Abstract
While vesicle transport is one of the principal functions of myosin motors in neurones, the role played by specific myosin subtypes in discrete vesicle trafficking is poorly understood. We conducted electrophysiological and morphological experiments to determine whether myosin isoforms II and V might be involved in the transport of small synaptic vesicles in presynaptic nerve terminals of a model cholinergic synapse. Electron microscopy revealed the presence of normal synaptic architecture and synaptic vesicle density in presynaptic terminals of cultured superior cervical ganglion neurones (SCGNs) from myosin Va null rats (dilute-opisthotonus, dop). Similarly, electrophysiological analyses of synaptic transmission and synaptic vesicle cycling at paired SCGN synapses failed to uncover any significant differences in synaptic development and function between normal and dop rats. Immunocytochemistry and in situ localization of green fluorescent protein (GFP)-fusion proteins in wild-type synapses revealed that myosins IIB and Va were distributed throughout the cell soma and processes of SCGNs, while myosins IIA and Vb were not detected in SCGNs. Myosin Va was conspicuously absent in presynaptic nerve terminals, but myosin IIB alone was found to be expressed. Furthermore, synaptic transmission was inhibited by introduction of myosin IIB heavy chain fragments into presynaptic terminals of SCGNs. Together these results suggest that only myosin IIB isoform participates in vesicle trafficking in presynaptic nerve terminals of cultured SCGNs.
Myosins belong to a multigene family of proteins that contains many isoforms which differ in their cellular distribution and function (Sellers, 2000; Berg et al. 2001). Among the 18 myosin classes identified so far, the class II (Kawamoto & Adelstein, 1991) and V (Espreafico et al. 1992) families have been best characterized in neurones. Both of these classes include multiple isoforms; thus, non-muscle myosin II is classified into subtypes IIA, IIB and IIC (Kawamoto & Adelstein, 1991; Golomb et al. 2004) and vertebrate myosin V has been subclassified into subtypes Va, Vb and Vc (Reck-Peterson et al. 2000; Berg et al. 2001). Myosin IIB was reported to be more abundant in the brain than myosin IIA (Kawamoto & Adelstein, 1991; Murakami & Elzinga, 1992). Myosin II was suggested to mediate nerve growth cone motility in cultured neurones (Cheng et al. 1992; Rochlin et al. 1995), while myosin Va has been implicated in the regulation of organelle transport (Evans et al. 1997; Suter et al. 2000). Although functional roles for myosin Vb and Vc in neurones have not been established, they have been reported to be involved in plasma membrane recycling in non-neuronal cells (Lapierre et al. 2001; Rodriguez & Cheney, 2002).
Several lines of evidence suggest that myosin may be involved in the regulation of synaptic vesicle trafficking. Myosin II modulates neurotransmitter release from synapses of cultured rat superior cervical ganglion neurones (SCGNs). Acetylcholine release from these neurones was reduced by the disruption of the interaction between myosin II and actin using anti-myosin antibodies or by blocking the catalytic activity of myosin with inhibitors of its light chain kinase (Mochida et al. 1994). Myosin Va was reported to interact with the synaptic vesicle proteins synaptobrevin and synaptophysin in a Ca2+-dependent manner (Prekeris & Terrian, 1997), and to colocalize with the synaptic vesicle protein SV2 (Evans et al. 1998). These findings led to the proposal that myosin Va mediates synaptic vesicle trafficking (Langford & Molyneaux, 1998; Reck-Peterson et al. 2000). However, unexpectedly, synaptic transmission was shown to be unaltered in hippocampal CA3–CA1 excitatory synapses in dilute-lethal mice, which bear a null mutation in the myosin Va gene (Schnell & Nicoll, 2001), though the possibility remained that other myosin isoforms, such as myosin Vb, might have substituted for the absent myosin Va (Mercer et al. 1991; Zhao et al. 1996). In contrast, a recent report demonstrated that spontaneous neurotransmitter release was reduced in cultured hippocampal neurones from dilute-lethal mice (Trinchese et al. 2003). Thus, it is probably safe to say that the exact role played by myosin Va in neurotransmitter release from hippocampal neurones has not as yet been clarified. Myosin Va has also been suggested to be a chromaffin vesicle motor that is involved in catecholamine secretion, since anti-myosin Va antibodies inhibited catecholamine release from stimulated cultured adrenal chromaffin cells (Rosé et al. 2003). Taken together, the above findings still support the notion that myosin Va is involved in vesicle trafficking in presynaptic nerve terminals.
In this study, we attempted to identify the specific myosin II and V isoforms that regulate synaptic vesicle trafficking at fast synapses that form between cultured rat superior cervical ganglion (SCG) sympathetic neurones (Mochida et al. 1994). Our electrophysiological measurements of synaptic development and transmission revealed indistinguishable differences in the formation and function of synapses between dilute-opisthotonus (dop) and wild-type rats. Ultrastructurally, dop synapses were found to be comparable to those found in wild-type neurones in terms of their synaptic membrane and synaptic vesicle density. Immunocytochemistry and in situ imaging, undertaken to examine the subcellular distribution of both endogenous myosin isoforms and recombinant myosins fused to green fluorescent protein (GFP), revealed differential localization patterns for myosins Va, Vb, IIA and IIB in the presynaptic terminals of SCGNs. Furthermore, synaptic transmission was analysed before and during inhibition of endogeneous myosin II function by cytoplasmic introduction of heavy chain fragments of myosins IIA and IIB. All of these results dictate a refinement in our notion of the myosin isoform specificity in synaptic nerve terminals such that it now appears that myosin IIB, rather than myosin Va, plays the predominant role.
Methods
SCGNs in culture
Wild-type (WT) and dilute-opisthotonus (dop) rats with a myosin Va null mutation (Futaki et al. 2000) were decapitated under diethylether anaesthesia on postnatal day 7 according to the guidelines of the Physiological Society of Japan. Their SCG neurones were isolated and maintained in culture for 5–6 weeks, as previously described (Mochida et al. 1994). Briefly, the SCGs were dissected free, desheathed, and incubated with collagenase (0.5 mg ml−1; Worthington Biochemical Corp., Lakewood, NJ, USA) in L-15 (Gibco Industries, Inc., Langley, OK, USA) at 37°C for about 10 min. Following enzyme treatment, the semi-dissociated ganglia were triturated gently through a small-pore glass pipette until a cloudy suspension was observed. After washing by low-speed centrifugation at 300 g for 3 min, the collected cells were plated onto coverslips in plastic dishes (Corning, New York, NY, USA; 35 mm diameter, approximately one ganglion per dish) containing 84% Eagle's minimal essential medium (Gibco Industries, Inc.), 10% fetal calf serum (Gibco Industries, Inc.), 1% penicillin/streptomycin (1: 1) (Gibco Industries, Inc.) and 25 ng ml−1 nerve growth factor (2.5 Svedburg, grade II; Alomone Laboratories, Ltd., Jerusalem, Israel). Cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2 and their media were changed twice a week.
