motoneurones following spinal cord transection (Beaumont et al. 2004). In the latter study, the transection-induced motoneuronal changes were prevented by daily ‘passive’ exercise of the paralysed hindlimbs. This signifies that these properties are situated on a continuum based on the level of chronic activity of the neurone. Our results, suggesting that some properties of slow motoneurones become more like those of fast motoneurones (AHP amplitude, cell capacitance, membrane time constant, minimum firing frequencies and currents, and shifting of the f–I relationship to the right), are also consistent with the interpretation that neurones experiencing the largest change in their normal activity patterns change the most in their properties (as is the case with muscles such as soleus, but not gastrocnemius). For example, in the increased exercise studies, motoneurone changes were restricted to lower-threshold motoneurones when exercise intensity was low, but were spread across the motoneurone pool when exercise was intense (Beaumont & Gardiner, 2002, 2003).
It was interesting to note that no significant change in motoneurone type was evident when using AHP half-decay time, which is normally a robust index for distinguishing fast and slow motoneurones (Zengel et al. 1985; Bakels & Kernell, 1993; Gardiner, 1993). However, f–I characteristics suggested a change in rhythmic firing characteristics from slow to fast (f–I relationship shifted upward and to the right in slow motoneurones). It may be that conductances involved in determining active properties are more sensitive than, and/or their adaptation precedes, conductances involved in determining passive properties, such as those which dictate the time course of the AHP. However, it is worth noting that although the time course of the AHP after a single spike was not changed following HU, AHP amplitude was diminished in both fast and slow motoneurones.
Such changes would be expected to have implications for the manner in which motoneurones are recruited to perform voluntary activity. For example, after single-legged hindlimb unweighting in humans, the normal pattern of motor unit recruitment changes, with a portion of this change most probably reflecting the loss in muscle mass that accompanied this intervention (Ploutz-Snyder et al. 1995). The alternating patterns of flexors and extensors during locomotion is disrupted as well following HU (Canu & Falempin, 1997, 1998). As a result of increased rheobase and a shift in the f–I relationship to the right, one would expect an increase in the effort required to recruit motoneurones, and to increase their firing rates to maximal. A common effect of decreased neuromuscular use is the reduced ability to achieve high enough frequencies to generate large voluntary forces. Indeed, several weeks of bed rest (Duchateau, 1995; Berg et al. 1997; Koryak, 1998), 9 days of wrist immobilization (Miles et al. 1994), 6–8 weeks of immobilization (Duchateau & Hainaut, 1990) and 10 days of lower limb unloading (Berg & Tesch, 1996) all result in a decrease in maximal voluntary contraction which cannot be explained totally by decreased muscle mass (Berg et al. 1997). After 6–8 weeks of immobilization, maximum firing frequencies are significantly reduced (Duchateau & Hainaut, 1990). Although maximal firing rates of motoneurones do not appear to be compromised by unweighting, maximum firing rates would become more difficult to achieve voluntarily due to the rightward shift in the f–I relationship (i.e. more current needed for the same frequency of firing).
All of these models (bed rest, immobilization, hindlimb unweighting, spinal cord transection) involve perturbations in normal neuromuscular activity that differ with respect to muscle length excursions, degree and patterns of activation of motoneurones, and afferent input onto motoneurones. In addition, these perturbations may not be the same across different species that have been examined. Nonetheless, the commonalities of muscle atrophy and muscle fibre conversions that have been reported for these models across different species, in conjunction with a clear pattern of neuromuscular performance adaptations which accompany these changes, suggest that the changes in basic motoneurone properties reported here represent a common adaptation of motoneurones to a decrease in neuromuscular activation.
In a motoneurone model, reductions in sodium conductance or increases in potassium conductance in the soma and initial segment produced responses that were similar to the observed changes. Manipulation of other conductances could not reproduce the experimental changes without producing other changes in spike height and duration, altered resting potential, large AHP amplitude and duration changes. This may suggest that changes in the amount, density and/or location of the ion channels responsible for these conductances may occur as a result of chronic changes in activation of the neuromuscular unit. Persistent inward currents, carried by calcium and sodium ions, may be involved in determining properties such as spike threshold rhythmic firing properties (Heckman et al. 2005). In particular, fast persistent inward sodium currents may be necessary for rhythmic firing, as blocking them with prolonged depolarization causes a deterioration of rhythmic firing and a depolarization of the spike threshold (Lee & Heckman, 2001). We are continuing our studies using pharmacological blockers and gene expression tools, to attempt to discern the molecular basis of these physiological adaptations.
