Abstract
Inward rectification is caused by voltage-dependent block of the channel pore by intracellular Mg2+ and polyamines such as spermine. In the present study, we compared inward rectification in the Kir3.1/Kir3.4 channel, which underlies the cardiac current IK,ACh, and the Kir2.1 channel, which underlies the cardiac current IK,1. Sustained outward current at potentials positive to the K+ reversal potential was observed through Kir3.1/Kir3.4, but not Kir2.1, demonstrating that Kir3.1/Kir3.4 exhibits weaker inward rectification than Kir2.1. We show that Kir3.1/Kir3.4 is more sensitive to extracellular spermine block than Kir2.1, and that intracellular and extracellular polyamines can permeate Kir3.1/Kir3.4, but not Kir2.1, to a limited extent. We describe a simple kinetic model in which polyamines act as permeant blockers of Kir3.1/Kir3.4, but as relatively impermeant blockers of Kir2.1. The model shows the difference in sensitivity to extracellular spermine block, as well as the difference in the extent of inward rectification between the two channels. This suggests that Kir3.1/Kir3.4 exhibits weaker inward rectification than Kir2.1 because of the difference in the balance of polyamine block and permeation of the two channels.
Kir2.1 and Kir3.1/Kir3.4 are inwardly rectifying K+ channels that are expressed in the heart. Kir2.1, along with Kir2.2 and Kir2.3, is thought to underlie the background inward rectifier K+ current IK,1 (Liu et al. 2001; Zaritsky et al. 2001; Zobel et al. 2003), whilst Kir3.1/Kir3.4 underlies the ACh-activated K+ current IK,ACh (Kubo et al. 1993; Krapivinsky et al. 1995). Comparison of IK,1 and IK,ACh in heart cells recorded in a number of studies (Kurachi, 1985; Zang et al. 1993; Dobrzynski et al. 2002; Ishihara et al. 2002; Lomax et al. 2003) suggests that there is a difference in the extent of inward rectification between these currents. The first aim of this study was to investigate inward rectification in the heterologously expressed Kir2.1 and Kir3.1/Kir3.4 channels.
Although inward rectification is due to voltage-dependent block of the channel by intracellular Mg2+ and polyamines such as spermine (Nichols & Lopatin, 1997), the regions of the channel involved with the coordination of Mg2+ and polyamines within the pore are debated. In Kir2.1, a number of residues within the pore lining second transmembrane domain and proximal C terminus have been shown to be important for inward rectification (Lu & MacKinnon, 1994; Stanfield et al. 1994; Yang et al. 1995; Kubo & Murata, 2001; Fujiwara & Kubo, 2002). Recent evidence suggests that these may not be the site of block, but instead shuttle the polyamines to their eventual binding site deeper within the pore (Kubo & Murata, 2001; Guo et al. 2003; Xie et al. 2003; Chang et al. 2003). Other recent evidence suggests that polyamines reach the selectivity filter (Guo & Lu, 2000; Dibb et al. 2003; Chang et al. 2003; Kurata et al. 2004). For example, extracellular polyamines can permeate Kir3.1/Kir3.4 to such an extent that significant polyamine currents can be measured (Dibb et al. 2003). In the present study, we have used extracellular polyamines as probes to demonstrate a difference in the extent of polyamine block and permeation between Kir3.1/Kir3.4 and Kir2.1. Using a simple kinetic model based on the experimental data, we show how the difference in the balance of polyamine block and permeation of the two channels can explain the difference in the extent of inward rectification.
Methods
Channel expression in mammalian cells
Kir2.1 and Kir3.1/Kir3.4 were expressed in Chinese Hamster Ovary (CHO-K1) cells that were stably transfected with hM2 (the human M2 muscarinic receptor), GRK2 (G-protein-coupled receptor kinase) and a selection marker for antibiotic-resistant growth in G418 sulphate, as previously described (Boyett et al. 2000). CHO cells were cultured in Ham's F-12 nutrient mixture (Invitrogen, Paisley, UK) supplemented with 10% fetal bovine serum, 50 units ml−1 penicillin G, 50 μg ml−1 streptomycin sulphate and 60 μg ml−1 G418 sulphate (Invitrogen) at 37°C in 95% air and 5% CO2. Approximately 1 × 105 cells were seeded onto glass slips and after 24 h transiently transfected with Kir2.1 (pcDNA3) or with Kir3.1 and Kir3.4 (pEF-BOS) using Fugene 6 transfection reagent (Roche Diagnostics, Lewes, UK). In all cases, cells were also transfected with green fluorescent protein (pEGFP-N1; Clontech Laboratories, Palo Alto, CA, USA) as a marker for successful transfection. All plasmids were used at a final concentration of 3 ng μl−1. Currents were recorded 24–72 h later using the whole-cell patch-clamp technique.
Whole-cell patch clamp
Transiently transfected CHO cells growing on glass slips were viewed using a Nikon TE300 Eclipse microscope. Cells were illuminated at 470–490 nm (and viewed through a 515 nm filter) to excite the GFP in successfully transfected cells. Cells that showed strong green fluorescence were chosen for study. Pipettes with a resistance of 5 MΩ were filled with (mm): potassium aspartate, 130; KCl, 20; KH2PO4, 1; MgCl2, 3.7 (free Mg2+, 1.8); EGTA, 5; Na2ATP, 3; GTP, 0.1; Hepes, 5; pH 7.4 (with KOH). Currents were recorded using an Axopatch 200A amplifier (filter frequency, 2 kHz; sampling frequency, 5 kHz; Axon Instruments, Union City, CA, USA). Eighty per cent of the series resistance (6–7 MΩ) of the electrode was compensated (therefore, at −60 mV, with a Kir2.1 current of ∼4 nA, the voltage error would be ∼6 mV). Currents were recorded during 750 ms voltage pulses from −60 to +100 mV (pulse frequency, 0.33 Hz) from a holding potential of 0 mV (computer-driven protocols using pCLAMP software and a Digidata 1322 interface; Axon Instruments). Experiments were performed at 20–25°C. Currents were recorded from cells expressing Kir2.1 or Kir3.1/Kir3.4 or from untransfected cells during perfusion of solution containing (mm): KCl, 140; MgCl2, 2.7; EGTA, 5; Hepes, 10; pH 7.4 (with KOH). In the case of cells expressing Kir3.1/Kir3.4, current was also recorded in the presence of 10 μm ACh. Kir3.1/Kir3.4 current was expressed as the difference between current recorded in the presence and absence of ACh. Kir2.1 current was expressed as the difference between current recorded from cells expressing Kir2.1 and mean current (n = 7) recorded from untransfected cells.
Channel expression in Xenopus oocytes
Plasmids containing Kir2.1 (pBluescript), Kir3.1, Kir3.4 or the hD2 receptor (human D2 dopamine receptor) (pTLNII) were linearized with HindIII or MluI (New England Biolabs, MA, USA), respectively, and transcribed in vitro using T3 (MEGAscript; Ambion, Austin, TX, USA) or SP6 (Riboprobe; Promega, Madison, WI, USA) RNA polymerase, respectively. Female Xenopus laevis (African clawed toads) were anaesthetized by immersion in 0.2% (w/v) ice-cold tricaine (3-aminobenzoic acid ethyl ester methanosulphate salt solution; pH 7.4 with NaOH; Sigma, Poole, UK) for 30–60 min. When unresponsive to a pinch applied to the forearm or leg, frogs were killed by severing the cervical spinal cord. Upon removal, oocytes were placed into sterile Ca2+-free ND96 solution (mm): NaCl, 96; KCl, 3; MgCl2, 1; Hepes, 5; pH 7.4 (with NaOH). Healthy stage V and VI oocytes were defolliculated using a combination of collagenase treatment (1 mg ml−1 for 1 h; Type 1A, Sigma) and manual defolliculation. Defolliculated oocytes were injected with 50 nl cRNA containing: Kir2.1 (30 ng μl−1); Kir3.1 (30 ng μl−1), Kir3.4 (30 ng μl−1) and hD2 (3.8 ng μl−1); hD2 only (38 ng μl−1); Kir3.4 (30 ng μl−1) and hD2 (3.8 ng μl−1) only; or Kir3.1 (300 ng μl−1), Kir3.4[E145Q] (300 ng μl−1) and hD2 (38 ng μl−1). The oocytes were then incubated for 1–2 days at 19°C in modified Barth's medium (mm): NaCl, 88; KCl, 1; NaHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; sodium pyruvate, 1.25; neomycin, 0.1 mg ml−1 (Sigma); penicillin–streptomycin mix, 100 units/0.1 mg ml−1 (Sigma); Hepes, 20; pH 7.4 (with NaOH). Control oocytes were from the same frog and were either water-injected or uninjected. All animal procedures were carried out according to national guidelines.
