Abstract
NMDA receptors regulate burst firing of dopaminergic neurones in the substantia nigra pars compacta (SNc) and may contribute to excitotoxic cell death in Parkinson's disease (PD). In order to investigate the subunit composition of functional NMDA receptors in identified rat SNc dopaminergic neurones, we have analysed the properties of individual NMDA receptor channels in outside-out patches. NMDA (100 nm) activated channels corresponding to four chord conductances of 18, 30, 41 and 54 pS. Direct transitions were observed between all conductance levels. Between 18 pS and 41 pS conductance levels, direct transitions were asymmetric, consistent with the presence of NR2D-containing NMDA receptors. Channel activity in response to 100 nm or 200 μm NMDA was not affected by zinc or TPEN (N,N,N′,N′-tetrakis-[2-pyridylmethyl]-ethylenediamine), indicating that SNc dopaminergic neurones do not contain functional NR2A subunits. The effect of the NR2B antagonist ifenprodil was complex: 1 μm ifenprodil reduced open probability, while 10 μm reduced channel open time but had no effect on open probability of channels activated by 100 nm NMDA. When the concentration of NMDA was increased to 200 μm, ifenprodil (10 μm) produced the expected reduction in open probability. These results indicate that NR2B subunits are present in SNc dopaminergic neurones. Taken together, these findings indicate that NR2D and NR2B subunits form functional NMDA receptor channels in SNc dopaminergic neurones, and suggest that they may form a triheteromeric NMDA receptor composed of NR1/NR2B/NR2D subunits.
Dopamine neurones of the substantia nigra pars compacta (SNc) provide a dopaminergic projection to the striatum that is crucial to the normal physiology of the basal ganglia and is lost in Parkinson's disease (PD). Excitatory afferents to SNc dopaminergic neurones activate NMDA glutamate receptors (Mereu et al. 1991; Wu & Johnson, 1996) to induce burst firing (Johnson et al. 1992). NMDA receptors may contribute to excitotoxic death of dopaminergic neurones in PD (Sonsalla et al. 1998; Doble, 1999; Blandini et al. 2000), and NMDA receptor antagonists have therapeutic potential in PD (Hallett & Standaert, 2004).
The functional and pharmacological properties of NMDA receptors are determined by their subunit composition (Stern et al. 1992; Wyllie et al. 1996; Vicini et al. 1998; see Cull-Candy & Leszkiewicz, 2004 for review). Although NR1 subunits consist of different alternatively spliced isoforms, NR2 subunits are the main determinants of NMDA receptor functional diversity. Four NR2 subunits are known, NR2A–NR2D, each with different properties. For example, cloned NMDA receptors containing the NR2D subunit have high affinity for glutamate, deactivate slowly, and show low sensitivity to blockade by magnesium ions (Monyer et al. 1994; Momiyama et al. 1996; Cull-Candy & Leszkiewicz, 2004; Qian et al. 2005). Triheteromeric NMDA receptors composed of NR1 subunits and more than one type of NR2 subunit show some unique properties (Cheffings & Colquhoun, 2000; Chazot et al. 2002; Brickley et al. 2003; Cull-Candy & Leszkiewicz, 2004; Hatton & Paoletti, 2005) providing scope for further diversity of function. A combination of single channel recording and pharmacological approaches can be used to infer the subunit composition of native NMDA receptors by comparison with recombinant receptors.
Although SNc dopaminergic neurones express functional NMDA receptors (Mereu et al. 1991; Wu & Johnson, 1996; Lin & Lipski, 2001), their subunit composition is not known. NR2D subunit mRNA (Monyer et al. 1994) and protein (Dunah et al. 1996) is expressed in the developing and adult rat brain, particularly in brainstem and diencephalon, and NR2D protein is present in SNc (Dunah et al. 1996). If NR2D-containing NMDA receptors are functional in SNc dopaminergic neurones and contribute to excitotoxicity, they could provide a novel therapeutic target in PD. The presence of functional NR2B subunits in SNc dopaminergic neurones is also an important question, because NR2B-selective NMDA receptor antagonists protect SNc dopaminergic neurones against neurotoxicity in experimental models of PD (Blanchet et al. 1999; Nash et al. 1999, 2000; Steece-Collier et al. 2000; Hallett & Standaert, 2004). NR2A and NR2B subunit proteins are present in low levels in the SNc of adult rats (Albers et al. 1999).
The objective of this study was to characterize functional NMDA receptor subtypes present in SNc dopaminergic neurones, and to determine whether NR2D and NR2B subunits form functional NMDA receptors, using single channel analysis of recordings from outside-out patches. The results support the idea that NR2B- and NR2D-containing NMDA receptors are present in SNc dopaminergic neurones, but demonstrate that they are not typical of diheteromeric recombinant receptors and are likely to be triheteromeric receptors composed of NR1, NR2B and NR2D receptors.
Methods
Preparation of brain slices
Sprague-Dawley rats (aged 14–16 days) were decapitated in accordance with the Animals Scientific Procedures Act, UK (1986) and the brain removed into ice-cold oxygenated slicing solution containing (mm): sucrose 206; KCl 2.5; CaCl2 1.0; MgCl2 1.0; NaHCO3 25; NaH2PO4 1; glucose 25 (all from BDH, UK). Coronal brain slices (300 μm thick) were prepared using a Dosaka DTK-1000 Vibroslicer (Kyoto, Japan). Slices containing the midbrain substantia nigra region were kept in oxygenated solution containing (mm): NaCl 125; KCl 2.5; CaCl2 1.0; MgCl2 1.0; NaHCO3 26; NaH2PO4 1.25; glucose 25 at room temperature for 1–6 h before use.
