Abstract
Protein synthesis in skeletal muscle is known to decrease during contractions but the underlying regulatory mechanisms are unknown. Here, the effect of exercise on skeletal muscle eukaryotic elongation factor 2 (eEF2) phosphorylation, a key component in protein translation machinery, was examined. Eight healthy men exercised on a cycle ergometer at a workload eliciting ∼67% peak pulmonary oxygen consumption (V˙O2peak) with skeletal muscle biopsies taken from the vastus lateralis muscle at rest as well as after 1, 10, 30, 60 and 90 min of exercise. In response to exercise, there was a rapid (i.e. < 1 min) 5- to 7-fold increase in eEF2 phosphorylation at Thr56 that was sustained for 90 min of continuous exercise. The in vitro activity of skeletal muscle eEF2 kinase was not altered by exercise indicating that the increased activity of eEF2 kinase to eEF2 is not mediated by covalent mechanisms. In support of this, the increase in AMPK activity was temporally unrelated to eEF2 phosphorylation. However, skeletal muscle eEF2 kinase was potently activated by Ca2+–calmodulin in vitro, suggesting that the higher eEF2 phosphorylation in working skeletal muscle is mediated by allosteric activation of eEF2 kinase by Ca2+ signalling via calmodulin. Given that eEF2 phosphorylation inhibits eEF2 activity and mRNA translation, these findings suggest that the inhibition of protein synthesis in contracting skeletal muscle is due to the Ca2+-induced stimulation of eEF2 kinase.
Very little is known about the control of protein turnover in working skeletal muscle during exercise. While it is recognized that both changes in gene transcription and RNA translation are likely to contribute to the altered phenotype of skeletal muscle in response to repetitive exercise, the relative importance of these two mechanisms remains to be elucidated (Hargreaves & Cameron-Smith, 2002). Studies in humans indicate that the protein synthesis rate is enhanced after exercise, particularly resistance exercise, but may be unchanged or depressed during exercise relative to resting conditions, probably depending on the exercise intensity and the methods used to measure protein metabolism (Rennie, 1996; Wagenmakers, 1998; Kimball et al. 2002). Furthermore, studies by Bylund-Fellenius et al. (1984) in perfused rat hindquarters demonstrated that protein synthesis rates are lower in contracting skeletal muscle, particularly in fast-twitch muscles. However, the mechanisms responsible for the putative decrease in protein synthesis are unknown.
While the synthesis of individual proteins is likely to be under the control of specific gene transcription, global protein synthesis is determined by the rate of messenger RNA translation. Translation is conventionally divided into three phases: initiation, elongation and termination controlled by proteins called eukaryotic initiation, elongation and release factors, respectively (Proud & Denton, 1997). Recent work has focused on the regulation of initiation factors after exercise (for a review see Bolster et al. 2004). By contrast, very few studies have focused on protein synthesis elongation in skeletal muscle. Eukaryotic elongation factor 2 (eEF2) mediates the translocation of the ribosome relative to the mRNA after addition of each amino acid to the nascent chain (Browne & Proud, 2002). The activity of eEF2 is regulated by reversible phosphorylation within the GTP-binding domain at Thr56 (Redpath et al. 1993). This inhibits eEF2 activity by preventing eEF2 binding to the ribosome (Carlberg et al. 1990) thereby impairing elongation rate (Ryazanov & Davydova, 1989; Redpath et al. 1993). Phosphorylation of eEF2 at Thr56 is catalysed by the dedicated Ca2+–calmodulin-dependent protein kinase called eEF2 kinase and can be reversed by specific protein phosphatases (Browne & Proud, 2002).
One study demonstrated that continuous stimulation of isolated rat extensor digitorum longus muscles at 10Hz, but not intermittent stimulation at 100 Hz, increased eEF2 phosphorylation (Atherton et al. 2005). In humans, it was recently demonstrated that eEF2 expression and eEF2 phosphorylation were higher in skeletal muscle of female subjects compared with males at rest (Roepstorff et al. 2005). However, the response to exercise was not studied. In order to examine the regulation of eEF2 and eEF2 kinase in humans, the present study was conducted in order to investigate the time effect of continuous moderate intensity exercise on skeletal muscle eEF2 and eEF2 kinase. eEF2 kinase is activated by factors (i.e. Ca2+, AMP-activated protein kinase (AMPK) and protein kinase A (PKA) activities; Browne & Proud, 2002) which increase in contracting muscle during exercise. It was therefore hypothesized that eEF2 phosphorylation would increase rapidly in response to exercise, as a consequence of the control of eEF2 kinase by allosteric and/or covalent regulation to increase its activity.