Two-electrode voltage clamp
Two-microelectrode voltage-clamp recordings from oocytes were performed using a GeneClamp 500B amplifier (Axon Instruments) filtering at 500 Hz and sampling at 2 kHz. Voltage protocols were generated using pClamp software (Axon Instruments) with a Digdata 1200 D/A converter (Axon Instruments). Oocytes were held at 0 mV, and 750 ms voltage pulses were applied from −130 to +60 mV in 10 mV increments. Electrodes were filled with 3 m KCl (tip resistance, 0.5–2.5 MΩ). Experiments were performed at 20–25°C. Control recordings were made in bath solution containing (mm): KCl, 90; CaCl2, 2; Hepes, 5; pH 7.4 (with KOH). In order to activate Kir3.1/Kir3.4, 10 μm dopamine was added (along with 10 μm ascorbic acid to prevent dopamine oxidation). To study spermine block, 1, 3, 10 or 30 mm spermine chloride was added to the bath solution. To study permeation, the KCl in the solution was replaced with 90 mm RbCl or 90 mm spermine chloride. To study K+ activation or spermine activation, KCl or spermine chloride was substituted by equimolar NMDG chloride. For the anomalous mole-fraction experiment, the KCl in the solution was progressively replaced with spermine chloride (sum of KCl and spermine chloride concentrations maintained at 90 mm). In all solutions containing spermine, free spermine (basic) was used and the pH was adjusted to pH 7.4 using HCl (resulting in the formation of spermine chloride). In other experiments, 1 μm Tertiapin Q, 1 mm BaCl2 or 50 mm iodoacetamide (IAA) was added to the recording solution to inhibit Kir3.1/Kir3.4. Tertiapin Q is an oxidation-resistant mutant of the inhibitor tertiapin, derived from honey-bee venom (Jin & Lu, 1999). Oocytes were perfused at ∼0.5 ml min−1 (equivalent to 3.3 bath vols min−1; bath volume ∼0.15 ml) and 2 min was allowed after a solution change before recordings were made. Because the bath electrode was a Ag–AgCl pellet, there was a change in the junction potential at the bath electrode when spermine chloride was added to the bath solution, or the KCl in the bath solution was replaced by spermine chloride. Junction potentials were measured with a flowing 3 m KCl electrode with reference to 90 mm K+ solution with 0 mm spermine, and were 0.4, 0.2, 2.4, 8 and 17 mV with 1, 3, 10, 30 and 90 mm spermine, respectively; when important, data were corrected for junction potentials and this is stated. Currents recorded from oocytes were not corrected for endogenous currents.
Tritiated-spermidine efflux measurements
Oocytes were injected with Kir2.1 or Kir3.1/Kir3.4 cRNA as described above. Uninjected oocytes were used as a control. Following cRNA injection, oocytes were incubated in Barth's medium at 19°C for 12–36 h. Channel expression was confirmed using the two-electrode voltage-clamp technique. Oocytes were then injected with 50 nl of 3H-labelled spermidine ([3H]spermidine; specific activity 15 Ci mmol−1) and incubated for a further 2 h. Following two brief washes, oocytes were incubated with ∼100 μl of experimental solution for a collection period of 15 min. At the end of the experiment, oocytes were lysed and counted. Radioactivity was measured using a scintillation spectrometer (Packard BioScience Company, Meriden, CT, USA). Optiphase ‘safe’ (PerkinElmer Life Sciences, Wellesley, MA, USA) scintillant was used in all experiments. [3H]Spermidine efflux is expressed as a percentage of the [3H]spermidine in the oocyte at the start of the 15 min collection period.
Data analysis
Data analysis was performed using Clampfit (Axon Instruments) and SigmaPlot (SPSS Science, Chicago, IL, USA) software. Dose–response curves were constructed using the following equation:
| (1) |
where [spermine]o is the extracellular spermine concentration and 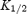 is the spermine concentration that blocks half of the current (the Hill coefficient was taken to be 1). Plots of
is the spermine concentration that blocks half of the current (the Hill coefficient was taken to be 1). Plots of 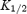 versus membrane potential (Vm) were fitted with the following exponential function:
versus membrane potential (Vm) were fitted with the following exponential function:
| (2) |
where 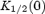 is the spermine concentration that blocks half the current at 0 mV, δ is the apparent fraction of the electrical field that spermine must cross in order to reach its binding site, z is the valency of spermine, and F, R and T have their usual meanings.
is the spermine concentration that blocks half the current at 0 mV, δ is the apparent fraction of the electrical field that spermine must cross in order to reach its binding site, z is the valency of spermine, and F, R and T have their usual meanings.
Permeability ratios for spermine and Rb+, PX/PK (where PX is the permeability of spermine or Rb+, and PK is the permeability of K+), were calculated from the Goldman-Hodgkin-Katz (GHK) current equation as previously described (Lewis, 1979; Bähring et al. 1997; Frazier et al. 2000). Although the GHK current equation assumes independence of flux of different ions, and permeation of Kir channels violates this assumption, we use PX/PK as a useful empirical measure. First, with K+ in the bathing medium (no extracellular spermine or Rb+), the Nernst equation was used to calculate [K+]i from the reversal potential of the Kir current. In the 19 oocytes used for the measurement of permeability ratios, with K+ in the bathing medium, the reversal potential was −5.8 ± 1.1 mV (mean ± s.e.m.) and calculated [K+]i was 115 ± 4.7 mm (mean ± s.e.m.); the calculated [K+]i is within the range of 93–150 mm reported previously for Xenopus oocytes (Zeuthen et al. 2002). The K+ in the bathing medium was then substituted by equimolar spermine or Rb+. Eqn (3) (derived from eqn (1) in Frazier et al. 2000) was then used to calculate PX/PK from the new reversal potential (Vrev):
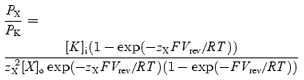 |
(3) |
where zX is the valency of spermine (+4) or Rb+, [K]i is the intracellular K+ concentration, and [X]o is the extracellular concentration of spermine or Rb+ (the intracellular spermine and Rb+ concentrations were taken to be zero). Note that because current recorded from oocytes was not corrected for endogenous current, the measured reversal potentials (and therefore permeability ratios) may be in error.
For conductance–voltage relationships, conductance (g) was calculated as i/(Vm−Vrev), where i is current. Measurement of reversal potentials is difficult in the case of inward rectifier K+ current, because conductance decreases as the reversal potential is approached and therefore the measured reversal potential is sensitive to endogenous current. If the wrong value is chosen for Vrev, calculated g changes dramatically in a non-smooth fashion positive and negative to Vrev (the behaviour of g is sensitive to small errors in Vrev). This provided a useful tool to estimate Vrev: the value of Vrev was adjusted to obtain a smooth decline in g (as Vm became more positive) around Vrev. The reversal potential for the calculation of the permeability ratio was measured in the same way.
All data are given as means ± s.e.m. (n is the number of cells). Where appropriate, results were compared using one-way ANOVA or Student's t test. Significance was assumed at P < 0.05.
Results
The extent of inward rectification is different in Kir2.1 and Kir3.1/Kir3.4
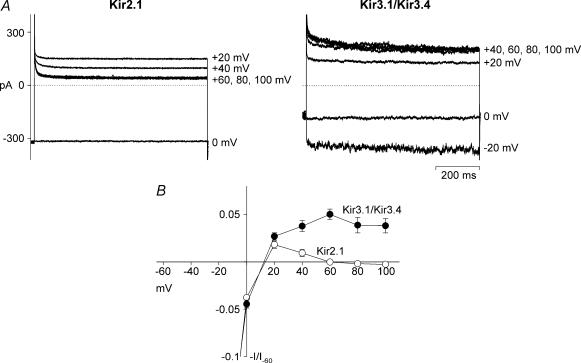
Figure 1 shows data recorded during whole-cell patch clamp of CHO cells expressing either Kir2.1 or Kir3.1/Kir3.4. This expression system was used for these experiments because the endogenous currents of CHO cells are small, allowing clear observation of outward current through the expressed channels. Kir2.1 and Kir3.1/Kir3.4 currents were recorded during 750 ms pulses from −60 to +100 mV from a holding potential of 0 mV in the presence of 140 mm extracellular K+. In Fig. 1A, Kir2.1 and Kir3.1/Kir3.4 currents recorded at 0 or −20 mV, and at more positive potentials, are shown. The extent of inward rectification was different in the two channels. In the case of Kir2.1, current was inward at 0 mV and outward at +20 mV, but, at more positive potentials, outward current decreased towards zero. In the case of Kir3.1/Kir3.4, current was again inward at 0 mV and outward at +20 mV, but, at more positive potentials, outward current increased further and was sustained even at +100 mV. The mean current–voltage relationships in Fig. 1B show more clearly that the extent of inward rectification was greater with Kir2.1 than with Kir3.1/Kir3.4 (current at +100 mV: n = 5–7; Student's t test, P < 0.01). This confirms that the cardiac current IK,1 (which Kir2.1 underlies) shows stronger inward rectification than the cardiac current IK,ACh (which Kir3.1/Kir3.4 underlies).
Figure 1. Inward rectification is stronger in Kir2.1 than Kir3.1/Kir3.4.
A, typical whole-cell currents recorded from CHO cells expressing Kir2.1 or Kir3.1/Kir3.4 during 750 ms voltage-clamp pulses from −60 to +100 mV. Only currents at 0 mV (−20 mV in the case of Kir3.1/Kir3.4) and more positive potentials are shown. The dotted lines show zero current. The Kir2.1 current is uncorrected for endogenous current. The Kir3.1/Kir3.4 current shown is ACh-dependent current (difference between current before and after the application of 10 μm ACh). B, mean normalized current–voltage relationships for Kir2.1 (○) and Kir3.1/Kir3.4 (•). Current (measured at the end of the pulse) is normalized to current at −60 mV. Kir2.1 current is corrected for endogenous current (measured in untransfected cells) and the Kir3.1/Kir3.4 current shown is ACh-dependent current. At −60 and +100 mV, current in Kir2.1-expressing cells was −4300 ± 400 and 80 ± 10 pA, whereas in untransfected cells it was −155 ± 39 and 168 ± 11 pA. At −60 and +100 mV, current in Kir3.1/Kir3.4-expressing cells in the presence of ACh was −2830 ± 520 and 240 ± 50 pA, whereas in Kir3.1/Kir3.4-expressing cells in the absence of ACh it was −258 ± 115 and 122 ± 19 pA. Means ± s.e.m. (n = 5–7) are shown.
