Abstract
Peripheral nerve inputs have an inhibitory effect on motor cortex excitability at short intervals (short-latency afferent inhibition, SAI). This can be tested by coupling electrical stimulation of peripheral nerve with transcranial magnetic stimulation (TMS) of the motor cortex. SAI is reduced by the anticholinergic drug scopolamine, and in patients with Alzheimer's disease. Therefore, it is possible that SAI is a marker of central cholinergic activity important for memory function. The benzodiazepine lorazepam also reduces SAI. Since benzodiazepines impair memory formation, but do not do so uniformly, with a maximum amnesic effect after lorazepam but less or no effect after diazepam, we were interested in testing in this non-behavioural study to what extent the effects of lorazepam and diazepam on circuits involved in SAI could be dissociated. In addition, and for control, we tested the effects of lorazepam and diazepam on short-interval intracortical inhibition (SICI), a motor cortical inhibition mediated through the GABAA receptor. Lorazepam markedly reduced SAI, whereas diazepam slightly increased it. In contrast, both benzodiazepines uniformly increased SICI. Our findings demonstrate opposite effects of lorazepam and diazepam on SAI, an inhibition modulated by central cholinergic activity, but the same effects on SICI, a marker of neurotransmission through the GABAA receptor. This dissociation suggests, for the first time, that TMS measures of cortical inhibition provide the opportunity to segregate differences of benzodiazepine action in human central nervous system circuits.
Muscle responses recorded in hand muscles after transcranial magnetic stimulation (TMS) of the motor cortex can be suppressed by electrical stimulation of the median nerve if the time interval between stimulation of the median nerve and motor cortex is 2–8 ms longer than the time needed for fast-conducting peripheral nerve afferent input to reach the cortex (Tokimura et al. 2000). This effect, named short-latency afferent inhibition (SAI) of the motor cortex, is produced by interactions within the cerebral cortex (Tokimura et al. 2000). Since this inhibitory phenomenon is reduced or abolished by the muscarinic antagonist scopolamine (Di Lazzaro et al. 2000a), we suggested that it might be a non-invasive way of testing cholinergic activity in the SAI pathway. More recently, we showed (Di Lazzaro et al. 2005) that also the benzodiazepine lorazepam results in a suppression of SAI, concomitantly with an increase in a different intracortical inhibitory phenomenon, the so-called short-latency intracortical inhibition (SICI) (Kujirai et al. 1993). SICI is believed to be mediated by GABAAergic inhibitory neuronal circuits (Ziemann et al. 1996a, b; Di Lazzaro et al. 2000b; Ziemann, 2004).
Lorazepam produces an impairment of memory function that is similar to the deficit produced by scopolamine (Mintzer & Griffiths, 2003). Several studies have suggested that the effect of different benzodiazepines on memory is not uniform. While lorazepam produced a maximum impairment of memory, in particular implicit memory, diazepam had minimal or no effects (Heisterkamp & Cohen, 1975; Healey et al. 1983; Sellal et al. 1992; Legrand et al. 1995; Vidailhet et al. 1996; Wagemans et al. 1998). These studies provided the rationale for the present investigation that aimed at comparing the effects of diazepam versus lorazepam on SAI. Previous studies in patients with Alzheimer's disease suggested that SAI is a marker of the cholinergic cortical deficit in these patients (Di Lazzaro et al. 2002), which in turn is thought to play a central role in their memory deficit (Hasselmo & Bower, 1993). Since diazepam, in contrast to lorazepam, has only minimal effects on memory, our hypothesis was that SAI should not be modified by diazepam, in contrast to the already known reduction of SAI by lorazepam. In a subgroup of subjects, we also evaluated the effects of diazepam and lorazepam on SICI, which we expected to be similar because diazepam and lorazepam enhanced this GABAAergic inhibition in previous studies (Ziemann et al. 1996a; Di Lazzaro et al. 2000b; Ilic et al. 2002). Similar effects of diazepam and lorazepam on SICI would serve as an important control for the expected dissociation of drug effects on SAI, because this would indicate that both drugs had reached similarly effective concentrations in the motor cortex to affect GABAAergic neurotransmission at the time of the TMS measurements.
Methods
Subjects
Eleven healthy volunteers (mean age, 27.9 ± 4.6 years) participated in the experiments; all subjects were right-handed according to the Edinburgh inventory (Oldfield, 1971). All gave their written informed consent. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the Catholic University of Rome and of the J.W. Goethe University of Frankfurt.
