Abstract
Reports differ as to whether reconstitution of telomerase activity alone is sufficient for immortalization of different types of human somatic cells or whether additional activities encoded by other “immortalizing” genes are also required. Here we show that ectopic expression of either the catalytic subunit of human telomerase (hTERT) or a temperature-sensitive mutant (U19tsA58) of simian virus 40 large-tumor antigen alone was not sufficient for immortalization of freshly isolated normal adult human mammary fibroblasts and endothelial cells. However, a combination of both genes resulted in the efficient generation of immortal cell lines irrespective of the order in which they were introduced or whether they were introduced early or late in the normal proliferative lifespan of the cultures. The order and timing of transduction, however, did influence genomic stability. Karyotype analysis indicated that introduction of both transgenes at early passage, with hTERT first, yielded diploid cell lines. Temperature-shift experiments revealed that maintenance of the immortalized state depended on continued expression of functional U19tsA58 large-tumor antigen, with hTERT alone unable to maintain growth at nonpermissive temperatures for U19tsA58 large-tumor antigen. Such conditional diploid lines may provide a useful resource for both cell engineering and for studies on immortalization and in vitro transformation.
Normal human somatic cells undergo a limited number of divisions before entering an irreversible growth-arrest state defined as senescence (1). This proliferative lifespan has been correlated with the age of the donor, although more recently it has been suggested that the disease status of the donor rather than age may be more significant (2). Most of this work has been done with fibroblasts, although other somatic cells such as epithelial or endothelial cells also have a finite mitotic lifespan. The lifespan varies between different cell types and also depends on culture conditions. Senescent cells can be maintained in a viable state for long periods but cannot be induced to divide by normal mitogenic stimuli (1, 3). However, overriding normal cell-cycle checkpoints with some viral oncogenes can result in an extension of growth potential beyond normal senescence with most cells finally terminating in a “crisis” phase in which abortive or abnormal mitosis continues to occur, leading to progressive cell death (4).
The acquisition of unlimited proliferative potential defined as true immortality is probably a critical step during tumorigenesis (5). Much work has been done to identify the underlying mechanisms that regulate the loss of replicative potential and how they are abrogated when cells become immortal either as the result of tumorigenesis in vivo or by in vitro manipulation of normal cells to generate cell lines (6). Two components regulate the finite lifespan: a mitotic “clock” that counts the number of cell divisions and the entry into the postmitotic state (7).
In human cells the progressive shortening of the telomeres with each cell division has been proposed as the mitotic clock (8). Telomeres are short repetitive DNA sequences at the ends of chromosomes synthesized by telomerase (9, 10). When adult somatic cells divide, their telomeres shorten by about 50 bp per division (11), because the cells do not contain functional telomerase. Telomerase is a multicomponent enzyme comprising a template RNA plus an essential catalytic subunit of human telomerase (hTERT) (12–18) which is absent from adult somatic cells. Consequently, functional telomerase activity can be reconstituted by ectopic expression of hTERT (18–20).
It has been proposed, mainly on the basis of experiments with fibroblasts, that reconstitution of telomerase activity alone is sufficient for immortalization of human somatic cells (19–22). Others have found that functional telomerase alone is not sufficient (23–25). Thus, Counter and colleagues (24, 25) used hTERT and simian virus 40 (SV40) large-tumor (LT) antigen to immortalize human fibroblasts and embryonic kidney cells before their transformation by ras. Kiyono and colleagues (23) have found that immortalization of neonatal keratinocytes and adult mammary epithelial cells required inactivation of the pRB/p16 INK4 pathway as well as functional telomerase. Thus, studies with human cells seem to be subject to variations that can result in apparent discrepancies between different laboratories and experiments.
To clarify the issue of whether reconstitution of telomerase activity alone is sufficient for immortalization of human somatic cells or whether additional activities encoded by “immortalizing” genes are required, we report here a systematic study using amphotropic retroviruses that transduce either hTERT (12–18) or a temperature-sensitive (ts) mutant (U19tsA58) of SV40 LT antigen (26, 27). These genes were transduced either singly or in combination in different orders and at different times in the finite lifespan of freshly isolated adult human mammary fibroblasts and endothelial cells. These cell types were chosen because U19tsA58 LT alone does not result in their immortalization, unlike human breast epithelial cells and embryonic fibroblasts in which immortal cell lines can arise at a low frequency from crisis cultures (27–30). Neither hTERT nor U19tsA58 LT antigen alone was sufficient for immortalization of either mammary fibroblasts or endothelial cells. A combination of both genes, in whatever order and at both early and late passage, however, resulted in their high-frequency immortalization. Importantly, temperature-shift experiments showed that in such lines, functional telomerase alone was unable to maintain the immortal state, which remained dependent on functional LT antigen.
Materials and Methods
Generation of Amphotropic Viruses.