Methods
Experimental protocol
Eight healthy young men (25 ± 1 year; mean ±s.e.m.) gave their informed consent to participate in the study, which conformed to the Declaration of Helsinki and was approved by the ethics committee of Copenhagen and Frederiksberg. Body height and weight averaged 1.83 ± 0.02 m and 77 ± 3 kg, respectively. The participants were physically active for at least 4 h per week. One to two weeks before the experiment, peak pulmonary oxygen consumption (V˙O2peak) was determined during an incremental cycle ergometer test to volitional exhaustion, where the average peak pulmonary oxygen uptake was 55 ± 1 ml kg −1 min−1. Respiratory measurements were made using the Douglas bag technique.
Four days before the experiment, participants consumed a standardized diet in which carbohydrate constituted 55–60%energy. On the morning of the experiment participants arrived at the laboratory at 08.00 h having abstained from foodstuffs for the last 8 h as well as heavy physical activity for the last 48 h before the experiment. Subjects rested in the supine position for at least 1 h, after which four incisions for the biopsies were performed under local anaesthesia. Two incisions were made in each leg, with two biopsies obtained from the same incision in each leg. The incisions were made in the mediolateral aspect of the vastus lateralis muscle 15–25 cm above the patella, at least 5 cm apart. After 5–10 min a resting biopsy was obtained, after which the subject commenced exercise on a cycle ergometer. Subjects worked for 90 min at a constant workload corresponding to 67 ± 2% (2.82 ± 0.13 l min−1) of their pre-estimated peak pulmonary oxygen consumption. Following 1, 10, 30, 60 and 90 min of exercise a muscle biopsy was obtained as quickly as possible with the subject still seated on the bike, after which the subject promptly continued cycling. The biopsy samples were frozen in liquid nitrogen between 15 and 30 s after cessation of activity and stored at −80°C until required.
Analytical techniques
All materials were purchased from Sigma-Aldrich (USA) unless otherwise stated. For extraction of tissue protein for analyses, a portion of the biopsy material from each sample was taken and freeze-dried, after which the tissue was dissected free of connective tissue and blood. The sample was then mechanically homogenized in ice-cold buffer (pH 7.5; 50 mm Hepes, 10% glycerol, 150 mm NaCl, 2 mm EDTA, 1 mm EGTA, 2 mm phenyl methyl sulphonyl fluoride, 20 mm sodium pyrophosphate, 20 mmβ-glycerophosphate, 10 mm NaF, 2 mm sodium orthovanadate, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 3 mm benzamidine, 1% (v/v) I gepal) until no visible particles remained. Homogenates were rotated end over end at 4°C for 60 min after which they were centrifuged at 4°C for 60 min at 15 000 g. The supernatants were harvested and total protein content was determined in lysates by the BCA method (Pierce Chemical Company, USA). The remaining extract was stored at −80°C until analysis.
To measure protein expression and phosphorylation, equal amounts of muscle extract proteins were resolved by SDS-PAGE, transferred to polyvinylidene fluoride membrane (Millipore, USA), after which membranes were incubated in a blocking buffer (2% skim milk or 3% BSA, pH 7.4) to reduce background signal. The membranes were then incubated with primary and secondary antibodies for optimized times and concentrations, and washed with Tris-buffered-saline (0.1% Tween-20; pH 7.4). Proteins were visualized by chemiluminescence (ECL plus, Amersham Biosciences, UK) and light detection under conditions of negligible ambient light (Kodak Image Station 2000MM, USA). The primary antibodies used were anti-eEF2 (Santa Cruz Biotechnology Inc., USA; sc-13003), anti-phospho-Thr56-eEF2 (Roepstorff et al. 2005), antieEF2 kinase (US Biological, USA; E2213-50), anti-phospho-Thr172-AMPKα (Cell Signalling Technology, Inc., USA; 2531), and anti-phospho-Ser221-ACC-β (Cell Signalling Technology, Inc., USA; 3661). Secondary antibodies were from DakoCytomation (Denmark). Band intensities were quantified by imaging software (Kodak 1D 3.5, USA). Preliminary experiments demonstrated that the amounts of protein loaded were within the dynamic range for the conditions used and the results obtained (data not shown). Preliminary experiments also showed that when tissue proteins were extracted in the absence of phosphatase inhibitors and lysates were incubated for 30 min at 30°C, the band intensities using the phosphospecific antibodies were negligible when compared with an equivalent sample prepared normally (data not shown). All antigens studied migrated at expected molecular weights.
