Abstract
We assessed segmental and global left ventricular (LV) diastolic function via tissue-Doppler imaging (TDI) as well as Doppler flow variables before and after a marathon race to extend our knowledge of exercise-induced changes in cardiac function. Twenty-nine subjects (age 18–62 year) volunteered to participate and were assessed pre- and post-race. Measurements of longitudinal plane TDI myocardial diastolic velocities at five sites on the mitral annulus included peak early myocardial tissue velocity (E′), peak late (or atrial) myocardial tissue velocity (A′) and the ratio E′/A′. Standard pulsed-wave Doppler transmitral and pulmonary vein flow indices were also recorded along with measurements of body mass, heart rate, blood pressures and cardiac troponin T (cTnT), a biomarker of myocyte damage. Pre- to post-race changes in LV diastolic function were analysed by repeated measures ANOVA. Delta scores for LV diastolic function were correlated with each other and alterations in indices of LV loading. Diastolic longitudinal segmental and mean TDI data were altered post-race such that the mean E′/A′ ratio was significantly depressed (1.51 ± 0.34 to 1.16 ± 0.35, P < 0.05). Changes in segmental and global TDI data were not related to an elevated post-race HR, a decreased post-race pre-load or an elevated cTnT. The pulsed wave Doppler ratio of peak early transmitral flow velocity (E)/peak late (or atrial) flow velocity (A) was also significantly reduced post-race (1.75 ± 0.46 to 1.05 ± 0.30, P < 0.05); however, it was significantly correlated with post-race changes in heart rate. The lack of change in E/E′ from pre- to post-race (3.4 ± 0.8 and 3.3 ± 0.7, respectively) suggests that the depression in diastolic function is likely to be due to altered relaxation of the left ventricle; however, the exact aetiology of this change remains to be determined.
The existence of a decline in left ventricular (LV) function after prolonged exercise remains controversial (Perrault et al. 1986; McGavock et al. 2002; McGavock et al. 2003). However, we (George et al. 2004; Whyte et al. 2005), and others (Manier et al. 1991; Lucia et al. 1999), have consistently observed a decline in LV diastolic function after running a marathon race, in the absence of changes in indices of LV systolic function. The explanation for such alterations has presented a significant challenge to previous studies for a variety of reasons. Firstly, most studies, including our own, have used pulsed-wave Doppler blood flow variables to assess LV diastolic function, such as the ratio of peak early transmitral flow velocity (E) to peak late (or atrial) flow velocity (A). Whilst these data may be informative they represents only one method of assessing LV diastolic function. Because LV diastolic function and its determinants are complex it would seem evident (Hees et al. 2004) that the use of a range of non-invasive techniques and variables might be more illuminating (Ommen, 2001). Secondly, evidence to support an exercise-induced decline in LV diastolic function has often come from studies that have involved exercise of extreme duration in severe environmental conditions (e.g. Douglas et al. 1987; Rifai et al. 1999). A common consequence of such exercise is a high heart rate and a change in LV loading (reduced pre-load) at the post-exercise assessment. These alterations provide a significant challenge to the interpretation of standard indices of LV diastolic function such as E/A, which is known to be pre-load or HR dependent (Dawson et al. 2003).
Tissue-Doppler imaging (TDI) has been developed over the past 10 years, as an adjunct to standard echocardiographic assessment of the left ventricle, to assess myocardial tissue velocities. Benefits of TDI include (a) high reproducibility and accuracy, (b) excellent feasibility even in subjects with poor 2-D echocardiograms, and (c) assessment of both regional and global LV function (Alam et al. 1999; Pellerin et al. 2003; Nagueh, 2003). Importantly, there is evidence to suggest it is less preload dependent that standard pulsed-wave Doppler imaging (Yalcin et al. 2002; Pela et al. 2004b), although this remains controversial (Firstenberg et al. 2001). To date no study has utilized TDI to assess segmental LV diastolic function after prolonged endurance exercise. Because of the aforementioned benefits of TDI it may have more value than conventional echocardiographic examinations, in the detection and understanding of exercise-induced changes in cardiac function.
The purpose of this study therefore was to assess LV diastolic function before and immediately after a marathon race in a heterogeneous cohort of runners utilizing TDI myocardial velocities. Secondly, the study sought to compare data gained from TDI with LV diastolic functional parameters gained from standard transmitral Doppler flow parameters. Finally, we also employed Doppler assessment of pulmonary vein flow in a further effort to help explain likely changes in diastolic filling (George et al. 2004) and to differentiate patterns of normal, impaired and pseudo-normal relaxation of the left ventricle that may be masked in standard transmitral Doppler flow patterns (Khouri et al. 2004). We hypothesized that post-race changes in pulsed-wave Doppler E/A will be mirrored by changes in the TDI-derived ratio of peak early myocardial tissue velocity (E′) to peak late (or atrial) myocardial tissue velocity (A′), and pulmonary vein flow variables.
