Abstract
Six young, healthy male subjects were each studied in two experiments: (1) during resting conditions before and for 360 min after a meal (54% of energy as carbohydrate, 30% of energy as lipid, and 16% of energy as protein) comprising 25% of their total daily energy intake (M→R); and (2) while exercising on a cycle ergometer for 60 min at 50% of the peak oxygen consumption commencing 60 min after the meal (M→E) and then for another 240 min. Regional metabolism was measured by Fick's Principle in a leg and in the splanchnic tissue. The combination of food intake and exercise led to increased plasma triacylglycerol (TAG) uptake and clearance in the exercising legs immediately and for at least 4 h post-exercise, while food intake per se did not change leg plasma TAG uptake or clearance for up to 6 h. It is hypothesized that the effect of exercise on leg plasma TAG metabolism is a result of capillary recruitment leading to exposure of the plasma lipoprotein particles to a larger amount of active LPL. In spite of the increased TAG uptake in the exercising legs the arterial plasma TAG concentration had a tendency to increase faster during exercise after a meal than during rest, but it also decreased faster implying that the total lipaemic response was the same whether exercise was performed or not. The amount of lipid taken up in the legs was higher than could be accounted for by whole body lipid oxidation during post-exercise recovery, indicating accumulation of lipid in skeletal muscle in this period. Neither food intake alone nor the combination of food and exercise affected the splanchnic net balance of TAG. Finally, there is an additive effect of exercise and food intake on splanchnic net glucose balance.
In a recent study we have found that the arterial triacylglycerol (TAG) concentration increases faster after a carbohydrate rich meal when exercise is performed 1 h after the meal compared with rest (Enevoldsen et al. 2004). This finding could not be explained by adipose tissue TAG or splanchnic lipoprotein metabolism (Enevoldsen et al. 2004) and it is in contrast to the attenuated increase, which has been demonstrated when exercise was performed 18–20 h before the meal (Aldred et al. 1994; Gill et al. 2003). The augmented lipaemia during exercise could be due to reduced muscle plasma TAG uptake in a situation with high carbohydrate availability. TAG molecules embedded in the core of circulating lipoprotein particles are hydrolysed by the enzyme lipoprotein lipase (LPL), which is attached to the endothelial cells in the capillary bed. Generally, it is believed that skeletal muscle LPL activity is inhibited by insulin (Lithell et al. 1978; Farese et al. 1991) implying that skeletal muscle LPL activity decreases postprandially. On the other hand, skeletal muscle LPL expression and activity are increased by exercise. However, this increase does not occur during exercise unless the exercise bout is vigorous and prolonged, but is rather found in the post-exercise recovery period (Kiens & Richter, 1998). Accordingly, this has led to the suggestion that one mechanism leading to the attenuated postprandial increase in plasma TAG found when exercise is performed 18–20 h before a meal could be an increased leg plasma TAG clearance (Aldred et al. 1994; Gill & Hardman, 2003). Nevertheless, the present knowledge of how lipoprotein TAG utilization by muscle is regulated is very limited, and to our knowledge the effect of exercise performed briefly after food intake on exercising skeletal muscle plasma TAG uptake has not been examined. Thus, one aim of the present experiments was to study how food ingestion 1 h before moderate intensity exercise affects skeletal muscle plasma TAG uptake in order to elucidate whether the exercising skeletal muscle plays a role in the regulation of arterial plasma TAG concentration in this situation. A second aim was to examine whether a mixed meal with a lower energy percentage of carbohydrate (50%) and a higher energy percentage of fat (30%) affects the splanchnic lipid metabolism differently from what we found in our previous studies, in which we used meals with higher carbohydrate contents (100 or 60%) and lower fat contents (0 or 20%) (Bülow et al. 1999; Enevoldsen et al. 2004).
Methods
Subjects
Six young, healthy male subjects were studied, of mean (range) age 25.0 (23–28) years, weight 74.8 (71–80) kg, and height 182 (180–183) cm. Body composition was determined by dual energy X-ray absorptiometry (DEXA) scanning as described below. The average percentage body fat was 15 (6–20)%. The lean body mass was 60.8 (56–66) kg and the fat mass was 11.2 (4–15) kg. Mean peak oxygen uptake was 3550 (3000–3900) ml min−1. The subjects were given a written and an oral description of the study and the possible discomfort and risks involved before giving their voluntary consent to participate. The study was approved by the Ethical Committee of Copenhagen, Denmark (project no. 01-039/02) and was in accordance with the Declaration of Helsinki.
Experimental protocols
Subjects participated in two experiments each, about 3–4 weeks apart. One was a rest experiment of 6 h duration in which the subjects ate a meal as described below, after an initial fasting period, and then rested throughout the experiment. The other was an exercise experiment in which the subjects exercised for 60 min commencing 60 min after food intake. Thereafter, the experiment was continued for another 240 min during rest. Prior to these experiments the maximum oxygen uptake of the subjects was determined. They exercised in the semirecumbent position on an electrically braked cycle ergometer (ergometrics er900L, ergoline, Bitz, Germany), initially at 50 W, and then, essentially, at a 50 W increase in load every 2 min, until exhaustion. Oxygen uptake and carbon dioxide output were measured continuously during the test by means of an Oxycon Champion system (Jaeger, Wuerzburg, Germany), using a face-mask and breath-to-breath technique. On the same day, body composition was determined by DEXA scanning (Lunar DPX-IQ, software version 4.6c, Lunar Corporation, Madison, WI, USA), using the medium scan mode and extended research analysis.
Habitual dietary intake was recorded for 2 days before the first experiment and replicated before the subsequent experiment. The subjects refrained from vigorous physical activity 24 h prior to the experiments. The experiments were initiated at 08.00 h when the subjects arrived in the laboratory after a 12-h overnight fast.
Main experiments
In the rest study subjects were examined during resting, supine conditions before and for 360 min after a meal. The meal was ingested within 20 min; this comprised an omelette with tomatoes, white bread, marmalade and orange juice (85 ± 6 g carbohydrate, 21 ± 1 g fat, and 25 ± 2 g protein (54% carbohydrate, 30% lipid and 16% protein), in total 2.8 ± 0.2 MJ energy) providing 25% of each participant's reported total daily dietary intake (10.48 ± 0.93 MJ energy), which was calculated using a database (Dankost, Danish Catering Centre, Copenhagen, Denmark). In the exercise experiment the subjects exercised for 60 min at about 50% of their peak oxygen uptake commencing 60 min after ingestion of their meal. All studies were initiated at least 60 min after infusion of indocyanine green (ICG) was begun. The test order was randomized to give a balanced design.