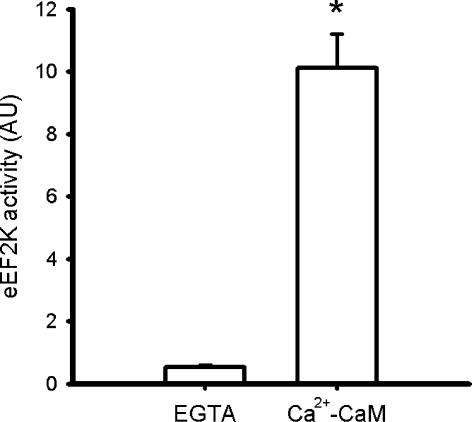
eEF2 kinase activity was measured according to Browne et al. (2004) with minor modifications. Briefly, eEF2 kinase was immunoprecipitated by incubating 3 μg of eEF2 kinase antibodies (US Biological, USA; E2213-50) with 20 μl of 33% (v/v) Protein-A-Sepharose (Amersham Biosciences, UK) and 50 μg of skeletal muscle lysate protein in 500 μl of immunoprecipitation buffer (IB; 10 mm Hepes, pH 7.2, 1 mm EGTA, 1 mm sodium pyrophosphate). The pellets were washed with IB, and the immune complex was assayed in a buffer containing 1 μg eEF2 (purified from HeLa cells; S.G. Finn) and 100 μm ATP (1.0 Ci mmol−1 5′[γ32P]ATP; Amersham Biosciences), with or without 1.2 mm CaCl2, 1.2 μm calmodulin (Upstate Biotechnology, USA). After 10 min at 30°C the reactions were stopped and subjected to SDS-PAGE followed by gel drying and autoradiography (Molecular Dynamics, USA). Samples on each gel were adjusted to an aliquot of an internal control reaction (i.e. immunopurification from rat heart extract). Preliminary experiments showed that the assay was within the dynamic range with respect to time and that there was negligible signal when substrate (i.e. eEF2) or eEF2 kinase antibodies were omitted from the assays (data not shown). In addition, it was shown that there was negligible activity when Ca2+ and calmodulin were omitted from the reaction mixture (Fig. 1), and hence activity of the samples was measured in the presence of Ca2+ and calmodulin.
Figure 1. Skeletal muscle eEF2 kinase activity is Ca2+–calmodulin dependent.
eEF2 kinase was immunoprecipitated from human skeletal muscle samples and activity was measured in vitro in the absence and presence of Ca2+–calmodulin (Ca2+-CaM). Data are mean ±s.e.m. from 4 samples, *P < 0.01 versus EGTA.
Calculations and statistics
Statistical testing was done with descriptive analyses (MS Excel), t tests (MS Excel) or one-way ANOVA with repeated measures with post hoc testing performed when differences were significant as appropriate (Sigma.Stat v. 2.1). Data are expressed as mean ±s.e.m. and differences were considered to be significant when P < 0.05.
Results
Physiological response to exercise
Exercise elicited an average V˙O2 of 2.73 ± 0.11 l min−1 at 10 min and averaged 2.92 ± 0.12 l min−1 at 90 min of exercise, which equated to 65 ± 1 and 69 ± 2% V˙O2peak, respectively. Respiratory exchange ratio was 0.96 ± 0.01 and 0.90 ± 0.01 at 10 and 90 min of exercise, respectively.