Recording single NMDA receptor channels
Slices were placed in a recording chamber on the stage of an Olympus upright microscope with Nomarski-DIC optics. Slices were continuously bathed in the same solution used for storing the slices (without magnesium), at room temperature. Patch pipettes were made from thick-walled borosilicate glass (GC150F, Harvard Apparatus, Kent, UK), fire polished to a final resistance of 10–15 MΩ and filled with solution containing (mm): CsCl 140; Hepes 10; EGTA 10; pH 7.2. Recordings were made from visually identified neurones in the SNc of the midbrain that had a clear time-dependent, hyperpolarization-activated inward current in whole-cell mode in response to a voltage step from −60 to −120 mV, typical of dopaminergic neurones (Johnson & North, 1992; Lin & Lipski, 2001). Outside-out patches were made from dopaminergic neurones and voltage-clamped at −80 to −40 mV (routinely −60 mV). NMDA 0.1 μm, 10 μm, or 200 μm (Tocris, UK) and glycine 10 μm were added to the solution perfusing the recording chamber in order to activate NMDA receptors. Patches showing spontaneous channel activity in the absence of NMDA and glycine were discarded. In seven patches, recordings were carried out in the presence of strychnine (10 μm) to block inhibitory glycine receptors, and no significant difference in channel open probability (Popen) was observed between control and strychnine patches. Single channel currents were amplified using an Axopatch 200B patch clamp amplifier (Axon Instruments, USA), filtered at 10 kHz by an 8 pole Bessel filter, and stored on tape (Biologic DAT recorder) for later analysis.
Data analysis
Acquired data were replayed from the DAT tape, filtered at 2 kHz and digitized at 20 kHz using a Micro1401 interface (Cambridge Electronic Design, Cambridge UK) and stored to computer using CONSAM, a continuous sampling program (Colquhoun & Sigworth, 1995). Single NMDA channel activity was analysed using SCAN (Colquhoun & Sigworth, 1995), a time course fitting programme (available at: http://www.ucl.ac.uk/Pharmacology/dc.html) and distributions for the channel current amplitudes, open times and shut times produced using EKDIST (Colquhoun & Sigworth, 1995). Amplitude distributions were made for openings longer than 2 filter rise times (Tr= 332 μs) and open time and shut time distributions for intervals longer than 100 μs. Amplitude distributions were fitted with the sum of 3–4 Gaussian components by the maximum likelihood method. Open and shut time distributions were fitted with a mixture of exponential components using the maximum likelihood method; mean open and shut times were calculated by integration of the mixture of exponential components. At low agonist concentration Popen was calculated by dividing the mean open time by the sum of the mean open time and mean shut time. Stability plots of channel amplitudes, mean open time, mean shut time and mean Popen were checked to ensure that data were stable throughout the recordings (Weiss & Magleby, 1989). At high NMDA concentrations (10 μm and 200 μm), multiple superimposed channel openings were common and so channel Popen was estimated assuming individual receptors behave independently, and fitting the binomial relation (assuming the number of channels in the patch to be one more than the maximum number of superimposed openings observed) to the relative areas of Gaussian components fitted to all-point amplitude distributions (Colquhoun & Sigworth, 1995).
For analysis of direct transitions, an amplitude-based separation of unitary currents was obtained by calculating critical amplitude values (Acrit) producing an equal percentage of misclassified events between the Gaussian components fitted to the amplitude distribution (Howe et al. 1991). Each amplitude level had a duration longer than 2.5 filter rise times (415 μs), without intervening closures longer than the shut time resolution (100 μs).
Data are shown as mean ± standard error (s.e.m.). Statistical significance was determined using paired t tests unless otherwise stated.
Results
To determine the subunit composition of NMDA receptors in rat SNc dopaminergic neurones, recordings of NMDA-activated single channels were made in 24 outside-out patches in slices from 15 rats. Dopaminergic neurones were identified by the presence of a slow hyperpolarization-activated cation conductance. Recordings were routinely performed at −60 mV, although the relationship between channel amplitude and membrane potential was determined in five patches (not shown) and found to correspond to that for NMDA receptor channels.
NMDA activates channels with four distinct conductance levels in SNc dopaminergic neurones
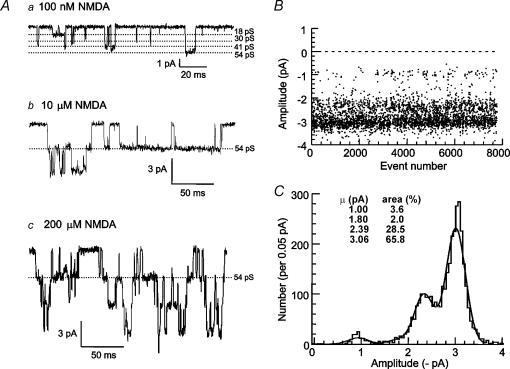
Application of NMDA (100 nm) and glycine (10 μm) to outside-out patches (n = 17) from rat SNc dopaminergic neurones activated individual NMDA receptor channels of four different amplitudes: 1.06 ± 0.04 pA, 1.83 ± 0.08 pA (in 15 patches), 2.48 ± 0.11 pA and 3.23 ± 0.12 pA. An example recording of NMDA receptor channel openings to all four amplitudes is shown in Fig. 1A, and the stability of channel openings to these four amplitudes throughout a recording is shown in Fig. 1B. In 17 patches, in the presence of 100 nm NMDA, the mean opening frequency was 12.8 ± 3.8 openings s−1, the mean open time was 2.83 ± 0.36 ms and the mean open probability (Popen) was 0.027 ± 0.006. The distribution of channel amplitudes activated by 100 nm NMDA in 15 of 17 patches was fitted with the sum of four Gaussian components (in two patches, only three components were evident). In addition to there being two patches where the 30 pS component of the amplitude distribution was not detected, in a further three patches the area of this component was less than 1%, while there were two patches where the area was greater than 50%. This makes it unlikely that the receptors are homogeneous between all patches. The measured channel amplitudes correspond to chord conductances (and relative areas) of 17.6 ± 0.74 pS (13.3 ± 4.0%, n = 17), 30.4 ± 1.30 pS (18.1 ± 6.2%, n = 15), 41.4 ± 1.76 pS (26.2 ± 4.8%, n = 17) and 53.9 ± 2.00 pS (43.0 ± 7.2%, n = 17). The distribution of amplitudes for the recording illustrated in Fig. 1B is shown in Fig. 1C, with the superimposed fit of the four Gaussian components. Channel activity in response to 10 μm and 200 μm NMDA is illustrated in Fig. 1A.