Antibodies
Polyclonal anti-myosin Va antibodies were a gift from Dr R. E. Cheney, University of North Carolina (Espreafico et al. 1992; Rodriguez & Cheney, 2002). The polyclonal anti-myosin Vb antibody was raised against bacterial-expressed fusion proteins containing the globular tail domain of myosin Vb that was tagged with maltose-binding protein (MBP), and was affinity purified against the Glutathione S-transferase (GST)-tagged myosin Vb globular tail domain. This antibody recognized a ∼200 kDa band on Western blot and specifically stained neurones in the rat hippocampus (E. M. Espreafico, unpublished observation). Polyclonal anti-myosin IIA and IIB antibodies were raised against synthetic peptides having sequences specific to myosin IIA and IIB (Murakami et al. 1991). Monoclonal anti-myosin IIB antibody was purchased from Developmental Studies Hybridoma Bank (Iowa City, IA, USA). Monoclonal anti-Bassoon (Stressgen Biotechnologies Corp., Victoria, Canada) and anti-synaptophysin antibodies (Sigma Bio-Science, St Louis, MO, USA) were also obtained from commercial sources, as noted.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 30 min or acetone for 5 min. After being washed in PBS, their non-specific binding sites were blocked using 3% BSA–5% normal goat serum. The cells were then treated with primary antibodies overnight at 4°C. After several washes, they were incubated with FITC-conjugated anti-mouse IgG and biotinylated antirabbit IgG for 1 h, followed by Texas Red–streptavidin for 1 h. Labelled cells were examined with a Zeiss LSM 510 laser-scanning confocal microscope.
Expression of enhanced GFP-myosin Va and IIB in SCGNs
Expression vectors for enhanced GFP (EGFP)-myosin IIB and Va were constructed as follows. Two cDNA fragments of myosin IIB (3.6 and 2.4 kb), producing the full-length myosin IIB heavy chain, were generated by PCR from a human lung cDNA library (Clontech, Palo Alto, CA, USA) and were ligated into the pEGFP-C1 vector (Clontech) at the Kpn I and Sac II sites. The EGFP-myosin Va expression construct was generated by inserting the cDNA coding EGFP into the pCB6 vector containing a cDNA encoding the chicken myosin Va heavy chain (Espreafico et al. 1992) between the Kpn I sites at the 5′ end of myosin Va. These vector–DNA constructs were microinjected into the nuclei of SCGNs through a microglass-pipette, as previously described (Mochida et al. 2003). For in situ localization studies, SCGNs were fixed with acetone for 5 min at 48 h after injection.
Electron microscopy
Cells were fixed with 2.5% glutaraldehyde in 0.1 m phosphate buffer (PB) for 1 h. After being washed in PB, they were postfixed with 1% OsO4 in PB for 1 h and processed for electron microscopy. Ultrathin sections were prepared and examined with a JEOL 1210 electron microscope. For quantification of synaptic vesicles in the presynaptic nerve terminal, random electron micrographs were taken (typically ×10 000–20 000) and scanned into a computer. The area of the presynaptic terminal was measured using NIH image 1.61 (NIH, Bethesda, MD, USA), synaptic vesicles were counted (entered manually via keyboard), and the density of synaptic vesicles per unit area was calculated.
Preparation of myosin IIA and IIB fragments
The C-terminal 46 and 47 kDa fragments from myosin IIA and IIB heavy chains, respectively, were prepared as previously described (Murakami et al. 2000). Purified heavy chain fragments, stored at −70°C in 6 m urea, were dialysed overnight at 4°C against 0.6 m NaCl, 10 mm Tris-HCl at pH 7.5, 1 mm dithiothreitol, 1 mm EDTA and 0.1 mm EGTA, and then extensively against distilled water. The dialysates were lyophilized and reconstituted in microinjection buffer (see below) at a concentration of 188 μm. This procedure did not alter in vitro assembly and disassembly properties of the fragments (data not shown).
Recombinant myosin fragments were dissolved in 150 mm potassium acetate, 5 mm Mg2+-ATP, 10 mm Hepes, pH 7.3 and introduced into the presynaptic cell body by diffusion from a suction glass pipette (15–20 MΩ tip resistance) (Mochida et al. 1996). Fast Green FCF (5%, Sigma Chemical Co.) was included in the peptide injection solution to confirm their entry into the presynaptic cell body. The injection pipette was removed 2–3 min after starting the injection.
Synaptic transmission between SCGNs
Excitatory postsynaptic potentials (EPSPs) were recorded as previously described (Mochida et al. 1994). In brief, conventional intracellular recordings were made from two neighbouring neurones using microelectrodes filled with 1 m potassium acetate (70–90 MΩ). EPSPs were then recorded from one of the neurones, while action potentials were generated in the other neurone by passage of current through an intracellular recording electrode. Synaptic couples with subthreshold EPSPs that did not produce postsynaptic action potentials were selected for further study. Neurones were superfused with a modified Krebs solution containing 136 mm NaCl, 5.9 mm KCl, 5.1 mm CaCl2, 1.2 mm MgCl2, 11 mm glucose, and 3 mm Na-Hepes (pH 7.4), except for the recordings of paired-pulse facilitation, which took place in a Krebs solution containing 1 mm CaCl2. Electrophysiological data were collected and analysed using software written by the late Dr Ladislav Tauc (CNRS, France; Mochida et al. 1996) and analysed with Origin 7.0 (Microcal Software Inc., Northampton, MA, USA). Ca2+-independent transmitter release induced by puff application of 0.5 m sucrose was recorded with Clampex (Pclamp 8.1; Axon Instruments, Union City, CA, USA) and analysed with the Mini Analysis Program (Synaptosoft, Inc., Decatur, GA, USA).
Statistical analyses
Data are expressed as the means ±s.e.m. Student's unpaired t test (2-tailed) was applied to compare synaptic transmission between dop and WT synapses.
Results
Normal synaptic transmission in myosin Va null SCGNs in culture
We first examined the functional involvement of myosin Va in fast synapses in cultured SCGNs prepared from myosin Va null mutant (dop) and WT rats by monitoring their synaptic transmission. The incidence of synapse formation between dop SCGNs (35 days in culture) was 80% (16/20 neurone pairs). This value was very similar to the 74% (20/27 neurone pairs) incidence of synaptic connections between WT SCGNs. In addition, the strength of the synaptic coupling between dop neurones was similar to that of WT SCGNs; in both cases, presynaptic action potentials elicited postsynaptic action potentials in 50% of the synapses (dop SCGNs, 8/16 neurone pairs; WT SCGNs, 10/20 neurone pairs). Reciprocal (bi-directional) synaptic transmission was observed in 19% (3/16 neurone pairs) of dop and in 20% (4/20 neurone pairs) of WT SCGNs, respectively. These results suggest that normal synapses were formed between dop SCGNs in culture. The membrane electrical properties of dop SCGNs were also normal: the resting potential was −55 ± 0.8 mV (n = 16) for dop and −54 ± 0.8 mV (n = 16) for WT SCGNs (not significant, P = 0.35, Student's unpaired t test) and the action potential waveforms of dop SCGNs appeared to be similar to those of WT SCGNs (Fig. 1E and F). Thus, synapse formation and function appeared to be normal in the absence of myosin Va.