Methods
Subjects
Twenty-nine runners participating in the London Marathon 2003 (distance 42.2 km) volunteered and provided written informed consent. Twenty-three were male and six female, with mean ±s.d. age 33 ± 10 years (range 18–62 years), body mass 76 ± 9 kg (range 57–87 kg), and height 1.73 ± 0.09 m (range 1.56–1.89 m). No specific fitness data were assessed but marathon finishing times were 256 ± 46 min (range 186–362 min) and these times can be used to demonstrate a broad spectrum of physical work capacities in this cohort. The study conformed to the standards set by the Declaration of Helsinki and ethical approval was obtained from Liverpool John Moores University Ethics Committee. Exclusion criteria included any personal and/or early family history of cardiopulmonary disease.
Design
The study employed a repeated measures design with the subject's initial assessment approximately 24 h prior to the completion of the race. For the post-race assessment subjects were asked to attend a testing area adjacent to the finish within 30 min of race completion. All test procedures, apart from a general health questionnaire, were administered on both occasions. All subjects were advised to abstain from hard training within the 48-h period prior to pre-testing. Subjects were also advised to drink water/sports drinks before participation and when required during the race, in order to maintain hydration status. Subjects were asked to avoid caffeine and alcohol consumption before both assessments, and the pre-race assessment occurred at least 3 h after their last meal. Race conditions were mild with a maximal temperature of 14°C, approximately 50% relative humidity, light winds and scattered cloud cover.
Procedures
Subjects were initially assessed for body mass (BM), in shorts and vest with footwear and T-shirt removed, on standard portable scales (Model A3JJT1K, Hanson, Sevenoaks, UK). Subjects then lay supine and after a 5 min resting period duplicate brachial artery systolic and diastolic blood pressures were assessed by standard auscultation. Also at this time a resting heart rate was recorded from the ECG within the echocardiography system.
Two-dimensional, M-mode, Doppler and pulsed tissue Doppler imaging (TDI) echocardiographic scans were performed using a commercially available ultrasound system (Siemens Medical, Acuson Sequoia, Mountain View, CA, USA) with a 2.5–4 MHz phased array transducer. All acquisitions were made with the subject lying in the left lateral decubitas position.
The mitral annulus tissue Doppler profile was obtained from five annular sites (lateral, septal, anterior, inferior and posterior walls), using the apical window, to assess longitudinal shortening. A 2 mm sample volume was employed ensuring the best alignment between wall motion and the ultrasound beam. The high pass filter was bypassed and gains set to a minimal value to obtain the best signal to noise ratio. The nyquist limit was set between 10 and 35 cm s−1. Peak early diastolic (E′) and late diastolic (A′) myocardial tissue velocities were recorded and the ratio E′/A′ was subsequently derived for all five mitral annular sites. Mean E′ and A′ and E′/A′ were calculated as the average value of all five annular sites.
Pulsed-wave Doppler recordings of mitral inflow were obtained from the apical four-chamber view. A 4 mm sample volume was placed at the tips of the mitral leaflets. Gain and filter settings were optimized to obtain best signal to noise ratio for all spectral Doppler flow recordings. Peak early (E) and late (or atrial) (A) flow velocities were measured and the ratio E/A calculated. Pulsed-wave Doppler and TDI data were combined in the calculation of the E/E′ ratio. The E/E′ ratio has been reported to be an excellent non-invasive estimate of left atrial pressure (Nagueh et al. 1998; Ommen et al. 2000) and thus an indirect assessment of preload. The E-wave deceleration time and A-wave duration was also measured. Atrial filling was estimated using a 5 mm sample volume placed 1 cm into the right upper pulmonary vein with colour flow guidance. Peak systolic pulmonary vein flow velocity, peak diastolic pulmonary vein flow velocity, peak atrial reverse pulmonary vein flow velocity (Ar) and peak atrial reverse flow duration were measured. The percentage of left atrial filling occurring in systole as well as the ratio A flow duration/Ar flow duration were subsequently derived.
M-mode and two-dimensional echocardiographic recordings were obtained from a parasternal long axis view in accordance with recommendations from the American Society of Echocardiography (Schiller et al. 1989). All system settings were optimized to produce best signal to noise ratio and provide optimal endocardial definition. Measurements obtained were left ventricular dimension at end-diastole and end-systole (LVIDd, LVIDs) and posterior wall thickness at end systole according to the guidelines set out by Sahn et al. (1978). Left ventricular meridonial wall stress (Reichek et al. 1982), an index of afterload, was calculated from M-mode data and blood pressure data.
Two experienced sonographers were used for all examinations. Each subject was assigned to a single sonographer for both pre- and post-race scans. Images were recorded digitally to magneto-optical disc and analysed off-line by a single experienced technician. Based on the heart rate differences pre- and post-race it was impossible to blind the technician to this aspect of analysis. A minimum of three consecutive cardiac cycles were measured and averaged.