Magnetic stimulation
Magnetic stimulation was performed with a high power Magstim 200 (Magstim Co., Whitland, Dyfed, UK). A figure-of-eight coil, with external loop diameters of 9 cm, was held over the dominant (five subjects) or non-dominant (six subjects) motor cortex at the optimum scalp position to elicit motor evoked potentials (MEPs) in the contralateral first dorsal interosseous (FDI) muscle. The induced current in the brain flowed in a posterior-to-anterior direction. Surface muscle responses were recorded with two 9 mm diameter Ag–AgCl electrodes with the active electrode over the motor point of the muscle, and the reference on the metacarpophalangeal joint of the index finger. EMG responses were amplified and filtered (bandwidth 3 Hz to 3 kHz) by D360 amplifiers (Digitimer, Welwyn Garden City, Herts, UK). Data were collected on a computer with a sampling rate of 10 kHz per channel, and stored for later analysis using a CED 1401 analog/digital converter (Cambridge Electronic Design, Cambridge UK). Resting motor threshold (RMT) was defined as the minimum stimulus intensity that produced a liminal MEP (>50 μV in at least 50% of 10 trials) at rest. Active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a small MEP (about 200 μV in 50% of 10 trials) during isometric contraction of the tested muscle, at about 20% of maximum voluntary contraction. A constant level of voluntary contraction was maintained with reference to an oscilloscope display of the EMG signal in front of the subject. Auditory feedback of the EMG activity was also provided.
In order to minimise the recording time for each protocol, both single and paired stimulation of the motor cortex were performed with the stimulator(s) connected to the BiStim Module (Magstim Co.) throughout all measurements.
Main experiment: effects of diazepam on homonymous SAI (inhibition produced by median nerve stimulation on MEPs in the FDI muscle)
All 11 subjects were tested. SAI was studied using the technique that we have recently described (Tokimura et al. 2000). Conditioning stimuli were single electrical pulses (duration, 200 μs) applied through a bipolar electrode to the median nerve at the wrist (cathode proximal). The intensity of the conditioning stimulus was set to just above motor threshold for evoking a visible twitch of the thenar muscles. The intensity of the unconditioned magnetic test pulse given to the contralateral motor cortex was adjusted to evoke a MEP in the relaxed FDI with a peak-to-peak amplitude of, on average, 1 mV.
The conditioning stimulus to the peripheral nerve preceded the test magnetic cortical stimulus. Interstimulus intervals (ISIs) were determined relative to the individual latency of the N20 component of the somatosensory evoked potential evoked by stimulation of the left (six subjects) or right (five subjects) median nerve. To record somatosensory evoked potentials, the active electrode was attached 3 cm posterior to C3 or C4 (International 10–20 EEG system) and the reference was placed at Fz. Five-hundred responses were averaged to identify the latency of the N20 peak.
ISIs corresponding to the latency of the individual N20 plus 2, 3, 4, 6 and 8 ms were investigated. Eight repeats were delivered at each ISI in pseudo-randomised order. The subjects were given audio-visual feedback at high gain (50 μV D−1) to assist in maintaining complete relaxation. The mean amplitudes of the conditioned EMG responses were expressed as a percentage of the mean amplitude of the unconditioned test EMG responses.
After drug intake the intensity of the test stimulus was adjusted whenever necessary, to ensure that the unconditioned test EMG response was matched in amplitude to the test EMG response recorded before drug intake (baseline).
Measurements were taken at baseline and 1.5 h after the administration of a single oral dose of 20 mg of diazepam. This dose was selected because one previous TMS study had demonstrated that it is effective to alter motor cortical excitability in healthy subjects (Ilic et al. 2002).
We call this protocol here homonymous SAI (in contrast to heteronymous SAI, see below) because the sensory territory of the hand supplied by the median nerve is contiguous to the target muscle (FDI). This takes into consideration that cutaneous afferents are important in mediating this form of motor cortical inhibition (Tokimura et al. 2000).
Effects of diazepam on the intensity curve of homonymous SAI
The effects of diazepam on homonymous SAI were further evaluated in three of the 11 subjects in experiment 1 (subjects 1, 2 and 3; mean age 29 ± 4.6 years). We used six different intensities of the conditioning stimulus corresponding to 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 times perceptual sensory threshold. Only one ISI was studied which corresponded to the individual N20 latency plus 2 ms. The rationale for this experiment was to increase the sensitivity for detecting subtle effects of diazepam on SAI, because the standard protocol (Tokimura et al. 2000) tests SAI with stimulus intensities that produce, at least in some subjects, maximal inhibition. This may result in floor effects due to saturated inhibition.
Comparison of the effects of diazepam and lorazepam on homonymous SAI and on SICI
Six of the 11 subjects in experiment 1 (subjects 4, 5, 6, 7, 8, 11; mean age 29 ± 4.9 years) received both diazepam and lorazepam in different sessions. At least one month passed between the administration of the two benzodiazepines. In this subgroup of subjects, in addition to homonymous SAI, we also evaluated short-latency intracortical inhibition (SICI).
SICI was studied using the technique of Kujirai et al. (1993). Two magnetic stimuli were given to the motor cortex through the same stimulating coil, using a Bistim module, and the effect of the first (conditioning) stimulus on the second (test) stimulus was investigated. The conditioning stimulus was set at an intensity of 5% (of maximum stimulator output) below AMT. The test stimulus intensity was adjusted to evoke an EMG response in the relaxed FDI with a peak-to-peak amplitude of, on average, 1 mV when given alone. Interstimulus intervals (ISIs) of 2 and 3 ms were investigated. Five stimuli were delivered at each ISI. For these recordings, muscle relaxation is very important and the subjects were given audio-visual feedback at high gain (50 μV D−1) to assist in maintaining complete relaxation.