Catheterizations
During ultrasound/colour-Doppler imaging, catheterization of the left femoral vein was performed retrogradely and under local anaesthesia (lidocaine 1%, 5–10 ml). The catheter was advanced 13 cm in the caudal direction, as this enables the correct positioning of the catheter tip distal to a set of valves situated below the point at which the great saphenous vein merges with the common femoral vein. Blood sampled from this position is derived mainly from the skeletal muscle compartments. The catheter (Check-Flo Performer, 5F, 13 cm, Cook, Denmark) was kept patent during the experiment by regular flushing with isotonic sodium chloride. The left femoral artery was catheterized retrogradely under local anaesthesia. Via this catheter (Certofix, 22G, 25 cm, Braun, Germany) a continuous infusion of ICG was given throughout the experiment for determination of leg and splanchnic blood flows. The right femoral vein was catheterized antegradely during local anaesthesia and a polyethylene catheter (outer diameter 2.0 mm) was advanced to a right-sided hepatic vein and left in situ with the tip positioned 1–2 cm from the wedge position during the rest of the experiment. The catheterization was done during fluoroscopic control. This catheter was kept patent throughout the study by continuous infusion of isotonic sodium chloride at a rate of 40 ml h−1. Another catheter was inserted percutaneously into the radial artery of the non-dominant arm during local anaesthesia (1 ml 1% lidocaine), with an Artflon (Ohmeda, Swindon, UK). The catheter was kept patent with regular flushing with isotonic sodium chloride.
Measurements
Leg and splanchnic blood flow
Leg (Jorfeldt & Wahren, 1971) and splanchnic (Henriksen & Winkler, 1987) blood flows were measured by continuous infusion of ICG. Immediately after the catheterization a priming dose (1 mg) of ICG was given followed by a continuous infusion (167 μg min−1) for the rest of the experiment. After 60 min of infusion a steady arterial concentration is normally achieved. The resting period was then begun.
Blood sampling
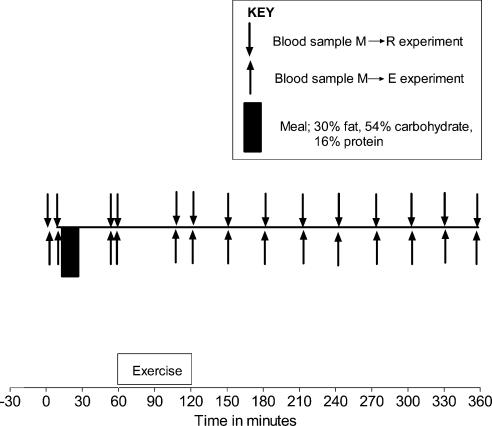
Blood samples were drawn simultaneously from the catheters positioned in the radial artery, the left femoral vein and the hepatic vein. Two sample sets were drawn from all three catheters during the initial fasting period and the means of these sets were used as basal levels (0 min). Another two sample sets were drawn about 3–5 min apart about 1 h after food intake and the means of these sets were used (60 min). Furthermore, blood was collected at 100, 120, 150, 180, 210, 240, 270, 300, 330 and 360 min after food intake (Fig. 1). Blood was collected from all three catheters for measurements of glycerol, fatty acids, total TAG, chylomicron- and very low density lipoprotein (VLDL)-TAG, and glucose. In addition, blood was collected from the artery for measurement of insulin. The blood was collected in vials at 4°C, and whole blood was immediately deproteinized or the plasma was separated by centrifugation at 4°C. The samples were then stored at −20°C until analysis except samples for fatty acids and insulin analysis, which were stored at −80°C and samples for chylomicron- and VLDL-TAG analysis, which were kept at 4°C until the next day, when separation by ultra-centrifugation took place.
Figure 1. Schematic representation of the study protocol.
Blood analysis
Glycerol was measured in perchloric acid extracts of whole blood, and fatty acids, TAG and glucose in EDTA–plasma with enzymatic methods as previously described (Humphreys et al. 1990; Bülow et al. 1999). The plasma glucose concentration was recalculated to whole blood concentration assuming that glucose is distributed in 70% of the total erythrocyte volume (Bülow & Madsen, 1981). Chylomicrons and VLDL were separated by ultra-centrifugation (Potts et al. 1994; Bülow et al. 1999). Approximately 1.5 ml of plasma was layered under a solution of density 1.006 g ml−1 in preweighed Beckman 6 ml polyallomer bell-top tubes. The tubes were re-weighed and the volume of plasma was obtained by difference. The tubes were heat sealed and ultra-centrifuged for 30 min at 26 000 r.p.m. in the outer ring in a 44-place Kontron TFT45.6 rotor giving on average 58 450 g. The chylomicrons were obtained by removing the top layer (1.5 ml) of the tube in a tube-slicer. For isolation of VLDL the total chylomicron infranate volume (4.5 ml) was then transferred to a Beckman 6 ml polyallomer bell-top tube and the necessary additional fluid volume, with density 1.006 g ml−1, was added to fill the tube. The tubes were heat sealed and ultra-centrifuged for 20 h at 40 700 r.p.m. in a 44-place Kontron TFT45.6 rotor in two concentric rings, inner ring 145 000 g (average), outer ring 182 000 g (average). For VLDL analysis 3.5 ml of the top layer was removed. All fractions were weighed to estimate their volume and thus to allow for calculation of the corresponding concentrations of chylomicron- and VLDL-TAG in the original plasma. Chylomicron- and VLDL-TAG were determined as previously described (Humphreys et al. 1990). Insulin concentrations in arterial EDTA plasma were determined using a commercial radioimmunoassay (RIA) kit (NOVO Nordic, Bagsvaerd, Denmark). The plasma ICG concentration was determined by spectrophotometry at 805 and 904 nm. To minimize effects of quasi steady state a mean of two ICG samples drawn within 10 min was used.
Whole body measurements
Whole body oxygen consumption and respiratory exchange ratio (RER) were measured by a ventilated hood system in the resting periods and by facemask and breath-by-breath technique during exercise. Heart rate and intra-arterial blood pressure were monitored continuously during the experiment via an Athena (S & W, Copenhagen, Denmark) interfaced to the Oxycon Champion system.