In an effort to assess whether tissue damage may underpin any changes in LV diastolic function post-race we collected venous blood samples (5 ml) that were allowed to clot; serum was drawn off then centrifuged and frozen (−20°C) for later analysis. Serum samples were assessed for cardiac troponin T (cTnT), a biomarker of cardiac myocyte damage, utilizing a validated 3rd generation assay (Hallermayer et al. 1999) via electrochemiluminesence (ECL) technology employed within the Elecsys 1010 automated batch analyser (Roche Diagnostics, Mannheim, Germany). All detectable values (> 0.01 μg l−1) represent minor myocardial injury (Collinson et al. 2001).
Statistics
Pre- and post-race values for BM, systolic and diastolic blood pressure, heart rate, M-mode and Doppler echocardiographic indices as well as mean TDI were analysed using repeated measures ANOVA. Data for segmental TDI were analysed via repeated measures two-way ANOVA. Δ (pre–post race) values for all TDI diastolic functional variables were correlated with changes in body mass, LVIDd, E/E′, HR, wall stress, finishing time and post-race cTnT via Pearson's product–moment analysis. Critical α level was set at 0.05 and all analyses were carried out on Statistica software (Statsoft Ltd, Tulsa, OK, USA). All data are reported as means ±s.d. (range).
Results
All 29 subjects completed the marathon and returned for the post-race tests within 30 min of race completion. Echocardiogram clarity was acceptable in all subjects. There was a significant reduction in BM (75.7 ± 9.4 to 74.1 ± 9.5 kg), systolic blood pressure (129 ± 12 to 119 ± 13 mmHg) and diastolic blood pressure (81 ± 6 to 76 ± 7 mmHg) after the race (P < 0.05). As a direct consequence left ventricular meridonial wall stress was significantly reduced post-race (124 ± 32 to 105 ± 34 dynes cm2 (1 dyn = 10 microN)). Not surprisingly HR was significantly increased post-race (57 ± 9 to 85 ± 10 beats min−1), whereas LVIDd was significantly reduced post-race (5.3 ± 0.4 to 5.0 ± 0.5 cm). The index E/E′ was not significantly altered post-race (3.4 ± 0.8 to 3.3 ± 0.7).
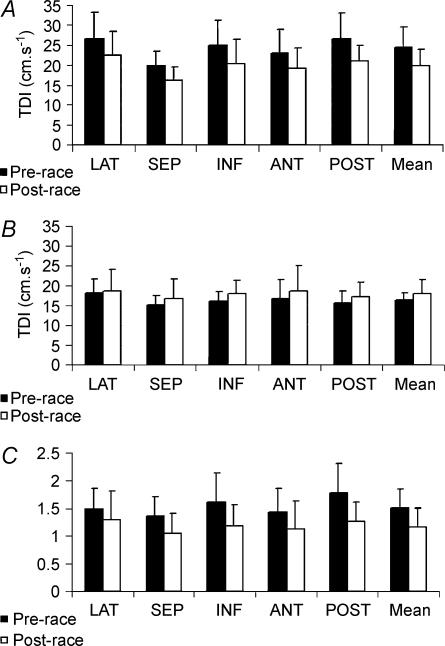
Left ventricular diastolic TDI data are reported in Fig. 1A–C. Inter-site differences in longitudinal TDI were apparent for E′ and A′ myocardial velocities with a general tendency for largest velocities at the lateral site and lowest velocities at the septal site (see Fig. 1A and B, P < 0.05). Fewer intersite differences were noted for TDI E′/A′ although mean data at the septal site were again lower than at the lateral, inferior and posterior sites (P < 0.05). Most TDI variables were significantly altered post-race. Most notably, E′ was significantly decreased and A′ was significantly increased with a subsequent significant reduction in the E′/A′ ratio.
Figure 1. Segmental and global longitudinal diastolic TDI myocardial velocities.
A, E′; B, A′; C, E′/A′. LAT, lateral; SEP, septal; INF, inferior; ANT, anterior; POST, posterior; TDI, tissue Doppler imaging; *Significant difference between pre- and post-race.