Extracellular polyamine block of Kir2.1 and Kir3.1/Kir3.4
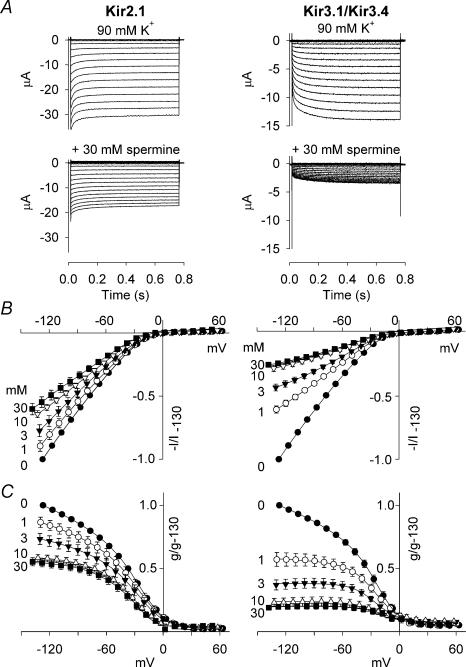
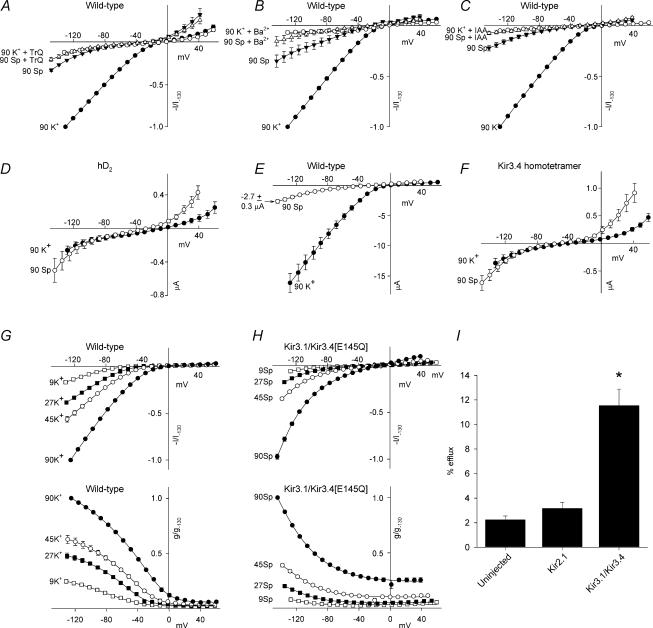
Owen et al. (1998) demonstrated a difference in extracellular Ba2+ block of Kir2.1 and Kir3.1/Kir3.4. We investigated block of both channels by extracellular spermine. These, and all further experiments, were performed using two-electrode voltage clamp of Xenopus oocytes. Figure 2A shows currents in the presence of 90 mm extracellular K+ under control conditions and on addition of 30 mm spermine. Addition of 30 mm spermine resulted in a significant decrease in current through both channels (n = 13–15; paired t test, P < 0.001). These data are summarized by the mean current– and conductance–voltage relationships in the absence and presence of various concentrations of spermine shown in Fig. 2B and C. Interestingly, at each concentration tested, extracellular spermine blocked Kir3.1/Kir3.4 to a greater extent than Kir2.1 (at −130 mV: n = 7–15; Student's t test, P < 0.001).
Figure 2. Polyamine block of Kir2.1 and Kir3.1/Kir3.4.
A, typical Kir2.1 and Kir3.1/Kir3.4 currents recorded under control conditions (top) and after the addition of 30 mm extracellular spermine (bottom). B and C, normalized current–voltage (B) and conductance–voltage (C) relationships for the two channels in the absence and presence of various concentrations of spermine (mm). Current/conductance (measured at the end of the pulse; corrected for junction potentials) is normalized to the current/conductance at −130 mV in the absence of spermine. Means ± s.e.m. (n = 7–15) are shown; some error bars are smaller than the size of the points.
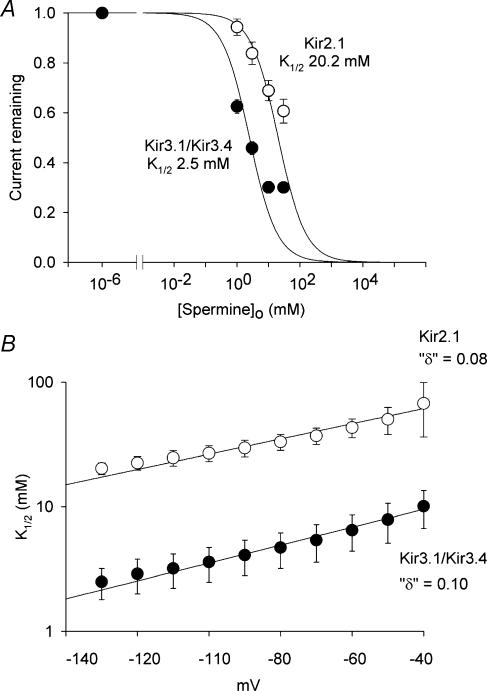
Figure 3A shows mean dose–response curves for extracellular spermine block of the two channels at −130 mV. The data have been fitted with the Hill equation, assuming a Hill coefficient of 1. In the case of Kir3.1/Kir3.4, the 30 mm data point deviates from the Hill curve (the block with 30 mm spermine was approximately the same as with 10 mm spermine). This could be the result of spermine permeation of Kir3.1/Kir3.4 at the highest extracellular spermine concentration – this deviation is more marked with mutant Kir3.1/Kir3.4 channels with a higher spermine permeability (Dibb et al. 2003). To minimize the effect of spermine permeation, the Hill curve was only fitted to data at spermine concentrations <30 mm. The data in Fig. 3A highlight the difference in sensitivity to spermine block between the two channels: the 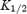 was 20.2 ± 2.2 mm for Kir2.1 (n = 15) and 2.5 ± 0.6 mm for Kir3.1/Kir3.4 (n = 13). Kir3.1/Kir3.4 was ∼8 times more sensitive to spermine block than Kir2.1 (t test, P < 0.001). Figure 3B shows the voltage dependence of spermine block of the two channels: the
was 20.2 ± 2.2 mm for Kir2.1 (n = 15) and 2.5 ± 0.6 mm for Kir3.1/Kir3.4 (n = 13). Kir3.1/Kir3.4 was ∼8 times more sensitive to spermine block than Kir2.1 (t test, P < 0.001). Figure 3B shows the voltage dependence of spermine block of the two channels: the 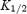 of spermine block (from data like those in Fig. 3A) is plotted against the membrane potential. From Fig. 3B, δ (the apparent fraction of the electrical field that spermine must cross to reach its binding site) was 0.08 ± 0.01 and 0.10 ± 0.01 (Student's t test, not significant) for Kir2.1 and Kir3.1/Kir3.4, respectively. The low values could suggest that spermine binds at a superficial site in the two channels (but see model below).
of spermine block (from data like those in Fig. 3A) is plotted against the membrane potential. From Fig. 3B, δ (the apparent fraction of the electrical field that spermine must cross to reach its binding site) was 0.08 ± 0.01 and 0.10 ± 0.01 (Student's t test, not significant) for Kir2.1 and Kir3.1/Kir3.4, respectively. The low values could suggest that spermine binds at a superficial site in the two channels (but see model below).
Figure 3. Characteristics of polyamine block of Kir2.1 and Kir3.1/Kir3.4.
A, dose–response curves for extracellular spermine block of Kir2.1 and Kir3.1/Kir3.4 at −130 mV (corrected for junction potentials). The current remaining (as a fraction of the current in the absence of spermine) is plotted against the spermine concentration. Data are fitted with the Hill equation (eqn (1)) assuming a Hill coefficient of 1. B, 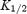 for spermine block plotted against membrane potential for the two channels. Data are fitted with eqn (2). The apparent electrical distance of the binding site (δ) is shown. Means ± s.e.m. (n = 7–15) are shown.
for spermine block plotted against membrane potential for the two channels. Data are fitted with eqn (2). The apparent electrical distance of the binding site (δ) is shown. Means ± s.e.m. (n = 7–15) are shown.