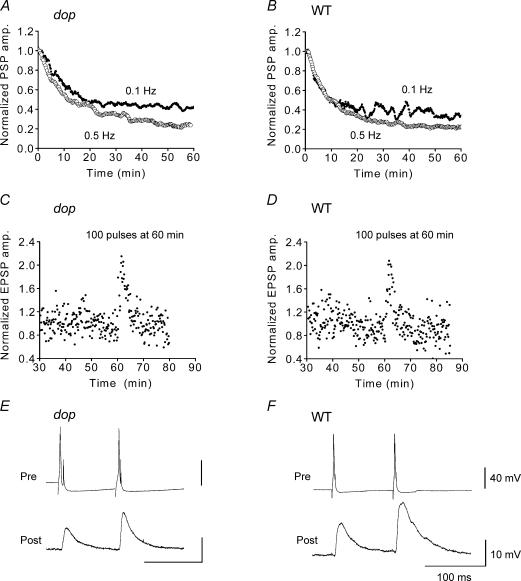
Figure 1. Synaptic transmission between cultured SCGNs from myosin Va null rats.
Synaptic transmission was examined by recording electrical postsynaptic responses in cultured superior cervical ganglion neurones (SCGNs) from wild-type (WT) and myosin Va null (dilute-opisthotonus; dop) rats. A–D, EPSP amplitudes recorded at dop (A and C) and WT synapses (B and D) were normalized and averaged. The resultant values were smoothed with a moving average algorithm and plotted against the recording time for A and B. Presynaptic neurones were stimulated at 0.1 Hz (•, A and B, n = 7; C, n = 5; D, n = 4) or 0.5 Hz (○; A, n = 5; B, n = 7) throughout the EPSP recordings. A tetanic stimulus of 10 Hz for 10 s was applied at 60 min for C and D. E and F, a paired-pulse facilitation recorded at dop (E) and WT SCGN synapses (F).
To further examine the putative role of myosin Va in synaptic vesicle trafficking, neurotransmission was monitored in the above neurones by recording EPSPs in synaptic couples that elicited subthreshold EPSPs for producing action potentials. We first examined whether synaptic depression was induced by high-frequency stimulation of presynaptic nerve terminals. Synaptic depletion is thought to be due to the depletion of synaptic vesicles from the readily releasable pool. If myosin Va participates in synaptic vesicle trafficking, synaptic depression should be stronger in dop SCGN synapses. We selected high stimulus frequencies, either 0.1 or 0.5 Hz, to examine synaptic depression in dop SCGNs. When presynaptic neurones were stimulated at 0.1 Hz, the EPSP amplitude decreased over 10–20 min and remained stable for 60 min during the stimulus train. At 30 min after the initial stimulus, the EPSP amplitude was 46 ± 9.8% (n = 7) of its initial value for dop synapses (Fig. 1A) and 39 ± 8.8% (n = 7) for WT synapses (Fig. 1B; not significant, P = 0.26, Student's unpaired t test). The rate of decrease was well fitted with a curve representing an exponential decay function, 9.0 ± 0.14 min for dop synapses (n = 7) and 6.2 ± 0.2 min for WT synapses (n = 7), suggesting that depletion of neurotransmitter with 0.1 Hz stimulation was not greater in the dop synapses than in WT synapses. When presynaptic terminals were stimulated at a frequency of 0.5 Hz, EPSP amplitude continually declined during the 60 min stimulus period. At 30 min after the initiation of stimulation, the EPSP amplitude was 36 ± 11% (n = 5) of the initial value for dop synapses (Fig. 1A) and 28 ± 5.1% (n = 7) for WT synapses (Fig. 1B; P = 0.25). This decrease was fitted with a curve representing a second order exponential decay, with time constants τ1= 5.6 ± 0.5 min, τ2= 44 ± 12 min for dop synapses and τ1= 6.9 ± 0.5 min, τ2= 46 ± 15 min for WT synapses (P = 0.26 and P = 0.53, respectively). These results suggest that activity-dependent depletion of synaptic vesicles from presynaptic nerve terminals was not significantly affected by the loss of myosin Va from dop SCGN synapses.
We next examined short-term plasticity that increases the efficacy of transmitter release, by analysing post-tetanic potentiation (PTP) and paired pulse facilitation (PPF). PTPs were elicited by tetanic stimulation of presynaptic terminals. If myosin Va is involved in synaptic vesicle trafficking, the PTP should be smaller in dop SCGN synapses. To monitor PTP, 100 stimuli were applied to dop SCGN synapses at 10 Hz. These tetanic stimuli were applied at 60 min after starting low-frequency (0.1 Hz) stimulation, to allow for the measurement of basal synaptic transmission. The maximum increase in EPSP amplitude produced by tetanic stimulation was 215 ± 28% (n = 5) of the basal level for dop synapses (Fig. 1C) and 207 ± 28% (n = 4) for WT synapses (Fig. 1D) (not significant, P = 0.64, Student's unpaired t test). Thus, the PTP was normal in dop SCGN synapses. When a synapse is activated twice within a brief period, the second response is larger than the first response; this phenomenon is referred to as the PPF. To elicit maximal PPF, pairs of presynaptic action potentials were elicited at 100 ms intervals in 1 mm external Ca2+ (Mochida et al. 1996). EPSP amplitudes in response to paired second pulses were increased to 173 ± 24% (n = 10) of the first control EPSP in dop synapses (Fig. 1E) and to 151 ± 16% (n = 5) in WT synapses (Fig. 1F; P = 0.55), suggesting that the PPF was not altered in the dop synapses. Taken together, these results suggest that the ability to produce short-term plasticity was normal in myosin Va null synapses.
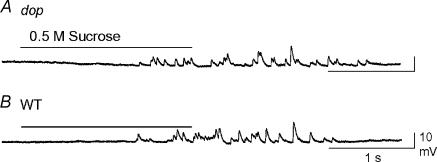
We also examined Ca2+-independent acetylcholine release in response to a hypertonic solution (Mochida et al. 1998) in dop (Fig. 2A) and WT SCGN synapses (Fig. 2B). Small EPSPs induced by focal application of 0.5 m sucrose were analysed in 16 synapses for each genotype. As summarized in Table 1, the amplitude, rise time, decay time and integral area of small hypertonic solution-induced EPSPs were not significantly different between dop and WT SCGN synapses. Spontaneous transmitter release was also observed sporadically in dop SCGN synapses in the absence of sucrose treatment (data not shown). The mean amplitude of the spontaneous postsynaptic responses observed in five SCGNs was 5.2 ± 1.9 mV. Collectively, these results indicate that the transmitter release machinery was intact in presynaptic terminals that were devoid of myosin Va.
Figure 2. Ca2+-independent transmitter release from dop and WT SCGNs in culture.
Ca2+-independent transmitter release was recorded by puff application of 0.5 m sucrose for 2 s at dop (A) and WT (B) SCGN synapses.
Table 1.
Response of dop and WT SCGN synapses to hypertonic sucrose
| Synapse | Number of experiments | Amplitude (mV) | Rise time (ms) | Decay time (ms) | Area (mV ms) |
|---|---|---|---|---|---|
| dop | 16 | 3.7 ± 0.4 | 7.4 ± 0.5 | 8.9 ± 1.2 | 58 ± 10 |
| WT | 16 | 3.5 ± 0.3 | 6.7 ± 0.2 | 8.4 ± 0.8 | 47 ± 6.5 |
| (P = 0.40) | (P = 0.24) | (P = 0.33) | (P = 0.35) |
Calcium-independent small excitatory postsynaptic potentials (sEPSPs) were induced by focal application of 0.5 m sucrose. Each value was evaluated using 4 or 5 recordings in each experiment, which were averaged (mean ±s.e.m.). No significant differences were observed between dop and WT synapses (P > 0.05, Student's unpaired t test).