Acknowledgments
This research was supported by grants from Natural Sciences and Engineering Research Council (NSERC) of Canada, the Canadian Space Agency, Canadian Institutes of Health Research (CIHR) and the Canada Research Chairs programme. The authors would like to thank Marcel Beaulieu, Gérard Ouellet and Pierre Corriveau at Université de Montréal, and Gilles Detillieux, Matt Ellis and Maria Setterbom at University of Manitoba for technical assistance, and Duane Button and Tanguy Marqueste for assistance in data analysis. We would especially like to thank Dr Yue Dai, University of Manitoba, for the modelling aspect of the study. P.F.G. is Canada Research Chair in Physical Activity and Health Studies at University of Manitoba, Canada.
References
- Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp Neurol. 1987;96:635–649. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- Anderson J, Almeida-Silveira MI, Pérot C. Reflex and muscular adaptations in rat soleus muscle after hindlimb suspension. J Exp Biol. 1999;202:2701–2707. doi: 10.1242/jeb.202.19.2701. [DOI] [PubMed] [Google Scholar]
- Bakels R, Kernell D. Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. J Physiol. 1993;463:307–324. doi: 10.1113/jphysiol.1993.sp019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Gardiner P. Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurones in rats. J Physiol. 2002;540:129–138. doi: 10.1113/jphysiol.2001.013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Gardiner PF. Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve. 2003;27:228–236. doi: 10.1002/mus.10308. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Houlé JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997;82:182–188. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand. 1996;157:63–70. doi: 10.1046/j.1365-201X.1996.476217000.x. [DOI] [PubMed] [Google Scholar]
- Blewett C, Elder GCB. Quantitative EMG analysis in soleus and plantaris during hindlimb suspension and recovery. J Appl Physiol. 1993;74:2057–2066. doi: 10.1152/jappl.1993.74.5.2057. [DOI] [PubMed] [Google Scholar]
- Canu MH, Falempin M. Effect of hindlimb unloading on two hindlimb muscles during treadmill locomotion in rats. Eur J Appl Physiol. 1997;75:283–288. doi: 10.1007/s004210050162. [DOI] [PubMed] [Google Scholar]
- Canu MH, Falempin M. Effect of hindlimb unloading on interlimb coordination during treadmill locomotion in the rat. Eur J Appl Physiol. 1998;78:509–515. doi: 10.1007/s004210050453. [DOI] [PubMed] [Google Scholar]
- Canu MH, Falempin M, Orsal D. Fictive motor activity in rat after 14 days of hindlimb unloading. Exp Brain Res. 2001;139:30–38. doi: 10.1007/s002210100734. [DOI] [PubMed] [Google Scholar]
- Cormery B, Marini JF, Gardiner PF. Changes in electrophysiological properties of tibial motoneurones in the rat following 4 weeks of tetrodotoxin-induced paralysis. Neurosci Lett. 2000;287:21–24. doi: 10.1016/s0304-3940(00)01110-1. [DOI] [PubMed] [Google Scholar]
- Correia RJ. Neuronal plasticity: adaptation and readaptation to the environment of space. Brain Res Rev. 1998;28:61–65. doi: 10.1016/s0165-0173(98)00043-5. [DOI] [PubMed] [Google Scholar]
- Czeh G, Gallego R, Kudo N, Kuno M. Evidence for the maintenance of motoneurone properties by muscle activity. J Physiol. 1978;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amelio F, Fox RA, Wu LC, Daunton NG. Quantitative changes of GABA-immunoreactive cells in the hindlimb representation of the rat somatosensory cortex after 14-day hindlimb unloading by tail suspension. J Neurosci Res. 1996;44:532–539. doi: 10.1002/(SICI)1097-4547(19960615)44:6<532::AID-JNR3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Dai Y, Jones KE, Fedirchuk B, McCrea DA, Jordan LM. A modelling study of locomotion-induced hyperpolarization of voltage threshold in cat lumbar motoneurones. J Physiol. 2002;544:521–536. doi: 10.1113/jphysiol.2002.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J. Bed rest induces neural and contractile adaptations in triceps surae. Med Sci Sports Exerc. 1995;27:1581–1589. [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. J Physiol. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton V, Roy RR, Recktenwald MR, Hodgson JA, Grindeland RE, Kozlovskaya I. Neural and neuroendocrine adaptations to microgravity and ground-based models of microgravity. J Gravit Physiol. 2000;7:45–52. [PubMed] [Google Scholar]
- Fitts R, Brimmer C, Heywood-Cooksey A, Timmerman R. Single muscle fiber enzyme shifts with hindlimb suspension and immobilization. Am J Physiol. 1989;25:C1082–C1091. doi: 10.1152/ajpcell.1989.256.5.C1082. [DOI] [PubMed] [Google Scholar]
- Foehring R, Sypert G, Munson J. Motor-unit properties following cross-reinnervation of cat lateral gastrocnemius and soleus muscles with medial gastrocnemius nerve. II. Influence of muscle on motoneurons. J Neurophysiol. 1987;57:1227–1245. doi: 10.1152/jn.1987.57.4.1227. [DOI] [PubMed] [Google Scholar]