Figure 1. Large and small-conductance NMDA receptor channels in SNc dopaminergic neurones.
A, example currents recorded from outside-out patches from SNc dopaminergic neurones at a membrane potential of −60 mV in the presence of a, 100 nm NMDA and 10 μm glycine showing four different current amplitudes corresponding to the four conductance levels; b, 10 μm NMDA and 10 μm glycine; and c, 200 μm NMDA and 10 μm glycine. B, stability plot of channel amplitudes for one patch throughout the duration of the recording. C, amplitude distribution for the patch illustrated in B, fitted with the sum of four Gaussian components. The standard deviations were constrained to be the same (0.205 pA) for each component. The mean amplitude and relative area of each component are shown on the histogram, and correspond to chord conductances of 16.7 pS, 30.5 pS, 39.8 pS and 51.2 pS. The amplitude distribution contains current amplitudes for all openings that were longer than two filter rise-times.
NMDA receptors in SNc dopaminergic neurones contain NR2D subunits
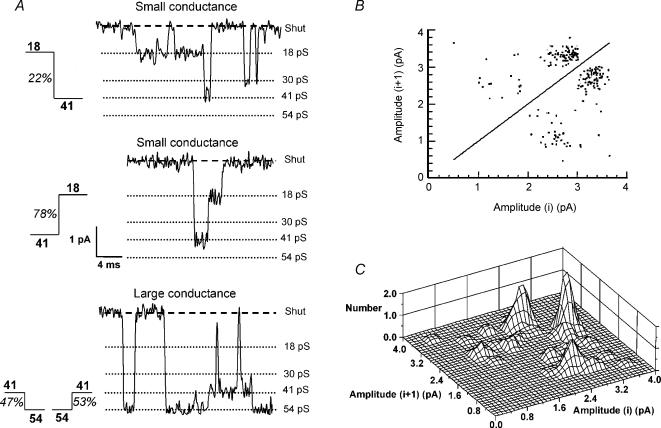
The 41 pS and 18 pS conductance levels are characteristic of small-conductance NMDA channels (composed of NR1/NR2C or NR1/NR2D), while 54 pS and 41 pS are characteristic of large-conductance NMDA channels (composed of NR1/NR2A or NR1/NR2B) in recombinant receptors (Stern et al. 1992; Wyllie et al. 1996). Direct transitions between conductance levels were analysed in 12 patches where there was a sufficient number of channel openings and sufficient distinction between the Gaussian components of the amplitude distribution to reliably determine critical amplitude values (Acrit) used to identify direct transitions between open channel states. Analysis of the frequency of direct transitions between the small-conductance states showed that transitions from 41 pS to 18 pS levels occur more frequently than transitions from 18 pS to 41 pS (Fig. 2). While 35.7 ± 7.8% of direct transitions were from 18 to 41 pS, 64.3 ± 7.8% were from 41 to 18 pS (P < 0.05, n = 12). This asymmetry of direct transitions is unique to NR2D-containing NMDARs (Wyllie et al. 1996; Chen et al. 2004), indicating that SNc dopaminergic neurones contain NR2D subunits. In the patch illustrated in Fig. 2, 53% of direct transitions between 54 and 41 pS levels were from 54 to 41 pS while 47% were from 41 to 54 pS. In contrast, 78% of transitions between 18 and 41 pS levels were from 41 to 18 pS while only 22% were from 18 to 41 pS (Fig. 2B and C).
Figure 2. NMDA receptors in SNc dopaminergic neurones contain NR2D subunits.
A, examples of direct transitions between 18 pS and 41 pS and 41 pS and 54 pS, with percentage occurrence observed in this patch indicated for each transition. Transitions were identified using Acrit values of 1.45 pA, 2.12 pA and 2.90 pA, calculated from the fit to the amplitude distribution shown in Fig. 1C. B, plot of channel amplitudes before and after direct transitions from the same patch illustrated in A. Each point on the graph represents a single direct transition. The density of points illustrates that direct transitions between 41 pS and 54 pS occur with equal frequency, while transitions between 18 pS and 41 pS are asymmetric, occurring more frequently from 41 pS to 18 pS than from 18 pS to 41 pS. C, Three-dimensional plot of the same data shown in B.