Normal presynaptic terminals of dop SCGNs
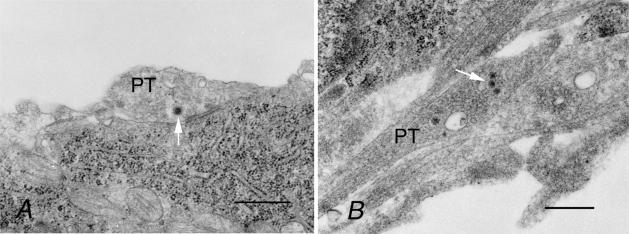
We examined the synapses formed between myosin Va null SCGNs in culture by electron microscopy. Our data showed that both dop and WT presynaptic boutons contained a large number of synaptic vesicles, the majority of which were round in shape, small in diameter (∼40–50 nm), and had clear centres (Fig. 3). Occasionally, dense-core vesicles (arrows in Fig. 3) were also observed in synaptic terminals. The number of synaptic vesicles was not significantly different between dop (91.2 ± 19.1 μm−2; mean ±s.e.m.; n = 11) and WT synapses (98.8 ± 15.3 μm−2; n = 9; P = 0.76, Student's unpaired t test), even though vesicle number varied between individual terminals from both genotypes. Thus, no perceptible difference in ultrastructural morphology was observed between dop and WT synapses. These findings are consistent with our demonstration of normal synaptic function in myosin Va null synapses by electrophysiology.
Figure 3. Electron micrographs of WT (A) and dop (B) SCGN synapses formed in culture.
Presynaptic terminals (PT) contained a large number of synaptic vesicles in both WT and dop SCGNs. Synaptic vesicles were generally small with clear interiors. Arrows indicate dense-core vesicles. Scale bars represent 500 nm.
Expression and localization of myosin isoforms in SCGNs
Following our functional and morphological assessment of myosin Va null synapses, we examined whether myosin Va was present in presynaptic nerve terminals of normal SCGNs and also investigated the distribution of myosin isoforms IIA, IIB and Vb in cultured SCGNs. We began by performing immunocytochemistry using isoform-specific antibodies. SCGNs that were maintained in culture for 5–6 weeks extruded numerous processes from their large, oval somata; there were two types of processes, one thick and short and the other long and thin (See Supplemental Fig. 1). Functional synapses are primarily formed between adjacent neurones and are predominantly axo-somatic in culture (Rees & Bunge, 1974; Mochida et al. 1994), while they are axo-dendritic or axo-spinotic in the SCG in vivo (Matthews, 1974; Kasa et al. 1991). We observed the presence of thin processes that tightly ensheathed the soma under both light microscopy (differential interference contrast microscope, DIC; Figs 4D and 5F and Supplemental Fig. 2) and electron microscopy (data not shown) and assessed synapse formation in cultured SCGNs using established neuronal markers. To identify presynaptic terminals, we stained the cells with anti-synaptophysin or anti-Bassoon antibodies; synaptophysin is a synaptic vesicle marker (Wiedenmann & Franke, 1985) and Bassoon is a component of the presynaptic cytomatrix at the active zone of conventional presynaptic nerve terminals (tom Dieck et al. 1998). Both of these components are important morphological markers of presynaptic terminals. Furthermore, both synaptophysin and Bassoon have been localized in presynaptic sites around the somata of cultured SCGNs (Takao-Rikitsu et al. 2004). Our results showed that Bassoon staining was restricted to thin process that tightly surrounded the soma and might be emitted from neighbouring neurones (see Supplemental Fig. 2). In contrast, synaptophysin staining was detected along the length of long processes as well as on the somata of the SCGNs (Figs 4B and C and 5B and C). Synaptophysin was reported to be highly concentrated in the presynaptic terminal and to also be present at lower concentrations in the soma, especially in the Golgi compartment of the neurone (Tixier-Vidal et al. 1988). GFP-synaptophysin was reported to be transported by endosomal compartments in cultured axons (Nakata et al. 1998). Taken together with our findings that Bassoon staining was not detected in long processes (Fig. 4E), these results suggest that the synaptophysin staining in long processes did not represent presynaptic sites but rather premature synaptic vesicles that were moving along the axons, as previously suggested by De Camilli et al. (2001). Thus, our data suggest that the punctate distribution of Bassoon and synaptophysin around the soma was indicative of the presence of synapses in SCGNs in culture.
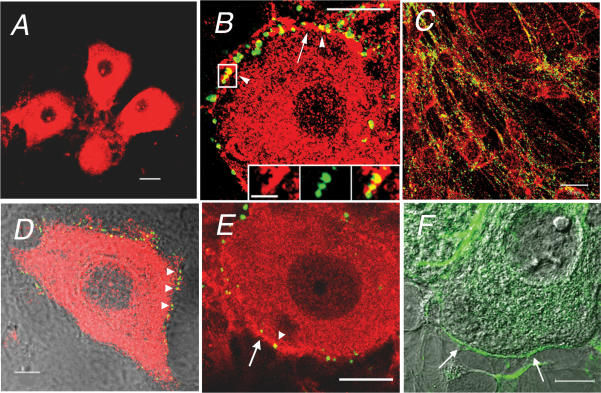
Figure 4. Distribution of myosin Va in cultured SCGNs.
Immunofluorescence of myosin Va in cultured SCGNs prepared from WT (A–E) and dop (F) rats. A, a low magnification image of myosin Va immunostaining. Arrows indicate thick processes of SCGNs. B and C, myosin Va (red) and synaptophysin (green) staining of the soma (B) and long processes (C). Arrows indicate presynaptic sites stained with the anti-synaptophysin antibody in B. No overlap of myosin Va and synaptophysin was observed around the somatic periphery, but overlap (yellow) was present on long processes of SCGNs in C. D, overlay of DIC (greyscale) and immunofluorescence images (red for myosin Va and green for Bassoon). Arrows indicate thin processes that ensheath the soma. Arrowheads indicate presynaptic sites stained with anti-Bassoon antibody. E, myosin Va (red) and Bassoon (green) staining of long processes. No processes stained positively for Bassoon. F, overlay of DIC, myosin Va (red) and synaptophysin (green) staining in a dop SCGN. Sacle bars represent 50 μm in A and 20 μm in B–F.
Figure 5. Distribution of myosin IIB in cultured SCGNs.
Immunofluorescence of myosin IIB in WT (A–D) and dop SCGNs (E and F). A, a low magnification image of myosin IIB immunostaining with polyclonal antibodies. B and C, myosin IIB (red) and synaptophysin (green) staining of the soma (B) and long processes (C). The arrow indicates intense myosin IIB staining around the soma. Arrowheads indicate overlap between myosin IIB and synaptophysin staining (yellow). The inset in B is the framed region shown at higher magnification; the image on the left is myosin IIB staining (red), the one in the middle is synaptophysin staining (green) and the one on the right is a merged image. Scale bar represents 5 μm. D, overlay of DIC (greyscale) and immunofluorescence (red for myosin IIB and green for Bassoon). Arrowheads indicate overlap of myosin IIB and Bassoon (yellow) staining. E, myosin IIB (red) and synaptophysin (green) in dop SCGNs. The arrow and arrowhead indicate intense myosin IIB staining around the soma and overlap between myosin IIB and synaptophysin (yellow), respectively. F, overlay of DIC (greyscale) and myosin IIB immunostaining with monoclonal antibodies (green). Intense staining was found in thin processes that ensheathed the soma (arrows). Scale bars represent 50 μm in A, 20 μm in B, C and E, and 10 μm in D and F.