Exercise increases skeletal muscle eEF2 phosphorylation
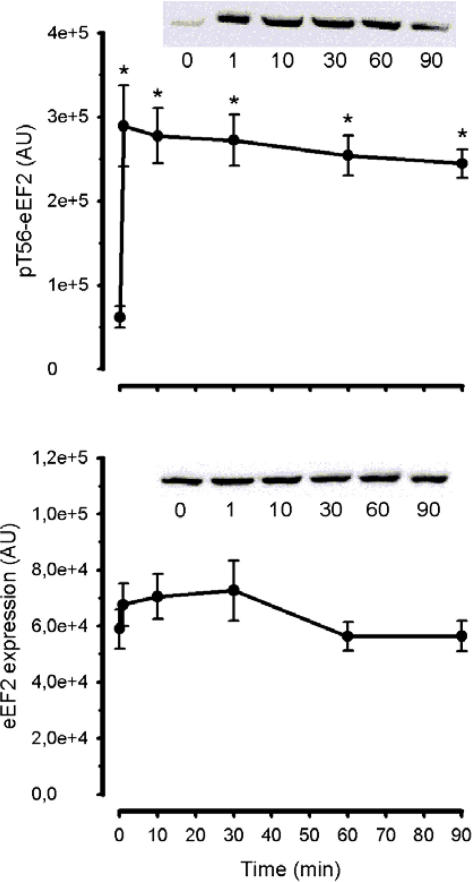
Phosphorylation of eEF2 at Thr56 in working skeletal muscle during exercise increased rapidly within 1 min and reached levels 5- to 7-fold higher than under basal conditions (Fig. 2). Moreover, eEF2 phosphorylation remained at this level over the 90 min of exercise (Fig. 2). There was no change in expression of skeletal muscle eEF2 at any time (Fig. 2).
Figure 2. Time course of the effect of exercise on eEF2 phosphorylation and expression.
Human skeletal muscle samples were subject to SDS-PAGE and immunoblotted with pT56-eEF2 (top panel) and eEF2 (bottom panel) antibodies. Data are mean ±s.e.m., n = 8; *P < 0.01 versus time 0. Representative immunoblots are shown. AU, arbitrary units.
Exercise does not increase in vitro eEF2 kinase activity
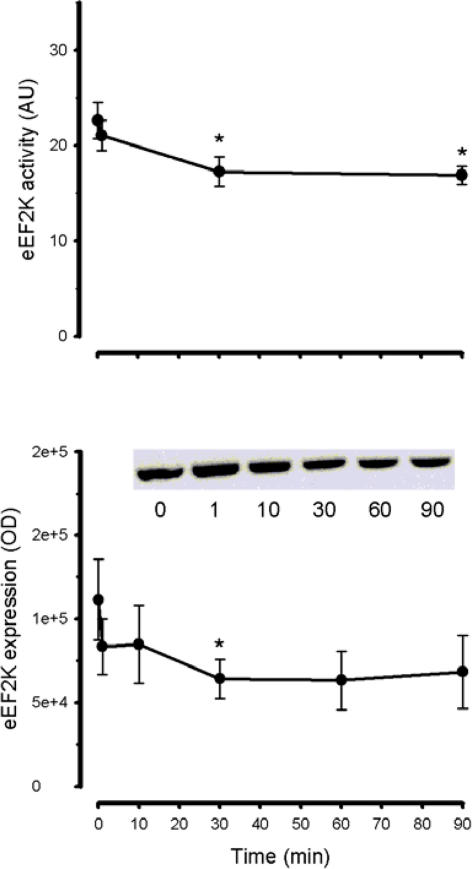
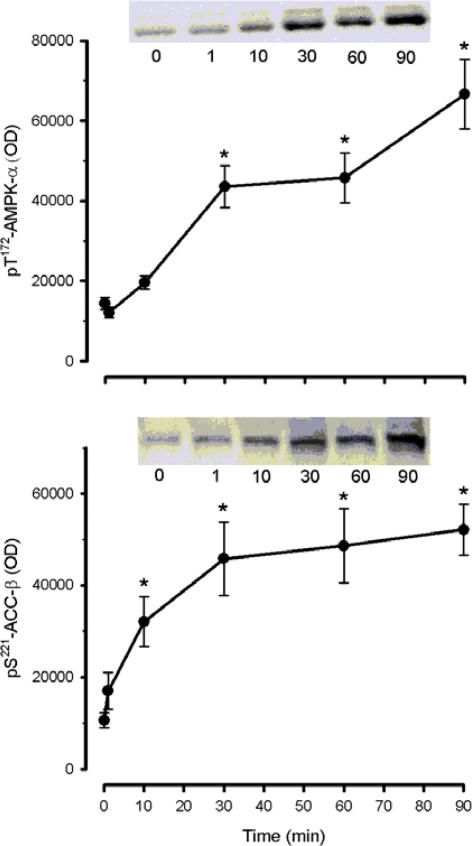
To explain the increase in eEF2 phosphorylation seen during exercise, total eEF2 kinase activity was measured in the presence of Ca2+ and calmodulin from immunoprecipitates of skeletal muscle extracts. Skeletal muscle eEF2 kinase activity was ∼20% lower at 30 and 90 min of exercise when compared with basal level (Fig. 3). This decrease in activity may reflect a decrease in eEF2 kinase protein levels in the tissue lysates (Fig. 3). In contrast to the rapid increase in eEF2 phosphorylation, exercise elicited a gradual and progressive increase in AMPK α-subunit phosphorylation at Thr172 with a slightly faster pattern of phosphorylation of the AMPK substrate acetyl CoA carboxylase-β at Ser221 (Fig. 4).
Figure 3. Time course of the effect of exercise on eEF2 kinase activity expression and phosphorylation.
