Abstract
ATP-sensitive K (KATP) channels are composed of Kir6, the pore-forming protein, and the sulphonylurea receptor SUR, a regulatory protein. We and others have previously shown that positively charged residues in the C terminus of Kir6.2, including R201 and K185, interact with the α and β phosphate groups of ATP, respectively, to induce channel closure. A positively charged residue in the N terminus, R50, is also important, and has been proposed to interact with either the γ or β phosphate group of ATP. To examine this issue, we systematically mutated R50 to residues of different size, charge and hydropathy, and examined the effects on adenine nucleotide sensitivity in the absence and presence of SUR1. In the absence of SUR1, only the size of residue 50 significantly altered ATP sensitivity, with smaller side chains decreasing ATP sensitivity. In the presence of SUR1, however, hydrophathy and charge also played a role. Hydrophilic residues decreased ATP sensitivity more than hydrophobic residues for small size residues, and, surprisingly, negatively charged residues E and D preserved ATP sensitivity and increased ADP sensitivity relative to the wild-type residue R. These observations suggest that a negative charge near position 50, due to either mutation of R50 or the interaction of the γ phosphate group of ATP with R50, facilitates closure of the ATP-dependent gate. Mutation of the nearby positively charged residue R54, known to be involved in stabilizing channel opening via electrostatic interactions with phosphatidylinositol 4,5-bisphosphate (PIP2), also caused increased ADP sensitivity as compared with ATP, suggesting a loss of function of ATP's γ phosphate. Based on these results, we propose that a phosphate group or a negative charge at position 50 initiates channel closure by destabilizing the electrostatic interactions between negative phosphate groups of PIP2 and residues such as R54.
ATP-sensitive K (KATP) channels act as metabolic sensors which couple cell function to changes in intracellular ATP and ADP levels in various organs. Channel activity is blocked by both ATP and ADP, but is also stimulated by MgADP. Stimulation by MgADP requires the presence of the sulphonylurea receptor SUR, a regulatory subunit for the pore-forming Kir6 subunit. SUR also selectively increases the ATP sensitivity of Kir6.2. In channels formed by Kir6.2 alone, ATP is two to three times more efficient at blocking the channel than ADP. However, when SUR1 is present, ATP becomes eight to ten times more efficient, while the blocking potency of ADP increases only modesty by two- to threefold (Ribalet et al. 2003). This observation suggests that SUR1 preferentially facilitates the channel's interaction with the γ phosphate group of ATP to promote channel closure. Experiments comparing the blocking potency of the adenine nucleotides ATP, ADP and AMP have suggested that in closing the channel, the β and α phosphate groups of ATP interact with K185 and R201 on Kir6.2, while the γ phosphate may interact with R50 on the N terminus (Trapp et al. 2003). However, based on the observation that the R50G mutant affected ATP and ADP sensitivity equally, with little change in AMP sensitivity, we (Ribalet et al. 2003) suggested that R50 also interacted with the β phosphate group common to both ATP and ADP. In the experiments described here, however, we found that when we substituted NaADP for KADP, R50G, as well as with other R50 substitutions with small residues, cause a lesser shift in ADP than ATP sensitivity, indicating that the apparent decrease in ADP sensitivity observed with NaADP could have been due to a stimulatory effect of Na+ (Ho & Murrell-Lagnado, 1999). To further investigate the potential role of R50 as a site of SUR1-dependent interaction of the channel with the γ phosphate of ATP, we have systematically mutated R50 to residues with different size, hydropathy and charge. We find that most R50 mutants, except for the negatively charged residues aspartate (D) and glutamate (E), demonstrate a large decrease in ATP sensitivity, but a minor shift in ADP sensitivity, supporting the Trapp et al. (2003) hypothesis that the γ phosphate of ATP interacts with R50. Negatively charged substitutions at R50 (R50D and R50E), on the other hand, retained high ATP sensitivity, suggesting that a specific interaction of the γ phosphate of ATP with R50 is not necessary for channel closure, and that the main requirement for this transition to the closed state is a negative charge in the region of position 50.
Stabilization of KATP channels in the open state may involve electrostatic interactions between the negative phosphate groups of phosphatidylinositol 4,5-bisphosphate (PIP2) and positively charged residues on Kir6.2, such as R54, R176 and R177 (Baukrowitz et al. 1998; Shyng & Nichols, 1998; Fan & Makielski, 1999; Ribalet et al. 2000; Schulze et al. 2003). Given the proximity of these residues to ATP binding residues, such as R50 and K185, one intriguing possibility is that the negatively charge phosphate groups of ATP may close the channel by inhibiting PIP2's electrostatic interaction with the channel. To test this hypothesis, we investigated whether, like R50 mutants, mutation of the R54 residue, which lowers PIP2 affinity, also increases the relative potency of ADP versus ATP in channel closure by rendering ATP's γ phosphate group irrelevant. Our findings support this conjecture.
Methods
The techniques for cDNA expression and patch-clamp recording have been described in detail (John et al. 1998), and are only briefly outlined here.