Calculations
Leg and splanchnic blood flow was calculated as previously described (Bülow, 1983; Jorfeldt & Wahren, 1971; Simonsen et al. 1995). Leg and splanchnic metabolite net fluxes were calculated by multiplication of the a–v or v–a concentration difference of the metabolite and the appropriate flow value (whole blood for calculation of glycerol and glucose fluxes, and plasma flow for calculation of fatty acid, and TAG fluxes). Leg plasma TAG clearance was calculated as leg plasma TAG uptake divided by arterial plasma TAG concentration.
Whole body lipid oxidation rate was calculated from the whole body oxygen consumption and carbon dioxide production corrected for an average protein combustion rate (100 μg kg −1 min−1) (Simonsen et al. 1993) using conventional principles for indirect calorimetry as described by Frayn (1983).
Splanchnic delivery of fatty acids was calculated as the arterial concentration multiplied by the splanchnic plasma flow. Areas under curves (AUCs) were calculated by the trapezoid method. Postprandial changes in areas under curves (ΔAUCs) were calculated by the trapezoid method as the increase from preprandial baseline.
Statistics
Data were analysed using SPSS for Windows release 11.5 (SPSS Inc., Chicago, IL, USA). All data are presented as the mean ± s.e.m. or mean (range). In Tables 1 and 3 only data every hour are presented. The full data set has been used for statistical analysis and AUC and ΔAUC calculations. Two-way ANOVA with repeated measures was used for analysis of changes with time using Bonferroni's method for post hoc testing. When appropriate Student's paired t test was used for analysis of differences between experiments. P < 0.05 was considered statistically significant.
Table 1.
Whole body oxygen uptake, RER, energy expenditure, and lipid oxidation rate at times (in minutes) after commencement of food ingestion
| 0 | 60 | 120 | 180 | 240 | 300 | 360 | |||
|---|---|---|---|---|---|---|---|---|---|
| Oxygen uptake (ml min−1) | |||||||||
| M→R | 307 ± 20 | 330 ± 17 | 347 ± 25* | 336 ± 23* | 342 ± 20* | 339 ± 18* | 334 ± 16 | ||
| M→E | 265 ± 8 | 302 ± 10* | 1625 ± 83*† | 309 ± 7* | 301 ± 7* | 304 ± 7* | 292 ± 5*† | ||
| Energy expenditure (kJ min−1) | |||||||||
| M→R | 6.14 ± 0.38 | 6.72 ± 0.33* | 7.00 ± 0.48* | 6.75 ± 0.43* | 6.87 ± 0.39* | 6.76 ± 0.34* | 6.65 ± 0.29 | ||
| M→E | 5.29 ± 0.15 | 6.16 ± 0.20* | 33.30 ± 1.70*† | 6.20 ± 0.14* | 6.03 ± 0.12* | 6.10 ± 0.15* | 5.84 ± 0.09*† | ||
| RER | |||||||||
| M→R | 0.80 ± 0.02 | 0.87 ± 0.02* | 0.84 ± 0.03 | 0.82 ± 0.04 | 0.82 ± 0.03 | 0.79 ± 0.04 | 0.77 ± 0.02 | ||
| M→E | 0.79 ± 0.02 | 0.89 ± 0.01* | 0.90 ± 0.01*† | 0.81 ± 0.02 | 0.80 ± 0.02 | 0.81 ± 0.02 | 0.80 ± 0.03 | ||
| Lipid oxidation rate (g min−1) | |||||||||
| M→R | 0.11 ± 0.01 | 0.07 ± 0.01* | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.02 | ||
| M→E | 0.09 ± 0.01 | 0.06 ± 0.01* | 0.27 ± 0.02*† | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01† | ||
Values are mean ± s.e.m. Bold data denotes exercise. M→R: Rest experiment; M→E: Postprandial exercise experiment.
Significant difference from basal.
Significant difference between the experiments.
Table 3.
Leg and splanchnic blood flows, and net substrate fluxes at time (in minutes) after commencement of food ingestion
| 0 | 60 | 120 | 180 | 240 | 300 | 360 | |
|---|---|---|---|---|---|---|---|
| Leg | |||||||
| Leg blood flow (ml min−1) | |||||||
| M→R | 495 ± 61 | 614 ± 74* | 587 ± 64 | 676 ± 135 | 619 ± 79* | 721 ± 104* | 716 ± 96* |
| M→E | 357 ± 30† | 442 ± 28*† | 3606 ± 327*† | 532 ± 86* | 595 ± 73* | 573 ± 66* | 591 ± 37* |
| Leg TAG clearance (l min−1) | |||||||
| M→R | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| M→E | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.08 ± 0.02*† | 0.03 ± 0.01* | 0.03 ± 0.01* | 0.02 ± 0.01* | 0.03 ± 0.01* |
| Splanchnic tissue | |||||||
| Splanchnic blood flow (ml min−1) | |||||||
| M→R | 1511 ± 74 | 2021 ± 132* | 1914 ± 151* | 1783 ± 105* | 1765 ± 91 | 1690 ± 67 | 1614 ± 74 |
| M→E | 1602 ± 116 | 2084 ± 116* | 1831 ± 139 | 2129 ± 194* | 1786 ± 84 | 1703 ± 101 | 1719 ± 76 |
| Fatty acid delivery (μmol min−1) | |||||||
| M→R | 439 ± 95 | 133 ± 23* | 68 ± 13* | 184 ± 55 | 427 ± 107 | 638 ± 112 | 696 ± 105* |
| M→E | 445 ± 108 | 106 ± 16* | 203 ± 24† | 532 ± 112 | 603 ± 87† | 696 ± 38 | 860 ± 76* |
| Fatty acid uptake (μmol min−1) | |||||||
| M→R | 115 ± 27 | 9 ± 7* | − 7 ± 5* | 9 ± 10* | 18 ± 60* | 112 ± 27 | 160 ± 36 |
| M→E | 120 ± 32 | 2 ± 4* | 10 ± 7* | 93 ± 31 | 110 ± 30 | 173 ± 22 | 191 ± 39 |
| Glycerol uptake (μmol min−1) | |||||||
| M→R | 55 ± 14 | 21 ± 6* | 19 ± 5* | 44 ± 12 | 55 ± 9 | 92 ± 20 | 71 ± 14 |
| M→E | 54 ± 12 | 27 ± 2* | 102 ± 18*† | 69 ± 16 | 90 ± 16 | 77 ± 8 | 82 ± 11 |
| Glucose output (mmol min−1) | |||||||
| M→R | 0.9 ± 0.1 | 1.7 ± 0.3* | 1.2 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| M→E | 0.9 ± 0.1 | 2.1 ± 0.5* | 2.6 ± 0.4*† | 1.3 ± 0.2 | 1.1 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 |
Values are mean ± s.e.m. Bolded data denotes exercise. M→R: Rest experiment; M→E: Postprandial exercise experiment.