A packaging cell clone producing helper-free amphotropic murine-leukemia-virus particles containing the Babe hygro-hTERT vector genome was obtained by using a packaging cell line, TEFLY-A, derived from human rhabdomyosarcoma TE671 cells containing CeB and AF plasmids coding for vector core and envelope proteins, respectively (31). pBabe-hygro-hTERT was constructed by inserting the EcoRI-to-SalI fragment containing the full-length hTERT cDNA from pCI-Neo-hTERT (18) into the EcoRI–SalI sites of pBabe-hygro (32). This plasmid was transfected into TEFLY-RD cells (a packaging cell line producing envelope proteins derived from feline endogenous retrovirus RD114) by using calcium phosphate. Cell supernatant was harvested 48 h later and plated on TEFLY-A cells. TEFLY-A colonies transduced with pBabe-hygro-hTERT, and therefore resistant to hygromycin B, were isolated and expanded, and a clone producing high-titer full-length pBabe-hygro-hTERT was selected.
Establishment and Transduction of Primary Cell Cultures.
Mammary fibroblasts and microvascular endothelial cultures were prepared from normal breast tissue obtained with consent from patients undergoing cosmetic surgery. Interlobular fibroblasts were set up as described (33) and maintained with DMEM, supplemented with 10% (vol/vol) FCS and antibiotics. Microvascular endothelial cells were isolated immunomagnetically by using the QBEND-40 mouse monoclonal antibody against thrombomodulin (34). Endothelial cells were cultured in microvascular endothelial growth medium (EGM-2; BioWhittaker). Unless specified otherwise, cultures were gassed with 5% CO2 in air. Midconfluence proliferating cultures were exposed to filtered (0.4 μm) supernatants from the retroviral packaging lines for 18 h with 8 μg/ml polybrene and grown to confluency (4–7 days). They were then selected with either 0.5 mg/ml G418 or 25 μg/ml hygromycin B until all untransduced cells had been killed (<14 days). Cultures transduced with tsLT either singly or in combination with hTERT were grown at 33.5°C; cultures transduced with hTERT only and untransduced cells were grown at 36.5°C. Temperature-arrest experiments were carried out at 39.5°C (±0.2°C). Growth measurements were carried out by replicate hemocytometer counting of triplicate cultures harvested when the fastest growing cells had nearly reached confluence.
Detection of Telomerase Activity by Telomerase-Detection Assay (TRAPeze).
Telomerase activity was assayed by the standard TRAPeze protocol by using 20 and 200 ng of cell extract and 200 ng of cell extract in which telomerase was heat inactivated at 85°C for 10 min before analysis. Radiolabeled forward TS primer (5′-AATCCGTCGAGCAGAGTT-3′), cell extracts, and PCR reagents were incubated at room temperature for 20 min then subjected to 30 PCR cycles of 95°C for 30 sec and 59°C for 30 sec. The resultant PCR products were separated on 10% 19:1 acrylamide/bisacrylamide gels and bands were detected on a Bio-Rad PhosphorImager using QUANTITY ONE software.
Telomere Length Measurement by Using the Terminal-Restriction Fragmentation (TRF) Methodology.
Cultured cells (106) were resuspended in 1% (wt/vol) low-melting-point agarose and incubated overnight at 50°C with Proteinase K before digestion of DNA with HinfI. DNA fragments were separated by pulse-field gel electrophoresis, transferred to Hybond N+ membranes, and hybridized with a radiolabeled 6-bp telomeric repeat (TTAGGG)4 oligonucleotide probe (35). Then, washed membranes were autoradiographed. Images were scanned by using a Bio-Rad GS700 imaging densitometer, analyzed by Bio-Rad QUANTITY ONE software, and mean telomere lengths were calculated.
Flow Cytometry.
Cultures were labeled with BrdUrd for 4 h (fibroblasts) or 6 h (endothelial cells) at mid-confluency at both permissive (33.5°C) and, after 5 days, nonpermissive (39.5°C) temperatures. After harvesting by trypsinization, cells were fixed in cold 70% (vol/vol) ethanol. After washing, cells were incubated in 2 M HCl and then labeled with mouse anti-BrdUrd antibody (Becton Dickinson) followed by FITC-conjugated rabbit anti-mouse F(ab′)2 (Dako). After counterstaining with 40 μg/ml propidium iodide (PI; Sigma), cells were analyzed on a FACScan (Becton Dickinson) with forward and right-angle light scatter to gate intact cells and pulse areas vs. pulse width of the PI signal to exclude clumps and debris. Then, 20,000 gated events were acquired and analyzed by using CELLQUEST software (Becton Dickinson). Cultures for LT-antigen staining were fixed also in 70% ethanol, and incubated with PAb412, a mouse monoclonal anti-LT antigen antibody, and FITC-labeled rabbit anti-mouse antiserum (Sigma).
Karyotype Analysis.
Metaphase spreads prepared from colchicine-arrested cells grown at the permissive temperature were stained with 4′,6-diamidino-2-phenylindole, and digital images were acquired and analyzed by using CYTOVISION software (Applied Imaging Systems, Santa Clara, CA).
Senescence-Associated (SA) β-Galactosidase Activity.