Molecular biology and cDNA expression in HEK 293 cells
In most experiments, HEK293 cells were transfected with cDNA for Kir6.2 mutants linked to GFP at the C terminus (Kir6.2-GFP) so that insertion of the constructs into the plasma membrane could be investigated. Our previous findings showed that linkage to GFP did not affect the kinetics or adenine nucleotide sensitivity of wild-type Kir6.2 + SUR1 channels (John et al. 1998). All wild-type cDNAs were subcloned into the vector pCDNA3amp (Invitrogen, Carlsbad, CA, USA). The cDNAs used to make the GFP chimeras were subcloned into the pEGFP vector. Both vectors used the CMV promoter. Single-site Kir6.2 mutations were constructed using the ‘QuikChange’ technique from Stratagene (La Jolla, CA, USA). The transfections were carried out using the calcium phosphate precipitation method (Graham & van der Eb, 1973). Expression of proteins linked to GFP was detected as early as 12 h after transfection. Patch-clamp experiments were started approximately 30 h after transfection. HEK 293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) high-glucose medium supplemented with 10% (v/v) fetal calf serum, penicillin (100 units ml−1), streptomycin (100 units ml−1) and 2 mm glutamine, and divided once a week by treatment with trypsin.
Patch-clamp methods
Currents were recorded in HEK293 cells using the inside-out patch-clamp configuration, with the pipette solution containing (mm): 140 KCl, 10 NaCl, 1.1 MgCl2, 10 Hepes, and pH adjusted to 7.2 with KOH. The bath solution consisted of 140 KCl, 10 NaCl, 1.1 MgCl2, 10 Hepes, 5 EGTA, with the pH adjusted to 7.2 with KOH. PIP2 (Calbiochem) was sonicated before use.
The data, filtered at 2 kHz with an eight-pole Bessel filter, was recorded with a List EPC 7 (Darmstadt, Germany) patch-clamp amplifier and acquired on videotape at a fixed frequency of 44 kHz after digitization with a digital audio processor. For analysis, the data were sampled at a rate of 5.5 kHz and analysed using pCLAMP 9 software (Axon Instruments, Inc., Union City, CA, USA).
ATP sensitivity measurements
Adenine nucleotides were added directly to the bath. Steady-state current values were used to assess the effects of adenine nucleotides and other interventions on channel activity. To measure ATP sensitivity prior to channel run-down, membrane patches were excised in the presence of 5 mm EGTA and 200 μm MgADP. To estimate the effect of run-down on ATP sensitivity, patches were exposed to Mg2+-free solutions or to 200 nm glibenclamide to decrease the open probability (Po). As previously reported (Ribalet et al. 2003), these methods provide similar estimates of ATP sensitivity before and after spontaneous channel run-down, with the advantage of allowing rapid assessment of the effects of Po on channel ATP sensitivity. The decrease in Po and increase in ATP sensitivity evoked by sulphonylureas occurs within 20 s, while run-down induced by Mg2+ removal is slow (10–30 min).
Results
KATP channel regulation by ATP and ADP
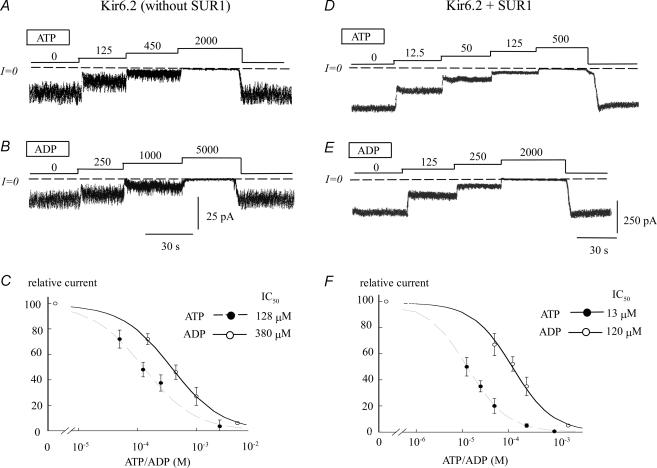
In the absence of SUR, Kir6.2 channels trafficked inefficiently to the plasma membrane, but still produced a small number of functional channels with a low Po (∼0.1). ATP blocked channel activity with only a two- to threefold greater efficiency than ADP, with half-maximal inhibition (IC50) values of 130 μm for ATP and 380 μm for ADP (Fig. 1A–C). Co-expression of SUR1 with Kir6.2 enhanced trafficking to the plasma membrane, and increased channel Po to 0.3–0.5 (Tucker et al. 1997; John et al. 1998). Moreover, ATP sensitivity increased 10-fold (IC50 13 μm), whereas ADP sensitivity (in the absence of Mg2+) increased only two- to threefold (IC50 120 μm) (Fig. 1C). Since ATP and ADP differ only by the γ phosphate group of ATP, this finding suggests that SUR1 preferentially facilitates the interaction of the channel with the γ phosphate group to promote channel closure.
Figure 1. ATP and ADP sensitivity of Kir6.2 expressed alone and with SUR1.