NMDA receptors containing NR2A subunits were not detected in SNc dopaminergic neurones
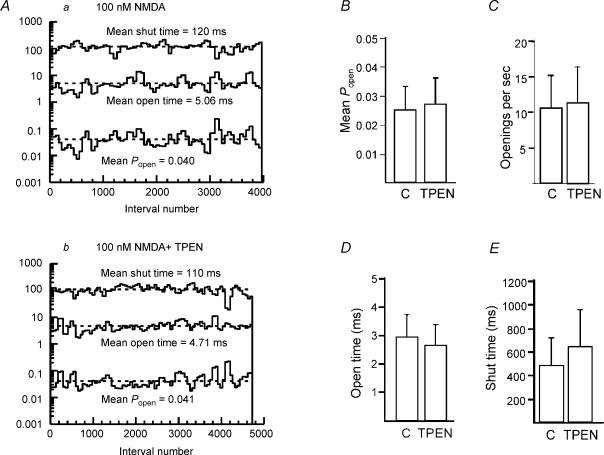
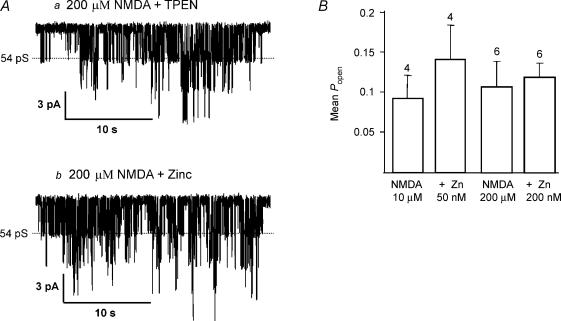
Cloned NMDA receptors composed of NR1 and NR2A subunits give large-conductance openings that are tonically inhibited by low levels of zinc present in extracellular solutions (Paoletti et al. 1997; Rachline et al. 2005). NR2A-containing NMDA receptors can therefore be potentiated by addition of the zinc chelating agent, TPEN (N,N,N′,N′-tetrakis-[2-pyridylmethyl]-ethylenediamine). In the presence of NMDA, prior to addition of TPEN (1 μm), opening frequency (10.6 ± 4.5 s−1), mean open time (2.96 ± 0.77 ms) and Popen (0.025 ± 0.008) were not significantly different from activity in the presence of TPEN (opening frequency 11.4 ± 5.1 s−1, mean open time 2.65 ± 0.72 ms, Popen 0.027 ± 0.009; n = 4). These data are summarized in Fig. 3. Furthermore, the proportion of large-conductance channel openings was not increased (control: 49.8 ± 8.1%, TPEN: 46.7 ± 11.4%, n = 4). These data suggest that either NR2A subunits are not present, or that zinc is not present in our extracellular solutions. In the same patches, when the concentration of NMDA was increased to 10 μm to increase channel activity, addition of zinc (50 nm) to the bath did not significantly reduce the Popen (control, 0.093 ± 0.029; Zn2+, 0.142 ± 0.043; n = 4) (Fig. 4), indicating that NMDA receptors in SNc dopaminergic neurones do not contain NR2A subunits. In order to ensure that an inhibitory effect of zinc was not being masked by an increase in receptor affinity (Chen et al. 1997; Paoletti et al. 1997; Zheng et al. 2001), a second set of experiments was carried out using a saturating concentration of NMDA (200 μm). In these experiments, Popen in the presence of 200 μm NMDA and 1 μm TPEN was 0.1071 ± 0.032 (n = 6). Addition of zinc (200 nm; no TPEN present) did not significantly reduce Popen (0.1195 ± 0.018; n = 6; P = 0.685) (Fig. 4). TPEN had no significant effect on channel activity in response to 200 μm NMDA (P = 0.47).
Figure 3. Large-conductance NMDA receptor channels in SNc dopaminergic neurones are unaffected by TPEN.
A, stability plots of Popen, mean open time and mean shut time for single-channel currents in the presence of a, 100 nm NMDA and 10 μm glycine and b, in the presence of NMDA, glycine and 1 μm TPEN show that TPEN did not affect the NMDA channel kinetics. Bar graphs summarizing data (mean ±s.e.m.) from four patches showing that TPEN had no significant effect on Popen (B), opening frequency (C) or open (D) and shut (E) times.
Figure 4. Large-conductance NMDA receptor channels in SNc dopaminergic neurones are unaffected by zinc.
A, example currents recorded in the presence of a, 200 μm NMDA and 1 μm TPEN; and b, 200 μm NMDA and 200 nm zinc, illustrating that zinc has no effect on channel activity. B, bar graph (mean ±s.e.m.) summarizes the lack of effect of zinc on channel activity in response to a low (10 μm, 4 patches) or saturating (200 μm, 6 patches) concentration of NMDA.
NMDA receptors in SNc dopaminergic neurones contain NR2B subunits
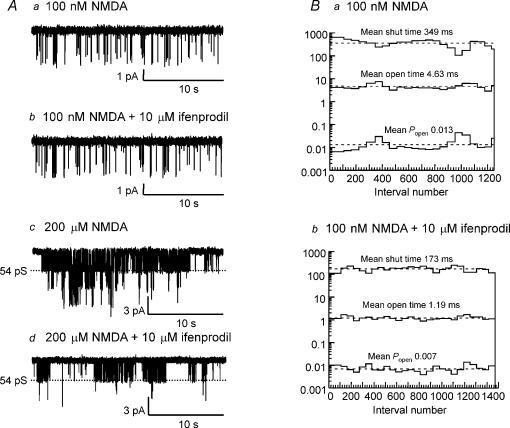
Ifenprodil is a non-competitive NMDA receptor antagonist that acts by increasing proton-inhibition of the receptor (Mott et al. 1998). Ifenprodil has approximately 350-fold higher affinity for NR2B-containing NMDA receptors than for other NR2 subunits (Williams 1993, 1995). The effect of 1 μm ifenprodil was investigated in five patches. In the presence of 100 nm NMDA and 10 μm glycine in control conditions, open probability (Popen) was 0.017 ± 0.005 (n = 5); open time was 3.16 ± 0.83 ms, opening frequency was 5.32 ± 1.03 s−1. Ifenprodil (1 μm) reduced Popen to 0.0099 ± 0.003 (60.5 ± 6.8% of control, n = 5, P = 0.04). Mean open time and opening frequency in ifenprodil were not significantly different (3.69 ± 1.37 ms, 4.1 ± 1.66 s−1; p = 0.65 and 0.15, respectively). Mean shut time was not significantly different between control (166.6 ± 31.4 ms) and ifenprodil 1 μm (426.5 ± 187.9 ms; P = 0.18): the decrease in Popen is accounted for by a significant increase in the time constant of the slowest component of the shut time distribution in the presence of 1 μm ifenprodil (1429 ± 345 ms) compared with control (536 ± 95 ms; P = 0.04). No other individual shut time component was significantly different in ifenprodil. Surprisingly, a higher concentration of ifenprodil (10 μm) had no effect on Popen in response to 100 nm NMDA (control 0.019 ± 0.004, ifenprodil 0.015 ± 0.006, n = 7; P = 0.98), although mean open time was significantly reduced (control 2.86 ± 0.67 ms, ifenprodil 1.48 ± 0.26 ms P = 0.05). This was probably due to a non-significant increase in opening frequency (control 17.5 ± 8.2 s−1, ifenprodil 26.8 ± 14.8 s−1; P = 0.12). Mean shut times were not significantly different between control (134.5 ± 41.8 ms) and 10 μm ifenprodil (134.3 ± 51.0 ms). The apparent lack of effect of 10 μm ifenprodil is illustrated in Fig. 5A. However, when a higher concentration of NMDA (200 μm) was used, control Popen was 0.085 ± 0.034 and ifenprodil reduced Popen to 0.019 ± 0.01 (a decrease of 77.9 ± 6.6%, n = 5). The effect of ifenprodil on 200 μm NMDA is illustrated in Fig. 5A.