Myosin Va antibodies intensely stained both the soma and processes of cultured SCGNs (Fig. 4A–E). The staining was diffuse in the somata and thick processes that extended from the somata (Fig. 4A), while it was punctate along the long processes (Fig. 4C and E). Unexpected findings were that double staining of myosin Va with anti-synaptophysin or anti-Bassoon antibodies failed to reveal any spatial overlaps around the somata (Fig. 4B and D). In contrast, there was overlap of myosin Va and synaptophysin staining along the long processes of the cells (Fig. 4C). It has been suggested that myosin Va plays a role in axonal transport in growing axons of cultured SCGNs, since myosin Va colocalized with synaptic vesicle protein SV2 (Bridgman, 1999); SV2 is a component of large rather than small vesicles that are typically found in mature fast synapses, suggesting that they may represent synaptic vesicle precursors (Evans et al. 1998). Taken together, these findings suggest that myosin Va was not present in the presynaptic nerve terminals in our cultured SCGNs. The distribution patterns of Bassoon and synaptophysin in dop SCGNs (Fig. 4F) were similar to those in WT SCGNs, suggesting that loss of myosin Va did not alter synapse formation; this finding was consistent with our electron microscopic observations.
Polyclonal anti-myosin IIB antibodies stained the cell bodies and processes of both WT and dop SCGNs (Fig. 5A–E). Myosin IIB staining was diffuse in the soma and thick processes of SCGNs, even along long processes (Fig. 5C), a pattern that was similar to that of myosin Va. However, at higher magnification, intense staining was frequently also noted in thin processes that ensheathed the somata in both WT and dop rats (Fig. 5B, D and E). Clearly, this staining overlapped with the punctate localization of synaptophysin or Bassoon (Fig. 5B, D and E). When we used mouse monoclonal anti-myosin IIB antibody that was raised against chicken brain myosin IIB, we confirmed that the more intense staining was restricted to the thin processes that ensheathed the soma in both WT and dop rats (Fig. 5F). Double staining for myosin Va and myosin IIB failed to reveal any overlap in thin processes that surrounded the soma of SCGNs (see Supplemental Fig. 3). Thus, myosin IIB appeared to be concentrated in presynaptic nerve terminals in both WT and dop SCGNs.
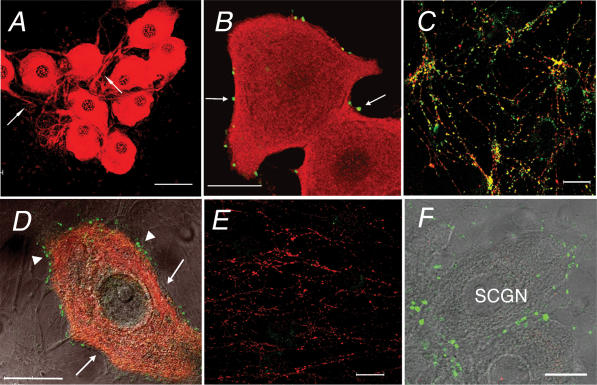
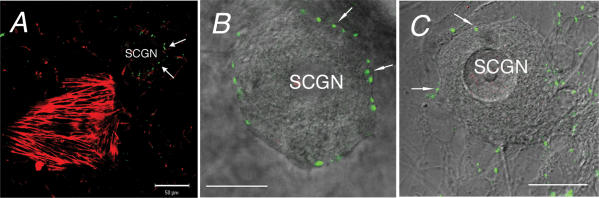
Anti-myosin IIA antibodies stained cells that were multishaped and contained filaments, especially stress fibres, thus suggesting that they were non-neuronal cells (Fig. 6A). Under the same conditions, these antibodies did not stain SCGNs from either WT (Fig. 6A) or dop rats (data not shown). Anti-myosin Vb antibodies did not stain presynaptic terminals, somata or processes of SCGNs from WT rats (Fig. 6B) and dop rats (Fig. 6C).
Figure 6. Myosin IIA and Vb immunofluorescence in cultured SCGNs.
A, myosin IIA (red) and synaptophysin (green) in WT SCGNs. Arrows indicate presynaptic sites around the soma. B and C, overlay of DIC, myosin Vb (red) and Bassoon (green) in B or synaptophysin (green) in C in WT (B) and dop SCGNs (C). Scale bars represent 50 μm in A, and 20 μm in B and C.
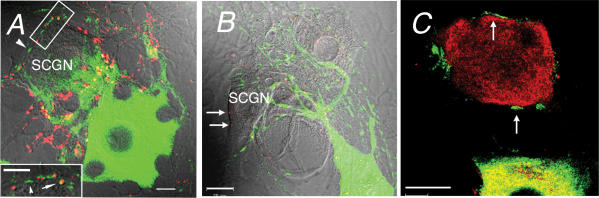
To confirm the differential distribution of myosin IIB and Va in presynaptic terminals, we introduced EGFP-fusion myosin IIB or Va heavy chains into individual cultured SCGNs. Between 24 and 48 h after injection, the EGFP-fusion proteins were detected in somata and processes (Fig. 7A–C). When the neurones expressing the EGFP-fusion protein for myosin Va were stained with anti-myosin Va antibodies, the EGFP signals completely overlapped with antibody labelling (see Supplemental Fig. 4), demonstrating that the distribution of the EGFP-myosin Va fusion protein represented the location of the endogenous protein. This was also true for EGFP-myosin IIB (data not shown). The EGFP-myosin IIB protein was found in both short and long processes of the SCGNs (Fig. 7A). Thin processes were often found to ensheath the soma of nearby SCGNs, suggesting that myosin IIB was targeted to axon terminals (inset in Fig. 7A); EGFP-myosin Va was not detected in these processes (arrows in Fig. 7B). Staining with anti-myosin Va antibodies failed to reveal the presence of myosin Va in the thin processes containing EGFP-myosin IIB (Fig. 7C). These results strongly support the contention that myosin IIB, but not Va, is localized in presynaptic nerve terminals of SCGNs.
Figure 7. Expression of EGFP-fusion proteins with myosin IIB and Va in cultured SCGNs.
A, overlay of EGFP-myosin IIB (green), synaptophysin immunostaining (red) and DIC images. The inset is the framed region shown at higher magnification. Arrowheads indicate thin processes containing EGFP-myosin IIB around the soma. Presynaptic sites that stained with the anti-synaptophysin antibody are located around the soma (red) and overlapped with EGFP-myosin IIB staining (arrow, yellow). Scale bar represents 10 μm. B, overlay of EGFP-myosin Va (green), Bassoon immunostaining (red) and DIC images. A projection image was constructed from an optical section series through the full thickness of an SCGN expressing EGFP-myosin Va. Arrows indicate presynaptic sites (Bassoon) around the soma. C, overlay of EGFP-myosin IIB (green) and myosin Va immunostaining (red). Arrows indicate thin processes containing EGFP-myosin IIB around the soma. Scale bars represent 20 μm.