A and D, current traces depict the ATP sensitivity of Kir6.2 alone and Kir6.2 + SUR1, respectively. These data illustrate the potent increase in ATP sensitivity elicited by coexpression with SUR1. In contrast, B and E, which illustrate the ADP sensitivity of Kir6.2 alone and Kir6.2 + SUR1, respectively, indicate a comparatively small SUR1-induced increase in ADP sensitivity. C, fitting of Kir6.2 data points to the Hill equation yielded IC50 values of 128 μm (n = 4) (•) and 380 μm (n = 4) (○) for ATP and ADP, respectively. In both cases, the Hill coefficients were close to unity. In this case channels were threefold more sensitive to ATP. F, fitting of Kir6.2 + SUR1 data points to the Hill equation yielded IC50 values of 13 μm (n = 5) (•) and 380 μm (n = 5) (○) for ATP and ADP, respectively. These data indicate that with SUR1, the channels were 10 times more sensitive to ATP than ADP and, thus, most of the increase in adenine nucleotide sensitivity evoked by SUR1 involves ATP γ phosphate group.
Positively charged residues that directly control adenine nucleotide sensitivity via electrostatic interaction with the phosphate groups of adenine nucleotides have been identified on the N terminus at R50, and on the C terminus at K185 and R201 (Tucker et al. 1998; Shyng et al. 2000; Ribalet et al. 2003; Trapp et al. 2003). From analysis of how mutations at these sites affected the relative sensitivity of the channel to inhibition by ATP, ADP and AMP, we previously proposed that K185 interacts with the β phosphate group of ATP to destabilize the open state, and R201 subsequently interacts with the α phosphate group to stabilize the closed state (Ribalet et al. 2003). To clarify the role of R50, we have systematically mutated this residue, and examined the effects on ATP and ADP sensitivity. Table 1 shows that when R50 was replaced with W, K, E, Q, V, N, D, C, S, A or G, ATP sensitivity was affected more than ADP sensitivity. That is, the ratio of the IC50 values for ATP and ADP fell from 9 in the wild-type R50 to 2.2–4.8 in these mutants (see Table 1). For instance, with the R50Q + SUR1 mutant channels, the sensitivity to ATP decreased by 10-fold, while ADP sensitivity decreased by only threefold. This result supports the hypothesis that R50 interacts primarily with the γ phosphate of ATP to close the channel. We previously reported that with R50G mutant channels, the ATP and ADP sensitivities decreased in a similar fashion (i.e. the ratio of IC50 values for ATP and ADP remained ≥10), which had led us to postulate that R50 interacted with the β phosphate of ATP (Ribalet et al. 2003). However, we originally performed these studies using the Na+ salt of ADP. When we replaced the Na+ salt with the K+ salt, we found that R50S, R50A and R50G behaved similar to the other R50 mutants, with ADP sensitivity increasing minimally relative to ATP sensitivity, and an ADP-to-ATP sensitivity ratio close to 3 (Table 1). We reckon that the potent decrease in ADP sensitivity seen with NaADP might be an effect of Na+, which has been found to stimulate Kir3 channels via interaction with D226 and increased channel regulation by PIP2 (Ho & Murrell-Lagnado, 1999). These data presented in Table 1 lead us to revise our former view in favour of the γ phosphate, as proposed by Trapp et al. (2003).
Table 1.
ATP and ADP sensitivity of R50X and R50X+ SUR1 channels as a function of charge, hydropathy and size
| X | ATP IC50 R50X + SUR1 | IC50 ratio ADP/ATP R50X + SUR1 | ATP IC50 R50X | IC50 ratio R50X/ R50X + SUR1 | Residue's hydropathy* | Residue's size |
|---|---|---|---|---|---|---|
| W | 51.5 ± 6.5 | 2.2 ± 0.4 | 130.0 | 2.5 | −0.9 | 1.2 |
| R | 13.2 ± 3 | 9 ± 0.9 | 135 ± 22 | 10.0 | −4.5 | 1.0 |
| K | 126 ± 27 | 2.8 ± 0.4 | 426 ± 75 | 3.4 | −3.9 | 0.8 |
| L | 425 ± 45† | 3.8 | 0.8 | |||
| E | 25.5 ± 7 | 2.6 ± 0.4 | 365 ± 80 | 14.3 | −3.5 | 0.7 |
| Q | 119 ± 12 | 2.9 | 375.0 | 3.1 | −3.5 | 0.7 |
| V | 143 ± 52 | 3 ± 0.5 | 215.0 | 1.5 | 4.2 | 0.7 |
| N | 167 ± 36 | 4.8 | 351.0 | 2.1 | −3.5 | 0.6 |
| D | 27 ± 6 | 2.7 ± 0.4 | 327.0 | 12.1 | −3.5 | 0.5 |
| C | 109 ± 12 | 4.4 ± 0.8 | 481 ± 88 | 4.4 | 2.5 | 0.5 |
| S | 420 ± 84 | 2.6 ± 0.2 | 796 ± 76† | 1.9 | −0.8 | 0.4 |
| A | 128 ± 27 | 3.8 | 880.0 | 6.9 | 1.8 | 0.4 |
| G | 1275 ± 195 | 3.3 ± 0.6 | 3400.0 | 2.7 | −0.4 | 0.3 |
Negative values indicate hydrophilic residues. Column 1 lists the residue (X) substituted at R50. Columns 2 and 4 show the ATP sensitivities (μM) of R50X + SUR1 and R50X, respectively. Column 3 shows the ratio of ADP to ATP sensitivities, and column 5 the ATP sensitivity ratio of R50X versus R50X + SUR1. Standard error values indicate that 3–5 experiments were performed; in cases where no standard error value is shown, only 2 experiments were performed.