Figure 5. Large-conductance NMDA receptor channels in SNc dopaminergic neurones contain NR2B subunits.
A, example currents recorded a, in the presence of 100 nm NMDA; b, in the presence of 100 nm NMDA and 10 μm ifenprodil; c, in the presence of 200 μm NMDA; and d, in the presence of 200 μm NMDA and 10 μm ifenprodil, showing that ifenprodil reduces channel activity in response to a higher but not a lower concentration of NMDA. B, stability plots of Popen, mean open time and mean shut time for single-channel currents in 100 nm NMDA and in the presence of 100 nm NMDA and 10 μm ifenprodil showing that, in this patch, in addition to decreasing channel open time, ifenprodil also decreased Popen and channel shut time.
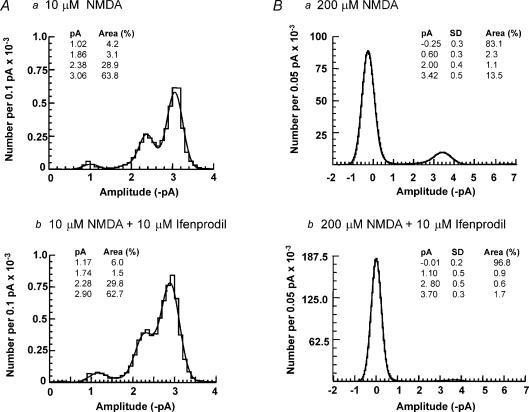
Neither 1 μm nor 10 μm ifenprodil significantly decreased the proportion of 54 pS channel openings observed in the amplitude distributions in response to 100 nm NMDA (control: 29.3 ± 9.5%, 1 μm ifenprodil: 43.1 ± 5.6%, n = 5; control 42.5 ± 12.8%, 10 μm ifenprodil: 47.1 ± 11.6%, n = 7), as would be expected if a proportion of receptors were NR1/NR2B diheteromers (Piña-Crespo & Gibb, 2002). The lack of effect of 10 μm ifenprodil is illustrated in Fig. 6A. However, when a higher concentration of NMDA (200 μm) was used, ifenprodil significantly decreased the proportion of 54 pS channel openings observed in amplitude distributions (control: 21.2 ± 7%, ifenprodil: 3.2 ± 1.5%, n = 4; P = 0.05) (illustrated in Fig. 6B). Ifenprodil caused a non-significant decrease in the proportion of lower conductance openings (control: 22.8 ± 7.9%, ifenprodil: 13.4 ± 6.0%, n = 4, P = 0.06). Together, these data suggest that NR2B subunits are present in SNc dopaminergic neurones.
Figure 6. Ifenprodil reduces NMDA receptor channel activity in response to a high agonist concentration in SNc dopaminergic neurones.
A, amplitude distributions in the presence of a, 10 μm NMDA; and b, 10 μm NMDA and 10 μm ifenprodil, fitted with the sum of four Gaussian components. The standard deviations were constrained to be the same (0.141 pA and 0.189 pA, respectively) for each component. Ifenprodil did not change the mean amplitude and relative area of each component. B, all-point amplitude distributions in the presence of a, 200 μm NMDA; and b, 200 μm NMDA and 10 μm ifenprodil, showing that ifenprodil reduces the channel activity.
NMDA receptors in SNc dopaminergic neurones are composed of NR1/NR2B/NR2D subunits
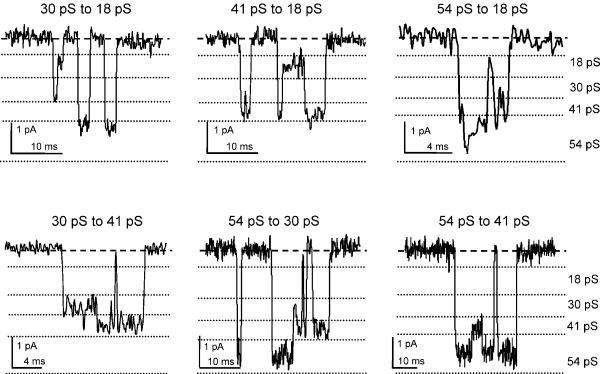
Transitions between different conductance levels were analysed to determine whether the four different conductance levels are due to a single type of NMDA receptor channel. Direct transitions between all conductance levels were analysed in 12 patches. In 9 of these 12 patches, direct transitions were evident between all conductance levels. In one patch, no transitions between 18 and 30 pS levels were evident, and in two other patches no transitions were observed between 18 and 54 pS. Examples of direct transitions between all open states are shown in Fig. 7, and the mean frequencies are summarized in Table 1. A significant number of transitions were observed between all conductance levels (Wilcoxon ranked sum test, P < 0.01). Only transitions between 18 and 41 pS showed significant asymmetry (Fig. 2 and Table 1). The observation that channel openings show direct transitions between large- and small-conductance levels suggests that both NR2B and NR2D subunits are present in the same NMDA receptor complex in SNc dopaminergic neurones.
Figure 7. Analysis of direct transitions between channel conductance levels.
Examples of direct transitions between conductance levels, as indicated above each trace. Broken lines indicate the boundaries of each conductance level, determined by the Acrit values calculated from the Gaussian components in the amplitude distributions for each patch. There were a significant number of transitions (Wilcoxon rank sum test, P < 0.01) for all 12 possible ways that four different current levels can be connected in pairs (see Table 1).
Table 1.