Kir3.1/Kir3.4, but not Kir2.1, conducts measurable inward spermine current
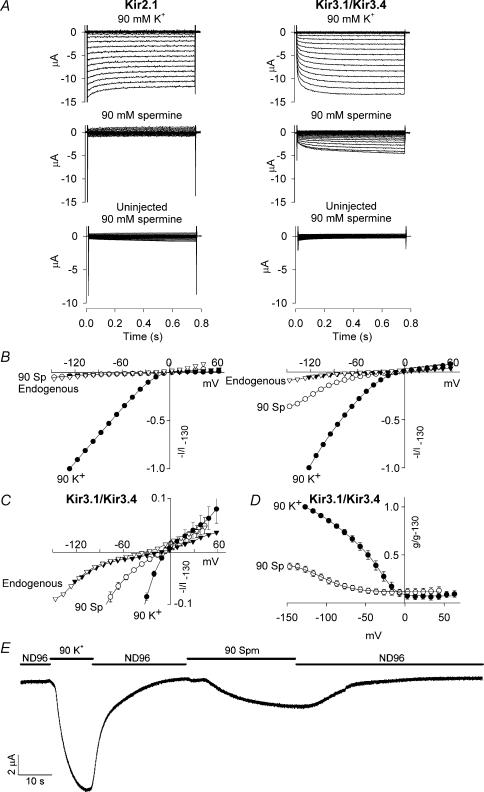
We have previously shown that extracellular polyamines, such as spermine, may permeate Kir3.1/Kir3.4 expressed in Xenopus oocytes (Dibb et al. 2003). The results in Fig. 4 suggest that extracellular spermine permeates Kir3.1/Kir3.4 more readily than Kir2.1. Figure 4A (top) shows control currents recorded through Kir2.1 and Kir3.1/Kir3.4 in the presence of 90 mm extracellular K+. We then replaced the extracellular K+ with 90 mm spermine (Fig. 4A, middle). In oocytes expressing Kir3.1/Kir3.4, extracellular spermine produced a substantial inward current presumably as a result of permeation of Kir3.1/Kir3.4 (see next section). This was significantly larger than endogenous current recorded from the same batch of oocytes (Fig. 4A, bottom; n = 8–11; ANOVA, P < 0.001). Figure 4E shows the time course of current recorded from an oocyte expressing Kir3.1/Kir3.4 when the bathing solution was switched between Na+ (ND96)-, K+- and spermine-containing solutions (membrane potential held at −80 mV); as expected, with Na+ as the charge carrier, there was no current, but with either K+ or spermine as the charge carrier there was inward current. In contrast, in oocytes expressing Kir2.1, extracellular spermine did not produce an inward current (current was not significantly different from endogenous current in the presence of spermine; Fig. 4A; n = 9; ANOVA, not significant). These data are summarized by the mean current–voltage relationships in Fig. 4B. In the case of Kir3.1/Kir3.4 only, magnified current–voltage relationships are shown in Fig. 4C (to show changes in reversal potential), and conductance–voltage relationships are shown in Fig. 4D.
Figure 4. Kir3.1/Kir3.4, but not Kir2.1, conducts measurable inward spermine current.
A, typical currents recorded from Xenopus oocytes expressing either Kir2.1 or Kir3.1/Kir3.4 or from uninjected oocytes. Top, control Kir2.1 and Kir3.1/Kir3.4 currents recorded in the presence of 90 mm extracellular K+. Middle, Kir2.1 and Kir3.1/Kir3.4 currents recorded when the extracellular K+ was replaced with 90 mm spermine. Bottom, currents recorded from uninjected cells in the presence of 90 mm extracellular spermine. B, mean normalized Kir2.1 and Kir3.1/Kir3.4 current–voltage relationships in the presence of 90 mm K+ or spermine (Sp). Endogenous current–voltage relationships in the presence of 90 mm spermine are also shown. C, magnified view of the Kir3.1/Kir3.4 current–voltage relationships (as well as the endogenous current–voltage relationships) in B to show changes in the reversal potential. D, mean normalized Kir3.1/Kir3.4 conductance–voltage relationships in the presence of 90 mm K+ or spermine. B–D, current/conductance (measured at the end of the pulse; corrected for junction potentials) is normalized to Kir current/conductance at −130 mV in the presence of K+, and means ± s.e.m. (n = 4–5) are shown; many error bars are smaller than the size of the points. •, Kir current with 90 mm K+; ○, Kir current with 90 mm spermine; ▾, endogenous current with 90 mm K+; ▿, endogenous current with 90 mm spermine. E, continuous recording of Kir3.1/Kir3.4 current at a holding potential of −80 mV (corrected for junction potentials) when the bathing solution was switched between Na+ (ND96), K+ (90 K+) and spermine (90 Spm) containing solutions as indicated. Dopamine (10 μm) was present in the K+- and spermine-containing solutions. Similar traces were recorded from four other oocytes.
Nature of the spermine current
To confirm that the inward current recorded from oocytes expressing Kir3.1/Kir3.4 in the presence of extracellular spermine was the result of spermine permeation of Kir3.1/Kir3.4, additional experiments were performed. The current–voltage relationships in Fig. 5A–C show the effect of 1 μm Tertiapin Q, 1 mm Ba2+ and 50 mm IAA on K+ and spermine current through Kir3.1/Kir3.4; Tertiapin Q is a specific inhibitor of Kir3.1/Kir3.4 (Jin & Lu, 1998), Ba2+ is a blocker of K+ channels and IAA is an irreversible non-specific alkylating reagent for cysteine residues and is known to act on ion channels (Schild & Moczydlowski, 1991). Tertiapin Q, Ba2+ and IAA significantly inhibited spermine as well as K+ current (Tertiapin Q, n = 7–8, ANOVA, P < 0.05; Ba2+, n = 8–9, ANOVA, P < 0.05; IAA, n = 7–20, ANOVA, P < 0.05), and this is consistent with the possibility that the spermine current is conducted by Kir3.1/Kir3.4. Ba2+ only blocked the spermine current when applied prior to the application of spermine, and this suggests that spermine impedes the access of Ba2+ to its blocking site.
Figure 5. Nature of the spermine current.
A–C, normalized Kir3.1/Kir3.4 current–voltage relationships in the presence of 90 mm K+ or spermine (Sp) in the absence and presence of Kir3.1/Kir3.4 blockers: 1 μm Tertiapin Q (A), 1 mm Ba2+ (B) and 50 mm iodoacetamide (IAA; C). The sequence of solution applications was: (1) 90 mm K+ (2) 90 mm K+ with blocker (Tertiapin Q, Ba2+ or IAA) and (3) 90 mm spermine with blocker. In each experiment, in a different set of oocytes (but from the same batch of oocytes and on the same day), currents were recorded in 90 mm K+ and then 90 mm spermine (both in the absence of blocker). Means ± s.e.m. (n = 7–20) are shown. D and E, current–voltage relationships from oocytes expressing human D2 dopamine receptor (hD2) alone (D) or hD2 and Kir3.1/Kir3.4 (E) in the presence of 90 mm K+ or spermine. Means ± s.e.m. (n = 6) are shown. F, current–voltage relationships from oocytes expressing Kir3.4 (but not Kir3.1) in the presence of 90 mm K+ or spermine. Means ± s.e.m. (n = 8) are shown. G and H, normalized Kir3.1/Kir3.4 (G) and Kir3.1/Kir3.4[E145Q] (H) current– and conductance–voltage relationships in the presence of the indicated K+ or spermine concentration (mm). Means ± s.e.m. (n = 6–9) are shown. A–H, current/conductance was measured at the end of the pulse and is corrected for junction potentials. A, B, C, G and H, current/conductance is normalized to the current/conductance at −130 mV in the presence of 90 mm K+ (or spermine in H) and absence of blockers. A–H, many error bars are smaller than the size of the points. I, efflux of injected [3H]spermidine from oocytes expressing Kir2.1 or Kir3.1/Kir3.4 or from uninjected oocytes during a 15 min collection period. Efflux is expressed as a percentage of the amount of [3H]spermidine in the oocyte at the start of the collection period. Means ± s.e.m. (n = 15–30) are shown. *Significantly different (P < 0.05) from uninjected oocytes.
Although endogenous currents were small in the presence of extracellular spermine in the present study (Fig. 4A), heterologous expression of membrane proteins (such as the hD2 receptor) has been reported, in Xenopus oocytes, to increase an endogenous hyperpolarization-activated non-specific cation current that superficially resembles Kir3.1/Kir3.4 current (Tzounopoulos et al. 1995). It is theoretically possible that the endogenous channel (rather than Kir3.1/Kir3.4) is permeable to spermine. The current–voltage relationships in Fig. 5D, recorded from oocytes expressing the hD2 receptor alone, show that the endogenous currents were small, and, furthermore, the inward current was not significantly altered in the presence of spermine (n = 6; paired t test, not significant). The current–voltage relationships in Fig. 5E show that spermine current recorded from oocytes (from the same batch of oocytes as used for Fig. 5D) expressing Kir3.1/Kir3.4 as well as the hD2 receptor was 6.1 times greater (at ∼−130 mV) than the current in the oocytes expressing the hD2 receptor alone. These results demonstrate that the spermine current was unlikely to have been a current through an endogenous channel. This conclusion is supported by the fact that the spermine current was also absent in oocytes expressing Kir2.1 (Fig. 4A). The spermine current was also absent in oocytes expressing homotetramer Kir3.4 channels (Fig. 5F), suggesting that only heterotetramer Kir3.1/Kir3.4 channels are permeable to spermine. Kir3.1 does not form homotetramer channels (Ma et al. 2002), and spermine current after expression of Kir3.1 alone was not investigated.
A well-known ‘signature’ of inward rectifier K+ channels is K+ activation. Figure 5G shows current–voltage (Fig. 5G, top) and conductance–voltage (Fig. 5G, bottom) relationships for Kir3.1/Kir3.4 in different extracellular K+ concentrations. As expected, Fig. 5G shows that as extracellular K+ was raised, Kir3.1/Kir3.4 current and conductance were increased. Figure 5H shows that raising the extracellular spermine concentration activates Kir3.1/Kir3.4 in the same way that K+ does: Fig. 5H shows current–voltage (Fig. 5H, top) and conductance–voltage (Fig. 5H, bottom) relationships for Kir3.1/Kir3.4 in different extracellular spermine concentrations. The Kir3.1/Kir3.4[E145Q] mutant channel was used for these experiments, because spermine currents are large (Dibb et al. 2003); at low spermine concentrations, spermine currents through the wild-type channel were too small to measure accurately.
On raising the extracellular K+ or spermine concentration from 9 to 90 mm there was the expected gradual change in the reversal potential from −51.0 ± 4.5 to −6.2 ± 1.1 mV in the case of K+ (Fig. 5G), and from −30.5 ± 3.2 to −16.0 ± 1.0 mV in the case of spermine (Fig. 5H). On raising the extracellular spermine concentration from 9 to 90 mm, the calculated equilibrium potential for Cl− (ECl) changed from −40.3 to −61.1 mV – neither the absolute values of ECl nor the direction of change in ECl corresponds to the observed changes, and this shows that the current observed in the presence of spermine is unlikely to be a Cl− current.