Values are taken from Table 1 in Proks et al. (1999). The values for the residue's size are based on measurements of the length of the side chain.
Role of residue size at position 50
Table 1 indicates that in the absence of SUR1, a correlation exists between ATP sensitivity and the size of the residue at position 50, with large residue substitutions such as R50W having little effect on ATP sensitivity, whereas small residue substitutions such as R50G decreased ATP sensitivity 25-fold compared with the wild-type. In contrast, charge or hydropathy at position 50 had almost no effect on ATP sensitivity. For example, the IC50 values for ATP were comparable for similarly sized R50K, R50L and R50E, despite markedly different hydropathy and charge. These findings indicate that in the absence of SUR1, a direct electrostatic interaction between the phosphate groups of adenine nucleotides and the residue at position 50 is not important for ATP inhibition. Furthermore, the dependence of ATP sensitivity upon the size of the residue size at position 50 is consistent with recent reports whereby the N terminus and R50, in particular, may control access of adenine nucleotides to the ‘binding pocket’ (Dabrowski et al. 2004; Antcliff et al. 2005).
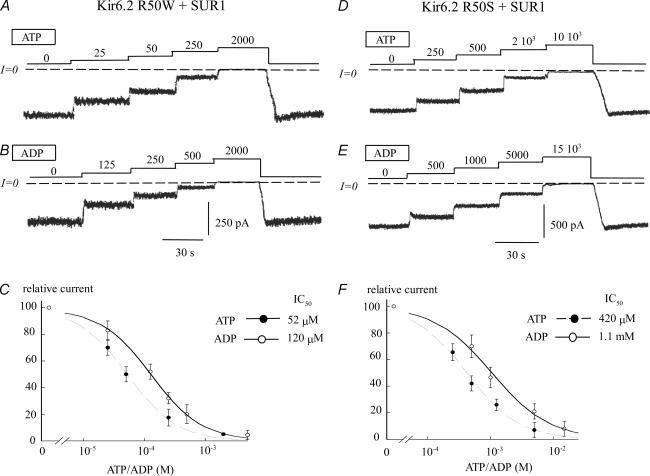
In the presence of SUR1, however, the hydropathy and charge of the residue at position 50 became important. Figure 2 illustrates that size still plays a role. For instance, despite similar charge and hydropathy, the small residue substitution R50S had an eightfold greater effect on ATP sensitivity than the large residue substitution R50W. ADP sensitivity also decreased, but to a lesser extent. The difference between ATP and ADP sensitivity was 2.2-fold for R50W + SUR1 channels, and 2.6-fold for R50S + SUR1. Similar results were obtained with R50A + SUR1 and R50G + SUR1.
Figure 2. ATP and ADP sensitivity of Kir6.2 R50W and Kir6.2 R50S coexpressed with SUR1: effect of the size of residue 50 on adenine nucleotide sensitivity.
A and D, current traces depict the ATP sensitivity of Kir6.2 R50W + SUR1 and Kir6.2 R50S + SUR1, respectively. These data illustrate the potent (almost ninefold) decrease in ATP sensitivity evoked by decreasing the size of residue 50. B and E, the ADP sensitivity of Kir6.2 R50W + SUR1 and Kir6.2 R50S + SUR1, respectively, shows a similar ninefold decrease in ADP sensitivity evoked by a small size residue at position 50. C, fitting of Kir6.2R50W + SUR1 data points to the Hill equation yielded IC50 values of 52 μm (n = 3) (•) and 120 μm (n = 3) (○) for ATP and ADP, respectively. In both cases the Hill coefficients were close to unity. In this case channels were 2.4 times more sensitive to ATP than ADP. F, fitting of Kir6.2R50S + SUR1 data points to the Hill equation yielded IC50 values of 420 μm (n = 5) (•) and 1100 μm (n = 5) (○) for ATP and ADP, respectively. These data indicate that with SUR1 the channels were 2.6 times more sensitive to ATP than ADP. These data illustrate how small size residues decrease adenine nucleotide sensitivity.
With SUR1 present, hydropathy also played a role. For instance, although R50S, R50G and R50A all involved substitutions with uncharged residues of similar size, the hydrophilic residues S and G decreased ATP sensitivity to a much greater extent than the hydrophobic residue A. These effects were not prevalent when Kir6.2 was expressed without SUR1, and suggest that in the presence of SUR1, a hydrophobic residue at position 50 selectively facilitated the interaction of the channel with the phosphate group(s) of ATP.