Significant difference from basal.
Significant difference between the experiments.
Results
Whole body
The mean oxygen uptake, RER, calculated energy expenditure and lipid oxidation rates before and for 360 min after commencement of food ingestion in the rest and exercise experiments are given in Table 1. Oxygen uptake and energy expenditure increased after food intake in both experiments and remained elevated for at least 300 min postprandially. Only during exercise and at 360 min postprandially did the oxygen uptake and energy expenditure differ significantly between experiments. RER increased within 60 min after food intake in both experiments and returned towards the fasting level at 180 min postprandially. Lipid oxidation rate decreased, accordingly, immediately after food intake and remained lowered for about 1 h postprandially. When exercise was performed RER and lipid oxidation rate were significantly higher compared with the rest experiment.
Arterial metabolite and insulin concentrations
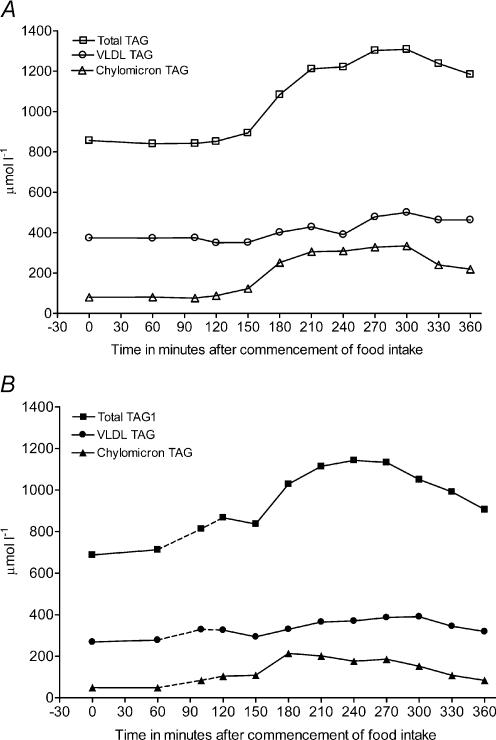
In both experiments the arterial total TAG concentrations increased with time (Table 2 and Fig. 2). When food intake was followed by exercise, the TAG concentration tended to increase (P = 0.08) already during exercise, i.e. between 100 and 120 min after the meal, as opposed to the rest experiment in which the TAG concentration first began to increase about 180 min postprandially. However, whereas the elevated total TAG concentration was maintained until the end of the rest experiment, it began to decrease towards the fasting level by the end of the exercise experiment. The AUC from 0 to 360 min did not differ between experiments, being 198 ± 54 and 242 ± 70 μmol l−1 min−1 in the rest and exercise experiments, respectively (P = 0.34). Arterial chylomicron-TAG concentrations increased postprandially in both experiments and began to decrease again towards the end of the experiments (Table 2 and Fig. 2). The AUC from 0 to 360 min tended to be lower in the exercise experiment compared with the rest experiment, being 75 ± 27 and 115 ± 21 μmol l−1 min−1, respectively (P = 0.08). The VLDL-TAG did not increase significantly above the fasting level in either experiment and there were no significant differences between the experiments (Table 2 and Fig. 2). Generally, the concentrations of glycerol and fatty acids decreased significantly immediately after the meal and tended to increase above fasting concentrations towards the end of the experiments. During exercise arterial glycerol and fatty acid concentrations differed significantly from the rest experiment. The glucose concentrations increased significantly after the meal and returned towards the fasting level within 100 min after food intake in both experiments. During exercise and at the end of the experiment it was significantly lower than during rest (Table 2). In both experiments the insulin concentrations peaked within the first hour after the meal and then decreased steadily (Table 2). During exercise the insulin concentration was increased about twofold compared to the fasting level whereas the increase was fourfold in the rest experiment.
Table 2.
Arterial metabolite and insulin concentrations at time (in minutes) after commencement of food ingestion
| 0 | 60 | 100 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 330 | 360 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total TAG (μmol l−1) | ||||||||||||||
| M→R | 857 ± 97 | 841 ± 90 | 842 ± 86 | 852 ± 96 | 895 ± 94 | 1084 ± 88 | 1212 ± 91* | 1221 ± 84* | 1303 ± 128* | 1308 ± 139* | 1238 ± 145* | 1185 ± 153* | ||
| M→E | 691 ± 57 | 713 ± 52 | 813 ± 83 | 867 ± 116 | 837 ± 66 | 1029 ± 115* | 1114 ± 108* | 1143 ± 98* | 1133 ± 83 | 1051 ± 72* | 991 ± 109 | 906 ± 95 | ||
| Chylomicron-TAG (μmol l−1) | ||||||||||||||
| M→R | 81 +10 | 80 ± 12 | 76 ± 11 | 88 ± 16 | 123 ± 21 | 252 ± 31* | 305 ± 23* | 309 ± 41* | 330 ± 54* | 334 ± 80* | 241 ± 50* | 219 ± 55* | ||