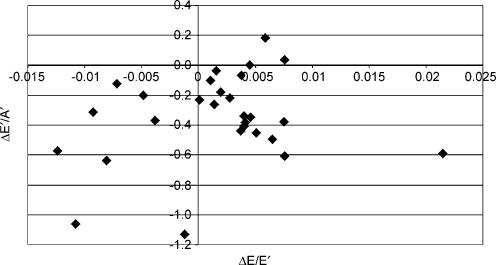
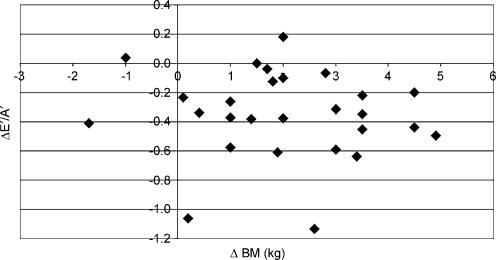
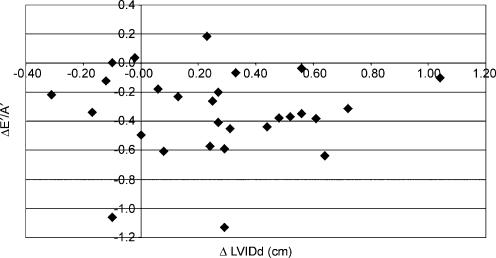
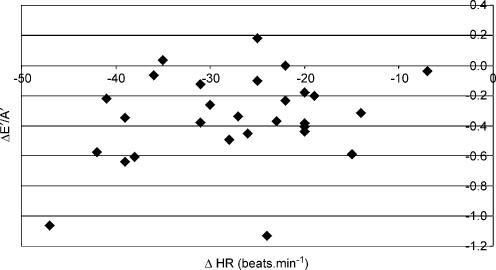
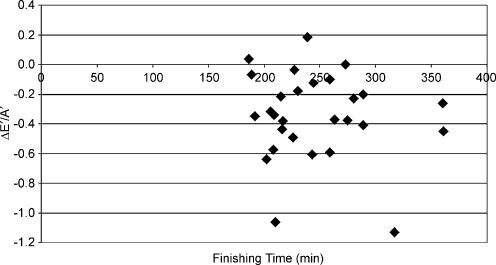
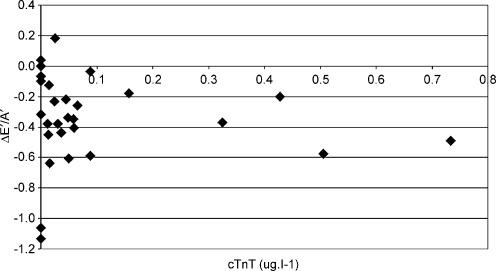
Longitudinal diastolic TDI data were not significantly associated with any changes in indirect indices of pre-load. The relationship between ΔE/E′ and ΔE′/A′ (see Fig. 2) was not statistically significant (r = −0.08) and similar data were reported for the association with changes in BM (r = 0.13, see Fig. 3) and LVIDd (r = −0.11, see Fig. 4). The relationships between ΔE′/A′ and wall stress (r = 0.03) and HR (r = 0.28, see Fig. 5) were also not statistically significant P > 0.05). Further, E′/A′ was not associated with either finishing time (r = −0.11, see Fig. 6) or post-race cTnT (r = −0.09, see Fig. 7).
Figure 2. ΔE′/A′ plotted against ΔE/E′.
Figure 3. ΔE′/A′ plotted against Δbody mass.
Figure 4. ΔE′/A′ plotted against ΔLVIDD.
Figure 5. ΔE′/A′ plotted against ΔHR.
Figure 6. ΔE′/A′ plotted against finishing time.
Figure 7. ΔE′/A′ plotted against cTnT.
The same pattern of post-race change was also apparent in Doppler flow data (see Table 1). A drop in E allied to an increase in A filling velocities resulted in a significant decline in E/A (P < 0.05). Of some concern, however, was the fact that Doppler E/A was significantly correlated with ΔHR (r = −0.40, P = 0.031) even though it did not significantly correlate with any index of afterload and/or preload. Other Doppler-derived diastolic functional indices (see Table 1) demonstrated a diverse pattern of responses pre–post race. Deceleration time for E flow, atrial flow duration, peak systolic pulmnary vein flow velocity and the ratio of A flow duration: Ar flow duration were not different pre- and post-race (P > 0.05). Conversely, peak diastolic pulmonary vein flow velocity, peak Ar pulmonary vein flow velocity, Ar flow duration and percentage of left atrial filling in systole were all significantly altered from pre- to post-race (P < 0.05).
Table 1.
Indices of left ventricular diastolic function derived from pulsed-wave Doppler
| Variable | Pre | Post | P-values |
|---|---|---|---|
| E (m s−1) | 0.80 ± 0.18 | 0.62 ± 0.15 | < 0.005 |
| E flow deceleration time (ms) | 185 ± 44 | 197 ± 51 | 0.397 |
| A (m s−1) | 0.48 ± 0.12 | 0.61 ± 0.13 | < 0.005 |
| A flow duration (ms) | 158 ± 28 | 153 ± 25 | 0.393 |
| E/A | 1.75 ± 0.46 | 1.05 ± 0.30 | < 0.005 |
| Peak systolic PV flow velocity (m s−1) | 0.65 ± 0.16 | 0.64 ± 0.15 | 0.890 |
| Peak diastolic PV flow velocity (m s−1) | 0.55 ± 0.11 | 0.48 ± 0.14 | 0.009 |
| Ar (m s−1) | 0.26 ± 0.04 | 0.29 ± 0.04 | 0.040 |
| Atrial reverse flow duration (ms) | 138 ± 28 | 115 ± 23 | 0.001 |
| A flow duration/A/Ar flow duration | 1.20 ± 0.20 | 1.34 ± 0.29 | 0.074 |
| Left atrial filling in systole (%) | 60 ± 7 | 66 ± 9 | 0.009 |
E, peak early transmitral flow veolicty; A, peak late flow veolicty; Ar, peak atrial reverse pulmonary artery flow velocity; PV, pulmonary vein.