Direct transitions between all conductance levels
| Sequence (pS) | % of total (total number) | % in class |
|---|---|---|
| 18–30 | 4.68 ± 3.21 (273) | 52 |
| 30–18 | 4.13 ± 2.22 (238) | 48 |
| 18–41 | 2.05 ± 0.45* (89) | 36 |
| 41–18 | 3.81 ± 0.87* (144) | 64 |
| 18–54 | 1.45 ± 0.59 (48) | 41 |
| 54–18 | 2.29 ± 0.46 (57) | 59 |
| 30–41 | 7.92 ± 1.75 (349) | 49 |
| 41–30 | 6.88 ± 1.80 (293) | 51 |
| 30–54 | 8.44 ± 2.56 (197) | 49 |
| 54–30 | 7.53 ± 1.99 (200) | 51 |
| 41–54 | 26.5 ± 3.51 (820) | 48 |
| 54–41 | 24.9 ± 3.51 (769) | 52 |
Left column shows direct transitions between conductance levels. Middle column shows occurrence of specified transitions expressed as a percentage of all transitions (mean ±s.e.m.), n = 12 patches,
P < 0.05). A significant number of direct transitions occurred between all levels (P < 0.01, Wilcoxon rank sum test). Transitions between 18 and 54 pS and 54 and 18 pS were not observed in two patches. The total number of the specified transitions is given in parentheses. Right column shows occurrence of specified transition expressed as a percentage of all transitions occurring between the specified conductance levels.
Discussion
We have used single channel analysis to characterize functional NMDA receptors present in SNc dopaminergic neurones. Dopaminergic neurones were found to contain both large- and small-conductance NMDA receptor channels that do not exhibit properties of a diheteromeric receptor. The data suggest that SNc dopaminergic neurones express functional triheteromeric NMDA receptors composed of NR1, NR2B and NR2D subunits.
Four NMDA receptor channel conductances are observed in SNc dopaminergic neurones
Functional NMDA receptors have previously been described in midbrain dopaminergic neurones (Mereu et al. 1991; Wu & Johnson, 1996; Lin & Lipski, 2001) although their subunit composition was not investigated. Here we show NMDA receptor channels of four different conductances are consistently observed in SNc dopaminergic neurones, although the proportion of 30 pS openings varied between patches suggesting the receptor population is not homogeneous. NMDA receptor properties are determined by the subunit composition (Stern et al. 1992; Monyer et al. 1994; Wyllie et al. 1996; Grant et al. 1997; Vicini et al. 1998; Rumbaugh et al. 2000; see Cull-Candy et al. 2001 or Cull-Candy & Leszkiewicz, 2004 for review). Recombinant NMDA receptors composed of NR1/NR2A subunits or NR1/NR2B subunits give rise to large-conductance channels of 38 pS and 50 pS (Stern et al. 1992), similar to the large-conductance channels observed in SNc dopaminergic neurones. Small-conductance channels are formed from NMDA receptors composed of NR1/NR2C subunits (19 pS and 36 pS; Stern et al. 1992) or NR1/NR2D subunits (17 pS and 35 pS; Wyllie et al. 1996), similar to the small-conductance channels observed in SNc dopaminergic neurones. Our observations suggest that functional NMDA receptors in SNc dopaminergic neurones are composed of NR1 with either NR2A or NR2B subunits, and NR1 with either NR2C or NR2D subunits, or a triheteromeric receptor composed of NR1, NR2A or NR2B and NR2C or NR2D.
Functional NMDA receptors in SNc dopaminergic neurones contain NR2D subunits
Currently, no well-characterized pharmacological agent is available that can distinguish between NR2C- and NR2D-containing NMDA receptor channels. However, NR2C and NR2D subunits can be distinguished by analysis of direct transitions between conductance levels (Wyllie et al. 1996, 1998; Cull-Candy & Leszkiewicz, 2004). In SNc dopaminergic neurones, NMDA receptor channels showed an asymmetry that is consistent with the presence of NR2D rather than NR2C subunits.
The presence of NR2D rather than NR2C subunits in NMDA receptor channels in SNc dopaminergic neurones is consistent with expression studies. NR2C mRNA was detected in rat brain SNc, but NR2D mRNA levels were much higher in the same study (Standaert et al. 1994). Similarly, intense levels of NR2D mRNA, with low or no levels of NR2C mRNA, were detected in human SNc postmortem (Counihan et al. 1998; Daggett et al. 1998). NR2D subunit mRNA (Monyer et al. 1994) and protein (Dunah et al. 1996) is expressed in the developing and adult rat brain, with highest levels of expression in the brainstem and diencephalon, peaking after the first postnatal week and decreasing in adulthood. Our findings support the idea that NR2D subunits form functional NMDA receptors in SNc dopaminergic neurones. Thus, NR2D-containing NMDA receptors could contribute to excitotoxicity, and may provide a novel therapeutic target in PD.
Functional NMDA receptors in SNc dopaminergic neurones do not contain NR2A subunits
The large-conductance channels in SNc dopaminergic neurones were not affected by pharmacological agents that interact with NR2A-containing receptors. Recombinant NMDA receptors containing NR2A subunits are inhibited by nanomolar concentrations of zinc ions, and TPEN selectively potentiates the response of NR2A-containing NMDA receptor channels, whereas NR2B-containing NMDA receptors are unaffected by these concentrations of zinc ions and TPEN (Paoletti et al. 1997; Rachline et al. 2005). Addition of zinc or the zinc chelator TPEN had no effect on NMDA receptor channels activated by 100 nm or 200 μm NMDA in SNc dopaminergic neurones. These data indicate that NR2A subunits are not present in functional NMDA receptors in SNc dopaminergic neurones. NR2A subunit protein is present at only low levels in the SNc dopaminergic neurones of adult rats (Albers et al. 1999), while NR2A mRNA was undetected in the rat SNc (Standaert et al. 1994). NR2A protein is not detectable in adult monkey SNc (Paquet et al. 1997), and only low levels of NR2A mRNA are found in human SNc (Counihan et al. 1998) suggesting that, like rats, primate SNc dopaminergic neurones express few or no functional NR2A-containing NMDA receptors. However, it is possible that NR2A subunits are concentrated at synaptic or dendritic sites that would not have been detected in the present study.