eEF2K was immunoprecipitated from skeletal muscle samples and in vitro activity was measured (top panel). Human skeletal muscle samples were subject to SDS-PAGE and immunoblotted with eEF2 kinase (bottom panel) antibodies. Data are mean ±s.e.m., n = 8; *P < 0.05 versus time 0. Representative immunoblots are shown. AU, arbitrary units; OD, optical density
Figure 4. Time course of the effect of exercise on AMPK-α and ACC-β phosphorylation.
Human skeletal muscle samples were subject to SDS-PAGE and immunoblotted with pT172-AMPK-α (top panel) and pS221-ACC-β (bottom panel) antibodies. Data are mean ±s.e.m., n = 8; *P < 0.01 versus time 0. Representative immunoblots are shown. OD, optical density.
Discussion
The major novel finding of this study was that exercise induced a rapid (i.e. < 1 min), potent (i.e. 5- to 7-fold) and sustained increase in Thr56 eEF2 phosphorylation in working skeletal muscle of humans (Fig. 2). The only other study that has examined eEF2 regulation in skeletal muscle showed that continuous stimulation of isolated rat extensor digitorum longus muscles at 10 Hz, but not intermittent stimulation at 100 Hz, led to an increase in eEF2 phosphorylation (Atherton et al. 2005). Although it is difficult to compare these two models, this does raise the possibility that eEF2 phosphorylation only occurs at low-frequency stimulation and that the increase in eEF2 phosphorylation may only occur in slow-twitch fibres that receive this type of stimulation in vivo (Hennig & Lømo, 1985). Clearly, further studies are required to delineate the fibre or stimulation type specificity of eEF2 phosphorylation in response to contraction, particularly in vivo.