SICI was calculated by normalising the mean amplitude of the conditioned MEPs to the mean amplitude of the unconditioned test MEPs. The SICI data were averaged across the two ISIs to obtain a grand mean amplitude.
After drug intake the intensity of the test stimulus was adjusted whenever necessary to ensure that the test EMG responses were matched in amplitude to the test EMG responses recorded before drug intake. This is important because SICI varies with the amplitude of the unconditioned test MEP (Stefan et al. 2002).
For SAI testing, ISIs corresponding to the latency of the N20 plus 2, 3, 4, 6 and 8 ms were investigated, using the same protocol as in experiment 1.
Measurements of SAI and SICI were done at baseline and 1.5 h after the administration of a single oral dose of 20 mg of diazepam, or 2 h after the administration of a single oral dose of 2.5 mg lorazepam. The dose of lorazepam was chosen because previous TMS studies proved it effective in altering motor cortical excitability (Ziemann et al. 1996a; Di Lazzaro et al. 2000b, 2005). Additional measurements of SAI were run 6 h and 24 h after drug administration for both drugs, to obtain information on the time course of drug effects.
The sedative effects of diazepam and lorazepam were evaluated at the presumed peak-plasma concentration time (1.5 h after diazepam administration and 2 h after lorazepam administration), using a visual analog scale (VAS) with a self-rating of the subjective state of sedation. The subjects marked a point on a 100 mm line that represented the full range of the subject's level of sedation (with 0 meaning ‘very alert’ and 100 meaning ‘very sedated’).
Effects of diazepam and lorazepam on heteronymous SAI (inhibition produced by ulnar nerve stimulation on MEPs in the FDI muscle)
Five of the 11 subjects in experiment 1 (subjects 1, 2, 3, 4 and 7; mean age 27.4 ± 4 years) received 20 mg of diazepam. Three subjects (subjects 6, 7 and 8; mean age 31.3 ± 6.1 years) received 2.5 mg of lorazepam.
The protocol of this study was the same as in the main experiment, with the difference that in this case the ulnar nerve was stimulated instead of the median nerve and the ISIs were determined from the individual latency of the N20 potential evoked by the stimulation of this nerve.
We call this heteronymous SAI because the sensory territory of the hand supplied by the ulnar nerve is non-contiguous to the target muscle (FDI). It was found in one recent study using conditioning digital cutaneous nerve stimulation that homonymous and heteronymous SAI are somatotopically organised: homonymous SAI is more readily elicited at lower intensities of the conditioning stimulus than heteronymous SAI (Tamburin et al. 2001).
The rationales of testing heteronymous SAI in the present study were to clarify (1) to what extent these somatotopic differences between homonymous and heteronymous SAI are also present when mixed nerves (median versus ulnar nerve) rather than digital cutaneous nerves are stimulated; and (2) whether the modulating effects of a given drug (e.g. diazepam) are the same or different on either type of SAI. If they were the same this would support the notion that the modulation takes place at or upstream of the site where somatotopic inhibitory integration takes place, but if they were different then this would point to a modulation at a more downstream site, possibly at the level of the stimulated afferents themselves.
Statistical analysis
Effects of diazepam and lorazepam on resting motor threshold, test MEP amplitude and SICI were tested using the two-tailed Student's t test for paired samples.
In experiment 1, the effects of diazepam on homonymous SAI were evaluated using a repeated measures analysis of variance (ANOVA) model with the within-subject effects of TIME (baseline, 1.5 h after diazepam intake) and ISI (5 intervals). Post hoc paired t tests were used to compare the diazepam effect at single ISIs.
The effects of diazepam on the SAI intensity curve were explored in another repeated measures ANOVA with the within-subject effects of TIME (baseline, 1.5 h after diazepam intake) and stimulus intensity (6 intensities).
For the comparison of the effects of diazepam and lorazepam on homonymous SAI and the evaluation of the time course of the effects, we used a repeated measures ANOVA with DRUG (diazepam versus lorazepam) and TIME (4 time points) as main within-subject factors. For SAI, only those ISIs (N20 + 4 ms and N20 +6 ms) at which a significant effect was observed in experiment 1 were included in this analysis. Post hoc paired t tests were applied to compare single time points after drug intake with baseline, and differences between drugs at the single time points.
For the comparison of the sedative effects of diazepam versus lorazepam as expressed on the VAS we used the Wilcoxon signed ranks test.
Significance was assumed whenever P < 0.05. Data are presented as means ± one standard deviation (s.d.), unless stated differently.