Functional NMDA receptors in SNc dopaminergic neurones contain NR2B subunits
Ifenprodil is a non-competitive antagonist of NR2B-containing NMDA receptors that acts by enhancing proton inhibition (Mott et al. 1998). Ifenprodil has a higher affinity for recombinant receptors containing NR2B subunits than those containing NR2A, NR2C or NR2D subunits (Williams 1993, 1995; Cull-Candy et al. 2001). In SNc dopaminergic neurones, 1 μm ifenprodil reduced the Popen of receptor channels activated by 100 nm NMDA by selectively increasing the duration of the slowest component of the shut time distribution. Interestingly, a higher concentration of ifenprodil (10 μm) had no effect on Popen, although it reduced the mean open time (the change in open time being compensated for by an increase in opening frequency). In addition to non-competitive antagonism, ifenprodil can also increase the apparent affinity of the receptor for the agonist, and potentiate the response to low concentrations of NMDA in cortical neurones (Kew et al. 1996; Zhang et al. 2000). Thus, 10 μm ifenprodil might have two opposing effects on receptors activated by a low concentration of NMDA (100 nm), both increasing proton inhibition and therefore decreasing mean open time, while at the same time increasing the apparent affinity of the receptor for NMDA, thereby increasing opening frequency. To test this, we used a saturating concentration of NMDA (200 μm), and found that ifenprodil did selectively reduce the proportion of the large-conductance openings. Our results indicate that functional NMDA receptors in SNc dopaminergic neurones contain NR2B subunits.
Interestingly, NR2B subunit protein is present in low levels in the SNc of adult rats (Albers et al. 1999) and NR2B mRNA was not detected in rat SNc (Standaert et al. 1994). NR2B protein is not detectable in adult monkey SNc (Paquet et al. 1997), and only low levels of NR2B mRNA are found in human SNc (Counihan et al. 1998; Hallett & Standaert, 2004). The presence of NR2B subunits in SNc dopaminergic neurones is an important question, because NR2B-selective NMDA receptor antagonists protect SNc dopaminergic neurones against neurotoxicity in experimental models of PD (Blanchet et al. 1999; Nash et al. 1999, 2000; Steece-Collier et al. 2000; Hallett & Standaert, 2004). Our finding that functional NMDA receptors in SNc dopaminergic neurones contain NR2B subunits is therefore of particular relevance to PD research.
NMDA receptors in SNc dopaminergic neurones may be triheteromeric receptors composed of NR1, NR2B and NR2D subunits
Our data suggest that both NR2D and NR2B subunits form functional NMDA receptors in SNc dopaminergic neurones, and several of our observations support the idea that NR2B and NR2D subunits form a triheteromeric NMDA receptor. Firstly, these NMDA receptors do not exhibit properties typical of diheteromeric NR1/NR2B receptors. For example, 1 μm ifenprodil would be expected to reduce the proportion of large-conductance openings by ∼75% in response to near-EC50 concentrations of NMDA (5 μm NMDA; Piña-Crespo & Gibb, 2002), but in SNc dopaminergic neurones, neither 1 μm nor 10 μm ifenprodil selectively reduced the proportion of large-conductance openings in response to 100 nm or 10 μm NMDA (Fig. 6). Although ifenprodil affected channel activity, these data suggest that ifenprodil potency is not consistent with an action at diheteromeric NR1/NR2B receptors (IC50, 300 nm; Williams, 1993). However, the interaction of ifenprodil-type ligands with the NMDA receptor is affected by the presence of other NR2 subunits in the complex, including NR2A (Chazot et al. 2002; Hatton & Paoletti, 2005) and NR2D (Brickley et al. 2003). Secondly, all 17 patches exhibited both large- and small-conductance openings. In 9 out of 12 patches, direct transitions were observed between all four conductance levels, including direct transitions between the largest (54 pS) and smallest (18 pS) conductance levels which although rare, were consistently observed. The presence of 30 pS channels may be characteristic of a triheteromeric receptor as observed by Cheffings & Colquhoun (2000) in the case of NR1/NR2A/NR2D receptors. It is known that native triheteromeric NMDA receptors can form from NR2D subunits with NR1 and NR2B subunits in rat brain (Dunah et al. 1998; Brickley et al. 2003; Cull-Candy & Leszkiewicz, 2004). Interestingly, triheteromers containing either NR2A (Cheffings & Colquhoun, 2000: 49 pS) or NR2B (Brickley et al. 2003: 53 pS) have large-conductance openings of similar conductance to those observed with diheteromeric NR1/NR2A or NR1NR2B receptors (Stern et al. 1992). Taken together these observations support the idea that NMDA receptors in SNc dopaminergic neurones may be triheteromeric assemblies of NR1, NR2B and NR2D subunits.
In summary, we have shown that NMDA receptors in SNc dopaminergic neurones contain NR2D and NR2B subunits, and exhibit properties consistent with a triheteromeric molecular composition. NMDA receptors mediate excitatory synaptic transmission (Mereu et al. 1991; Wu & Johnson, 1996), induce long-term potentiation (Bonci & Malenka, 1999), and determine the firing properties (Johnson et al. 1992) in midbrain dopamine neurones. Our study identifies specific NR2 subunits in SNc dopaminergic neurones that form a relatively rare NMDA receptor.
Acknowledgments
This work was supported by The Wellcome Trust and a Parkinson's Disease Foundation/National Parkinson's Foundation Joint Project Grant.