Several but not all studies indicate that protein synthesis rates in active muscle decline during exercise in humans (Rennie, 1996; Wagenmakers, 1998; Kimball et al. 2002). A study using the perfused rat hindquarter, where the hindlimb muscles were stimulated at a frequency of 4 Hz with 14C-phenylalanine incorporation into muscle proteins used to trace protein synthesis, demonstrated that protein synthesis was decreased in muscles containing fast-twitch fibres (i.e. tibialis anterior, gastrocnemius, plantaris) with no change in the slow-twitch soleus muscle (Bylund-Fellenius et al. 1984). Given that eEF2 phosphorylation increases in fast-twitch muscle during slow frequency stimulation (Atherton et al. 2005), and that Thr56 eEF2 phosphorylation decreases eEF2 activity (Redpath et al. 1993) and hence peptide chain elongation, the increase in eEF2 phosphorylation observed in the present study is likely to be one mechanism by which protein synthesis is inhibited in working muscle. However, in other models of cellular stress using non-muscle cells, it has been shown that protein synthesis can be decreased in the absence of a rise in eEF2 phosphorylation (Patel et al. 2002). Under these conditions, the inhibition of protein synthesis might be due to control at initiation and thus in the present study the putative decrease in muscle protein synthesis rate during exercise may be partially independent of eEF2 inhibition. Indeed, a study using rat skeletal muscle suggested that the decrease in protein synthesis during contraction involved regulation both at the initiation and elongation phases (Bylund-Fellenius et al. 1984). Nevertheless, as the elongation phase is the step that accounts for the vast majority of the energy consumed during protein synthesis, the increase in eEF2 phosphorylation in working muscle may be an important mechanism to lower energy consumption in contracting myocytes to spare ATP for the activated ATPases during contraction (Browne & Proud, 2002). Furthermore, the inhibition of elongation rather than initiation during contraction may preserve polysome integrity (Wong et al. 1991), allowing a rapid induction of translation once the period of contraction is over (Browne & Proud, 2002).
Phosphorylation of eEF2 at Thr56 is catalysed by eEF2 kinase which, when purified from non-muscle cells, was shown to be stimulated by Ca2+–calmodulin (Mitsui et al. 1993; Redpath & Proud, 1993). Likewise, eEF2 kinase activity immunoprecipitated from skeletal muscle was strongly dependent on Ca2+–calmodulin (Fig. 1). In addition to this allosteric stimulation, eEF2 kinase is regulated covalently by multisite phosphorylation, some sites being inhibitory and some sites being activating (Browne & Proud, 2002). eEF2 kinase activity is enhanced by phosphorylation via cAMP-dependent protein kinase (McLeod et al. 2001) and AMP-activated protein kinase (Horman et al. 2002; Browne et al. 2004), and the inhibition of protein synthesis correlates positively with the magnitude of the decrease in energy charge in working rat skeletal muscle (Bylund-Fellenius et al. 1984). However, in vitro eEF2 kinase activity was not increased by exercise (Fig. 3) indicating that the putative increase in the in vivo eEF2 kinase activity was not mediated by phosphorylation at activating sites. In support of these data, the increase in eEF2 phosphorylation occurred rapidly after the onset of exercise, whereas the increase AMPK activity was more gradual, indicating a dissociation of AMPK activity and eEF2 phosphorylation. The lack of apparent effect of heightened AMPK activity is surprising given that it has been repeatedly shown that eEF2 kinase is a substrate of AMPK in cardiac muscle (Horman et al. 2002, 2003; Browne et al. 2004) and that changes in eEF2 phosphorylation appear to mimic that of AMPK phosphorylation in electrically stimulated rat skeletal muscle (Atherton et al. 2005). However, AMPK could still contribute to the lowering of protein synthesis in skeletal muscle during exercise by signalling to translation initiation, as treatment of muscle with the AMPK activator AICAR reduces phosphorylation/activity of eukaryotic initiation factor eIF4E-binding protein at Thr37 (Bolster et al. 2002). The lack of association of AMPK and eEF2 kinase activities in skeletal muscle in the present study may be explained by the expression of an eEF2 kinase isoform in skeletal muscle that is not an AMPK substrate, or to the in vivo exercise compared with previous in vitro studies. Indeed, tissue differences in eEF2 kinase are not unprecedented (Browne & Proud, 2002). It has also been observed that higher eEF2 phosphorylation occurs after only 10–15 s of electrically stimulated contractions in rat gastrocnemius muscle (A. J. Rose, B. Kiens & E. A. Richter, unpublished observation). Such a rapid rise in eEF2 phosphorylation is consistent with stimulation of eEF2 kinase by Ca2+–calmodulin, as rises in intracellular free [Ca2+] are a requirement of excitation–contraction coupling in skeletal muscle (Bozler, 1954), and hence must occur at the immediate onset of contraction. Indeed, there are many examples showing that an increase in intracellular Ca2+ and increases in eEF2 phosphorylation are linked to protein synthesis inhibition (for review see Nairn et al. 2001). On the other hand, the increase in eEF2 phosphorylation could also be the result of a decrease in protein phosphatase activity for eEF2, as has been suggested to occur in working hearts (Horman et al. 2003). Clearly further work is required to fully delineate the roles of eEF2 kinase and phosphatases in this process.