Results
Effects of diazepam on homonymous SAI (inhibition produced by median nerve stimulation on MEPs in the FDI muscle)
The RMT was not significantly modified by diazepam (39.9 ± 7.8% of maximum stimulator output at baseline versus 40.5 ± 8.1% after diazepam, P > 0.05; paired t test). Test MEP amplitude was 1.0 ± 0.3 mV at baseline versus 1.2 ± 0.6 mV after diazepam intake (P > 0.05; paired t test).
A repeated measures analysis of variance (ANOVA) model with the within-subject effects of TIME (baseline, 1.5 h after diazepam intake) and ISI (5 intervals) showed a significant effect both of TIME (F1,50= 9.72, P < 0.05) and of ISI (F4,50= 4.76, P < 0.05).
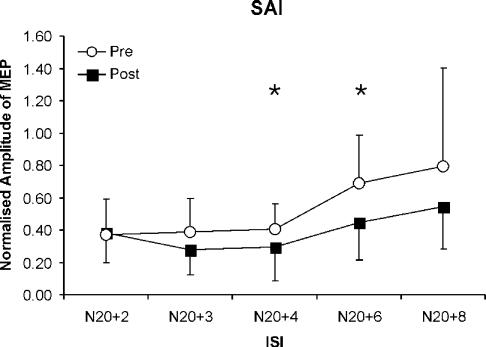
Post hoc comparisons revealed that diazepam significantly increased SAI at ISIs of N20 + 4 ms (at baseline: 0.4 ± 0.16, 1.5 h after diazepam: 0.29 ± 0.21) and N20 +6 ms (at baseline: 0.69 ± 0.3, 1.5 h after diazepam: 0.45 ± 0.23) (Fig. 1).
Figure 1. Effects of diazepam on homonymous short-latency afferent inhibition (SAI) produced by median nerve stimulation at different interstimulus intervals (ISIs).
Data are means (n = 11) at baseline (○) and 1.5 h after diazepam administration (▪), error bars are standard deviations. SAI is reported by the amplitude of the conditioned motor evoked potential (MEP) normalised to the unconditioned test MEP. A repeated measures analysis of variance (ANOVA) model with the within-subject effects of TIME (baseline, 1.5 h after diazepam intake) and ISI (5 intervals) shows a significant effect both of TIME (F1,50= 9.72, P < 0.05) and of ISI (F4,50= 4.76, P < 0.05). Diazepam increases the amount of SAI at ISIs of N20 +4 ms and N20 +6 ms (*P < 0.05). The mean N20 latency was 18.5 ± 1.3 ms.
Effects of diazepam of the intensity curve of homonymous SAI
In this experiment, intensity of the median nerve stimulus was set relative to the sensory perceptual threshold, that ranged between 0.2 and 0.5 times motor threshold (mean, 0.36 ± 0.12). Test MEP amplitude was 1.02 ± 0.40 mV at baseline versus 1.16 ± 0.58 mV after diazepam intake (P > 0.05, paired t test).
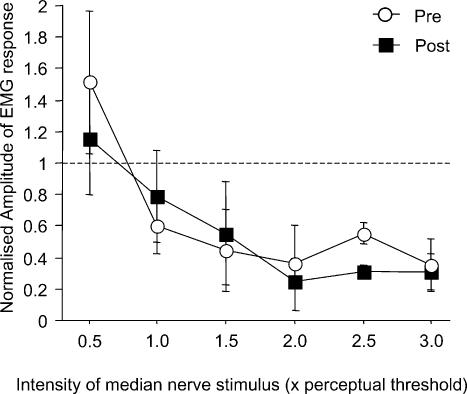
Homonymous SAI increased with the intensity of the median nerve stimulus (P = 0.0009), but diazepam had no significant effect on the SAI intensity curve (Fig. 2).
Figure 2. Effects of diazepam on the intensity curve of homonymous SAI.
Data are means (n = 3) of homonymous SAI tested at a single interstimulus interval of N20 +2 ms and different intensities of the conditioning electrical pulse applied to the median nerve, ranging from 0.5 to 3.0 times perceptual sensory threshold (x-axis) at baseline (○) and 1.5 h after diazepam intake (▪). The mean N20 latency was 19.9 ± 1.6 ms. Error bars are standard deviations. SAI is expressed by the amplitude of the conditioned motor evoked potential (MEP) normalised to the unconditioned test MEP (y-axis). SAI increased with the intensity of the conditioning electrical stimulus applied to the median nerve (P = 0.0009). Diazepam had no significant effect on the SAI intensity curve.
Comparison of the effects of diazepam and lorazepam on homonymous SAI and on SICI
In this subgroup of six subjects, the RMT was not significantly modified by diazepam (44.0 ± 7.6% of maximum stimulator output at baseline versus 44.7 ± 8.1% after diazepam, P > 0.05; paired t test), or by lorazepam (43.5 ± 7.5% at baseline versus 42.7 ± 6.9% after lorazepam, P > 0.05; paired t test). Test MEP amplitudes before and at the different time points after drug intake were matched in the diazepam experiment (baseline: 1.0 ± 0.2 mV, 1.5 h: 1.0 ± 0.5 mV, 6 h: 0.9 ± 0.1 mV, 24 h: 0.9 ± 0.3 mV, P > 0.05, repeated measures ANOVA) and in the lorazepam experiment (baseline: 1.1 ± 0.3 mV, 2 h: 1.3 ± 0.7 mV, 6 h: 1.0 ± 0.3 mV, 24 h: 1.1 ± 0.4 mV, P > 0.05, repeated measures ANOVA).