Pre-race Doppler E/A was significantly correlated with pre-race longitudinal TDI E′/A′ at the lateral wall (r = 0.43), septal wall (r = 0.51), inferior wall (r = 0.65), anterior wall (r = 0.55), posterior wall (r = 0.52) and the mean (r = 0.64). The pre–post race change in Doppler E/A was only significantly correlated with Δ longitudinal TDI E′/A′ at the inferior wall (r = 0.47) and anterior wall (r = 0.38).
All pre-race blood samples had no detectable levels of cTnT. One subject declined a post-race blood sample. Post-race the average cTnT was 0.101 ± 0.178 μg l−1. This reflected a range from 0 μg l−1 (undetectable in 7 subjects) to a highest value of 0.733 μg l−1 with measurable cTnT levels found in 75% of subjects (21/28).
Discussion
To the authors' knowledge this is the first study to utilize segmental TDI and pulmonary vein Doppler flow data in the investigation of exercise-induced changes in LV diastolic function. The key finding from this study was that running a marathon had a significant impact upon diastolic longitudinal TDI myocardial velocities at all segments post-race. With no post-prolonged exercise segmental TDI data available in the literature we can only compare the current information with global indices of systolic and diastolic function derived from pulsed-Doppler measures in this and previous research. It is then pertinent to speculate on possible reasons why an alteration in diastolic function was observed.
Mean TDI data for all sites were slightly higher than normal values (Alam et al. 1999). This suggests a slightly augmented LV diastolic function in our subjects compared to sedentary controls as has been reported in other cross-sectional athlete-control comparisons (e.g. Pela et al. 2004a). The average change in diastolic TDI data was similar site to site, suggestive of a global decrease in diastolic function. This interpretation is supported by the concomitant drop in pulsed-wave Doppler E/A and the correlational data relating E′/A′ to E/A. The relatively close correlation between these variables supports data from previous literature that has demonstrated positive correlations between TDI and Doppler diastolic data at rest (Farias et al. 1999; Firstenberg et al. 2001). Likewise the current data reinforce the finding of a reduced E/A following a marathon race (Manier et al. 1991; Lucia et al. 1999; George et al. 2004; Whyte et al. 2005) as well as prolonged activity of shorter (Eysmann et al. 1996) and longer duration (Douglas et al. 1987).
The current study's observation of a decline in global diastolic function after prolonged exercise is difficult to fully explain partially due to the fact that diastolic function is complex and relatively poorly understood compared to systolic function (Libonati, 1999). We can, however, offer some further insights based upon the combination of echocardiographic data obtained, the data analysis performed and reference to previous literature as well as an assessment of potential limitations of the study and assessment techniques. Early diastolic function is effectively driven by the pressure gradient between the left atrium and the left ventricle. An alteration in early diastolic function could therefore originate in altered left atrial pressure changes in diastole and/or a disturbance of the pressure decay in the LV during the diastolic period (Hees et al. 2004). Without direct catheterization a clear explanation of changes in left atrial and LV pressure, and thus their pressure gradient, throughout diastole are not available. However, we utilized other non-invasive indices in an attempt to inform this scenario. The ratio E/E′, a non-invasive indicator of left atrial pressure (Nagueh et al. 1998; Ommen et al. 2000), did not change pre–post race, which would suggest the depression in TDI and pulsed-wave Doppler flow were predominantly due to changes in LV pressure decay. Further, our study data were augmented, uniquely, by pulmonary vein flow variables. Changes in pulmonary vein flow post-race were indicative of impaired relaxation (Khouri et al. 2004). Key characteristics of impaired relaxation as defined by Khouri et al. (2004), peak systolic pulmonary vein flow velocity > peak diastolic pulmonary vein flow velocity, pulmonary vein atrial flow duration > pulmonary vein atrial reversal flow duration, and a significant decline in peak diastolic pulmonary vein flow velocity from pre- to post-race were apparent in our data. The decrease in peak diastolic pulmonary vein flow velocity from pre- to post-race would suggest that the left ventricle is not relaxing as well as at rest and thus the suction effect of the decline in LV pressure to promote transmitral flow is reduced, relative to pre-race data. The pulmonary vein flow data suggest a moderate but significant reduction in diastolic function that is manifested in reduced early diastolic relaxation compensated for by an increased atrial emptying during the late, active contraction portion of diastole.