In conclusion, skeletal muscle eEF2 phosphorylation at Thr56 is rapidly induced by exercise and may play a role in the reduction in protein synthesis rate in active muscle during exercise. It is hypothesized that the increase in eEF2 phosphorylation is mediated by a Ca2+–calmodulin–eEF2 kinase cascade.
Acknowledgments
The authors acknowledge the technical assistance of Irene Beck Nielsen and Betina Bolmgren and financial support from the Copenhagen Muscle Research Centre, from the Danish Natural Science Research Council, an Integrated Project (contract number LSHM-CT-2004-005272) from the European Union, and the British Heart Foundation (to C.G.P.). A.J.R. was supported by a postdoctoral fellowship from the Carlsberg Foundation and from the European Union.
Reference
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Bozler E. Relaxation in extracted muscle fibers. J General Physiol. 1954;38:149–159. doi: 10.1085/jgp.38.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygard O. Functional properties of phosphorylated elongation factor 2. Eur J Biochem. 1990;191:639–645. doi: 10.1111/j.1432-1033.1990.tb19169.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Cameron-Smith D. Exercise, diet, and skeletal muscle gene expression. Med Sci Sports Exerc. 2002;34:1505–1508. doi: 10.1097/00005768-200209000-00017. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- McLeod LE, Wang L, Proud CG. β-Adrenergic agonists increase phosphorylation of elongation factor 2 in cardiomyocytes without eliciting calcium-independent eEF2 kinase activity. FEBS Lett. 2001;489:225–228. doi: 10.1016/s0014-5793(01)02100-7. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Brady M, Palfrey HC, Nairn AC. Purification and characterization of calmodulin-dependent protein kinase III from rabbit reticulocytes and rat pancreas. J Biol Chem. 1993;268:13422–13433. [PubMed] [Google Scholar]
- Nairn AC, Matsushita M, Nastiuk K, Horiuchi A, Mitsui K, Shimizu Y, Palfrey HC. Elongation factor-2 phosphorylation and the regulation of protein synthesis by calcium. Prog Mol Subcell Biol. 2001;27:91–129. doi: 10.1007/978-3-662-09889-9_4. [DOI] [PubMed] [Google Scholar]
- Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem. 2002;269:3076–3085. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- Proud CG, Denton RM. Molecular mechanisms for the control of translation by insulin. Biochem J. 1997;328:329–341. doi: 10.1042/bj3280329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath NT, Price NT, Severinov KV, Proud CG. Regulation of elongation factor-2 by multisite phosphorylation. Eur J Biochem. 1993;213:689–699. doi: 10.1111/j.1432-1033.1993.tb17809.x. [DOI] [PubMed] [Google Scholar]
- Redpath NT, Proud CG. Purification and phosphorylation of elongation factor-2 kinase from rabbit reticulocytes. Eur J Biochem. 1993;212:511–520. doi: 10.1111/j.1432-1033.1993.tb17688.x. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Influence of exercise on protein and amino acid metabolism. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology; Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 995–1035. [Google Scholar]
- Roepstorff C, Schjerling P, Vistisen B, Madsen M, Steffensen CH, Rider MH, Kiens B. Regulation of oxidative enzyme activity and eukaryotic elongation factor 2 in human skeletal muscle: influence of gender and exercise. Acta Physiol Scand. 2005;184:215–224. doi: 10.1111/j.1365-201X.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Davydova EK. Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation. Phosphorylated EF-2 is unable to catalyze translocation. FEBS Lett. 1989;251:187–190. doi: 10.1016/0014-5793(89)81452-8. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJ. Protein and amino acid metabolism in human muscle. Adv Exp Med Biol. 1998;441:307–319. doi: 10.1007/978-1-4899-1928-1_28. [DOI] [PubMed] [Google Scholar]
- Wong WL, Brostrom MA, Brostrom CO. Effects of Ca2+ and ionophore A23187 on protein synthesis in intact rabbit reticulocytes. Int J Biochem. 1991;23:605–608. doi: 10.1016/0020-711x(87)90055-3. [DOI] [PubMed] [Google Scholar]