Because the results of experiment 1 showed that diazepam significantly increased SAI at ISIs of N20 +4 ms and N20 +6 ms, only these intervals were considered in the analysis. The data were averaged across these two ISIs to obtain a grand mean amplitude.
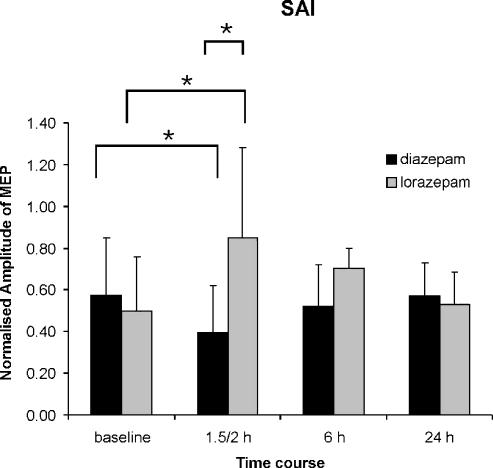
A repeated measures ANOVA with DRUG (diazepam versus lorazepam) and TIME (4 time points) as main within-subject factors showed a significant interaction between DRUG and TIME (F3,10= 5.15, P < 0.05). At the first interval after drug intake, SAI was significantly increased by diazepam, whereas it was reduced by lorazepam (P < 0.05) (Fig. 3). In addition, SAI was less after lorazepam (mean ratio of conditioned to unconditioned test MEP, 0.85 ± 0.44) than after diazepam (0.40 ± 0.22) (P < 0.05) at this time point (Fig. 3).
Figure 3. Effects and time course of the effects of lorazepam and diazepam on homonymous short-latency afferent inhibition (SAI).
Bar graphs show grand means across N20 +4 ms and N20 +6 ms interstimulus intervals at baseline and 1.5 (diazepam) or 2 (lorazepam), and 6 and 24 h after diazepam and lorazepam administration. Error bars are standard deviations. SAI is expressed by the amplitude of the conditioned MEP normalised to the unconditioned test MEP (y-axis). A repeated measures ANOVA with DRUG (diazepam versus lorazepam) and TIME (4 time points) as main within-subject factors shows a significant interaction between DRUG and TIME (F3,10= 5.15, P < 0.05). At the first interval after drug intake the amount of inhibition is significantly increased by diazepam (*P < 0.05) while it is reduced by lorazepam (*P < 0.05). In addition, there is significantly less SAI after lorazepam than after diazepam at this time point (*P < 0.05). The mean N20 latency was 18 ± 0.6 ms.
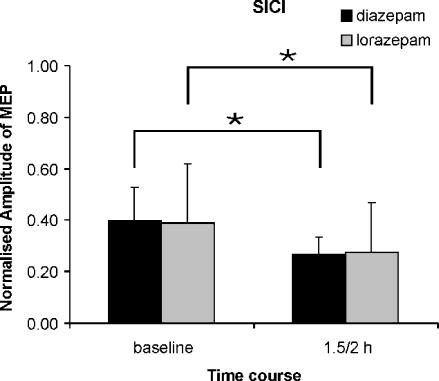
SICI was significantly increased after diazepam (mean ratio of conditioned to unconditioned test MEP, 0.4 ± 0.13 at baseline versus 0.27 ± 0.1 after diazepam, P < 0.05), and after lorazepam (mean ratio of conditioned to unconditioned test MEP, 0.39 ± 0.23 at baseline versus 0.28 ± 0.19 after lorazepam, P < 0.05) (Fig. 4). There was no significant difference of SICI between diazepam and lorazepam before or after drug intake.
Figure 4. Effects of diazepam and lorazepam on short latency intracortical inhibition (SICI).
Bar graphs show grand means of SICI across interstimulus intervals of 2 and 3 ms at baseline and 1.5 h (diazepam) and 2 h (lorazepam) after drug administration. Error bars are standard deviations. SICI is expressed as the amplitude of the conditioned MEP normalised to the unconditioned test MEP (y-axis). Lorazepam and diazepam increase SICI significantly and to similar extents (*P < 0.05).
The mean VAS sedation score was slightly higher after diazepam (55 ± 14) than after lorazepam (49.5 ± 18.4), however, the difference was not significant (P > 0.05, Wilcoxon signed ranks test). There was no correlation between the individual differences in sedation levels produced by diazepam and lorazepam, and the individual differences in change of SAI produced by the two drugs (r2= 0.047, P = 0.5).