To confirm the difference in polyamine permeation between Kir2.1 and Kir3.1/Kir3.4, we measured efflux of injected [3H]spermidine from oocytes expressing either Kir2.1 or Kir3.1/Kir3.4, or from uninjected oocytes (Fig. 5I). During incubation with solution containing 90 mm K+, which depolarized the cell membrane to ∼0 mV, only 2.2 ± 0.3% of the injected [3H]spermidine effluxed from uninjected oocytes (no channel cRNA injected) over 15 min (n = 15; Fig. 5I). In contrast, in oocytes expressing Kir3.1/Kir3.4, [3H]spermidine efflux was significantly greater: 11.5 ± 1.3% over 15 min (n = 30; ANOVA, P < 0.001; Fig. 5I). However, [3H]spermidine efflux from oocytes expressing Kir2.1 was not significantly different from uninjected cells: only 3.2 ± 0.5% effluxed over 15 min (n = 19; ANOVA, not significant; Fig. 5I).
In conclusion, the data shown in Fig. 5 confirm that (1) the spermine current shown in Fig. 4 is the result of spermine permeation of Kir3.1/Kir3.4, and (2) polyamines can measurably permeate Kir3.1/Kir3.4, but not Kir2.1.
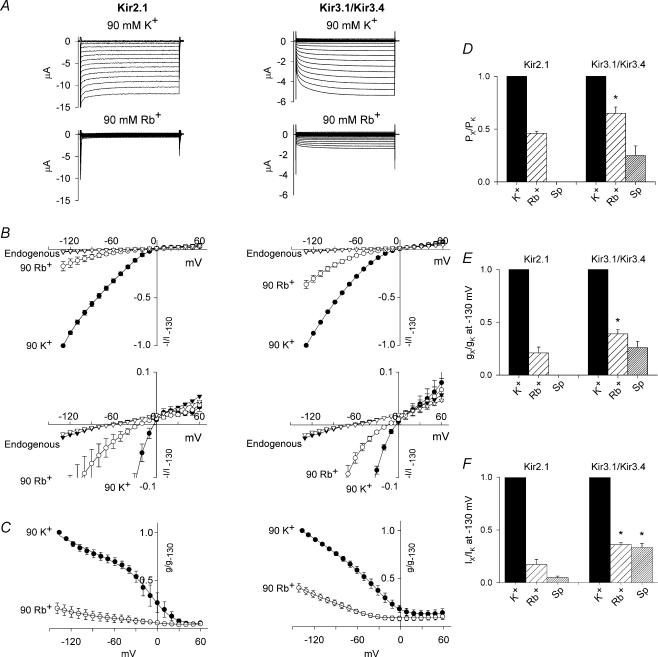
Ion selectivity is different in Kir2.1 and Kir3.1/Kir3.4
The difference in polyamine permeation between Kir2.1 and Kir3.1/Kir3.4 suggests that there is a difference in ion selectivity between the two channels, and this is confirmed by Fig. 6. Figure 6A shows Kir2.1 and Kir3.1/Kir3.4 currents recorded in the presence of 90 mm extracellular K+ (Fig. 6A, top) or 90 mm extracellular Rb+ (Fig. 6A, bottom). Rb+ permeated both channels, but permeation was significantly greater with Kir3.1/Kir3.4. This is shown more clearly by the mean current– and conductance–voltage relationships in Fig. 6B and C. For example, at −130 mV, Rb+ current was 18 ± 4% of the K+ current through Kir2.1, but 36 ± 4% in the case of Kir3.1/Kir3.4 (n = 5; Student's t test, P < 0.05). From these data, conductance (gX/gK) and current (IX/IK) ratios at −130 mV for Rb+ and spermine were calculated for Kir2.1 and Kir3.1/Kir3.4 (Fig. 6E and F). On replacing K+ with Rb+, there was a change in the reversal potential from −6.8 ± 2.3 to −26.6 ± 1.7 mV (n = 5) in the case of Kir2.1, and from −3 ± 3.4 to −14.3 ± 2.1 mV (n = 5) in the case of Kir3.1/Kir3.4 (Fig. 6B, bottom). On replacing K+ with spermine, there was a change in the reversal potential from −6.6 ± 0.7 to −5.9 ± 4.4 mV (n = 5) in the case of Kir3.1/Kir3.4 (Fig. 4C); in the case of Kir2.1, spermine current was immeasurable and therefore it was not possible to estimate a reversal potential. From these data, apparent permeability ratios (PX/PK) were calculated using eqn (3) and are plotted in Fig. 6D. The permeability ratio for Rb+ was 0.46 ± 0.02 and 0.65 ± 0.06 for Kir2.1 and Kir3.1/Kir3.4, respectively, whereas the permeability ratio for spermine was 0.25 ± 0.09 for Kir3.1/Kir3.4; the permeability ratio for Rb+ was significantly greater in Kir3.1/Kir3.4 than Kir2.1 (n = 5; Student's t test, P < 0.05). The gX/gK and IX/IK ratios in Fig. 6E and F support these data.
Figure 6. Ion selectivity of Kir2.1 and Kir3.1/Kir3.4.
A, typical Kir2.1 and Kir3.1/Kir3.4 currents recorded in the presence of 90 mm extracellular K+ (top) and when K+ was replaced with 90 mm Rb+ (bottom). B, normalized current–voltage relationships in the presence of 90 mm K+ or Rb+ (top, full current–voltage relationships; bottom, expanded view showing reversal potentials). endogenous current–voltage relationships in the presence of 90 mm K+ or Rb+ are also shown. Current (measured at the end of the pulse) is normalized to Kir current at −130 mV in the presence of 90 mm K+. C, normalized conductance–voltage relationships in the presence of 90 mm K+ or Rb+. Conductance (measured at the end of the pulse) is normalized to Kir conductance at −130 mV in the presence of 90 mm K+. B and C, •, Kir current/conductance with 90 mm K+; ○, Kir current/conductance with 90 mm Rb+; ▾;, endogenous current with 90 mm K+; ▿, endogenous current with 90 mm Rb+. D, permeability ratios for K+, Rb+ and spermine (Sp) for Kir2.1 and Kir3.1/Kir3.4. E and F, conductance (E) and current (F) ratios at −130 mV in the presence of 90 mm extracellular K+, Rb+ or spermine. Conductance/current (corrected for junction potentials) is normalized to the K+ conductance/current in the same oocyte. Means ± s.e.m. (n = 4–5) are shown; in B and C, some error bars are smaller than the size of the points. *Significantly different (P < 0.05) from the corresponding value in Kir2.1.
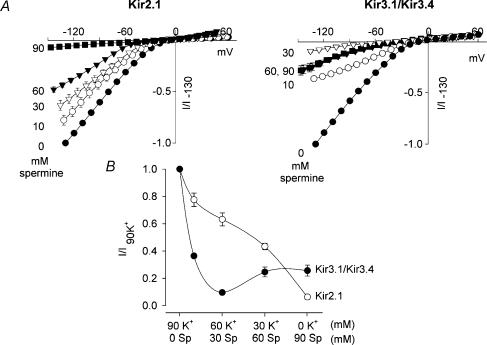
Kir3.1/Kir3.4 experiences an anomalous mole-fraction effect
Because spermine is a permeant blocker of Kir3.1/Kir3.4, there may be an anomalous mole-fraction effect. Figure 7A shows mean current–voltage relationships for Kir2.1 and Kir3.1/Kir3.4 when the 90 mm extracellular K+ was replaced gradually with equimolar concentrations of spermine. Raising the spermine concentration (and reducing the K+ concentration) in the bath solution resulted in a monophasic reduction of current through Kir2.1. In contrast, Kir3.1/Kir3.4 exhibited a biphasic response. Current decreased with a K+: spermine concentration ratio of 60: 30, but further decreases of the K+: spermine concentration ratio to 30: 60 and 0: 90 caused an increase in current. The mean data at −130 mV (Fig. 7B) demonstrate this more clearly. In the case of Kir2.1, increasing the spermine concentration caused a monotonic block of the channel. In the case of Kir3.1/Kir3.4, the biphasic response may be explained if spermine blocked and then permeated the channel, with relief from block at spermine concentrations above ∼30 mm.
Figure 7. Anomalous mole-fraction effect with Kir3.1/Kir3.4.
A, normalized Kir2.1 and Kir3.1/Kir3.4 current–voltage relationships recorded in 90 mm extracellular K+ and when the K+ was gradually replaced with equimolar concentrations of spermine. Concentrations of spermine are shown (mm). B, normalized current at −130 mV plotted against the spermine/K+ concentrations for the two channels. The data are fitted with lines to guide the eye. Current (measured at the end of the pulse; corrected for junction potentials) is normalized to current at −130 mV in the presence of 90 mm K+. Means ± s.e.m. (n = 5–6) are shown; some error bars are smaller than the size of the points.