| M→E | 50 ± 7† | 49 ± 7 | 84 ± 23 | 104 ± 41 | 109 ± 26 | 214 ± 67* | 202 ± 72*† | 176 ± 52*† | 186 ± 29*† | 152 ± 23† | 108 ± 20† | 84 ± 17† | ||
| VLDL-TAG (μmol l−1) | ||||||||||||||
| M→R | 374 ± 81 | 373 ± 76 | 375 ± 73 | 350 ± 81 | 352 ± 68 | 402 ± 74 | 429 ± 78 | 391 ± 51 | 478 ± 76 | 501 ± 84 | 464 ± 79 | 464 ± 82 | ||
| M→E | 269 ± 29 | 278 ± 31 | 329 ± 54 | 326 ± 52 | 294 ± 39 | 330 ± 45 | 365 ± 54 | 370 ± 49 | 387 ± 27 | 391 ± 42 | 345 ± 29 | 319 ± 31 | ||
| Glycerol (μmol l−1) | ||||||||||||||
| M→R | 43 ± 7 | 20 ± 4* | 22 ± 3 | 27 ± 3 | 24 ± 6 | 40 ± 8 | 53 ± 10 | 47 ± 8 | 58 ± 8 | 66 ± 11 | 58 ± 5 | 64 ± 6 | ||
| M→E | 41 ± 5 | 16 ± 1* | 66 ± 10*† | 67 ± 12*† | 26 ± 3 | 44 ± 10 | 55 ± 7 | 62 ± 12 | 51 ± 7 | 59 ± 4 | 56 ± 7 | 62 ± 4 | ||
| Fatty acids (μmol l−1) | ||||||||||||||
| M→R | 468 ± 91 | 110 ± 22* | 68 ± 14* | 82 ± 16* | 103 ± 23* | 188 ± 48* | 324 ± 116 | 398 ± 99 | 478 ± 114 | 594 ± 110 | 645 ± 60 | 689 ± 84 | ||
| M→E | 465 ± 94 | 89 ± 15* | 175 ± 25*† | 243 ± 40† | 218 ± 29* | 437 ± 115 | 534 ± 104† | 583 ± 103† | 592 ± 85 | 686 ± 48 | 704 ± 53* | 820 ± 45* | ||
| Glucose (mmol l−1) | ||||||||||||||
| M→R | 4.41 ± 0.13 | 6.53 ± 0.40* | 4.86 ± 0.31 | 4.83 ± 0.35 | 4.29 ± 0.09 | 4.39 ± 0.18 | 4.55 ± 0.16 | 4.51 ± 0.11 | 4.46 ± 0.05 | 4.67 ± 0.12 | 4.67 ± 0.27 | 4.71 ± 0.17 | ||
| M→E | 4.21 ± 0.05 | 6.53 ± 0.31* | 3.89 ± 0.20† | 3.96 ± 0.05† | 4.47 ± 0.13 | 4.29 ± 0.10 | 4.50 ± 0.08 | 4.53 ± 0.15 | 4.50 ± 0.11 | 4.50 ± 0.11 | 4.43 ± 0.10 | 4.34 ± 0.10† | ||
| Insulin (pmol l−1) | ||||||||||||||
| M→R | 36 ± 11 | 259 ± 46* | 141 ± 40* | 114 ± 45* | 75 ± 24 | 78 ± 19 | 57 ± 10 | 50 ± 9 | 36 ± 6 | 42 ± 11 | 52 ± 12 | 46 ± 12 | ||
| M→E | 35 ± 11 | 264 ± 23* | 67 ± 15† | 44 ± 5† | 79 ± 19 | 48 ± 11† | 35 +5 | 41 +10 | 35 ± 5 | 42 ± 10 | 31 ± 6 | 38 ± 8 | ||
Values are mean ± s.e.m. Bolded data denotes exercise. M→R: Rest experiment; M→E: Postprandial exercise experiment.
Significant difference from basal.
Significant difference between the experiments.
Figure 2. Trend curves of mean arterial plasma concentrations of total TAG, VLDL-TAG and chylomicron-TAG in the rest (A) and exercise (B) experiments.
Leg metabolism
Table 3 shows the leg blood flow before and for 360 min after commencement of food ingestion in the rest and exercise experiments. In both experiments leg blood flow was increased significantly by 25 ± 6% within 1 h after food ingestion. In the exercise experiment leg blood flow increased about tenfold during exercise. Post-exercise (150–360 min) the blood flow stayed relatively more increased than in the rest experiment (63%± 11% compared to 39 ± 6%) (P = 0.01).
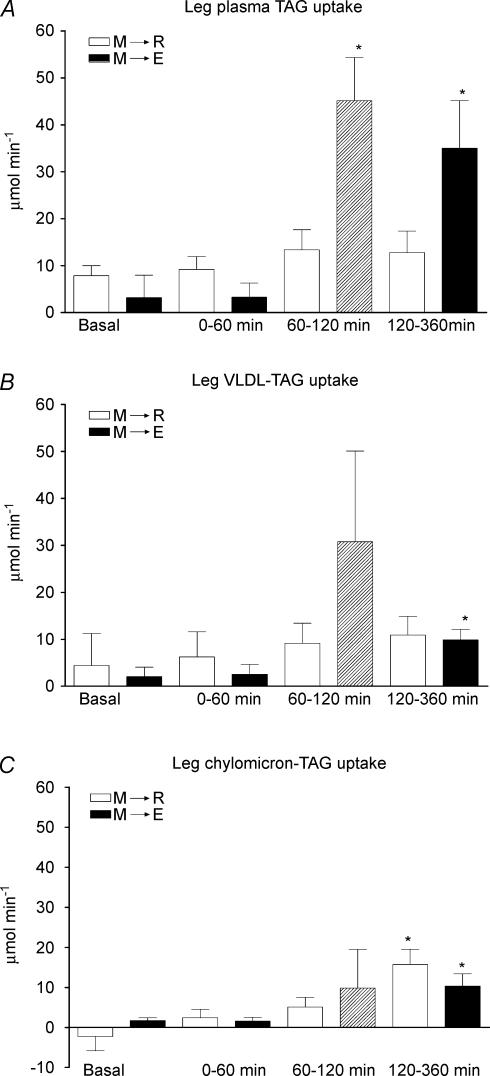
Leg plasma TAG uptake and clearance did not change significantly during the rest experiment (Fig. 3A and Table 3). In contrast, during exercise leg plasma TAG uptake was increased to a level about fourfold and clearance about eightfold higher than in the corresponding period in the rest experiment (60–120 min). Furthermore, during the 4-h post-exercise resting period, leg TAG uptake and clearance were on average about 2.5-fold higher than in the corresponding period in the rest experiment (120–360 min). The average ΔAUCs of plasma TAG uptake at 60–360 min were 13 ± 4 μmol min−1 in the rest experiment and 37 ± 8 μmol min−1 in the exercise experiment (P = 0.05). Leg VLDL-TAG uptake did not change significantly with time in the rest experiment. In the exercise experiment it increased during exercise and stayed increased post-exercise (Fig. 3B). On average the chylomicron A–V differences across the leg were not significantly different from zero until about 120 min after food intake; from 120 to 360 min the average integrated ΔAUCs were 14 ± 3 μmol min−1 in the rest experiment and 10 ± 4 μmol min−1 in the exercise experiment (P = 0.55) (Fig. 3C).