SA β-galactosidase activity was detected as described by Dimri et al. (36), with >1,000 cells counted in replicate to determine the percentage of positive cells.
Results
Retroviral Transduction of Fibroblasts and Endothelial Cells with U19tsA58 LT and hTERT.
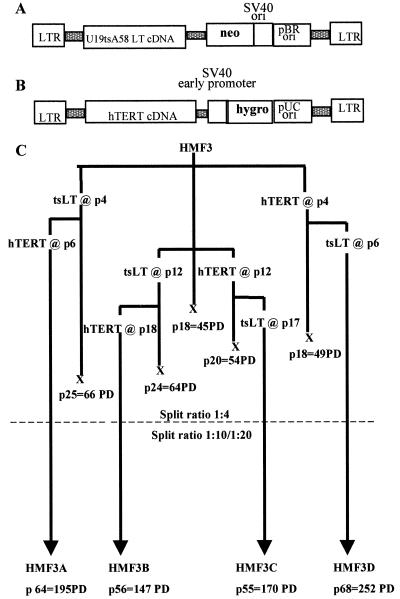
Freshly prepared proliferating cultures of human mammary interlobular fibroblasts (HMF; one donor) and human microvascular endothelial cells (HMME ; two donors) were transduced with the recombinant retroviruses shown in Fig. 1 A and B. Viruses from stable helper-free producer cell lines were used singly and in sequential combination at both early and late passages in the normal proliferative lifespan of the cells. After infection and selection with the appropriate drug, successfully transduced cells were passaged as bulk cultures to determine the proliferative lifespan of each population. In each case, subcultures of the single gene transductions were used for sequential infection with the second virus. Infection frequencies were typically 5–10% with both viruses, resulting in cultures initially composed of many tens of thousands of individually transduced cells. The experimental design and summary of results with the mammary fibroblasts are shown in Fig. 1C.
Figure 1.
Retroviral transduction with tsLT and hTERT. (A) The U19tsA58 LT virus (27) was constructed by using the pZipNeoSV(X)1 backbone and encodes a full-length LT cDNA from U19tsA58 and confers resistance to G418. The U19tsA58 LT is a combination of two mutants, U19, which encodes a LT that does not bind specifically to SV40-origin DNA sequences, and tsA58, which encodes a thermolabile LT antigen that is wild type at 33.5°C but inactive at 39.5°C. (B) The hTERT virus was constructed by using the pBabe–hygro backbone and encodes a full-length hTERT cDNA as well as the hygromycin resistance gene. (C) Sequence of retroviral gene transduction and resulting population-doubling (PD) potential of human adult mammary fibroblasts from a single 19-year-old donor (HMF3).
Cultures of untransduced HMF3 cells underwent irreversible growth arrest after 45 PDs at 36.5°C. In contrast to previous reports that hTERT alone was sufficient for immortalization (19–22), we observed only a minimal extension in lifespan after infection with hTERT at either early or late passages with final PDs of 49 and 54, respectively, at 36.5°C. The appearance of the late-stage hTERT cultures was similar to cells undergoing normal senescence with no accumulation of grossly abnormal cells or abortive attempts at continued division, unlike cells transduced with LT antigen. Introduction of U19tsA58 LT antigen into HMF3 cells either at early or late passage significantly extended the lifespan to the same extent in both cases (total of 66 and 64 PDs, respectively) when grown at 33.5°C. These cultures progressively accumulated cells with aberrant morphologies and foci of abortive proliferation consistent with the definition of the crisis phase of culture. They remained in crisis with continuous cell loss during a period of 6 months and, as observed previously with cells of this type from other donors, gave rise to no immortal variants.
In contrast, senescence was overcome, and no subsequent crisis was observed when both genes were introduced, irrespective of the order and timing of infection, provided that the retroviral infections were carried out before the cells had stopped dividing. The doubly transduced cultures have continued to proliferate at 33.5°C for over 70 weeks in all four combinations by using cells from the same donor. Thus far, all four cultures (HMF3A, -B, -C, and -D) have undergone >145 PDs at a constant proliferation rate. All have been cloned by serial dilution from late-passage stocks [colony-forming efficiency (CE) ≈50%], accumulating upwards of 200 PDs after explantation and are thus functionally immortal.
Transduction of the same genes into the mammary microvascular endothelial cells gave the same results. In the cases of HMME2 (donor age, 57) and HMME7 (donor age, 25), the uninfected cells underwent 25 and 42 PDs, respectively, before irreversible growth arrest. U19tsA58 LT-antigen transduced HMME2 cells grew to 49 PDs, and HMME7 cells had their growth extended to 64 PDs before cessation of net growth and crisis with cell loss. HMME7 cells transduced with hTERT alone have not progressed beyond 58 PDs but have remained viable for >4 months with a senescent phenotype. Introduction of both genes in either order (tsLT followed by hTERT in HMME2 or hTERT followed by tsLT in HMME7) resulted in abrogation of senescence and no visible crisis. These cells have now been in continuous culture for over 1 year (>130 PDs over 50 serial passages). The late-passage endothelial cells are also clonogenic (CE, ≈50%), growing through multiple rounds of culture at clonal densities equivalent to >200 PDs after explantation and thus are also functionally immortal by the most stringent criteria.