Function of myosin II isoforms on neurotransmitter release
Finally, to determine a functional role of myosin II isoforms in presynaptic nerve terminals of SCGNs, we introduced C-terminal fragments from myosin IIA or IIB heavy chain (Murakami et al. 2000) in cultured SCGNs from WT rats. These fragments were predicted to perturb function of endogenous myosin II by forming cofilaments with native filaments (Burns et al. 1995; S. Mochida & N. Murakami, unpublished observations). First we examined the effect of 47 kDa myosin IIB fragments (MIIBF) on synaptic transmission in cultured SCGNs from WT rats. After recording control EPSPs every 20 s for more than 30 min, MIIBF was microinjected into presynaptic neurones from a suction pipette (94 μm in the pipette, for 2–3 min). MIIBF gradually decreased the amplitude of evoked EPSPs without affecting the EPSP time course (Figs 8A and B). At 55 min after the start of MIIBF injection, the mean EPSP amplitude was reduced by 28 ± 8.0% (n = 4; Fig. 8C). These results suggest a role for myosin IIB in the maintenance of normal transmitter release from SCGNs. In contrast, introduction of 46 kDa fragments of myosin IIA (MIIAF) produced no significant reduction in synaptic transmission (Fig. 8B). At 55 min after the start of MIIAF injection, the reduction in mean EPSP amplitude was 3.0 ± 8.3% (n = 5; Fig. 8C). This reduction is significantly smaller than the reduction for MIIBF (P < 0.01, Student's unpaired t test, MIIAF versus MIIBF). Thus, myosin IIA does not appear to participate in transmitter release, which is consistent with the absence of immunocytochemical staining for this isoform in cultured SCGNs.
Figure 8. Effects of myosin II fragments on neurotransmitter release.
Myosin II heavy chain fragments, MIIAF or MIIBF, were injected into presynaptic neurones at time 0, at a concentration in the injection pipette of 94 μm. Presynaptic neurones were stimulated at 0.05 Hz. A shows EPSPs from one representative experiment (a) and the first order derivative representing the rate of rise and fall for EPSPs (b). Black line represents control before injection; blue line is at 55 min after injection. The myosin IIB fragment, MIIBF was injected. B, normalized EPSP amplitudes are averaged. The resultant values are smoothed with a moving average algorithm and plotted against recording time. MIIAF (○, n = 5) or MIIBF (•, n = 4) were injected. C, bar graph illustrating the decrease in EPSP amplitude at 55 min after injection of MIIAF and MIIBF (*P < 0.01, Student's unpaired t test, MIIBF versus MIIAF).
Discussion
In previous studies that involved the use of cultured SCG neurones, myosin IIB was shown to be highly concentrated in the peripheral and marginal zone of growth cones, suggesting that it participated in growth cone motility (Rochlin et al. 1995). Myosin Va was also reported to be present in the growth cones of these cells (Evans et al. 1997). However, comparative localization of myosin II and V isoforms in presynaptic nerve terminals has not been examined previously.
Our data showed that both endogenous and EGFP-tagged myosin Va were present throughout the cell soma and within distal processes of cultured SCGNs, but were notably absent from presynaptic nerve terminals (Fig. 5). We used anti-myosin Va antibodies that were raised against the peptide that was encoded by the tail region of chicken brain myosin Va. In fact, myosin Va differs somewhat between chickens and rats in terms of its amino acid sequence, even though its globular tail domain shows 98% sequence identity in these species (Reck-Peterson et al. 2000). The chicken myosin Va antibodies were reported to be able to detect the cellular distribution of myosin Va in the rat brain (Calliari et al. 2002; Casaletti et al. 2003; Tilelli et al. 2003; Sotelo-Silveira et al. 2004). EGFP-myosin Va encodes chicken brain myosin Va heavy chain and was used to express the fusion protein in cultured rat SCGNs. The expression pattern of EGFP-tagged myosin Va was quite similar to that found for endogenous myosin Va protein that was labelled with anti-myosin Va antibodies. When we stained the cells expressing EGFP-tagged myosin Va with anti-myosin Va antibodies, we found that the staining patterns completely overlapped (Supplemental Fig. 4). These findings suggest that both of these techniques successfully localized the distribution of myosin Va in somata and long processes, but not in presynaptic terminals, in our cultured SCGNs.
Our findings are consistent with the reported immuno-electron microscopic observation that myosin Va was present in postsynaptic terminals, but not presynaptic boutons, of cerebellar parallel fibre synapses (Petralia et al. 2001). However, in growing SCGN neurites in culture, endogenous and EGFP-tagged myosin Va was reported to colocalize with SV2 (Bridgman, 1999). Double labelling of brain microsomal fractions with anti-SV2 and anti-myosin Va antibodies revealed that the majority of vesicles containing both myosin Va and SV2 were much larger (the average size: 90 ± 45 nm) than smaller synaptic vesicles (∼50 nm) that mostly lacked myosin Va (Evans et al. 1998). This finding suggested that myosin Va does not regulate small synaptic vesicle trafficking at nerve terminals, but may instead be involved in large dense-core or constitutive vesicle trafficking. We observed that the majority of synaptic vesicles were small (40–50 nm) and clear cored in axo-somatic synapses formed between SCGNs in culture (Fig. 3); thus, specific effects on large dense-core vesicle trafficking would probably have remained undetected in our study.
In contrast, we found that both endogenous and recombinant myosin IIB was highly concentrated in presynaptic nerve terminals of SCGNs in culture (Fig. 5). Myosin IIB is known to be restricted to the marginal and peripheral actin-rich regions of growth cones (Cheng et al. 1992; Rochlin et al. 1995), suggesting that it functions in growth cone motility and neurite outgrowth. Myosin IIB mRNA was also shown to increase during neurite outgrowth in cultured neurones, suggesting that myosin IIB is required for driving neuritic processes (Wylie et al. 1998). However, studies in myosin IIB null mutant mice suggested that myosin IIB might play a role in brain development, since these animals exhibited hydrocephalus; furthermore, mice with a point mutation in their myosin IIB gene showed impaired neuronal migration in distinct areas of the brain (Tullio et al. 2001; Ma et al. 2004). Our immunohistochemical analyses revealed intense staining for myosin IIB in thin processes that ensheathed somata (Fig. 5B and F). However, EGFP-tagged myosin IIB was found to be expressed broadly throughout neuronal cell bodies and processes, including the above-mentioned thin processes. In our study, we used myosin IIB cDNA from a lung cDNA library to express EGFP-tagged proteins in cultured SCGNs. Neuronal isoforms of myosin IIB have inserts (IIB1 and IIB2) as a result of alternative splicing, and either or both are expressed in embryonic and adult brains. However, rat SCG tissues were reported to express markedly low levels of myosin IIB with inserts (Itoh & Adelstein, 1995). We expect that the majority of the myosin IIB isoform that was endogenously expressed in our cultured SCGNs lacked inserts, like the isoform expressed in the lung. Therefore, we believe that the use of myosin IIB cDNA from lung was appropriate in our study. Furthermore, the distribution pattern of myosin IIB EGFP-signals was similar to that seen by immunostaining using the antibody raised against brain myosin IIB. Thus, we conclude that EGFP-tagged as well as endogenous myosin IIB was located in the presynaptic nerve terminals of our cultured SCGNs. Our electrophysiological analyses also suggest that myosin IIB might be important in the regulation of transmitter release from cultured SCGNs, since myosin IIB fragments reduced synaptic transmission (Fig. 8). Taken together, these data suggest that myosin IIB is involved in vesicle trafficking at presynaptic nerve terminals.