Role of the charge at position 50
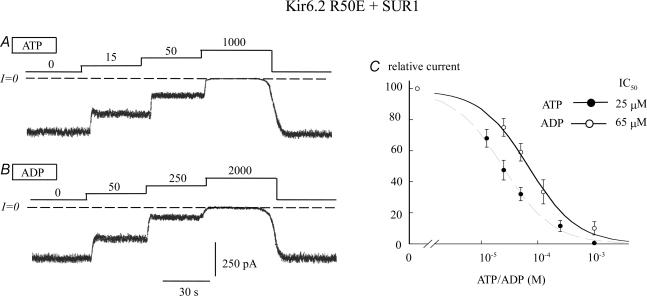
In the presence of SUR1, the charge at position 50 had striking effects. For instance, despite their similar sizes, charge reversal in the R50D mutation increased adenine nucleotide sensitivity, whereas charge neutralization in R50N and R50C mutations both decreased ATP sensitivity. The effect of the N substitution was greater than with the C substitution, consistent with the latter being more hydrophobic. Figure 3 also shows that the negatively charged glutamate was also a very good substitute for positively charged arginine in preserving channel block by ATP, while ADP sensitivity was actually enhanced compared to wild-type R50 + SUR1 channels (Fig. 1B). In contrast, substituting the positively charged lysine for arginine decreased ATP sensitivity almost 10-fold, while ADP sensitivity was little affected.
Figure 3. ATP and ADP sensitivity of Kir6.2 R50E coexpressed with SUR1: effect of charge on adenine nucleotide sensitivity.
A and B, current traces depict the ATP and ADP sensitivity of Kir6.2 R50E + SUR1. C, fitting of Kir6.2R50E + SUR1 data points to the Hill equation yielded IC50 values of 25 μm (n = 5) (•) and 65 μm (n = 3) (○) for ATP and ADP, respectively. In both cases the Hill coefficients were close to unity. This data show that the ATP sensitivity of R50E + SUR1 is similar to that of wild-type channels, while that for ADP is higher. Thus, a negative residue at position 50 mimics the interaction of the γ phosphate with R50.
These results suggest that a negative charge at position 50 is sufficient to mimic the closing effect of the γ phosphate group of ATP, and that interaction of this phosphate group with R (but not K) at this position has the same effect as introducing a negative charge by mutating R50 to D or E.
Destabilization of a channel open state by a negative charge at position 50: role of PIP2
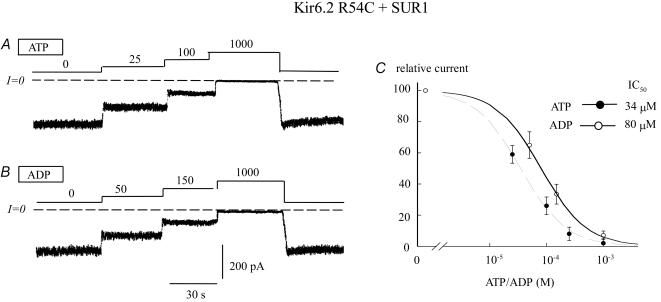
It is generally accepted that KATP channels are stabilized in the open state via electrostatic interaction between negative phosphate groups of PIP2 and positively charged residues on Kir6.2 N terminus (R54) (Schulze et al. 2003) and C terminus (R176 and R177) (Fan & Makielski, 1999; Enkvetchakul et al. 2000). Thus, if a negative charge at position 50 initiates channel closure by destabilizing the open state, a possible mechanism might involve destabilization of the electrostatic interaction between PIP2 and the nearby positive residue R54 on Kir6.2. In this case, mutating R54, which lowers PIP2 affinity (Schulze et al. 2003), should minimize the effect of the γ phosphate group of ATP so that ATP and ADP inhibitory potency should become comparable. To test this idea, we examined how mutations at R54 affected adenine nucleotide sensitivity in patches treated with sulphonylureas or after Mg2+ removal to prevent channel stimulation by ADP. Figure 4A–C indicates that in the presence of SUR1, the ratio of ADP to ATP sensitivity of R54C channels was about 2.4 (IC50 80 μm and 34 μm, respectively), similar to the ratio for R50 mutant channels, but different from that for wild-type Kir6.2 + SUR1 channels (see Table 1). Thus, in the presence of SUR1, when the channel's interaction with PIP2 at the R54 site was suppressed, the ability of the γ phosphate of ATP to promote channel closure by interacting with R50 was greatly diminished. These observations support the hypothesis that in the presence of SUR1, the interaction of the γ phosphate of ATP with R50 may destabilize the channel's interaction with PIP2 at the nearby R54 site. Similar results were obtained with R176/R177 mutant channels (data not shown), consistent with the hypothesis that the Kir6.2 PIP2 binding site, which stabilizes the channel in the open state, involves multiple positively charged residues. The low ADP/ATP ratio was specific for R54 mutants, since other positively charged residues on the N terminus, such as R31, R32 K39, K47 and K67, exhibited ADP/ATP ratios ranging from 7 to 10, indicating that these residues are not involved in the PIP2-dependent gating process.
Figure 4. ATP and ADP sensitivity of Kir6.2 R54C coexpressed with SUR1: effect of charge on adenine nucleotide sensitivity.
A and B, current traces depict the ATP and ADP sensitivity of Kir6.2 R54C + SUR1. C, fitting of Kir6.2R50E + SUR1 data points to the Hill equation yielded IC50 values of 34 μm (n = 5) (•) and 80 μm (n = 5) (○) for ATP and ADP, respectively. In both cases the Hill coefficients were close to unity.