Telomerase Activity.
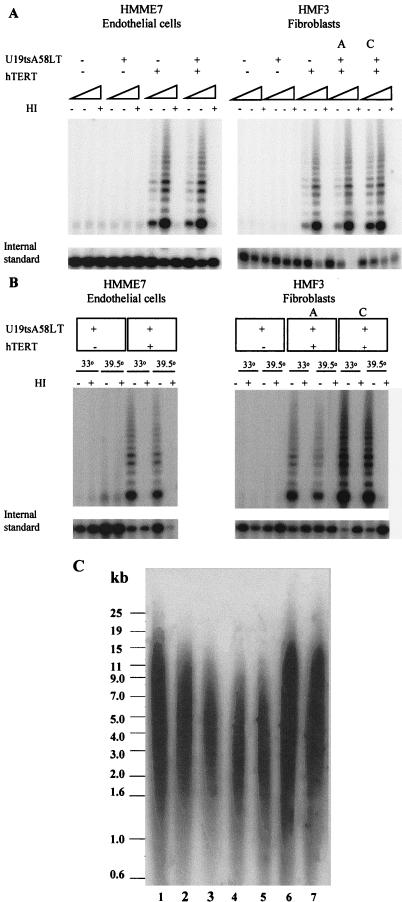
To demonstrate that both genes were active in the transduced cultures, we first examined whether infection with the hTERT retrovirus reconstituted telomerase activity. The results in Fig. 2A show that both cell types were positive for TRAP (37) assay after transduction with hTERT, with uninfected cells and U19tsA58 LT-antigen only transduced cells being negative. Because it has been shown that it is possible for cells to be TRAP positive but not to maintain telomeres (24), the mean telomere lengths (TRF length) of different representative cultures were determined. Results are shown in Table 1 and Fig. 2C. As expected, serial passaging of normal adult fibroblasts resulted in a decrease in TRF from 7.3 kb at p3 to ≈6 kb at p18 (the onset of senescence; see Fig. 2C, lanes 1–3). Further shortening was observed when lifespan was extended by U19tsA58 LT antigen introduced at early or late passages (final TRF values were 4.1 and 3.5 kb, respectively; see Fig. 2C, lanes 4 and 5). In comparison to late-passage control HMF cultures that exhibited a TRF value <6 kb, the hTERT-only infected cultures had longer telomeres (6.8 and 7.0 kb, respectively; see Fig. 2C , lanes 6 and 7). Analysis of the TRF lengths in the HMF3 cultures transduced with both U19tsA58 LT and hTERT showed that after an initial decrease in TRF length, the telomeres stabilized at around 6–8 kb and remained at this length during continuous culture. TRF length of the HMME2 and HMME7 cultures was also examined after infection with both viruses and was found to be maintained at ≈6 kb in both cultures.
Figure 2.
Telomerase activity. (A) 20- and 200-ng aliquots of cellular extracts were assayed for the presence of telomerase activity by the telomeric repeat amplification protocol (TRAP) assay. Extracts (200 ng each) were heat-treated (HI) to inactivate telomerase and assayed as a negative control. An internal PCR standard was included in each PCR to demonstrate that an absence of telomerase did not result from PCR inhibitors in the cell extracts. (B) Telomerase activity measured by the TRAP assay was detected in cellular extracts (20 and 200 ng) from HMF3 and HMME7 cells containing ectopic hTERT and U19tsA58 LT cultured at both 33.5°C (permissive temperature for tsA58 LT) and at 39.5°C (nonpermissive temperature) for 7 days. Activity was not detected in HMF3 and HMME7 cells containing U19tsA58 LT alone. (C) Southern blot showing TRF lengths for the fibroblast cultures HMF3 at p3 (lane 1), p12 (lane 2), p18 (lane 3), p24 after early transduction of tsLT (lane 4), p23 after late transduction of tsLT (lane 5), and p17 after both early (lane 6) and late (lane 7) transduction of hTERT. Numerical values for mean TRF lengths of these and other cultures are shown in Table 1.
Table 1.
TRF determination
| HMF3 samples | TRF length, kb |
|---|---|
| HMF3 p3 | 7.3 |
| HMF3 p12 | 6.3 |
| HMF3 p18 | 6.0 |
| HMF3 LT (early) p24 | 4.1 |
| HMF3 LT (late) p23 | 3.5 |
| HMF3 TERT (early) p17 | 7.0 |
| HMF3 TERT (late) p17 | 6.8 |
| HMF3A p36 | 6.8 |
| HMF3B p31 | 7.7 |
| HMF3C p33 | 6.0 |
| HMF3D p27 | 8.0 |
Analysis of T Antigen Expression.