Such speculation is plausible but must be tempered by the knowledge that any exercise-induced changes in pre-load could produce similar alterations in TDI and Doppler flow data. Again without direct assessment of filling pressures we can only speculate on what happened to pre-load in the current study; however, further analysis of the data and previous research may shed some light on this issue. Firstly, the exercise-induced change in TDI data lacked any significant association with changes in indirect indices of LV loading such as E/E′, body mass loss, and LVIDd. The conclusion from these data would be that most of the change in LV diastolic function could not be explained by altered preload. The use of E/E′, to represent left atrial filling pressure, is worthy of specific comment as it is derived from both Doppler flow and TDI parameters, both of which could be altered by a reduced pre-load leading to a ‘pseudo-maintenance’. Whilst we have to acknowledge this as a potential explanation it is clear from previous literature that E/E′ is a fairly robust parameter that has demonstrated in clinical studies a high (> 0.8) and significant correlation with directly assessed filling pressures in circumstances where LV loading has changed (Sundereswaran et al. 1998; Nagueh et al. 1999; Dokainish et al. 2004). Another point of interest derived from recent literature is that TDI velocities from specific segments may provide useful information. Srivastava et al. (2005) suggested that TDI velocities from the septal annulus are potentially more diagnostically sensitive and less affected by load than those derived from the free wall. In our data septal E′/A′ was significantly reduced post-race, again providing support for a load-independent change in E′/A′.
A further concern when interpreting our post-race TDI data was the influence of a significantly increased HR as small alterations in TDI have been reported with tachycardia in an animal model (Nagueh et al. 2004). We reported, however, no significant correlation between ΔE′/A′ and ΔHR casting doubt on any significant impact of HR upon the post-race TDI data. This is supported by other recent research such as that from Peverill et al. (2004) who reported in subjects at rest, across a HR range of 40–90, that neither septal nor lateral E′ was significantly correlated with HR. Another study by Nagueh et al. (1998) stated that the association of E/E′ with pulmonary capillary wedge pressure was significant in both patients with sinus tachycardia and normal subjects. On the basis of current data and past research it would seem that a post-race increase in HR cannot explain a substantial component of the change in TDI data
Taken together, our data provide some support for an intrinsic impairment of LV relaxation as a mechanism to explain the post-race changes in diastolic function witnessed in the TDI and pulsed-wave Doppler data. Despite such insight the current data do not point to a specific mechanism to explain the impaired relaxation of the left ventricle. We assessed a biomarker of cardiac damage, cTnT, whose appearance in 75% of the cohort post-race was a similar finding to previous marathon data (George et al. 2004; Whyte et al. 2005). However, this evidence of minor cardiac damage was not associated with changes in LV diastolic filling. It would appear that a depression in cardiac function after prolonged exercise and exercise-induced cardiac damage represent two different and unrelated phenomena. Alternatively we might speculate that an altered passive compliance of the left ventricle post-race would influence early diastolic function. Factors that might influence passive compliance such as oedema were not evaluated. In tandem with passive compliance, we could speculate that active relaxation was impaired possibly due to energy store depletion resulting in incomplete or a slowed removal of intracellular Ca2+ from the cytosol to the sarcoplasmic reticulum (Dawson et al. 2003). This, however, remains unsubstantiated but may be the focus of future research. Other factors that might impair active relaxation include an alteration of β-adrenergic responsiveness with prolonged exercise (Eysmann et al. 1996). This has been speculated previously to underpin post-race changes in LV contractile function but not relaxation. Again, this may serve as the focus for future research efforts.
It is pertinent to recognize some study-specific limitations and suggest future pathways for research. Whilst the question of pre-load independence of TDI exists, as discussed above, it may be useful to search for other parameters that do not suffer from such problems. Recent research has suggested that tissue velocity characteristics during the isovolumic relaxation period of diastole before any transmitral flow occurs may be pre-load independent (Hashimoto et al. 2005). It may be useful to include such data in future studies. Normal myocardial motion is affected by three components: radial contraction, longitudinal contraction and rotation. We acknowledge that TDI suffers from an inability to account for any cardiac rotation and translation effect that is superimposed on the muscle velocity data. The use of strain and strain rate, looking at deformation rather than velocity, as well as the myocardial velocity gradient in future studies could overcome the problems caused by rotation and translation. Specifically the use of LV propagation rate (Vp) via colour M-mode would provide another method for assessing LV pressure decline in early diastole, although again pre-load dependency is still being debated (Garcia et al. 1998). This would, however, facilitate a further non-invasive index of left atrial pressure: the E/Vp ratio (Firstenberg et al. 2000). Some insight might also be gained in future studies to compare post-race data collected supine and with legs raised. We did not include a comparative short-exercise bout alongside the marathon race. This would have served to assess whether exercise per se and associated changes in HR and blood pressures could explain some or all of the changes in diastolic function. Previous research, however, from our group (Shave et al. 2002) and others (Douglas et al. 1987; Seals et al. 1988) has employed this strategy and reported no effect of short duration exercise on LV diastolic function even with an elevated HR at the post-exercise assessment. We were also unable to document the reversibility of the current changes with recovery post-marathon. Although such data exists for transmittal flow (Lucia et al. 1999) future research should evaluate this issue with TDI data.