A negative charge at position 50 decreases Kir6.2 affinity for PIP2
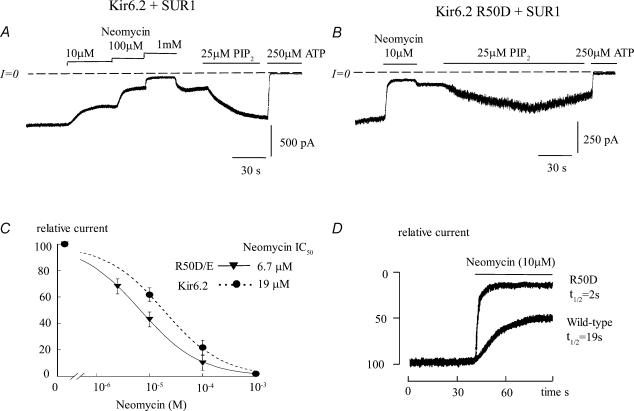
If the interaction of the γ phosphate of ATP with R50 facilitates channel closure by inhibiting PIP2 binding at R54, then placing a negative residue at position 50 should induce this inhibition permanently. In this case, R50D/E + SUR1 channels would be predicted to have a reduced PIP2 affinity compared with wild-type Kir6.2 + SUR1 channels. This is consistent with the observation by Reimann et al. (1999), who found that the R50E mutant ran-down very quickly upon patch excision and that this effect could be reversed by addition of MgATP, since Kir6.2 channel run-down is thought to be related to PIP2 hydrolysis (see for reference Lin et al. 2003). To test this hypothesis, we compared the reactivation of wild-type and R50E/D mutant channels by PIP2 and their inhibition by neomycin, which has been shown to antagonize interaction with PIP2 in a time- and concentration-dependent manner, reflecting the channels' affinity for PIP2 (Fan & Makielski, 1997; Schulze et al. 2003). Figure 5A and B compares the effects of neomycin and PIP2 on wild-type and R50D channels, respectively, coexpressed with SUR1. In wild-type Kir6.2 + SUR1 channels, 10 μm neomycin inhibited channel activity gradually and partially, reaching 50% inhibition in ∼19 s. In contrast, inhibition of R50D + SUR1 channels by 10 μm neomycin was rapid and almost complete, reaching 50% inhibition in 2 s. The high neomycin sensitivity of R50D is consistent with a low PIP2 affinity as compared with wild-type channels. This observation is further supported by data obtained with PIP2. After inhibition by neomycin, reactivation of wild-type Kir6.2 + SUR1 channels was rapid (within <1 min) and was almost complete. By comparison the reactivation of R50D + SUR1 channels was much slower, partial and often transitory. On average, reactivation of R50D + SUR1 channels by PIP2 was 32 ± 15% of control and was sustained in only two out of four patches in which reactivation of channel activity occurred. These results strongly suggest that a negative charge at position 50 weakens the channel's interaction with PIP2.
Figure 5. Reduced PIP2 affinity in Kir6.2 R50E/D + SUR1 channels.
A and B, current traces depict the partial inhibitory effect of neomycin, an antagonist of PIP2, and the reversal of inhibition by exogenous phosphatidylinositol 4,5-bisphosphate (PIP2) with wild-type Ki6.2 + SUR1 (A) and R50D + SUR1 mutant (B) channels. R50D/E mutant channels had higher neomycin sensitivity, indicating lower PIP2 affinity, than wild-type channels. The IC50 for R50D/E channel inhibition by neomycin was about threefold lower than that of control (C) and the rate at which channels closed in the presence of 10 μm neomycin was almost 10 times faster in mutant channels. C, fitting of the neomycin data points to the Hill equation yielded IC50 values of 19 μm (n = 6) for wild-type Kir6.2 + SUR1 (•) and 6.7 μm (n = 5) for R50D/E + SUR1 (○). In both cases the Hill coefficients were less than unity and there was no statistical difference between R50D and R50E sensitivity to neomycin.
Discussion
The first important result is that in the absence of SUR1, the size of the residue at position 50, rather than its charge or hydropathy, is the factor that influences ATP sensitivity. As the length of the residue's side chain became shorter, ATP sensitivity decreased dramatically (Table 1). The observation that the residue's size at position 50 may play a role in determining ATP sensitivity was first reported by Trapp et al. (2003), and is consistent with recent reports indicating that ATP binding is favoured when the width of the ‘binding pocket’ increases (Dabrowski et al. 2004). Thus, it may be envisioned that a small residue at position 50, causing a collapse of the ‘binding pocket’, may weaken binding of adenine nucleotides to this site. Alternatively, it has been suggested that Kir6.2 N terminus and R50 may close the pocket formed by the C terminus, and stabilize ATP binding at its site (Antcliff et al. 2005). If this were the case, then it is possible that a small residue at position 50 may not trap ATP efficiently and thus weaken its binding to the site.