The HMF and HMME cultures were analyzed for T antigen expression by flow cytometry. The levels of T antigen expressed by the different lines varied by a factor of 2, as measured by indirect immunofluorescence. However, there was no correlation between the level of T antigen and the order or timing of infection, their individual growth rates, or the temperature dependence of the different cell types. Levels, however, were consistent with the different ploidies of individual cultures.
Temperature Sensitivity For Growth.
We have shown previously that rodent cell lines immortalized with SV40 LT antigen depend on LT antigen for maintenance of growth (7, 38). Similar results have been obtained with human cell lines derived by immortalization with SV40 LT antigen after crisis (27, 29). We therefore determined the effect of inactivating the U19tsA58 LT antigen in the hTERT-positive cells by culturing them at 33.5°C (permissive temperature), 36.5°C (semipermissive temperature), and 39.5°C (nonpermissive temperature).
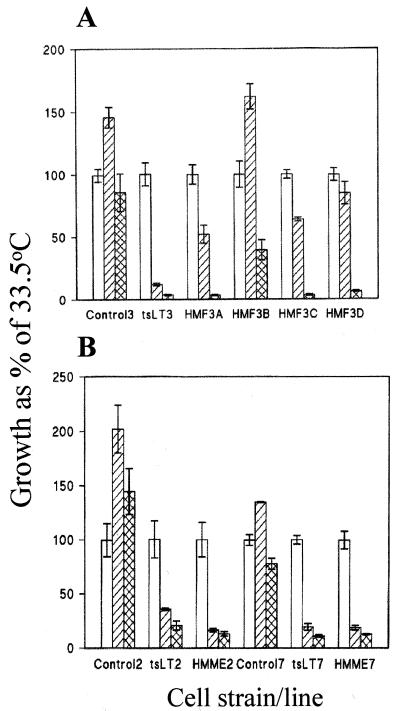
Fig. 3A shows that midpassage (p10) untransduced fibroblasts grew at all three temperatures but maximally at 36.5°C. In contrast, the majority of doubly transduced and the tsLT-only transduced fibroblasts grew best at 33.5°C and less at 36.5°C and ceased to grow at 39.5°C. An identical pattern of temperature sensitivity was observed when the fibroblast lines were cultured in a serum-free defined medium (Clonetics, San Diego; data not shown). The results obtained with the endothelial cells were similar. Untransduced endothelial cells grow at all three temperatures but maximally at 36.5°C, whereas the hTERT-tsLT positive cells were even more temperature sensitive than the corresponding fibroblasts, and arrest completely at 36.5°C (Fig. 3B).
Figure 3.
Effects of U19tsA58 LT inactivation on growth. (A) Growth of HMF3A, -B, -C and -D at late passage (>p30) compared with that of untransduced cells (control 3 at p10) and cells transduced with only the tsLT gene (tsLT3 at p18). Cells were grown at permissive (33.5°C; open bars), semipermissive (36.5°C; hatched bars), and nonpermissive (39.5°C; cross-hatched bars) temperatures. Relative growth rates (means ± SD, n = 3) are expressed as a percentage of cell numbers at permissive temperature. (B) Growth of HMME2 and HMME7 at late passage (>p30) compared with that of corresponding untransduced controls and tsLT-only transduced cells at 33.5°C (open bars), 36.5°C (hatched bars), and 39.5°C (cross-hatched bars). Relative growth rates (means ± SD, n = 3) are expressed as a percentage of cell numbers at permissive temperature.
Loss of proliferative potential when cultures were shifted to 39.5°C with inactivation of U19tsA58 LT antigen was not caused by loss of functional telomerase activity. HMME7, HMF3A, and HMF3C cells were still positive for TRAP activity after 7 days at 39.5°C (Fig. 2B).
PI/BrdUrd Cytofluorimetry.
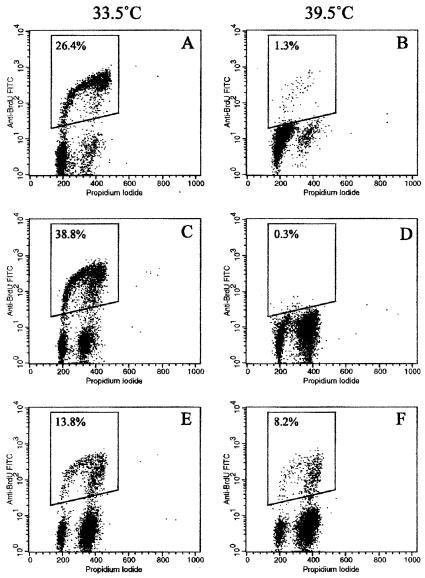
To determine whether the lack of increase in cell numbers at nonpermissive temperatures was due to reduced cell proliferation or to increased cell death, cultures were analyzed by PI and PI/BrdUrd flow cytometry before and after temperature shift. PI analysis showed that although there was a small (<3%) sub G1 peak in some cultures after 5 days at nonpermissive temperature, indicative of apoptosis, most of the cells were arrested in G1 and G2 phases of the cell cycle (data not shown). This was confirmed on selected cultures (HMME7, HMF3A, and HMF3B) by using BrdUrd and PI double labeling to quantify the proportion of cells in S phase (Fig. 4). The S phase fraction in HMME7 and HMF3A after 5 days at 39.5°C was reduced to 1.3 and 0.3%, respectively. In the least-conditional culture, HMF3B, cells in S phase were detectable at 39.5°C, but the proportion was lower than at 33.5°C (8.2 vs. 13.8%).