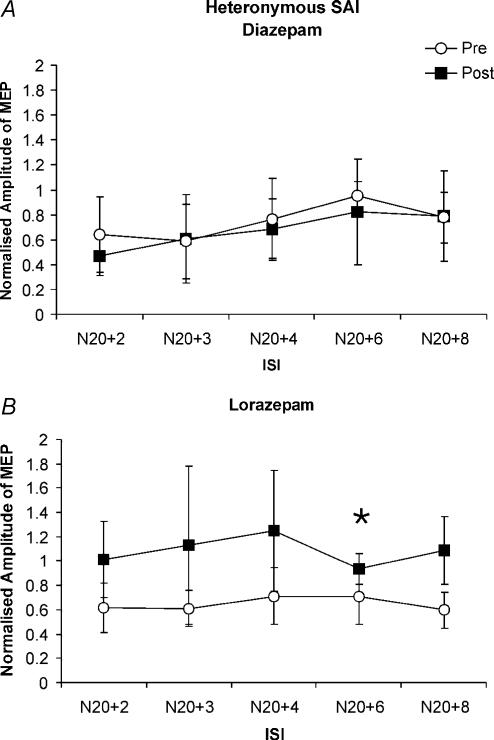
Effects of diazepam and lorazepam on heteronymous SAI (inhibition produced by ulnar nerve stimulation on MEPs in the FDI muscle)
Unconditioned test MEP amplitudes were matched before and after drug intake in the diazepam experiment (baseline: 0.9 ± 0.3 mV, 1.5 h: 1.0 ± 0.3 mV, P > 0.05, paired t test) and in the lorazepam experiment (baseline: 1.0 ± 0.2 mV, 2 h: 0.9 ± 0.4 mV, P > 0.05, paired t test). MEPs were inhibited when the ulnar nerve stimulus was given before TMS of the motor cortex, but the inhibition was less pronounced than homonymous SAI after median nerve stimulation and did not reach statistical significance at baseline or 1.5 h after diazepam (Fig. 5A). Diazepam did not lead to a significant change in heteronymous SAI (Fig. 5A). As observed for homonymous SAI, lorazepam resulted in a suppression of heteronymous SAI (Fig. 5B). The comparison between the baseline data and the corresponding data after lorazepam showed a significant decrease of SAI at the ISI of N20 +6 ms (mean ratio of conditioned to unconditioned test MEP, 0.69 ± 0.2 at baseline versus 0.93 ± 0.13 after lorazepam) (Fig. 5B, P < 0.05).
Figure 5.
A, effects of diazepam on heteronymous short-latency afferent inhibition (SAI) produced by ulnar nerve stimulation at different interstimulus intervals (ISIs). Data are means (n = 5) at baseline (○) and 1.5 h after diazepam administration (▪). Error bars are standard deviations. SAI is expressed as amplitude of the conditioned MEP normalised to the unconditioned test MEP (y-axis). MEPs are inhibited by preceding ulnar nerve stimulation at baseline and after diazepam, but this inhibition is not significant if all ISIs are included in the analysis. Diazepam does not significantly modify heteronymous SAI. Mean ulnar nerve N20 latency was 20 ± 1.8 ms. B, effects of lorazepam on heteronymous SAI produced by ulnar nerve stimulation at different ISIs. Data are means (n = 3) at baseline (○) and 2 h after lorazepam administration (▪). Error bars are standard deviations. Lorazepam reduces heteronymous SAI. This decrease is significant at the ISI of N20 +6 ms (*P < 0.05). Mean ulnar nerve N20 latency was 19.3 ± 0.6 ms.
Discussion
The present results provide the first evidence in humans that two different benzodiazepines produce dissociable excitability changes in central nervous system circuits. Diazepam resulted in an enhancement of short-latency intracortical inhibition (SICI), a form of inhibition that is believed to be mediated by neurotransmission through the GABAA receptor. In addition, diazepam led to a slight enhancement of homonymous short-latency afferent inhibition (SAI), a form of inhibition that is modulated by muscarinic cholinergic activity. In contrast, lorazepam resulted in an enhancement of SICI but a suppression of homonymous SAI, as reported in a recent study (Di Lazzaro et al. 2005).
Why diazepam increased SAI only at longer ISIs of N20 +4 ms and N20 +6 ms but not the short ISIs of N20 +2 ms (cf. Figs 1 and 2) is unclear. This phenomenon resembles the increase of SICI by benzodiazepines, which is strongest at relatively long ISIs of 4–5 ms while shorter ISIs were not or were less affected (Ziemann et al. 1996a; Di Lazzaro et al. 2000b). One explanation for these observations is that the increase in the inhibitory postsynaptic potential (IPSP) by benzodiazepines takes a few milliseconds after IPSP onset to develop. Therefore, it is plausible that excitatory inputs during the early phase of the IPSP (i.e. at short ISIs) are less affected by a benzodiazepine-mediated increase in inhibition compared to a slightly later phase (i.e. at longer ISIs).