- Frank K, Fuortes G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956;134:451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J Neurophysiol. 1993;69:1160–1170. doi: 10.1152/jn.1993.69.4.1160. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Collins WF., III Modulation of motoneuron excitability by brain-derived neurotrophic factor. J Neurophysiol. 1997;77:502–506. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. Quantitative aspects of repetitive firing of mammalian motoneurones, caused by injected currents. J Physiol. 1963;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC, Misulis KE, Dettbarn WD. Changes in the cholinergic system of rat sciatic nerve and skeletal muscle following suspension-induced disuse. Exp Neurol. 1985;89:622–633. doi: 10.1016/0014-4886(85)90012-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Katz R, Malmsten J. Effects of chronic partial deafferentation on the electrical properties of lumbar alpha-motoneurones in the cat. Brain Res. 1982;246:23–33. doi: 10.1016/0006-8993(82)90138-x. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini M, Bennett DLH. Persistent inward currents in motoneuron dendrites: implications for motor control. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. I. Composite monosynaptic Ia EPSPs in four motoneuron pools. J Neurophysiol. 1994a;71:1452–1467. doi: 10.1152/jn.1994.71.4.1452. [DOI] [PubMed] [Google Scholar]
- Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. II. Motoneuron electrical properties. J Neurophysiol. 1994b;71:1468–1479. doi: 10.1152/jn.1994.71.4.1468. [DOI] [PubMed] [Google Scholar]
- Hodgson J, Riazansky S, Goulet C, Badakva A, Kozlovskaya I, Recktenwald MR, McCall GE, Roy RR, Fanton JW, Edgerton V. Rhesus leg muscle emg activity during a foot pedal pressing task on Bion 11. J Gravit Physiol. 2000;7:S87. [PubMed] [Google Scholar]
- Inglis FM, Zuckerman KE, Kalb RG. Experience-dependent development of spinal motor neurons. Neuron. 2000;26:299–305. doi: 10.1016/s0896-6273(00)81164-2. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Ohira Y, Roy RR, Nagaoka S, Sekiguchi C, Hinds WE, Edgerton VR. Effects of 14 days of spaceflight and nine days of recovery on cell body size and succinate dehydrogenase activity of rat dorsal root ganglion neurons. Neuroscience. 1997;81:275–279. doi: 10.1016/s0306-4522(97)00097-3. [DOI] [PubMed] [Google Scholar]
- Ito M, Oshima T. Electrical behavior of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965;180:607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koryak Y. Electromyographic study of the contractile and electrical properties of the human triceps surae muscle in a simulated microgravity environment. J Physiol. 1998;510:287–295. doi: 10.1111/j.1469-7793.1998.287bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol. 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne CS, McDonald PV, Bloomberg JJ. Neuromuscular activation patterns during treadmill walking after space flight. Exp Brain Res. 1997;113:104–116. doi: 10.1007/BF02454146. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Miles MP, Clarkson PM, Bean M, Ambach K, Mulroy J, Vincent K. Muscle function at the wrist following 9 d of immobilization and suspension. Med Sci Sports Exerc. 1994;26:615–623. [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- Picquet F, Canu MH, Falempin M. Phenotypic changes in the composition of muscular fibres in rat soleus motor units after 14 days of hindlimb unloading. Pflugers Arch. 2000;440:229–235. doi: 10.1007/s004240000288. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Tesch PA, Crittenden DJ, Dudley GA. Effect of unweighting on skeletal muscle use during exercise. J Appl Physiol. 1995;79:168–175. doi: 10.1152/jappl.1995.79.1.168. [DOI] [PubMed] [Google Scholar]
- Recktenwald MR, Hodgson JA, Roy RR, Riazansky S, McCall GE, Kozlovskaya I, Washburn DA, Fanton JW, Edgerton V. Quadripedal locomotion in Rhesus monkeys after 14 days of spaceflight. J Gravit Physiol. 2000;7:S71. [PubMed] [Google Scholar]
- Roy RR, Bello MA, Bouissou P, Edgerton VR. Size and metabolic properties of fibers in rat fast-twitch muscles after hindlimb suspension. J Appl Physiol. 1987;62:2348–2357. doi: 10.1152/jappl.1987.62.6.2348. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Edgerton VR. Distribution of myosin heavy chain isoforms in non-weight-bearing rat soleus muscle fibers. J Appl Physiol. 1996;81:2540–2546. doi: 10.1152/jappl.1996.81.6.2540. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol. 1997;105:269–279. doi: 10.1016/s0924-980x(97)00024-6. [DOI] [PubMed] [Google Scholar]
- Zengel J, Reid S, Sypert G, Munson J. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]