Figure 4.
Cell-cycle analysis. Flow cytograms showing cell-cycle parameters of HMME7 (A and B), HMF3A (C and D), and HMF3B (E and F). Cells were grown at either 33.5°C (A, C, and E) or 39.5°C (B, D, and F) for 5 days and pulse labeled with BrdUrd. Plots show PI fluorescence (x axis) vs. FITC fluorescence from BrdUrd (y axis). The proportion of cells in S phase is shown, being reduced to nearly zero in HMME7 and HMF3A but not HMF3B cells, at 39.5°C.
Irreversibility of Temperature-Dependent Growth Arrest.
To determine whether the temperature-induced growth arrest was irreversible, the CE at permissive temperature (33.5°C) was measured after different periods of growth arrest in all four fibroblast and the two endothelial cultures. Cells were cloned under conditions (5% oxygen in both cases and with irradiated HMF fibroblast feeders in the case of endothelial cells) in which the CE of control cells not exposed to 39.5°C was ≈50%. In all cultures (Fig. 5A), there was a reduction in CE that was proportional to the time at the nonpermissive temperature. In HMF3C, for example, the CE after 14 days at 39.5°C had dropped to <5% of control value, with most colonies being small and abortive. HMF3B, the least-conditional fibroblast culture in terms of overall growth (see Fig. 3A), showed the smallest decrease in clonogenic potential as a result of exposure to 39.5°C. Progressive loss of clonogenic potential at 33.5°C was also seen in the endothelial cultures when they were exposed to temperatures of both 36.5°C and 39.5°C (Fig. 5B). These results demonstrate clearly that the temperature-dependent growth arrest seen in the tsT-hTERT-containing cells, and its progressive irreversibility, is due to thermal inactivation of tsT antigen and not a nonspecific effect of heat shock.
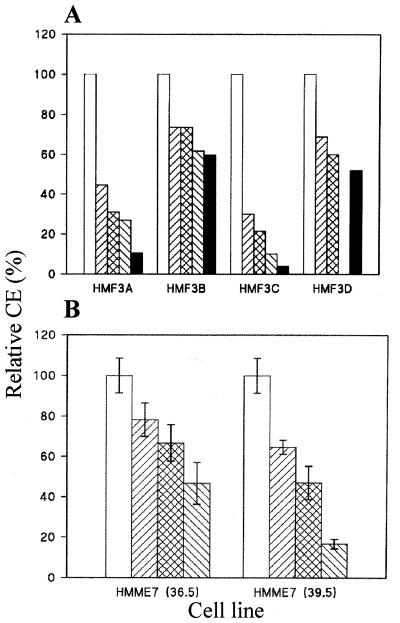
Figure 5.
Clonal analysis of growth arrest. (A) Relative CE of the four fibroblast cultures at 33.5°C after 0 days (open bars), 3 days (▨), 7 days (cross-hatched bars), 10 days (▧), and 14 days (solid bars) at 39.5°C. Results are means of duplicate determinations (variance of replicate CE < ± 10%). (B) Relative colony-forming efficiency at 33.5°C of the endothelial culture HMME7 after 0 days (open bars), 3 days (▨), 6 days (cross-hatched bars), and 9 days (▧) at 36.5°C and at 39.5°C, respectively. Results are means ± SD (n = 4). The corresponding numbers of SA β-galactosidase positive cells were 5% (day 0), 11% (day 3), 54% (day 6), and 79% (day 9) in cultures at 36.5°C, compared with 3% (day 3), 6% (day 6), and 12% (day 9) in cultures at 39.5°C.
Induction of SA β-galactosidase.
We next determined whether cells undergo biochemical senescence after inactivation of T antigen by staining cultures for SA β-galactosidase (36). Both HMME2 and HMME7 gave ≈80% positive cells after 9 days at 36.5°C, although numbers were lower (≈10%) at 39.5°C, indicating that the higher temperature may partially inhibit induction of SA β-galactosidase activity (see legend to Fig. 5B). After 6 days at 39.5°C, all the fibroblast cultures showed positive cells (1–10%), whereas none were detected in the corresponding cultures at 33.5°C (<0.1%).
Karyotype Analysis.
The cultures were karyotyped to determine whether the order and timing of retroviral gene transduction affected the chromosomal status. A normal modal number of chromosomes was observed in both HMME7 and HMF3D cells that had been derived by introducing both genes during the early phase with hTERT first. HMF3A, the culture in which both genes were also introduced early but with LT antigen first, showed a bimodal karyotype with near-diploid and near-tetraploid modes. Cultures in which hTERT was introduced late after LT antigen (HMME2 and HMF3B) showed significant aneuploidy.