The differences of diazepam versus lorazepam on SAI cannot be attributed to relevant differences in pharmacokinetics because excitability measurements were performed at the expected plasma peak levels (1.5 h for diazepam (Shader et al. 1984), 2.0 h for lorazepam (Kyriakopoulos et al. 1978)). In addition, diazepam and lorazepam produced very similar enhancing effects on SICI. It is very likely that this reflects an increase of neurotransmission through GABAA receptors in output circuits of the motor cortex (Ziemann et al. 1996a; Di Lazzaro et al. 2000b; Ilic et al. 2002). Therefore, this suggests that diazepam and lorazepam were available in the motor cortex in sufficient concentration to modulate neurotransmission through the GABAA receptor. Furthermore, the dissociated effects of diazepam and lorazepam on SAI cannot be attributed to the level of sedation because both drugs resulted in a similar amount of sedation, and there was no correlation between the individual differences in the level of sedation and the individual differences in change of SAI caused by diazepam versus lorazepam.
The consistency of modulating drug effects across homonymous and heteronymous SAI (both types of SAI unaffected or slightly enhanced by diazepam, both types suppressed by lorazepam) indicates that the modulation of SAI takes place at or ‘upstream’ of the site where mutual inhibition of somatotopically organised afferent inputs coming from the median and ulnar nerve is expressed, rather than affecting the afferent inputs per se. Since this inhibitory integration between the median and ulnar nerve is expressed at the level of the spinal cord (cuneate nucleus), thalamus, and sensory and motor cortex (Hsieh et al. 1995; Tinazzi et al. 2000), it is possible that the effects of diazepam and lorazepam on SAI occurred at any of these sites. It should be noted that we define heteronymous SAI by the inhibitory effect of ulnar nerve stimulation on MEP amplitude in the FDI. This might not be entirely correct because it is implicitly assumed that SAI is largely mediated by the cutaneous fibres of the ulnar nerve, while the ulnar nerve also carries muscle afferents which are ‘homonymous’ for the FDI muscle. However, Tokimura et al. (2000) showed that digital nerve stimulation is equally effective compared to mixed nerve stimulation in producing SAI (cf. Figs 2B and 3A in that paper). Therefore, it is rather likely that cutaneous fibres play a major role in producing the effect, though we cannot completely exclude a contribution from muscle afferents.
Why the effects of diazepam and lorazepam on SAI dissociate cannot be answered with this experimental approach. But there is one important possibility that should be briefly discussed. Benzodiazepines act at the GABAA receptor as positive allosteric agonists. GABAA receptors can be differentiated into a variety of different subtypes (for review, see Olsen & Homanics, 2000). Of nearly 20 identified GABAA receptor subunits, just four (α1, α2, α3, and α5), together with an obligatory γ2 subunit, contribute to the benzodiazepine binding site (Sieghart, 1995). Diazepam is the prototype of a classical benzodiazepine that binds non-selectively to all four GABAA receptor subtypes (Möhler et al. 2002). Lorazepam is a 3-hydroxy benzodiazepine derivative, and its affinity to the GABAA receptors containing the four different alpha-subunits is not known. Therefore, a possible explanation for parts of the present results is that different GABAA receptor subtypes are involved in SICI and SAI, and that diazepam and lorazepam have similar affinity to the ones that mediate SICI, while they show different affinity to the ones involved in SAI. This explanation is well suited in the setting of other recent studies that demonstrated the existence of GABAA receptor subtype-specific circuits in the central nervous system (Freund, 2003) and the differential role of these circuits in regulating cortical excitability and plasticity (Fagiolini et al. 2004). However, different affinity of diazepam and lorazepam for the GABAA receptor subtype involved in SAI is not sufficient to explain an oppositely directed effect, i.e. the increase of SAI by diazepam and the decrease of SAI by lorazepam. The maximum possible difference would be a certain effect by one drug and no effect by the other drug. Therefore, at least one more process needs to come into play to explain the observed dissociation. Animal experiments showed consistently that diazepam and other benzodiazepines decrease ACh release at various subcortical and cortical sites (Petkov et al. 1983; Imperato et al. 1993). The anticholinergic drug scopolamine reduces SAI (Di Lazzaro et al. 2000a). Thus, it may be speculated that both diazepam and lorazepam similarly decrease SAI through suppression of ACh release, but diazepam more than compensates for this by increasing neurotransmission through the GABAA receptor subtype that mediates inhibition in the SAI circuit, whereas lorazepam has not sufficient affinity for this receptor to reverse the decrease of SAI produced by its anticholinergic effect. The net effects would be a modest increase of SAI by diazepam and a more conspicuous decrease of SAI by lorazepam.
It was reported that lorazepam and the anticholinergic drug scopolamine produce similar impairment of memory function (Curran et al. 1991; Mintzer & Griffiths, 2003), whereas diazepam has less pronounced effects, in particular on implicit memory (Heisterkamp & Cohen, 1975; Healey et al. 1983; Sellal et al. 1992; Legrand et al. 1995; Vidailhet et al. 1996; Wagemans et al. 1998). ACh has a central role in memory function (Hasselmo & Bower, 1993). The findings of the present work, demonstrating a depression by lorazepam but not diazepam in an Ach-dependent central pathway, as evaluated with SAI testing, might contribute to our understanding of why these two benzodiazepines impair memory function differently.