Figure 3. ΔAUCs of leg skeletal muscle total plasma TAG (A), VLDL-TAG (B) and chylomicron-TAG (C) uptake in six healthy male subjects before (basal), and after (0–60 min, 60–120 min and 120–360 min) food intake.
M→R: rest experiment; M→E: exercise experiment. A hatched bar indicates exercise. Values are the mean ± s.e.m.*P < 0.05, significant difference from basal.
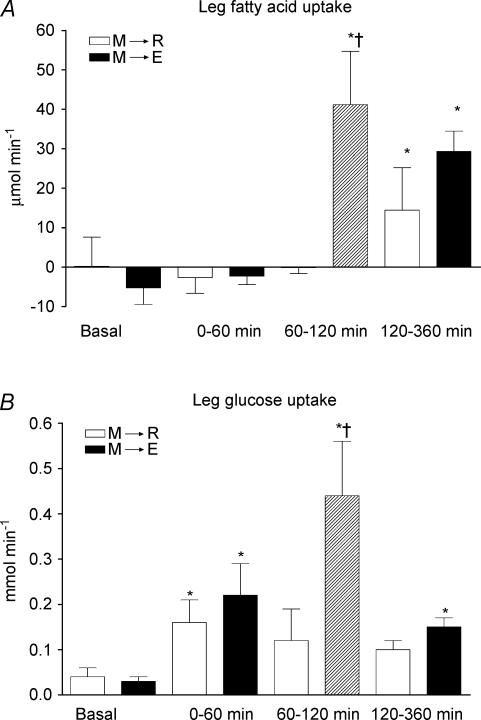
In the rest experiment the leg fatty acid uptake did not differ significantly from zero (Fig. 4A). In the exercise experiment there was a significant uptake of 41 ± 14 μmol min−1 during exercise and 29 ± 5 μmol min−1 post-exercise.
Figure 4. ΔAUCs of leg skeletal muscle plasma fatty acid (A) and glucose uptake (B) in 6 healthy male subjects before (basal), and after (0–60 min, 60–120 min and 120–360 min) food intake.
M→R: rest experiment; M→E: exercise experiment. A hatched bar indicates exercise. Values are the mean ± s.e.m.*P < 0.05, significant difference from basal. †P < 0.05, significant difference between experiments.
Regarding leg net glycerol balance it was not different from zero in either of the experiments (data not shown).
In both experiments food ingestion led to an increased uptake of glucose within 60 min. In the exercise experiment it increased further during exercise. Post-exercise it was still increased compared to the preprandial uptake (Fig. 4B).
Splanchnic metabolism
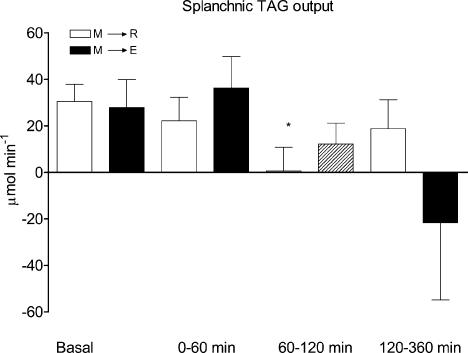
In both experiments blood flow increased significantly within the first 60 min after food intake and this increase remained for at least 180 min postprandially (Table 3). In the fasting state, there was a significant release of TAG (Fig. 5). Sixty minutes after food intake the release of TAG decreased in both experiments. We were not able to measure v–a differences significantly different from zero regarding the VLDL-TAG subfraction in either of the experiments (data not shown).
Figure 5. ΔAUCs of splanchnic TAG output in 6 healthy male subjects before (basal), and after (0–60 min, 60–120 min and 120–360 min) food intake.
M→R: rest experiment; M→E: exercise experiment. A hatched bar indicates exercise. Values are the mean ± s.e.m.*P < 0.05, significant difference from basal.
The splanchnic glycerol uptake decreased within 60 min after food intake in both experiments and in the rest experiment it increased again to reach the premeal level about 180 min after food intake (Table 3). In the exercise experiment the splanchnic glycerol uptake was increased above the pre-meal level during exercise and in this period it was significantly differerent from that found in the rest experiment. Similarly, the splanchnic uptake of fatty acids decreased after food intake in both experiments to increase again towards the end of experiments. The net fatty acid uptake during exercise stayed decreased in spite of an increased hepatic delivery (Table 3). The splanchnic glucose output increased significantly within 60 min after food intake in both experiments (Table 3) and during exercise it was significantly higher than in the corresponding period in the rest experiment.
Discussion
The major finding of the present study is that 1 h of moderate-intensity exercise early after food intake increases leg plasma TAG net uptake and clearance severalfold during as well as for at least 4 h post-exercise. In contrast, when exercise is not performed postprandially leg plasma TAG uptake and clearance do not change up to 6 h after food intake. The exercise induced increases in plasma TAG clearance and uptake suggest that the skeletal muscle LPL activity is increased in this situation. The increased TAG uptake in the post-exercise recovery period is in accordance with a study on fasting subjects, in which muscle LPL expression and activity were both increased (Kiens & Richter, 1998). However, the mechanisms which regulate the LPL activity in skeletal muscle have not been described in detail (Seip & Semenkovich, 1998; Hamilton et al. 2004). Insulin is generally considered to have a suppressive effect on muscle LPL activity (Lithell et al. 1978; Farese et al. 1991). Although exercise elicited a suppression of the insulin concentration, it was higher during exercise as well as in the recovery period compared to when exercise is performed in the fasted state (Wigernæs et al. 2001). Thus, our findings suggest that acute exercise per se may increase the muscle LPL activity and overrule the suppressive effects of insulin.