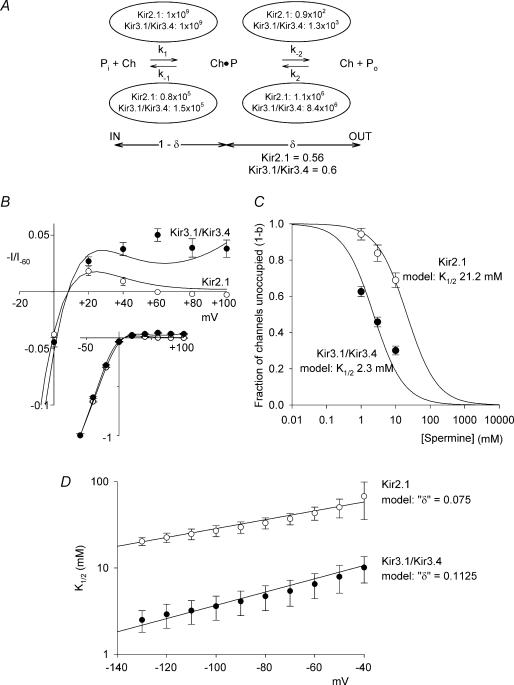
A kinetic model of polyamine permeation and block describes the experimental data
We constructed a simple (proof-of-principle) two barrier–one binding site model of polyamine block and permeation based on that of Woodhull (1973) and Guo & Lu (2000). A schema of the model is shown in Fig. 8A. On the left hand side, the channel (Ch) is open and intracellular polyamine (Pi) is unbound. Binding of polyamine to the channel (governed by the rate constant, k1) causes channel block (Ch · P). From the blocked state (Ch · P), polyamine can unbind and return to the intracellular fluid (governed by the rate constant k−1) or, as we have shown in Fig. 4 for the Kir3.1/Kir3.4 channel at least, permeate the channel and exit to the extracellular fluid (Ch + Po; governed by the rate constant k−2). As we have shown in Figs 2 and 3, extracellular polyamine (Po) can block both channels (governed by the rate constant k2) and unbind to either the intracellular or extracellular fluids (governed by the rate constants k−1 and k−2, respectively). The fraction of channels blocked, b (i.e.  where square brackets denote concentration) is given by eqn (4):
where square brackets denote concentration) is given by eqn (4):
| (4) |
where
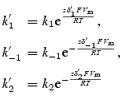 |
and
[P]i and [P]o are the intracellular and extracellular polyamine concentrations, and z is the valency of the polyamine. k1, k−1, k2 and k−2 are the rate constants of the four transitions at 0 mV and δ′1, δ′−1, δ′2 and δ′−2 are the electrical distances of the four transitions. The barriers were assumed to be symmetrical, i.e. 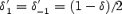 and
and 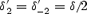 , where δ is the electrical distance of the binding site (0.56 for Kir2.1 and 0.6 for Kir3.1/Kir3.4). [P]i was set to 2 × 10−4m (Nichols et al. 1996). k1 was set to 109m−1 s−1 (Guo & Lu, 2003), and the other rate constants and δ were chosen to obtain the best fit ‘by eye’ of the model to the experimental data. Figure 8B–D shows that the fit of the model (smooth lines) to the experimental data (points) is reasonable.
, where δ is the electrical distance of the binding site (0.56 for Kir2.1 and 0.6 for Kir3.1/Kir3.4). [P]i was set to 2 × 10−4m (Nichols et al. 1996). k1 was set to 109m−1 s−1 (Guo & Lu, 2003), and the other rate constants and δ were chosen to obtain the best fit ‘by eye’ of the model to the experimental data. Figure 8B–D shows that the fit of the model (smooth lines) to the experimental data (points) is reasonable.
Figure 8. Kinetic model of polyamine block and permeation of Kir2.1 and Kir3.1/Kir3.4.
A, schematic diagram of the model. Pi, intracellular polyamine; Ch, channel; Ch · P, channel–polyamine complex (blocked); Po, extracellular polyamine. Values of the rate constants k1, k−1, k−2 and k2, are shown. B, normalized current–voltage relationships from −20 to +100 mV (main panel) or −60 to +100 mV (inset) for the two channels. The symbols show experimental data (from Fig. 1B) and the smooth lines show the prediction of the model. Current is normalized to current at −60 mV. C, plot of 1 −b (fraction of binding sites unoccupied by spermine) against the extracellular spermine concentration for the two channels at −130 mV. Smooth lines, model data. The symbols show equivalent experimental data (fractional current remaining in the presence of spermine from Fig. 3A). 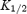 values (obtained by fitting eqn (1) to the model data) are shown. D,
values (obtained by fitting eqn (1) to the model data) are shown. D, 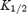 for 1 −b plotted against membrane potential for the two channels. Smooth lines, model data. The symbols show equivalent experimental data (
for 1 −b plotted against membrane potential for the two channels. Smooth lines, model data. The symbols show equivalent experimental data (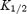 values for spermine block of the two channels from Fig. 3B). The apparent electrical distance of the binding site (δ; obtained by fitting eqn (2) to the model data) is shown.
values for spermine block of the two channels from Fig. 3B). The apparent electrical distance of the binding site (δ; obtained by fitting eqn (2) to the model data) is shown.
The inset in Fig. 8B shows the current–voltage relationships for Kir2.1 and Kir3.1/Kir3.4 over a wide voltage range under conditions designed to emulate Fig. 1 (K+, not spermine, present in the extracellular solution). Current was calculated as (1 −b) × (Vm−Vrev), where Vrev is the reversal potential (+ 8 mV); current was then normalized to the current at −60 mV as in experiments (the normalization negates the need for a conductance term; current carried by intracellular polyamine was not included, but is likely to be negligible). The overall shapes of these are largely determined by the left-hand transition in Fig. 8A, i.e. by the binding and unbinding of intracellular polyamines (Pi+ Ch → Ch · P) and therefore by k1, k−1 and 1 −δ. The negative limbs of the current–voltage relationships of the two channels are similar and, according to the model, k1, k−1, and 1 −δ are similar: k1= 109 and 109m−1 s−1, k−1= 0.8 × 105 and 1.5 × 105 s−1, and 1 −δ= 0.44 and 0.4 for Kir2.1 and Kir3.1/Kir3.4, respectively. The equivalent valency for the negative limb of the model current–voltage relationships for Kir2.1 and Kir3.1/Kir3.4 was measured (using the Boltzmann equation from conductance–voltage relationships) to be 1.78 and 1.67 (approximately equal to z(1 −δ), i.e. 1.76 for Kir2.1 and 1.60 for Kir3.1/Kir3.4). These values are close to experimentally measured values of the equivalent valency for Kir2.1 (CHO cells, 2.12 ± 0.05, n = 5; oocytes, 1.98 ± 0.06, n = 14) and Kir3.1/Kir3.4 (CHO cells, 1.69 ± 0.18, n = 7; oocytes, 2.19 ± 0.09, n = 7). Data from others have yielded similar values of equivalent valency (Adrian & Freygang, 1962; Hagiwara & Takahashi, 1974; Hagiwara et al. 1977; Lopatin et al. 1995).
Figure 8C shows a plot of 1 −b (the fraction of channels not blocked by spermine) against the extracellular spermine concentration for the two channels at −130 mV. If spermine permeation is not significant, 1 −b will be equal to the fractional current remaining in the presence of extracellular spermine (shown in Fig. 3A). Above we suggest that spermine permeation is not significant at extracellular spermine concentrations of <30 mm, and in Fig. 8C we have plotted the fractional current remaining in the presence of 1, 3 and 10 mm extracellular spermine − the fit of the model (smooth lines) to the experimental data (points) is reasonable. The dose–response curves (i.e. plots of 1 −b against the extracellular spermine concentration) are greatly influenced by the transition Ch + Po→ Ch · P in Fig. 8A, and therefore by k2. Note that the dose–response curves are little influenced by k−2 and instead are influenced by k−1 (which was determined by the negative limb of the current–voltage relationships as discussed above); this is because k−1 is ∼100–1000 times larger than k−2 (at 0 mV). According to the model, binding of extracellular polyamine is almost 10 times greater in Kir3.1/Kir3.4 than in Kir2.1: k2= 1.1 × 106 and 8.4 × 106m−1 s−1 for Kir2.1 and Kir3.1/Kir3.4, respectively. This is consistent with the greater permeability of Kir3.1/Kir3.4 to extracellular polyamines (Figs 4, 5, 6). The difference in k2 explains the greater sensitivity of Kir3.1/Kir3.4 to extracellular spermine block (Fig. 8C). Figure 3B shows that the voltage dependence of extracellular spermine block of both Kir2.1 and Kir3.1/Kir3.4 was weak. In the model, the voltage dependence of extracellular spermine block was also weak and the apparent value of δ (obtained by fitting the model data with eqn (2)) was ∼0.1 for Kir2.1 and Kir3.1/Kir3.4 (Fig. 8D). It could be argued that the low value of ‘δ’ indicates a superficial binding site, but the model demonstrates that in the case of a permeant blocker such a conclusion is inappropriate. In the model, δ for the binding site is substantially greater (∼0.6 for Kir2.1 and Kir3.1/Kir3.4; Fig. 8A). The apparent value ofδ is low because it represents the balance of two opposing voltage-dependent processes: spermine binding dependent on the true value of δ (∼0.6) and spermine unbinding dependent on 1 −δ (∼0.4). In the model, with δ < 1 −δ, it is even possible to reverse the voltage dependence of extracellular spermine block (this general property was first noted by Woodhull, 1973).
The main part of Fig. 8B focuses on the current–voltage relationships of the two channels at positive potentials, and this shows the characteristic difference in inward rectification. The extent of inward rectification at these potentials is largely determined by the unbinding of polyamine from the channel to the extracellular fluid, i.e. the transition Ch · P → Ch + Po in Fig. 8A and therefore by k−2. In Kir3.1/Kir3.4, k−2 is ∼10 times greater than in Kir2.1: k−2= 0.9 × 102 and 1.3 × 103 s−1 for Kir2.1 and Kir3.1/Kir3.4, respectively. This is consistent with the greater permeability of Kir3.1/Kir3.4 to intracellular polyamines (Fig. 5I). The difference in k−2 explains the weaker inward rectification of Kir3.1/Kir3.4 and the persistence of substantial outward current at positive potentials (Fig. 8B). These results show that the model, which in essence treats polyamines as permeant blockers of Kir3.1/Kir3.4 but as relatively impermeant blockers of Kir2.1, can explain both the difference in the extent of inward rectification between the two channels and the difference in sensitivity to extracellular spermine block.