Knowledge of the physiological basis of the effects of the two different benzodiazepines on the intact human brain may be useful for selecting the best treatment in different clinical settings. Indeed, the administration of lorazepam is more appropriate in all situations in which an amnesic effect is desired together with sedation such as for surgery premedication, or for patients on the intensive care unit, whereas the use of diazepam is more appropriate when only sedative effects are required. The present experiments show, for the first time, that TMS measures of cortical inhibition provide an opportunity to segregate differences of benzodiazepine action in human central nervous system circuits.
Acknowledgments
This work was partially supported by Programma di ricerca cofinanziato MIUR anno 2003 ‘Strategie Innovative Bio-ispirate per il controllo di Sistemi di Movimentazione’.
References
- Curran HV, Schifano F, Lader M. Models of memory dysfunction? A comparison of the effects of scopolamine and lorazepam on memory, psychomotor performance and mood. Psychopharmacology (Berl) 1991;103:83–90. doi: 10.1007/BF02244079. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000b;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000a;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16:218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- Healey M, Pickens R, Meisch R, McKenna T. Effects of clorazepate, diazepam, lorazepam, and placebo on human memory. J Clin Psychiatry. 1983;44:436–439. [PubMed] [Google Scholar]
- Heisterkamp DV, Cohen PJ. The effect of intravenous premedication with lorazepam (ativan), pentobarbitone or diazepam on recall. Br J Anaesth. 1975;47:79–81. doi: 10.1093/bja/47.1.79. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Shima F, Tobimatsu S, Sun SJ, Kato M. The interaction of the somatosensory evoked potentials to simultaneous finger stimuli in the human central nervous system. A study using direct recordings. Electroencephalogr Clin Neurophysiol. 1995;96:135–142. doi: 10.1016/0168-5597(94)00251-9. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Dazzi L, Obinu MC, Gessa GL, Biggio G. Inhibition of hippocampal acetylcholine release by benzodiazepines: antagonism by flumazenil. Eur J Pharmacol. 1993;238:135–137. doi: 10.1016/0014-2999(93)90518-m. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos AA, Greenblatt DJ, Shader RI. Clinical pharmacokinetics of lorazepam: a review. J Clin Psychiatry. 1978;39:16–23. [PubMed] [Google Scholar]
- Legrand F, Vidailhet P, Danion JM, Grange D, Giersch A, Van der Linden M, Imbs JL. Time course of the effects of diazepam and lorazepam on perceptual priming and explicit memory. Psychopharmacology (Berl) 1995;118:475–479. doi: 10.1007/BF02245949. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: a single-dose comparison of effects on human memory and attentional processes. Exp Clin Psychopharmacol. 2003;11:56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Homanics GE. Functions of GABAA receptors: insights from mutant and knock out mice, in GABA in the nervous system. In: Martin DL, Olsen RW, editors. The View at Fifty Years. Philadelphia: Lippincott. Williams & Wilkins; 2000. pp. 81–96. [Google Scholar]
- Petkov V, Georgiev V, Getova D, Petkov VV. On the effects of diazepam, hyoscine and oxotremorine on acetylcholine release from the cerebral cortex. Acta Physiol Pharmacol Bulg. 1983;9:3–13. [PubMed] [Google Scholar]
- Sellal F, Danion JM, Kauffmann-Muller F, Grange D, Imbs JL, Van der Linden M, Singer L. Differential effects of diazepam and lorazepam on repetition priming in healthy volunteers. Psychopharmacology (Berl) 1992;108:371–379. doi: 10.1007/BF02245126. [DOI] [PubMed] [Google Scholar]
- Shader RI, Pary RJ, Harmatz JS, Allison S, Locniskar A, Greenblatt DJ. Plasma concentrations and clinical effects after single oral doses of prazepam, clorazepate, and diazepam. J Clin Psychiatry. 1984;45:411–413. [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acid A receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburin S, Manganotti P, Zanette G, Fiaschi A. Cutaneomotor integration in human hand motor areas: somatotopic effect and interaction of afferents. Exp Brain Res. 2001;141:232–241. doi: 10.1007/s002210100859. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguiere F, Fiaschi A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain. 2000;123:42–50. doi: 10.1093/brain/123.1.42. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet P, Kazes M, Danion JM, Kauffmann-Muller F, Grange D. Effects of lorazepam and diazepam on conscious and automatic memory processes. Psychopharmacology (Berl) 1996;127:63–72. doi: 10.1007/BF02805976. [DOI] [PubMed] [Google Scholar]
- Wagemans J, Notebaert W, Boucart M. Lorazepam but not diazepam impairs identification of pictures on the basis of specific contour fragments. Psychopharmacology (Berl) 1998;138:326–333. doi: 10.1007/s002130050678. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhof BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptics drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]