Exercise performed ∼16–18 h before food intake renders muscle LPL resistant to the effects of insulin, and when measured in muscle biopsies the LPL activity does not increase during exercise unless the exercise bout is vigorous and prolonged (Kiens & Richter, 1998). This finding is in contrast to the present findings. This emphasizes that it may be difficult to extrapolate from in vitro measurements of muscle LPL activity in biopsies to in vivo measurements of net plasma TAG flux in the leg. In the biopsy, the total LPL activity present is measured. In contrast, only the lipolytic effect of the accessible fraction of LPL (i.e. the amount found in the perfused capillaries) is measured in the arterio-venous in vivo measurements. Not all nutritive capillaries are perfused simultaneously in the resting skeletal muscle but the functional hyperaemia elicited by the increased metabolic demand in the exercising muscle increases the number of perfused capillaries, so-called capillary recruitment (Rattigan et al. 2005). When not all capillaries in the muscle are perfused, as is the case during rest and low intensity exercise, differences between the results emerging from biopsy and arterio-venous difference studies must arise. Accordingly, an explanation for the present findings is that capillary recruitment during exercise exposes the lipoprotein particles to a larger amount of active LPL. Thus, plasma TAG hydrolysis will be increased in spite of the same level of LPL activity measured per muscle weight during and before exercise. However, capillary recruitment alone cannot explain the present findings.
In a previous study on fasting subjects, we have found that exercise at 40% or 60% of peak power output does not result in significant changes in leg plasma TAG uptake either during exercise or post-exercise (Mulla et al. 2000) irrespective of the applied exercise intensity. It is likely that the diet plays a role for the skeletal muscle metabolism of plasma TAG during exercise as it has been demonstrated that subjects, who have been on a 65 energy percentage high-fat diet for 7 weeks before an exercise experiment comparable to the above mentioned utilize VLDL-TAG as an important energy source in skeletal muscle during exercise (Helge et al. 2001). As found in the present study, the hormonal changes induced by acute food intake alone do not induce changes in the resting skeletal muscle plasma TAG uptake and clearance. It is therefore hypothesized that it is the combination of the exercise-induced capillary recruitment and the meal- and/or exercise-induced hormonal changes which elicits this response.
Regarding the lipoprotein-TAG subfractions, we found that most of the leg TAG uptake during exercise could be explained by an increased uptake of VLDL-TAG and only a smaller fraction by chylomicron-TAG uptake, which is in accordance with the delivery of VLDL-TAG being more than threefold higher than that of chylomicron-TAG (600 versus 180 μmol min−1). In the post-exercise recovery period a significant and increased portion of the total plasma TAG uptake in the leg came from the chylomicrons in keeping with a greater fraction of the TAG delivered at this time point stemming from absorbed fat from the meal. An enhanced removal into skeletal muscle of TAG from TAG-rich lipoproteins has been suggested as a plausible mechanism for the reduced concentration of circulating TAG seen when a meal is consumed several hours after a single exercise bout (Aldred et al. 1994; Malkova et al. 2000; Gill & Hardman, 2003). In the present study the enhanced leg TAG uptake and clearance found during exercise did not, however, result in a decreased early postprandial lipaemia compared with the rest experiment. On the contrary, the arterial TAG concentration tended to increase faster during exercise than during rest, as we have also found in a recent study performed under almost identical experimental conditions (Enevoldsen et al. 2004). However, after this initial faster increase, the concentration also decreased faster implying that the integrated areas under the TAG concentration curves were not different between the two experiments. Thus, while increased removal of TAG by muscle during post-exercise recovery seems to lead to a decrease in circulating TAG postprandially this is not the case during exercise. Furthermore, the augmented lipaemia found during exercise could not be explained by increased splanchnic TAG output (Fig. 5) and in our previous study (Enevoldsen et al. 2004) changes in adipose tissue TAG metabolism could also not explain the response. A possible tissue which may be responsible for the difference between the rest and exercise situation is the non-exercising muscle mass. However, this hypothesis needs to be elucidated in further experiments.
During exercise whole body lipid oxidation increased by about 0.20 g min−1. With an average molecular weight of triacylglycerol of 856 g mol−1 this corresponds to 700 μmol min−1 of fatty acids. If it is assumed that all the fatty acids liberated by the degradation of TAG in the exercising muscles were taken up in addition to the uptake of plasma free fatty acids, then about 600 μmol min−1 of fatty acids would have been taken up in the two legs during exercise. Thus, it seems that the lipid content in the legs by and large stays constant during exercise. However, during post-exercise recovery about 3 times as much fatty acid seems to be taken up in the legs than can be accounted for by the increase in whole body fatty acid oxidation, suggesting that during post-exercise recovery in the postprandial state fatty acids accumulate inside the muscle either as long chain acyl-CoA esters, which are intermediates in the oxidative pathway, or esterified into TAG. This has been suggested to affect the skeletal muscle insulin sensitivity (Yu & Ginsberg, 2004). Yet, in light of the increased glucose uptake as compared to the preprandial uptake, this does not seem to be the case in the present experiments because the insulin concentrations after exercise were not different from the value during fasting.
As in our previous study (Enevoldsen et al. 2004) we were not able to demonstrate changes in the splanchnic net balance of total TAG. A likely explanation is that changes in the fatty acid and TAG metabolism in the intra-abdominal adipose tissue depots and in the liver are opposite and by and large balance each other. Another possibility is that a hepatic net release of VLDL-TAG is balanced out by a concomitant hepatic uptake of TAG present in chylomicron remnants the concentration of which is increased after a mixed meal. Additionally, it has been well described that it may be difficult to detect changes in the splanchnic TAG net balance due to small venous–arterial differences approaching the detection limit of the TAG analysis (Jensen, 2003). The present finding is in contrast to our previous study during rest after an oral glucose load (Bülow et al. 1999) and other studies (Havel et al. 1970; Lewis et al. 1995) in which it has been demonstrated that the splanchnic TAG output is positively correlated to fatty acid uptake. The present results suggest that the liver for some reason is not able to deal with the entire blood-borne surplus of lipid metabolites resulting when lipids are being mobilized from endogenous depots simultaneously with ongoing lipid absorption from the gastrointestinal channel. In this situation is seems that other tissues, apart from adipose tissue, e.g. skeletal muscle, participate in the deposition of lipid.
On the other hand, the present experiments confirm that there is an additive effect of exercise and a carbohydrate rich meal on the splanchnic net glucose balance as we have recently described (Enevoldsen et al. 2004). This is probably a result of a larger decrease in the blood glucose concentration during exercise as compared to rest (Table 2) due to a threefold higher leg glucose uptake during exercise. Furthermore, with respect to this response it does not seem to play a quantitatively important role whether the carbohydrate content of the meal is 50 or 60%.