Discussion
Based on experimental and modelling data, it is concluded that inward rectification of Kir3.1/Kir3.4 is weaker than that of Kir2.1, because polyamines can act as a permeant blocker of Kir3.1/Kir3.4 but not Kir2.1. A corollary is that Kir3.1/Kir3.4 is more sensitive than Kir2.1 to extracellular spermine block, because spermine has better access to its binding site in the channel pore.
Variable inward rectification
The present study has shown that inward rectification of Kir3.1/Kir3.4 is weaker than that of Kir2.1. In the case of Kir2.1, outward current declined to zero at positive potentials and, in the heart, IK,1 (for which Kir2.1 is in part responsible) shows the same behaviour (Kurachi, 1985; Dobrzynski et al. 2002; Ishihara et al. 2002). In the case of Kir3.1/Kir3.4, outward current was maintained at positive potentials and, in the heart, IK,ACh (for which Kir3.1/Kir3.4 is responsible) shows the same behaviour (Zang et al. 1993; Dobrzynski et al. 2002; Lomax et al. 2003).
Site of polyamine block underlying inward rectification
Inward rectification is caused by voltage-dependent block of the pore by intracellular Mg2+ and polyamines (Nichols & Lopatin, 1997). Despite much effort, the structural regions of the channel that are involved with the coordination of polyamines within the pore remain unclear. Intracellular polyamine block has been extensively studied in Kir2.1 and has been shown to involve negatively charged glutamate residues (E224 and E299 in the proximal C terminus) within the cytoplasmic pore, and aspartate and serine residues (D172 and S165 in the second transmembrane domain) in the inner vestibule of the transmembrane pore (Lu & MacKinnon, 1994; Stanfield et al. 1994; Yang et al. 1995; Kubo & Murata, 2001; Fujiwara & Kubo, 2002; Xie et al. 2003; Guo & Lu, 2003; Guo et al. 2003). The steep voltage dependence of block by polyamines suggests that they enter deep within the pore (Fakler et al. 1994; Ficker et al. 1994; Lopatin et al. 1994, 1995). It has been proposed that polyamines are shuttled by the cytoplasmic negative charges (E224 and E299) to their eventual blocking site at D172 (Kubo & Murata, 2001; Guo et al. 2003; Xie et al. 2003). But is D172 the eventual blocking site? In the case of Kir2.1, Chang et al. (2003) showed that D172 is unlikely to be the spermine binding site and that spermine binds above D172 (in the direction of the selectivity filter). Because it is a heterotetramer and its subunits are not identical, Kir3.1/Kir3.4 possesses fewer of these negatively charged residues: the residue equivalent to D172 in Kir3.1 is D173, but in Kir3.4 there is an uncharged asparagine residue (N179) at this position; the residue equivalent to E224 in Kir3.4 is E231, but in Kir3.1 there is a serine residue (S225) at this position. Furthermore, neutralization of the residues equivalent to D172 or E224 in Kir3.1/Kir3.4 has little effect on inward rectification (Lancaster et al. 2000; Dibb et al. 2003). This work suggests that other parts of the channel may be important for polyamine block. It is possible that the function of D172, as well as that of E224 and E299, is to ‘shuttle’ polyamines to their eventual binding site deeper in the pore. Guo & Lu (2000) provided evidence that polyamines are permeant blockers of Kir2.1: intracellular polyamines block Kir2.1, but at positive potentials current does not reduce to zero as expected for an impermeant blocker. Interestingly, Guo & Lu (2000) showed that philanthotoxin-343 (spermine with a bulky chemical group at one end) behaves in the expected manner for an impermeant blocker. In this study (Figs 4, 6 and 7), we have demonstrated that small but significant polyamine currents through Kir3.1/Kir3.4, but not Kir2.1, can be measured when the extracellular polyamine concentration is increased above 30 mm. In addition, [3H]spermidine efflux from oocytes expressing Kir3.1/Kir3.4 is greater than from oocytes expressing Kir2.1 and uninjected oocytes (Fig. 5I). Finally, we have shown that various mutations that are expected to affect the selectivity filter (for example, mutations that disrupt a salt bridge behind the selectivity filter) greatly reduce inward rectification (Dibb et al. 2003) – there is a significant correlation between selectivity and inward rectification. These results suggest therefore that polyamines can actually interact with the selectivity filter of the channel. This is plausible given the dimensions of a spermine molecule: cylindrical diameter of 4.4 Å and length of 20 Å (Nichols & Lopatin, 1997; Bähring et al. 1997). We raise the possibility that polyamines block the channel and generate inward rectification by binding within the selectivity filter of the channel. Molecular dynamics simulations support the possibility that polyamines bind within the selectivity filter (Dibb et al. 2003).
Nature of the spermine current
It is possible that the substantial inward current recorded when Kir3.1/Kir3.4 is heterologously expressed in Xenopus oocytes and the bathing medium contains 90 mm spermine (instead of K+) could be spermine activation of endogenous current, or a spermine current through an endogenous channel. However, evidence suggests that it is a spermine current through Kir3.1/Kir3.4. (1) In uninjected oocytes, no such current is recorded (Fig. 4A). (2) In H2O-injected oocytes, no such current is recorded (Dibb et al. 2003). (3) It has been reported that heterologous expression (of a channel or a non-channel, e.g. the D2 receptor) in Xenopus oocytes results in an increase in a hyperpolarization-activated non-specific cation current (Tzounopoulos et al. 1995). This endogenous current superficially resembles Kir3.1/Kir3.4 current, and perhaps (for the sake of argument) this channel is permeable to spermine. However, in Xenopus oocytes expressing Kir2.1 or the hD2 receptor alone there was no substantial spermine current (Figs 4 and 5D). Furthermore, Tzounopoulos et al. (1995) report that there is only significant activation of the endogenous current after 4–8 days of heterologous expression. All currents recorded in this study were recorded after 1–2days of heterologous expression. Finally, although the spermine current in the present study superficially resembled the hyperpolarization-activated non-specific cation current, substantial spermine current through the Kir1.1 channel was also observed (data not shown) and this showed kinetics characteristics of Kir1.1 (i.e. different from the hyperpolarization-activated non-specific cation current). (4) Tertiapin Q, a specific Kir3.1/Kir3.4 blocker (Jin & Lu, 1998), blocked the spermine current (Fig. 5A). (5) Ba2+, a well-known blocker of K+ channels, blocked the spermine current (Fig. 5B). (6) IAA, which inhibited K+ current through Kir3.1/Kir3.4, also inhibited the spermine current (Fig. 5C). (7) Raising extracellular spermine activated Kir3.1/Kir3.4 just as K+ does (Fig. 5G and H). (8) The change in the reversal potential on changing the extracellular spermine concentration was inconsistent with the involvement of a Cl− current (Fig. 5H). (9) Zero (Dibb et al. 2003) or low (data not shown) extracellular Ca2+ does not affect the current in the presence of extracellular spermine. This makes the involvement of the endogenous Ca2+-activated Cl− current unlikely. (10) Cx38 antisense blocks current through the connexin hemichannels in Xenopus oocytes, but it does not block the spermine current (Dibb et al. 2003). (11) Significant [3H]spermidine (Fig. 5I; Dibb et al. 2003) and [14C]spermine (T. W. Claydon, unpublished data) flux through Kir3.1/Kir3.4 has been observed. (12) The amplitude of the spermine current is sensitive to site-specific mutations in Kir3.1 and Kir3.4 (Dibb et al. 2003).
Both intracellular and extracellular polyamines also both block and permeate the GluR6 glutamate receptor channel (Bähring et al. 1997): putrescine: Na+, spermidine: Na+ and spermine: Na+ permeability ratios were measured to be 0.42, 0.07 and 0.02 (in the present study the spermine: K+ permeability ratio for Kir3.1/Kir3.4 was measured to be 0.25 ± 0.09).
Fine-tuning inward rectification
In the present study, although both extracellular and intracellular polyamines could permeate Kir3.1/Kir3.4, they could not measurably permeate Kir2.1 (Figs 4 and 5I). We conclude from this that polyamines are more easily able to traverse the selectivity filter of Kir3.1/Kir3.4 than Kir2.1. In addition, in the present study, extracellular spermine blocked both Kir2.1 and Kir3.1/Kir3.4, but the affinity of Kir3.1/Kir3.4 for extracellular spermine was ∼8 times greater than that for Kir2.1 (Fig. 3A). This suggests that extracellular spermine has better access to its binding site in Kir3.1/Kir3.4 than in Kir2.1. If extracellular spermine is blocking the channel by binding within the selectivity filter, this is consistent with spermine being better able to traverse the selectivity filter of Kir3.1/Kir3.4 than Kir2.1. The difference in polyamine permeation between the two channels is presumably the result of a subtle difference in the structure of the selectivity filter, consistent with the observation that ion selectivity is also different between the two channels (Fig. 6). The ability of spermine to act as a permeant blocker of Kir3.1/Kir3.4 is further highlighted by the anomalous mole-fraction effect shown in Fig. 7. The kinetic model of polyamine interaction with Kir2.1 and Kir3.1/Kir3.4 (Fig. 8A) shows that the difference in polyamine permeation between the two channels not only explains the difference in sensitivity to extracellular spermine block, but also the difference in the extent of inward rectification between the two channels (Fig. 8). This suggests therefore that the weaker inward rectification of Kir3.1/Kir3.4 compared with that of Kir2.1 is the result of greater polyamine permeation (rather than block). We propose that the polyamine permeability of the selectivity filter fine tunes the extent of inward rectification amongst strong inward rectifier K+ channels (such as Kir2.1 and Kir3.1/Kir3.4). In this respect, it is interesting that different degrees of agonist activation of Kir3.1/Kir3.4 affect the selectivity filter (as shown by changes in sensitivity to blockers that are known to bind within the selectivity filter) and the extent of inward rectification (Hommers et al. 2003).