Myosin IIA has not been detected in SCGNs under conditions in which non-neuronal cells were stained. Rochlin et al. (1995) detected myosin IIA throughout the soma and growth cones of cultured SCGNs, but the staining intensity was relatively weak and required the use of a sensitive CCD camera. We used myosin IIA antibodies raised against human macrophage myosin IIA heavy chain (Murakami et al. 1991). Rat myosin IIA heavy chain differs from that in the humans by three amino acids. We previously reported that there were no significant differences in the reactivity of human, bovine, rat and mouse myosin IIA heavy chain (Murakami et al. 1991, 1993; Murakami & Elzinga, 1992; Nikol et al. 1997). Our antibody certainly stained cultured non-neuronal cells that were derived from rat SCG tissues. Furthermore, it was reported that the antibody for human myosin IIA yielded immunoblots and immunofluorescence that was indistinguishable from that obtained using anti-rat myosin IIA antibody (Rochlin et al. 1995). In addition, our electrophysiological study demonstrated that the injection of myosin IIA fragments did not result in a significant reduction in synaptic transmission (Fig. 8). Based on these data, we suggest that myosin IIA in presynaptic terminals of SCGNs is probably expressed at much lower levels than myosin IIB, and does not function in synaptic transmission.
Our electrophysiological studies revealed that myosin Va was not essential for synaptic vesicle trafficking in SCGNs, since synaptic transmission was normal in myosin Va null rats. This finding is consistent with that of Schnell & Nicoll (2001), who demonstrated that transmission in hippocampal CA3–CA1 synapses in myosin Va null mice was normal. We also observed that basal synaptic transmission was normal in dop nerve terminals. However, this finding contradicts the report of Trinchese et al. (2003), who showed that basal synaptic transmission was reduced in cultured hippocampal neurones from myosin Va null mice. This discrepancy might have been due to the fact that myosin Va is present in the presynaptic nerve terminals of hippocampal neurones (Trinchese et al. 2003) but not in those of cultured SCGNs. The assumption is that myosin Va is essential for some aspect of transmitter release from hippocampal neurones. Our data suggest the possibility that other myosin V isoforms compensate for the absence of myosin Va in SCGN nerve terminals. This notion is supported by the fact that myosin Vb was not expressed at significant levels in presynaptic nerve terminals from either normal (Fig. 6B) or mutant SCGNs (Fig. 6C). Furthermore, brain expression of myosin Vb was found to be restricted to areas such as the hippocampus, and there was no compensatory increase in expression in myosin Va null mice (Zhao et al. 1996). Finally, the myosin Vc isoform was reported to be particularly abundant in epithelial and glandular tissues and not in the brain (Rodriguez & Cheney, 2002), and is therefore perhaps an unlikely substitute for myosin Va. All of the above notwithstanding, we are not able to rule out the minor possibility that a different class of myosin may functionally compensate for the loss of myosin Va in dop rats.
Class V myosin isoforms have been proposed as strong candidates for synaptic vesicle transport motors in nerve terminals. However, based on our direct assessment of myosin Va expression and function in dop and wild-type neurones in the context of our broader examination of myosin II and V isoforms, we are forced to conclude that myosin Vs are not involved in synaptic vesicle trafficking at the cholinergic synapse in cultured SCGNs. Alternatively, we have found that myosin IIB plays such a role. Our extended work demonstrates that myosin IIB appears to regulate mobilization of synaptic vesicles from the reserve pool to the readily releasable pool in presynaptic nerve terminals of cultured SCG neurones (S. Mochida & N. Murakami, unpublished observations). Thus, our present study proposes the idea that expression of myosin isoform and its function in presynaptic terminals differs with type of neurones and properties of neurotransmitter release.
Acknowledgments
We thank Dr Y. Hayashi for constructing the EGFP-tagged myosin Va cDNA, Ms S. R. Banzi for producing the myosin Vb antibodies and Drs G. J. Augustine and C. T. Yokoyama for reading the manuscript. This work was supported by the Nagoya University Foundation (to Y.T.), Fundação de Amparo à Pesquisa do Estado de São Paulo, and Conselho Nacional de Desenvolvimento Cient'fico e Tecnológico (to E.M.E.), the New York State Office of Mental Retardation and Developmental Disabilities (to N.M.) and a Grant-in-Aid for Scientific Research on Priority Areas (A), for Scientific Research (B), the Toyota Fund (to S.M.).
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2005.095943
jp.physoc.org/cgi/content/full/jphysiol.2005.095943/DC1 and contains supplemental material consisting of four figures.
This material can also be found as part of the full-text HTML version available from www.blackwell-synergy.com
References
- Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CG, Reedy M, Heuser J, De Lozanne A. Expression of light meromyosin in Dictyostelium blocks normal myosin II function. J Cell Biol. 1995;130:605–612. doi: 10.1083/jcb.130.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calliari A, Sotelo-Silveira J, Costa MC, Nogueira J, Cameron LC, Kun A, Benech J, Sotelo JR. Myosin Va is locally synthesized following nerve injury. Cell Motil Cytoskeleton. 2002;51:169–176. doi: 10.1002/cm.10017. [DOI] [PubMed] [Google Scholar]
- Casaletti L, Tauhata SB, Moreira JE, Larson RE. Myosin-Va proteolysis by Ca2+/calpain in depolarized nerve endings from rat brain. Biochem Biophys Res Commun. 2003;308:159–164. doi: 10.1016/s0006-291x(03)01350-0. [DOI] [PubMed] [Google Scholar]
- Cheng TP, Murakami N, Elzinga M. Localization of myosin IIB at the leading edge of growth cones from rat dorsal root ganglionic cells. FEBS Lett. 1992;311:91–94. doi: 10.1016/0014-5793(92)81374-u. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Haucke V, Takei KEM. The structure of synapses. In: Cowan WM, Sudhof TC, Stevens CF, editors. Synapses. Baltimore: Johns Hopkins University Press; 2001. pp. 89–133. [Google Scholar]
- Espreafico EM, Cheney RE, Matteoli M, Nascimento AA, De Camilli PV, Larson RE, Mooseker MS. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J Cell Biol. 1992;119:1541–1557. doi: 10.1083/jcb.119.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LL, Hammer J, Bridgman PC. Subcellular localization of myosin V in nerve growth cones and outgrowth from dilute-lethal neurons. J Cell Sci. 1997;110:439–449. doi: 10.1242/jcs.110.4.439. [DOI] [PubMed] [Google Scholar]
- Evans LL, Lee AJ, Bridgman PC, Mooseker MS. Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J Cell Sci. 1998;111:2055–2066. doi: 10.1242/jcs.111.14.2055. [DOI] [PubMed] [Google Scholar]