In conclusion, the present experiments show that exercise performed after a meal results in an immediate increase in plasma TAG uptake and clearance in the exercising legs as well as for at least 4 h post-exercise. It is hypothesized that this is a result of capillary recruitment leading to exposure of the circulating lipoprotein particles to a larger amount of active LPL in combination with the hormonal changes induced by food intake and exercise. In spite of the increased plasma TAG uptake and clearance in the exercising legs the lipaemic response during 6 h postprandially is the same whether exercise is performed or not. Furthermore, there is an additive effect of exercise and food intake on splanchnic net glucose balance regardless of the carbohydrate content of the meal being 50 or 60 energy per cent. Finally, the present results suggest that skeletal muscle participates in the deposition of the surplus of lipid metabolites resulting from endogenous lipid mobilization together with ongoing lipid absorption from the gastrointestinal channel elicited by the applied combination of food intake and exercise.
Acknowledgments
We thank Inge Rasmussen for excellent technical assistance. This study was supported by grants from the Novo Nordic Foundation, The John and Birthe Meyer Foundation, The Danish Medical Research Council (22-01-0235) and from The Danish Heart Foundation (02-2-3-34-22016).
References
- Aldred HE, Perry IC, Hardman AE. The effect of a single bout of brisk walking on postprandial lipemia in normolipidemic young adults. Metabolism. 1994;43:836–841. doi: 10.1016/0026-0495(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Bülow J. Adipose tissue blood flow during exercise. Dan Med Bull. 1983;30:85–100. [PubMed] [Google Scholar]
- Bülow J, Madsen J. Influence of blood flow on fatty acid mobilization form lipolytically active adipose tissue. Pflugers Arch. 1981;390:169–174. doi: 10.1007/BF00590202. [DOI] [PubMed] [Google Scholar]
- Bülow J, Simonsen L, Wiggins D, Humphreys SM, Frayn KN, Powell D, Gibbons GF. Co-ordination of hepatic and adipose tissue lipid metabolism after oral glucose. J Lipid Res. 1999;40:2034–2043. [PubMed] [Google Scholar]
- Enevoldsen LH, Simonsen L, Macdonald IA, Bülow J. The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J Physiol. 2004;561:871–882. doi: 10.1113/jphysiol.2004.076588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV Jr, Yost TJ, Eckel RH. Tissue-specific regulation of lipoprotein lipase activity by insulin/glucose in normal-weight humans. Metabolism. 1991;40:214–216. doi: 10.1016/0026-0495(91)90178-y. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gill JM, Hardman AE. Exercise and postprandial lipid metabolism: an update on potential mechanisms and interactions with high-carbohydrate diets (review) J Nutr Biochem. 2003;14:122–132. doi: 10.1016/s0955-2863(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Gill JM, Herd SL, Vora V, Hardman AE. Effects of a brisk walk on lipoprotein lipase activity and plasma triglyceride concentrations in the fasted and postprandial states. Eur J Appl Physiol. 2003;89:184–190. doi: 10.1007/s00421-002-0788-9. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32:161–166. doi: 10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel RJ, Kane JP, Balasse EO, Segel N, Basso LV. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970;49:2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Watt PW, Richter EA, Rennie MJ, Kiens B. Fat utilization during exercise: adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J Physiol. 2001;537:1009–1020. doi: 10.1111/j.1469-7793.2001.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen JH, Winkler K. Hepatic blood flow determination. A comparison of 99mTc-diethyl-IDA and indocyanine green as hepatic blood flow indicators in man. J Hepatol. 1987;4:66–70. doi: 10.1016/s0168-8278(87)80011-9. [DOI] [PubMed] [Google Scholar]
- Humphreys SM, Fisher RM, Frayn KN. Micro-method for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem. 1990;27:597–598. doi: 10.1177/000456329002700613. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Fate of fatty acids at rest and during exercise: regulatory mechanisms. Acta Physiol Scand. 2003;178:385–390. doi: 10.1046/j.1365-201X.2003.01167.x. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol. 1998;275:E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H, Boberg J, Hellsing K, Lundqvist G, Vessby B. Lipoprotein-lipase activity in human skeletal muscle and adipose tissue in the fasting and the fed states. Atherosclerosis. 1978;30:89–94. doi: 10.1016/0021-9150(78)90155-7. [DOI] [PubMed] [Google Scholar]
- Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR, Hardman AE. Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 2000;279:E1020–E1028. doi: 10.1152/ajpendo.2000.279.5.E1020. [DOI] [PubMed] [Google Scholar]
- Mulla NA, Simonsen L, Bülow J. Postexercise adipose tissue and skeletal muscle lipid metabolism in humans: the effects of exercise intensity. J Physiol. 2000;524:919–928. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JL, Fisher RM, Humphreys SM, Gibbons GF, Frayn KN. Separation of lipoprotein fractions by ultracentrifugation: investigation of analytical recovery with sequential flotation and density gradient procedures. Clin Chim Acta. 1994;230:215–220. doi: 10.1016/0009-8981(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Wheatley C, Richards SM, Barrett EJ, Clark MG. Exercise and insulin-mediated capillary recruitment in muscle. Exerc Sport Sci Rev. 2005;33:43–48. [PubMed] [Google Scholar]
- Seip RL, Semenkovich CF. Skeletal muscle lipoprotein lipase: molecular regulation and physiological effects in relation to exercise. Exerc Sport Sci Rev. 1998;26:191–218. [PubMed] [Google Scholar]
- Simonsen L, Bülow J, Madsen J, Hermansen F, Astrup A. Local forearm and whole-body respiratory quotient in humans after an oral glucose load: methodological problems. Acta Physiol Scand. 1993;147:69–75. doi: 10.1111/j.1748-1716.1993.tb09473.x. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Ryge C, Bülow J. Glucose-induced thermogenesis in splanchnic and leg tissues in man. Clin Sci (Lond) 1995;88:543–550. doi: 10.1042/cs0880543. [DOI] [PubMed] [Google Scholar]
- Wigernæs I, Høstmark AT, Strømme SB, Kierulf P, Birkeland K. Active recovery and postexercise white blood cell count, free fatty acids, and hormones in endurance athletes. Eur J Appl Physiol. 2001;84:358–366. doi: 10.1007/s004210000365. [DOI] [PubMed] [Google Scholar]
- Yu YH, Ginsberg HN. The role of acyl-CoA: diacylglycerol acyltransferase (DGAT) in energy metabolism. Ann Med. 2004;36:252–261. doi: 10.1080/07853890410028429. [DOI] [PubMed] [Google Scholar]