Yang et al. (1996) and Dobrzynski et al. (2002) have shown that on application of ACh to dog and ferret ventricle there is an activation of IK,ACh as expected, but there is also an inhibition of IK,1. In this way, a stronger inward rectifier K+ current (IK,1) is exchanged for a weaker inward rectifier K+ current (IK,ACh). Dobrzynski et al. (2002) argued that this is physiologically important, because it results in an increase of outward current at systolic potentials (leading to a shortening of the action potential and therefore a decrease in contraction), but little change in current at diastolic potentials (leading to little change in the input resistance during diastole and therefore cell excitability, i.e. action potential threshold).
The kinetic model of polyamine permeation and block
Is the model able to predict the spermine current through Kir3.1/Kir3.4 measured experimentally? If the membrane potential is −100 mV, the single-channel conductance of Kir3.1/Kir3.4 with K+ as the charge carrier is 36.7 pS (Shui et al. 2001) and the spermine current is 25% of the K+ current, then one spermine molecule must exit the selectivity filter to the cytosol every 0.7 μs. If this is taken as the residence time, then k−1 (reciprocal of the residence time; Hille, 1992) must be ∼1.5 × 106 s−1 at −100 mV. The model predicts a value of k−1 at −100 mV of 1.5 × 105 s−1 for Kir3.1/Kir3.4 – this is comparable to the experimental estimate. Calculation shows that k2 is more than adequate to supply such a spermine flux. It is concluded that the model in Fig. 8 is consistent with the spermine current through Kir3.1/Kir3.4 measured experimentally. Analogous calculations for Kir2.1 show that k2 at −100 mV is ∼10 times lower than that for Kir3.1/Kir3.4; this may result in an immeasurable spermine current through Kir2.1. However, the spermine current is proportional to b(k−1−k−2), and (k−1−k−2) is similar for Kir2.1 and Kir3.1/Kir3.4. With ∼1 m extracellular spermine, b should approach 1 for both channels (Fig. 8C), and therefore the model predicts a substantial inward spermine current through Kir2.1 as well as Kir3.1/Kir3.4.
The model is a simple proof-of-principle model only and it does not consider K+–polyamine competition or block by Mg2+. In the future, the model could be used to examine related phenomena, for example channel kinetics in response to voltage pulses, and the behaviour of mutant channels with altered inward rectification (Dibb et al. 2003).
References
- Adrian RH, Freygang WH. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962;163:61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol. 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR, Shui Z, Khan IA. Use of electrophysiology to monitor and study receptor desensitization. In: Haga T, Berstein G, editors. G Protein-Coupled Receptors. Boca Raton: CRC Press; 2000. [Google Scholar]
- Chang HK, Yeh SH, Shieh RC. The effects of spermine on the accessibility of residues in the M2 segment of Kir2.1 channels expressed in Xenopus oocytes. J Physiol. 2003;553:101–112. doi: 10.1113/jphysiol.2003.052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb KM, Rose T, Makary SY, Claydon TW, Enkvetchakul D, Leach R, Nichols CG, Boyett MR. Molecular basis of ion selectivity, block and rectification of the inward rectifier Kir3.1/Kir3.4 K+ channel. J Biol Chem. 2003;278:49537–49548. doi: 10.1074/jbc.M307723200. [DOI] [PubMed] [Google Scholar]
- Dobrzynski H, Janvier NC, Leach R, Findlay JB, Boyett MR. Effects of ACh and adenosine mediated by Kir3.1 and Kir3.4 on ferret ventricular cells. Am J Physiol Heart Circ Physio. 2002;283:H615–630. doi: 10.1152/ajpheart.00130.2002. [DOI] [PubMed] [Google Scholar]
- Fakler B, Brandle U, Bond C, Glowatzki E, Konig C, Adelman JP, Zenner HP, Ruppersberg JP. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994;356:199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, George EG, Jones SW. Apparent change in ion selectivity caused by changes in intracellular K+ during whole-cell recording. Biophys J. 2000;78:1872–1880. doi: 10.1016/S0006-3495(00)76736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. Ser165 in the second transmembrane region of the Kir2.1 channel determines its susceptibility to blockade by intracellular Mg2+ J Gen Physiol. 2002;120:677–693. doi: 10.1085/jgp.20028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Lu Z. Mechanism of IRK1 channel block by intracellular polyamines. J Gen Physiol. 2000;115:799–814. doi: 10.1085/jgp.115.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Lu Z. Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels. J Gen Physiol. 2003;122:485–500. doi: 10.1085/jgp.200308890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Ramu Y, Klem AM, Lu Z. Mechanism of rectification in inward-rectifier K+ channels. J Gen Physiol. 2003;121:261–276. doi: 10.1085/jgp.200208771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Krasne S, Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+–Tl+ mixtures. J Gen Physiol. 1977;70:269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2nd edn. Sunderland, MA, USA: Sinauer; 1992. [Google Scholar]
- Hommers LG, Lohse MJ, Bünemann M. Regulation of the inward rectifying properties of G protein-activated inwardly rectifying K+ (GIRK) channels by Gβγ subunits. J Biol Chem. 2003;278:1037–1043. doi: 10.1074/jbc.M205325200. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Yan DH, Yamamoto S, Ehara T. Inward rectifier K+ current under physiological cytoplasmic conditions in guinea-pig cardiac ventricular cells. J Physiol. 2002;540:831–841. doi: 10.1113/jphysiol.2001.013470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- Jin W, Lu Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1999;38:14286–14293. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+ channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Murata Y. Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel. J Physiol. 2001;531:645–660. doi: 10.1111/j.1469-7793.2001.0645h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata HT, Phillips LR, Rose T, Loussouarn G, Herlitze S, Fritzenschaft H, Enkvetchakul D, Nichols CG, Baukrowitz T. Molecular basis of inward rectification: polyamine interaction sites located by combined channel and ligand mutagenesis. J Gen Physiol. 2004;124:541–554. doi: 10.1085/jgp.200409159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MK, Dibb KM, Quinn CC, Leach R, Lee JK, Findlay JB, Boyett MR. Residues and mechanisms for slow activation and Ba2+ block of the cardiac muscarinic K+ channel, Kir3.1/Kir3.4. J Biol Chem. 2000;275:35831–35839. doi: 10.1074/jbc.M006565200. [DOI] [PubMed] [Google Scholar]
- Lewis CA. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Rose RA, Giles WR. Electrophysiological evidence for a gradient of G protein-gated K+ current in adult mouse atria. Br J Pharmacol. 2003;140:576–584. doi: 10.1038/sj.bjp.0705474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. The mechanism of inward rectification of potassium channels: ‘long-pore plugging’ by cytoplasmic polyamines. J Gen Physiol. 1995;106:923–955. doi: 10.1085/jgp.106.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 1994;371:243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circ Res. 1996;78:1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- Owen JM, Leach R, Quinn C, Findlay JBC, Boyett MR. Effect of extracellular cations on the inward-rectifier K+ channels, Kir 2.1 and Kir 3.1/Kir 3.4. Exp Physiol. 1998;84:471–488. [PubMed] [Google Scholar]
- Schild L, Moczydlowski E. Competitive binding interaction between Zn2+ and saxitoxin in cardiac Na+ channels. Evidence for a sulfhydryl group in the Zn2+/saxitoxin binding site. Biophys J. 1991;59:523–537. doi: 10.1016/S0006-3495(91)82269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui Z, Yamanushi TT, Boyett MR. Evidence of involvement of GIRK1/GIRK4 in long-term desensitization of cardiac muscarinic K+ channels. Am J Physiol Heart Circ Physiol. 2001;280:H2554–2562. doi: 10.1152/ajpheart.2001.280.6.H2554. [DOI] [PubMed] [Google Scholar]
- Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994;478:1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Maylie J, Adelman JP. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys J. 1995;69:904–908. doi: 10.1016/S0006-3495(95)79964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, John SA, Weiss JN. Inward rectification by polyamines in mouse Kir2.1 channels: synergy between blocking components. J Physiol. 2003;550:67–82. doi: 10.1113/jphysiol.2003.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZK, Boyett MR, Janvier NC, McMorn SO, Shui Z, Karim F. Regional differences in the negative inotropic effect of acetylcholine within the canine ventricle. J Physiol. 1996;492:789–806. doi: 10.1113/jphysiol.1996.sp021346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 1995;14:1047–1054. doi: 10.1016/0896-6273(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Zang WJYuXJ, Honjo H, Kirby MS, Boyett MR. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993;464:649–679. doi: 10.1113/jphysiol.1993.sp019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T, Zeuthen E, Klaerke DA. Mobility of ions, sugar and water in the cytoplasm of Xenopus oocytes expressing Na+-coupled sugar transporters (SGLT1) J Physiol. 2002;542:71–87. doi: 10.1113/jphysiol.2001.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel C, Cho HC, Nguyen TT, Pekhletski R, Diaz RJ, Wilson GJ, Backx PH. Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2. J Physiol. 2003;550:365–372. doi: 10.1113/jphysiol.2002.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]