- Futaki S, Takagishi Y, Hayashi Y, Ohmori S, Kanou Y, Inouye M, Seo H, Iwaikawa Y, Murata Y. Identification of a novel myosin-Va mutation in an ataxic mutant rat, dilute-opisthotonus. Mamm Genome. 2000;11:649–655. doi: 10.1007/s003350010121. [DOI] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Itoh K, Adelstein RS. Neuronal cell expression of inserted isoforms of vertebrate nonmuscle myosin heavy chain II-B. J Biol Chem. 1995;270:14533–14540. doi: 10.1074/jbc.270.24.14533. [DOI] [PubMed] [Google Scholar]
- Kasa P, Dobo E, Wolff JR. Cholinergic innervation of the mouse superior cervical ganglion: light- and electron-microscopic immunocytochemistry for choline acetyltransferase. Cell Tissue Res. 1991;265:151–158. doi: 10.1007/BF00318149. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Adelstein RS. Chicken nonmuscle myosin heavy chains: differential expression of two mRNAs and evidence for two different polypeptides. J Cell Biol. 1991;112:915–924. doi: 10.1083/jcb.112.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford GM, Molyneaux BJ. Myosin V in the brain: mutations lead to neurological defects. Brain Res Rev. 1998;28:1–8. doi: 10.1016/s0165-0173(98)00020-4. [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bahler M, Goldenring JR. Myosin Vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Kawamoto S, Hara Y, Adelstein RS. A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons. Mol Biol Cell. 2004;15:2568–2579. doi: 10.1091/mbc.E03-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR. Ultrastructure of ganglionic junctions. In: Hubbard JJ, editor. The Peripheral Nervous System. New York: Plenum Press; 1974. pp. 111–150. [Google Scholar]
- Mercer JA, Seperack PK, Strobel MC, Copeland NG, Jenkins NA. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Mochida S, Kobayashi H, Matsuda Y, Yuda Y, Muramoto K, Nonomura Y. Myosin II is involved in transmitter release at synapses formed between rat sympathetic neurons in culture. Neuron. 1994;13:1131–1142. doi: 10.1016/0896-6273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-class Ca2+ channels. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Mochida S, Westenbroek RE, Yokoyama CT, Itoh K, Catterall WA. Subtype-selective reconstitution of synaptic transmission in sympathetic ganglion neurons by expression of exogenous calcium channels. Proc Natl Acad Sci U S A. 2003;100:2819–2824. doi: 10.1073/pnas.262787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Yokoyama CT, Kim DK, Itoh K, Catterall WA. Evidence for a voltage-dependent enhancement of neurotransmitter release mediated via the synaptic protein interaction site of N-type Ca2+ channels. Proc Natl Acad Sci U S A. 1998;95:14523–14528. doi: 10.1073/pnas.95.24.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Elzinga M. Immunohistochemical studies on the distribution of cellular myosin II isoforms in brain and aorta. Cell Motil Cytoskeleton. 1992;22:281–295. doi: 10.1002/cm.970220408. [DOI] [PubMed] [Google Scholar]
- Murakami N, Kotula L, Hwang YW. Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry. 2000;19:11441–11451. doi: 10.1021/bi000347e. [DOI] [PubMed] [Google Scholar]
- Murakami N, Mehta P, Elzinga M. Studies on the distribution of cellular myosin with antibodies to isoform-specific synthetic peptides. FEBS Lett. 1991;278:23–25. doi: 10.1016/0014-5793(91)80074-d. [DOI] [PubMed] [Google Scholar]
- Murakami N, Trenkner E, Elzinga M. Changes in expression of nonmuscle myosin heavy chain isoforms during muscle and nonmuscle tissue development. Dev Biol. 1993;157:19–27. doi: 10.1006/dbio.1993.1108. [DOI] [PubMed] [Google Scholar]
- Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J Cell Biol. 1998;140:659–674. doi: 10.1083/jcb.140.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol S, Murakami N, Pickering JG, Kearney M, Leclerc G, Hofling B, Isner JM, Weir L. Differential expression of nonmuscle myosin II isoforms in human atherosclerotic plaque. Atherosclerosis. 1997;130:71–85. doi: 10.1016/s0021-9150(96)06047-9. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Sans N, Worley PF, Hammer IJ, Wenthold RJ. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. Eur J Neurosci. 2001;135:1722–1732. doi: 10.1046/j.0953-816x.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Terrian DM. Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex. J Cell Biol. 1997;137:1589–1601. doi: 10.1083/jcb.137.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Provance DW, Jr, Mooseker MS, Mercer JA. Class V myosins. Biochim Biophys Acta. 2000;1496:36–51. doi: 10.1016/s0167-4889(00)00007-0. [DOI] [PubMed] [Google Scholar]
- Rees R, Bunge RP. Morphological and cytochemical studies of synapses formed in culture between isolated rat superior cervical ganglion neurons. J Comp Neurol. 1974;157:1–11. doi: 10.1002/cne.901570102. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Cheney RE. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J Cell Sci. 2002;115:991–1004. doi: 10.1242/jcs.115.5.991. [DOI] [PubMed] [Google Scholar]
- Rosé DR, Lejen T, Casaletti L, Larson RE, Pene TD, Trifaró JM. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J Neurochem. 2003;85:287–298. doi: 10.1046/j.1471-4159.2003.01649.x. [DOI] [PubMed] [Google Scholar]
- Schnell E, Nicoll RA. Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J Neurophysiol. 2001;85:1498–1501. doi: 10.1152/jn.2001.85.4.1498. [DOI] [PubMed] [Google Scholar]
- Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Sotelo-Silveira JR, Calliari A, Cardenas M, Koenig E, Sotelo JR. Myosin Va and kinesin II motor proteins are concentrated in ribosomal domains (periaxoplasmic ribosomal plaques) of myelinated axons. J Neurobiol. 2004;60:187–196. doi: 10.1002/neu.20015. [DOI] [PubMed] [Google Scholar]
- Suter DM, Espindola FS, Lin CH, Forscher P, Mooseker MS. Localization of unconventional myosins V and VI in neuronal growth cones. J Neurobiol. 2000;42:370–382. [PubMed] [Google Scholar]
- Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, Takai Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J Cell Biol. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilelli CQ, Martins AR, Larson RE, Garcia-Cairasco N. Immunohistochemical localization of myosin Va in the adult rat brain. Neuroscience. 2003;121:573–586. doi: 10.1016/s0306-4522(03)00546-3. [DOI] [PubMed] [Google Scholar]
- Tixier-Vidal A, Faivre-Bauman A, Picart R, Wiedenmann B. Immunoelectron microscopic localization of synaptophysin in a Golgi subcompartment of developing hypothalamic neurons. Neuroscience. 1988;26:847–861. doi: 10.1016/0306-4522(88)90104-2. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Rao M, Peterhoff C, Kumar A, Liu S, Nixon R, Arancio O. Myosin Va is required for neurotransmitter release during basal synaptic transmission and synaptic plasticity. 43rd Annual Meeting for Amer Soc for Cell Biol; 2003. Presentation no. 998. [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wylie SR, Wu PJ, Patel H, Chantler PD. A conventional myosin motor drives neurite outgrowth. Proc Natl Acad Sci U S A. 1998;95:12967–12972. doi: 10.1073/pnas.95.22.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LP, Koslovsky JS, Reinhard J, Bahler M, Witt AE, Provance DW, Jr, Mercer JA. Cloning and characterization of myr 6, an unconventional myosin of the dilute/myosin-V family. Proc Natl Acad Sci U S A. 1996;93:10826–10831. doi: 10.1073/pnas.93.20.10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.