When coexpressed with SUR1, however, regulation of adenine nucleotide sensitivity by R50 was more complex. In addition to size, residue hydropathy and charge also took on important roles. Specifically, hydrophobicity at position 50 preserved high ATP sensitivity. Thus, the hydrophobic substitutions R50C + SUR1 and R50A + SUR1 exhibited high ATP sensitivity compared with hydrophilic substitutions R50S + SUR1 and R50G + SUR1. However, the most striking effect of residue substitution at position 50 was due to insertion of a negative charge.
A negative charge at position 50 accounts for SUR1-induced increase in ATP sensitivity
In the presence of SUR1, both ATP and ADP sensitivities increased, but ATP sensitivity increased about 10-fold while ADP sensitivity increased only threefold. Since ATP differs from ADP only by its γ phosphate group, this indicates that SUR1 selectively promotes an interaction with ATP's γ phosphate group to facilitate channel closure. It is important to note that this differential effect could not be attributed to the stimulatory effect of ADP, since the lack of increase in ADP sensitivity with SUR1 was observed in the absence of Mg2+ or after addition of sulphonylureas, both of which prevented channel stimulation by ADP. In the case of the wild-type channel, the interaction of the γ phosphate of ATP with R50 increased the ATP sensitivity by 10-fold when SUR1 was present. However, the data in Table 1 indicate that most of the increased ATP sensitivity induced by SUR1 could be attributed to a negative charge in the region of position 50, rather than the presence of an ATP phosphate group per se, for the following reasons. First, in the presence of SUR1, the R50E and R50D mutants had similar ATP sensitivities as wild-type channels, although neither of these residues could have interacted electrostatically with the γ phosphate of ATP. Second, the ADP sensitivities of R50E + SUR1 and R50D + SUR1 channels were dramatically increased and approached that of ATP. We interpret these findings to indicate that a negative charge in the region of position 50 mimics the effects of the ATP's γ phosphate group interaction with R50 to facilitate channel closure evoked by the α and β phosphate groups of the ATP or ADP molecule interacting with R201 and K185, respectively, within the binding pocket.
The interaction of R50 and PIP2 with R54
A decrease in burst duration has been observed with R54 mutants (Ribalet et al. 2005), which decreases Kir6.2's interaction with PIP2 (Schulze et al. 2003), suggesting that interaction of PIP2 with R54 is required to stabilize the channel in the bursting state. Similar results are observed with R176/R177 mutants, suggesting that multiple positively charged residues interact with PIP2 to stabilize the open state (Ribalet et al. 2005). Since bursting behaviour in wild-type Kir6.2 channels also requires SUR1, SUR1 may normally acts in concert with the PIP2–R54–R176/R177 interaction to promote bursting. These observations can be integrated with the effects of adenine nucleotides in the present study as follows. In the absence of SUR1, Kir6.2 channels do not burst, and adenine nucleotides suppress openings primarily via the interactions of their α and β phosphate groups with K185 and R201 in the ATP binding pocket. In the presence of SUR1, however, the charge near R50 becomes important in regulating the bursting state. Specifically, the introduction of a negative charge at position 50, either via interaction with the γ phosphate of ATP or by mutation of R50 to a negatively charged residue, destabilizes the channel's bursting state and initiates the channel closure by favouring interaction of the other phosphates groups of ATP or ADP within the ATP binding pocket near K185 and R201. In this model, our findings with R54 mutants, which exhibited a small ratio of ATP relative to APD sensitivity when coexpressed with SUR1, can be understood as follows. Since loss of the PIP2–R54 interaction already prevented stabilization of the bursting state, the ability of negative charge at R50 (provided by its interaction with the γ phosphate) to suppress bursting was lost. Thus, the effect of negative charge at R50 may be to destabilize the channel's interaction with PIP2 at R54, thereby suppressing bursting and promoting channel closure. Since this requires the presence of SUR, the effect of SUR may be to position residue 50 near R54 and/or perhaps R176/R177 so that interaction with PIP2 can be destabilized. In the absence of SUR, the low ATP sensitivity may be accounted for by assuming that residue 50 is too far from R54 or R176/R177 to destabilize the interaction with PIP2. Such a scheme is further supported by recent modelling (Antcliff et al. 2005), suggesting that the complex formed by R50 and the γ phosphate sticks out of the binding pocket and is able to flex. This renders SUR-induced motion of R50-γ phosphate plausible.
Our proposal that the effects of SUR1 on ATP sensitivity are mediated via R50 does not conflict with the hypothesis recently proposed by Dabrowski et al. (2004) that SUR1 increases ATP affinity by widening the ATP binding groove. Indeed, we find that, independent of R50 mutations, both ATP and ADP sensitivity were two to three times higher with than without SUR1. This implicates a mechanism independent of the interaction of ATP's γ phosphate with R50, for instance by SUR1 directly broadening the ATP binding groove.