References
- Albers DS, Weiss SW, Iadarola MJ, Standaert DG. Immunohistochemical localization of N-methyl-d-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor subunits in the substantia nigra pars compacta of the rat. Neuroscience. 1999;89:209–220. doi: 10.1016/s0306-4522(98)00328-5. [DOI] [PubMed] [Google Scholar]
- Blanchet PJ, Konitsiotis S, Whittemore ER, Zhou ZL, Woodward RM, Chase TN. Differing effects of N-methyl-d-aspartate receptor subtype selective antagonists on dyskinesias in levodopa-treated 1-methyl-4-phenyl-tetrahydropyridine monkeys. J Pharmacol Exp Ther. 1999;290:1034–1040. [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the VTA. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot PL, Lawrence S, Thompson CL. Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B-selective NMDA receptor antagonists. Neuropharmacology. 2002;42:319–324. doi: 10.1016/s0028-3908(01)00191-5. [DOI] [PubMed] [Google Scholar]
- Cheffings CM, Colquhoun D. Single channel analysis of a novel NMDA channel from Xenopus oocytes expressing recombinant NR1a, NR2A and NR2D subunits. J Physiol. 2000;526:481–491. [PubMed] [Google Scholar]
- Chen PE, Johnston AR, Mok MH, Schoepfer R, Wyllie DJ. Influence of a threonine residue in the S2 ligand binding domain in determining agonist potency and deactivation rate of recombinant NR1a/NR2D NMDA receptors. J Physiol. 2004;558:45–58. doi: 10.1113/jphysiol.2004.063800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- Counihan TJ, Landwehrmeyer GB, Standaert DG, Kosinski CM, Scherzer CR, Daggett LP, Velicelebi G, Young AB, Penney JB., Jr Expression of N-methyl-d-aspartate receptor subunit mRNA in the human brain: mesencephalic dopaminergic neurons. J Comp Neurol. 1998;390:91–101. [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science STKE. 2004;255:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Daggett LP, Johnson EC, Varney MA, Lin FF, Hess SD, Deal CR, Jachec C, Lu CC, Kerner JA, Landwehrmeyer GB, Standaert DG, Young AB, Harpold MM, Velicelebi G. The human N-methyl-d-aspartate receptor 2C subunit: genomic analysis, distribution in human brain, and functional expression. J Neurochem. 1998;71:1953–1968. doi: 10.1046/j.1471-4159.1998.71051953.x. [DOI] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB. Subunit composition of N-methyl-d-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol. 1998;53:429–437. doi: 10.1124/mol.53.3.429. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang YH, Luo J, Davila-Garcia M, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J Neurochem. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Grant ER, Bacskai BJ, Pleasure DE, Pritchett DB, Gallagher MJ, Kendrick SJ, Kricka LJ, Lynch DR. N-methyl-d-aspartate receptors expressed in a nonneuronal cell line mediate subunit-specific increases in free intracellular calcium. J Biol Chem. 1997;272:647–656. doi: 10.1074/jbc.272.1.647. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Howe JR, Cull-Candy SG, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat VTA and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-d-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Kew JN, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol. 1996;497:761–772. doi: 10.1113/jphysiol.1996.sp021807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY-L, Lipski J. Dopaminergic substantia nigra neurones express functional NMDA receptors in postnatal rats. J Neurophysiol. 2001;85:1336–1339. doi: 10.1152/jn.2001.85.3.1336. [DOI] [PubMed] [Google Scholar]
- Mereu G, Costa E, Armstrong DM, Vicini S. Glutamate receptor subtypes mediate excitatory synaptic currents of dopamine neurones in midbrain slices. J Neuroscience. 1991;11:1359–1366. doi: 10.1523/JNEUROSCI.11-05-01359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A, Feldmeyer D, Cull-Candy SG. Identification of a native low-conductance NMDA channel with reduced sensitivity to Mg2+ in rat central neurones. J Physiol. 1996;494:479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendly MJ, Lyuboslavsky P, Traynelis S, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nature Neurosci. 1998;1:659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, Maneuf Y, Hille C, Brotchie JM, Crossman AR. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol. 2000;165:136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- Nash JE, Hill MP, Brotchie JM. Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Exp Neurol. 1999;155:42–48. doi: 10.1006/exnr.1998.6963. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1–NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, Tremblay M, Soghomonian JJ, Smith Y. AMPA and NMDA glutamate receptor subunits in midbrain dopaminergic neurons in the squirrel monkey: an immunohistochemical and in situ hybridization study. J Neurosci. 1997;17:1377–1396. doi: 10.1523/JNEUROSCI.17-04-01377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña-Crespo JC, Gibb AJ. Subtypes of NMDA receptors in new-born rat hippocampal granule cells. J Physiol. 2002;541:41–64. doi: 10.1113/jphysiol.2001.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian A, Buller AL, Johnson JW. NR2 subunit dependence of NMDA receptor channel block by external Mg2+ J Physiol. 2005;562:319–331. doi: 10.1113/jphysiol.2004.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JF, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol. 2000;83:1300–1306. doi: 10.1152/jn.2000.83.3.1300. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Albers DS, Zeevalk GD. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids. 1998;14:69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young AB, Penney JB., Jr Organization of N-methyl-d-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP_101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol. 2000;163:239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Roy Soc Lond B. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Weiss DS, Magleby KL. Gating scheme for single GABA-activated Cl−channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J Neurosci. 1989;9:1314–1324. doi: 10.1523/JNEUROSCI.09-04-01314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N–methyl-d-aspartate (NMDA) receptors containing the epsilon 4 (NR2D) subunit. Neurosci Lett. 1995;184:181–184. doi: 10.1016/0304-3940(94)11201-s. [DOI] [PubMed] [Google Scholar]
- Wu YN, Johnson SW. Pharmacological characterization of inward current evoked by N-methyl-d-aspartate in dopamine neurons in the rat brain slice. J Pharmacol Exp Ther. 1996;279:457–463. [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc Roy Soc Lond B. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Zhang XX, Bunney BS, Shi WX. Enhancement of NMDA-induced current by the putative NR2B selective antagonist ifenprodil. Synapse. 2000;37:56–63. doi: 10.1002/(SICI)1098-2396(200007)37:1<56::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Zheng F, Erreger K, Low CM, Banke T, Lee CJ, Conn PJ, Traynelis SF. Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat Neurosci. 2001;4:894–901. doi: 10.1038/nn0901-894. [DOI] [PubMed] [Google Scholar]