Discussion
Amphotropic retroviruses that transduce either hTERT or U19tsA58 LT antigen have been used to determine whether reconstitution of telomerase activity alone is sufficient for immortalization of freshly isolated adult human mammary fibroblasts and microvascular endothelial cells, or whether additional activities are required. The results show clearly that the latter is the case with cells from three separate donors, cultured under conditions that are used widely and are appropriate for each cell type. Although ectopic expression of hTERT alone resulted in extension or stabilization of telomeres, this activity was not sufficient for immortalization of these cells in our hands. Some extension of growth potential was observed with both cell types, but all cultures with hTERT alone ultimately ceased growth and remained arrested with a senescent phenotype. Sequential transduction of both genes resulted in immortal cultures that grew without either a senescent or crisis phase being observed.
We have shown previously that rodent cell lines immortalized with SV40 LT antigen depend on LT antigen for maintenance of growth (7, 38). Similar results have been obtained with human cell lines derived by immortalization with SV40 LT antigen after crisis (27, 29). To test the hypothesis that additional factors are required in telomerase-positive lines to maintain as well as to initiate the immortal phenotype, LT antigen was inactivated in the doubly transduced cells by temperature shift. Both cell types clearly depended on functional LT antigen, with the results showing lack of growth at physiological temperatures by the endothelial cells, excluding any possibility of arrest being due to heat-shock effects. As observed previously with LT-immortalized rodent cells (7, 38), growth arrest occurs at both G1 and G2 phases of the cell cycle.
The results obtained in this study are, at face value, at variance with experiments on human fibroblasts and endothelial cells reported from other laboratories. These studies seem to show that hTERT alone is sufficient to immortalize such cells (19–22). The role of hTERT alone in maintaining the immortal phenotype in adult human somatic cells is controversial. Some studies show that its removal by Cre-lox reinstates telomere shortening, with eventual loss of proliferative potential, although a recent report has shown that in some instances, such cells can continue to divide (39–45). The reason for these discrepancies is not immediately obvious. The most plausible hypothesis is that a “second event” is involved. Stress-associated proliferative block(s) may prevent hTERT from exerting its full immortalizing potential, requiring the intervention of LT, or functionally equivalent viral genes, to overcome the block(s). One such stress could involve free-radical damage associated with high levels of ambient O2 in conventional culture conditions. Oxidative stress has been shown to enhance single-strand damage to telomeres triggering p53-dependent cell-cycle arrest (46). However, our cultures of adult microvascular endothelial cells have been maintained in the same commercial medium, which contains the antioxidant l-ascorbic acid, as that used in a study in which hTERT alone apparently was successful in immortalizing this cell type (21). Furthermore, the continued dependence of the fibroblast cultures on LT antigen when cultured in serum-free defined medium suggests that culture conditions may not be a major factor.
Another type of second event could involve the abrogation in some hTERT cultures of the intrinsic cell-cycle regulating pathways that are inhibited by LT, independently of the external milieu, or viral immortalizing genes. This hypothesis is testable by comparing patterns of gene expression in early- and late-stage hTERT cultures. It may be significant that recent studies on human T cells have failed to show immortalization when early-passage cultures were transduced with hTERT (47), although late-passage cells were immortalized (48).
Irrespective of the precise reason for such differences, the present results show that it is possible to use a combination of hTERT and tsLT to generate conditionally immortal human cells in a reproducible and convenient manner. Such cells are not only of value in understanding the fundamental mechanisms of cellular immortalization, but also provide potential sources of cells that can undergo terminal differentiation for medical cell-engineering purposes. Because delay in introducing immortalizing genes seems to result in significant aneuploidy, the use of a bicistronic retroviral vector containing both hTERT and tsLT should, in principle, provide the best means of generating conditionally immortal diploid human lines from freshly isolated cells.
Acknowledgments
We thank R. Weinberg for providing pCI-Neo-hTERT and R. Weinberg and K. Relph for critical reading of the manuscript. This work was funded partially by financial support to P.S.J. from ReNeuron LTD (London, United Kingdom). A.S. and J.M. are supported by the Commission of European Communities Contract Grant F14P-CT95-0008.