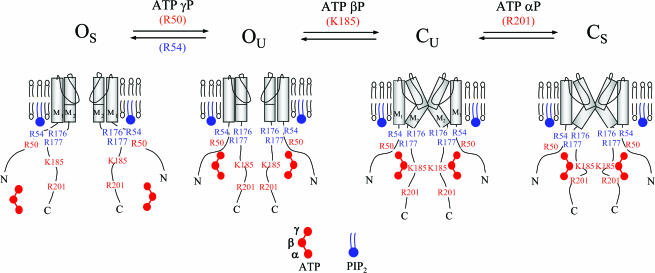
In summary, our data suggest that SUR1 increases ATP sensitivity by allowing a negative charge at position 50, provided either exogenously by ATP's γ phosphate group or endogenously by mutation, to destabilize an open state of the channel, promoting the interaction of ATP's α and β phosphate groups with K185 and R201 to close the channel, independent of SUR1, as we and others have proposed previously (Tucker et al. 1998; Shyng et al. 2000; Ribalet et al. 2003) (Fig. 6). In addition, the negative charge at R50 may initiate channel closure by destabilizing the electrostatic interaction PIP2 with nearby positively charged residues such R54, and possibly R176/R177.
Figure 6. Allosteric model for interaction of PIP2 and phosphate groups of adenine nucleotides with positively charged residues in Kir6.2.
In the absence of ATP, R54 and R176/R177 interact with PIP2 to stabilize the channel in the stable open configuration (OS). Binding of the ATP γ phosphate group with R50, or a negatively charged residue at this position, destabilizes channel interaction with PIP2, causing transition to the unstable open state (OU). As previously suggested (John et al. 2003), destabilization of the open state leads to unstable closure of the channel gate within M2 (CU), and binding of the ATP β phosphate to K185 allosterically promotes transition from OU to CU. In this model binding of ATP β phosphate to K185 is state independent. The interaction of the ATP β phosphate with K185 is favoured by a ‘large’ residue at position 50, which broadens the ATP binding pocket. Finally, once the channel is in the CU state, the ATP α phosphate group interacts with R201 to stabilize the channel in a stable closed state (CS).
Acknowledgments
This work was supported by NIH grants R37HL60025 and NIH SCOR in Sudden Cardiac Death P50 HL52319, and Laubisch and Kawata Endowments to J.N.W.
References
- Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the K(ATP) channel Kir6.2 subunit. EMBO J. 2005;24:229–239. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Dabrowski M, Tarasov A, Ashcroft FM. Mapping the architecture of the ATP-binding site of the KATP channel subunit Kir6.2. J Physiol. 2004;557:347–354. doi: 10.1113/jphysiol.2003.059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvetchakul D, Loussouarn G, Makhina E, Shyng SL, Nichols CG. The kinetic and physical basis of K(ATP) channel gating: toward a unified molecular understanding. Biophys J. 2000;78:2334–2348. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Phosphoinositides decrease ATP sensitivity of the cardiac ATP-sensitive K+ channel. A molecular probe for the mechanism of ATP-sensitive inhibition. J Gen Physiol. 1999;114:251–269. doi: 10.1085/jgp.114.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, van der Eb AJ. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;54:536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Ho IH, Murrell-Lagnado RD. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J Physiol. 1999;520:645–651. doi: 10.1111/j.1469-7793.1999.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Monck JR, Weiss JN, Ribalet B. The sulphonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K+ channels linked to the green fluorescent protein in human embryonic kidney cells (HEK 293) J Physiol. 1998;510:333–345. doi: 10.1111/j.1469-7793.1998.333bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Weiss JN, Xie L-H, Ribalet B. Molecular mechanism for ATP-dependent closure of the K+ channel Kir6.2. J Physiol. 2003;552:23–34. doi: 10.1113/jphysiol.2003.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Jia T, Weinsoft AM, Shyng SL. Stabilization of the activity of ATP-sensitive potassium channels by ion pairs formed between adjacent Kir6.2 subunits. J Gen Physiol. 2003;122:225–237. doi: 10.1085/jgp.200308822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Gribble FM, Adhikari R, Tucker SJ, Ashcroft FM. Involvement of the N-terminus of Kir 6.2 in the inhibition of the KATP channel by ATP. J Physiol. 1999;514:19–25. doi: 10.1111/j.1469-7793.1999.019af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Tucker SJ, Proks P, Ashcroft FM. Involvement of the N terminus of Kir6.2 in coupling to the sulphonylurea receptor. J Physiol. 1999;518:325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, John SA, Weiss JN. Regulation of cloned ATP-sensitive K channels by phosphorylation, MgADP, and phosphatidylinositol bisphosphate (PIP2): a study of channel rundown and reactivation. J Gen Physiol. 2000;116:391–410. doi: 10.1085/jgp.116.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, John SA, Weiss JN. Molecular basis for Kir6.2 channel inhibition by adenine nucleotides. Biophys J. 2003;84:266–276. doi: 10.1016/S0006-3495(03)74847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, John SA, Xie L-H, Weiss JN. The sulfonylurea receptor, SUR, and PIP2 in KATP channel regulation. Biophys J. 2005;88:1395. [Google Scholar]
- Schulze D, Krauter T, Fritzenschaft H, Soom M, Baukrowitz T. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J Biol Chem. 2003;278:10500–10505. doi: 10.1074/jbc.M208413200. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP2 regulation of inward rectifier K(ATP) channels. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Trapp S, Haider S, Jones P, Sansom MS, Ashcroft FM. Identification of residues contributing to the ATP binding site of Kir6.2. EMBO J. 2003;22:2903–2912. doi: 10.1093/emboj/cdg282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO J. 1998;17:3290–3296. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]