In conclusion, marathon running in recreational runners resulted in a significant change in diastolic longitudinal TDI myocardial velocities, in all segments, suggestive of a global reduction in LV diastolic function. These alterations in diastolic function were supported by pulsed-Doppler flow data across the mitral valve and in the pulmonary vein. These changes were not significantly related to changes in indices of LV loading or HR and thus may represent an exercise-induced alteration in diastolic function. Whilst indirect evidence points towards impairment in LV pressure decay in early diastole the clear aetiology of the changes remains to be fully determined and requires further study.
Acknowledgments
We would like to thank all those associated with Cancer Research UK and Cardiac Risk in the Young at the London Marathon, especially those who volunteered to participate in this study. We would also like to thank Siemens Medical Ltd, UK for technical assistance.
References
- Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed-wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–628. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- Collinson PO, Boa FG, Gaze DC. Measurement of cardiac troponins. Ann Clin Biochem. 2001;38:423–449. doi: 10.1177/000456320103800501. [DOI] [PubMed] [Google Scholar]
- Dawson E, George K, Shave R, Whyte G, Ball D. Does the heart fatigue subsequent to prolonged exercise in humans? Sports Med. 2003;33:365–380. doi: 10.2165/00007256-200333050-00003. [DOI] [PubMed] [Google Scholar]
- Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, et al. Optimal non-invasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–2439. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- Douglas PS, O'Toole ML, Hiller DB, Hackney K, Reichek N. Cardiac fatigue after prolonged exercise. Circulation. 1987;76:1206–1213. doi: 10.1161/01.cir.76.6.1206. [DOI] [PubMed] [Google Scholar]
- Eysmann SB, Gervino E, Vatner DE, Katz SE, Decker L, Douglas PS. Prolonged exercise alters β-adrenergic responsiveness in healthy sedentary humans. J Appl Physiol. 1996;80:616–622. doi: 10.1152/jappl.1996.80.2.616. [DOI] [PubMed] [Google Scholar]
- Farias C, Rodriguez L, Garcia MJ, Sun JP, Klein AL, Thomas JD. Assessment of diastolic function by tissue Doppler echocardiography: Comparison with standard transmitral and pulmonary venous flow. J Am Soc Echocardiogr. 1999;12:609–617. doi: 10.1053/je.1999.v12.a99249. [DOI] [PubMed] [Google Scholar]
- Firstenberg MS, Greenberg NL, Main ML, Drinko JK, Odabashian JA, Thomas JD, et al. Determinants of diastolic myocardial tissue Doppler velocities: Influences of relaxation and preload. J Appl Physiol. 2001;90:299–307. doi: 10.1152/jappl.2001.90.1.299. [DOI] [PubMed] [Google Scholar]
- Firstenberg MS, Levine BD, Garcia MJ, Greenburg NL, Cardon L, Morehead AJ, et al. Relationships of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36:1664–1669. doi: 10.1016/s0735-1097(00)00909-8. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- George KP, Whyte G, Stephenson C, Shave R, Dawson E, Edwards B, et al. Post-exercise left ventricular function and cTnT in recreational marathon runners. Med Sci Sports Exerc. 2004;36:1709–1715. doi: 10.1249/01.mss.0000142408.05337.49. [DOI] [PubMed] [Google Scholar]
- Hallermayer K, Klenner D, Vogel R. Use of recombinant human cardiac troponin T for the standardization of third generation troponin T methods. Scand J Clin Laboratory Invest. 1999;59:128–131. [PubMed] [Google Scholar]
- Hashimoto I, Li X-K, Hejmadi Bhat A, Jones M, Sahn DJ. Quantitative assessment of regional peak myocardial acceleration during isovolumic contraction and relaxation times by tissue Doppler imaging. Heart. 2005;91:811–816. doi: 10.1136/hrt.2004.033845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hees PS, Fleg JL, Dong S-J, Shapiro EP. MRI and echocardiographic assessment of the diastolic dysfunction of normal aging: altered LV pressure decline or load. Am J Physiol. 2004;286:H782–H788. doi: 10.1152/ajpheart.01092.2002. [DOI] [PubMed] [Google Scholar]
- Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–297. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Libonati JR. Myocardial diastolic function and exercise. Med Sci Sports Exerc. 1999;31:1741–1747. doi: 10.1097/00005768-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Lucia A, Serratosa L, Saborido A, Pardo J, Boraita A, Moran M, et al. Short-term effects of marathon running: no evidence of cardiac dysfunction. Med Sci Sports Exerc. 1999;31:1414–1421. doi: 10.1097/00005768-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Manier G, Wickers F, Lomenech AM, Cazorla G, Roudaut R. Echocardiographic assessment of myocardial performance after prolonged strenuous exercise. Eur Heart J. 1991;12:183–1188. doi: 10.1093/eurheartj/12.11.1183. [DOI] [PubMed] [Google Scholar]