Abbreviations
- hTERT
catalytic subunit of human telomerase
- SV40
simian virus 40
- LT
large-tumor
- ts
temperature-sensitive
- TRF
terminal-restriction fragmentation
- PI
propidium iodide
- SA
senescence-associated
- PD
population-doubling
- TRAP
telomeric repeat amplification protocol
- CE
colony-forming efficiency
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hayflick L, Moorhead P S. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Cristofalo V J, Allen R G, Pignolo R J, Martin B G, Beck J C. Proc Natl Acad Sci USA. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayreuther K, Rodemann H P, Homenel R, Dittmann K, Albeiz M, Francz P I. Proc Natl Acad Sci USA. 1988;85:5112–5166. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay J W, Wright W E, Werbin H. Biochim Biophys Acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg R A. Cell. 2000;7:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein S. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 7.Gonos E S, Burns J S, Mazars G R, Kobrna A, Riley T E, Barnett S C, Zafarana G, Ludwig R L, Ikram Z, Powell A J, Jat P S. Mol Cell Biol. 1996;16:5127–5138. doi: 10.1128/mcb.16.9.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allsopp R C, Vaziri H, Patterson C, Goldstein S, Younglai E V, Futcher A B, Greider C W, Harley C B. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn E H, Gall J G. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 10.Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 11.de Lange T. Proc Natl Acad Sci USA. 1994;91:1882–1885. [Google Scholar]
- 12.Zhu J, Bishop J M, Blackburn E H. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, et al. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 14.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 15.Harrington L, Zhou W, McPhail T, Oulton R, Yeung D S, Mar V, Bass M B, Robinson M O. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilian A, Bowtell D D, Abud H E, Hime G R, Venter D J, Keese P K, Duncan E L, Reddel R R, Jefferson R A. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 18.Counter C M, Meyerson M, Eaton E N, Ellisen L W, Caddle S D, Haber D A, Weinberg R A. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 19.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Science. 1998;279:349–252. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 20.Vaziri H, Benchimol S. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Chang E, Cherry A M, Bangs C D, Oei Y, Bodnar A, Bronstein A, Chiu C P, Herron G S. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 22.Ouellette M M, Liao M, Herbert B S, Johnson M, Holt S E, Liss H S, Shay J W, Wright W E. J Biol Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- 23.Kiyono T, Foster S A, Koop J I, McDougall J K, Galloway D A, Klingelhutz A J. Nature (London) 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 24.Counter C M, Hahn W C, Wei W, Caddle S D, Beijersbergen R L, Lansdorp P M, Sedivy J M, Weinberg R A. Proc Natl Acad Sci USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Nature (London) 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 26.Almazan G, McKay R. Brain Res. 1992;579:234–245. doi: 10.1016/0006-8993(92)90056-f. [DOI] [PubMed] [Google Scholar]
- 27.Stamps A C, Davies S C, Burman J, O'Hare M J. Int J Cancer. 1994;57:865–874. doi: 10.1002/ijc.2910570616. [DOI] [PubMed] [Google Scholar]
- 28.Small M B, Gluzman Y, Ozer H L. Nature (London) 1982;296:671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- 29.Radna R L, Caton Y, Jha K K, Kaplan P, Li G, Traganos F, Ozer H L. Mol Cell Biol. 1989;9:3093–3096. doi: 10.1128/mcb.9.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha K K, Banga S, Palejwala V, Ozer H L. Exp Cell Res. 1998;245:1–7. doi: 10.1006/excr.1998.4272. [DOI] [PubMed] [Google Scholar]
- 31.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K L. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgenstern J P, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atherton A J, O'Hare M J, Buluwela L, Titley J, Monaghan P, Paterson H F, Warburton M J, Gusterson B A. J Cell Sci. 1994;107:2931–2939. doi: 10.1242/jcs.107.10.2931. [DOI] [PubMed] [Google Scholar]
- 34.Drake B L, Loke Y W. Hum Reprod. 1991;6:1156–1159. doi: 10.1093/oxfordjournals.humrep.a137502. [DOI] [PubMed] [Google Scholar]
- 35.Counter C M, Avilion A A, LeFeuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O, et al. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 38.Jat P S, Sharp P A. Mol Cell Biol. 1989;9:1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohmura H, Tahara H, Suzuki M, Ide T, Shimizu M, Yoshida M A, Tahara E, Shay J W, Barrett J C, Oshimura M. Jpn J Cancer Res. 1995;86:899–904. doi: 10.1111/j.1349-7006.1995.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuthbert A P, Bond J, Trott D A, Gill S, Broni J, Marriott A, Khoudoli G, Parkinson E K, Cooper C S, Newbold R F. J Natl Cancer Inst. 1999;91:37–45. doi: 10.1093/jnci/91.1.37. [DOI] [PubMed] [Google Scholar]
- 41.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H, Meyerson M, Weinberg R A. Nat Genet. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 42.Herbert B, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Hannon G J, Beach D H. Nature (London) 2000;405:755–756. doi: 10.1038/35015674. [DOI] [PubMed] [Google Scholar]
- 45.Ouellette M M, McDaniel L D, Wright W E, Shay J W, Schultz R A. Hum Mol Genet. 2000;12:403–411. doi: 10.1093/hmg/9.3.403. [DOI] [PubMed] [Google Scholar]
- 46.Saretzki G, Sitte N, Merkel U, Wurm R E, von Zglinicki T. Oncogene. 1999;18:5148–5158. doi: 10.1038/sj.onc.1202898. [DOI] [PubMed] [Google Scholar]
- 47.Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini J C, Romero P, Nabholz M. J Immunol. 2000;15:4978–4984. doi: 10.4049/jimmunol.165.9.4978. [DOI] [PubMed] [Google Scholar]
- 48.Hooijberg E, Ruizendaal J J, Snijders P J, Kueter E W, Walboomers J M, Spits H. J Immunol. 2000;15:4239–4245. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]