- McGavock JM, Haykowsky MJ, Warburton DER, Taylor D, Quinney HA, Welsh RC. Left ventricular systolic performance during prolonged strenuous exercise in female triathletes. Dyn Med. 2003;2:279–292. doi: 10.1186/1476-5918-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavock JM, Warburton DER, Taylor D, Welsh RC, Quinney HA, Haykowsky MJ. The effects of prolonged strenuous exercise on left ventricular function: a brief review. Heart Lung. 2002;31:279–292. doi: 10.1067/mhl.2002.126106. [DOI] [PubMed] [Google Scholar]
- Nagueh SF. Search for non-invasive load-independent indices of left ventricular relaxation. Clin Sci. 2003;105:395–397. doi: 10.1042/CS20030214. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, III, Zogbhi WA, Quniones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Mitaki I, Kopelen HA, Middleton KJ, Quinones MA, Zoghibi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Rao L, Soto J, Middleton KJ, Khoury DS. Haemodynamic insights into the effects of ischemia and cycle length on tissue Doppler-derived mitral annulus diastolic velocities. Clin Sci. 2004;106:147–154. doi: 10.1042/CS20030204. [DOI] [PubMed] [Google Scholar]
- Ommen SR. Echocardiogrpahic assessment of diastolic function. Curr Opin Cardiol. 2001;16:240–245. doi: 10.1097/00001573-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and Doppler tissue imaging in the estimation of left ventricular filling pressures. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- Pela G, Bruschi G, Montagna L, Manara M, Manca C. Left and right ventricular adaptation assessed by Doppler tissue echocardiography I athletes. J Am Soc Echocardiogr. 2004a;17:205–211. doi: 10.1016/j.echo.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Pela G, Regolisti G, Coghi P, Cabassi A, Basile A, Cavatorta A, et al. Effects of the reduction of preload on left and right ventricular myocardial velocities analysed by Doppler tissue echocardiography in healthy subjects. Eur J Echocardiogr. 2004b;5:262–271. doi: 10.1016/j.euje.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Pellerin D, Sharma R, Elliot P, Veyrat C. Tissue Doppler, strain and strain rate echocardiography for assessment of left and right systolic ventricular function. Heart. 2003;89:9–17. doi: 10.1136/heart.89.suppl_3.iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault H, Peronnet F, Lebeau R, Nadeau RA. Echocardiographic assessment of left ventricular performance before and after marathon running. Am Heart J. 1986;112:1026–1031. doi: 10.1016/0002-8703(86)90316-9. [DOI] [PubMed] [Google Scholar]
- Peverill RE, Gelman JS, Mottram PM, Moir S, Jankelowitz C, Bain JL, et al. Factors associated with mitral annular systolic and diastolic velocities in healthy humans. J Am Soc Echocardiogr. 2004;17:1146–1154. doi: 10.1016/j.echo.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Reichek N, Wilson J, Sutton M, Plappert TA, Goldberg S, Hirshfeld JW. Non-invasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation. 1982;65:99–108. doi: 10.1161/01.cir.65.1.99. [DOI] [PubMed] [Google Scholar]
- Rifai N, Douglas PS, O'Toole ML, Rimm E, Ginsburg GS. Cardiac troponin T and I, echocardiographic wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon. Am J Cardiol. 1999;83:1085–1089. doi: 10.1016/s0002-9149(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimesional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- Seals DR, Rogers MA, Hagberg JM, Yamamoto C, Cryer PE, Ehsani AA. Left ventricular dysfunction after prolonged strenuous exercise in healthy subjects. Am J Cardiol. 1988;61:875–879. doi: 10.1016/0002-9149(88)90362-1. [DOI] [PubMed] [Google Scholar]
- Shave R, Dawson E, Whyte G, George K, Ball D, Collinson P, et al. The cardiospecificity of the third-generation cTnT assay after exercise-induced muscle damage. Med Sci Sports Exerc. 2002;34:651–654. doi: 10.1097/00005768-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Srivastava PM, Burrell LM, Calafiore P. Lateral vs medial mitral annular tissue Doppler in the echocardiographic assessment of diastolic function and filling pressures: Which should we use? Eur J Echocardiogr. 2005;6:97–106. doi: 10.1016/j.euje.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sundereswaran L, Nagueh SF, Vardan S, Middleton KJ, Zogbhi WA, Quinones MA, et al. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998;82:352–357. doi: 10.1016/s0002-9149(98)00346-4. [DOI] [PubMed] [Google Scholar]
- Whyte G, George K, Shave R, Dawson E, Stephenson C, Edwards B, et al. Impact of marathon running on cardiac structure and function in recreational runners. Clin Sci. 2005;108:1–8. doi: 10.1042/CS20040186. [DOI] [PubMed] [Google Scholar]
- Yalcin F, Kaftan A, Muderrisoglu H, Korkmaz ME, Flachskampf F, Garcia M, et al. Is Doppler tissue velocity during early left ventricular filling preload independent? Heart. 2002;87:336–339. doi: 10.1136/heart.87.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]