About 25 years ago some colleagues and I carried out long and complicated studies on ourselves to attempt to find out what happened to human protein metabolism during exercise (Rennie et al. 1981). We dosed ourselves with 15N-glycine and measured the production of labelled ammonia in urine before and during nearly 4 h of uphill walking on a treadmill and during the 24 h afterwards. What we found suggested that whole-body protein synthesis was depressed during exercise and that although there was an increase in the utilization of protein as a metabolic fuel this was almost insignificant compared with the use of fat and carbohydrate. We also found some evidence, somewhat to our surprise, that muscle protein breakdown was not increased during exercise but we lacked the appropriate techniques to make direct measurements of protein synthesis and breakdown in human muscle although that did not stop us speculating that muscle protein turnover was probably depressed during exercise: teleologically, muscle's metabolic machinery had more important things to do than worry about maintenance during contractile activity. (In fact no convincing fall in human muscle protein synthesis has yet been demonstrated during resistance exercise – probably for technical reasons although Bob Wolfe and colleagues did show that treadmill walking at about 40% of V˙O2max resulted in a downward trend in quadriceps protein synthesis (Carraro et al. 1990). Nevertheless measurements made over periods of time which include a period of exercise and a period post-exercise, suggest that the average rate of protein synthesis is depressed (Cuthbertson et al. 2002).
In the middle 1980's Ann-Christin Bylund-Fellenius, on sabbatical with Jim Jefferson at Penn State, did experiments in perfused rat hind limb demonstrating that contractile activity was associated with marked depression of muscle protein synthesis and that the effect appeared to be associated with a fall in the ATP/ADP ratio (Bylund-Fellenius et al. 1984). This suggested that muscular contraction imposed some energetic limitation – or at least flipped some allosteric switches which might turn off protein synthesis. However, the rapid report by Rose et al. (2005) in this issue of The Journal of Physiology suggests a much more direct and elegant explanation for why muscle protein synthesis would fall during exercise. What Rose has demonstrated is that one of the factors which controls the rate of translation of pre-existing mRNA in muscle – the eukaryotic elongation factor eEF2 – shows increased phosphorylation (indicating decreased activity) in human muscle during dynamic exercise; the effect is apparently not mediated via what Graham Hardie, its discoverer, has described as the fuel gauge of the cell, AMP kinase but instead by a Ca2+–calmodulin-dependent process activating eEF2 kinase. This is a more direct way of shutting off protein synthesis than the mechanism which would operate through alteration of the ATP/ADP ratio.
It is a pity that the authors did not simultaneously measure protein synthesis (which with modern methods is now feasible) during their studies and indeed, nowadays, I would submit that it is becoming mandatory that studies of signalling should include measurement of the physiological readout as well as indirect measures of signalling activity such as phosphorylation.
Nevertheless, these results raise a number of interesting questions such as what other control mechanisms in muscle protein turnover are regulated by Ca2+–calmodulin-dependent processes? Do any of the processes stay switched off immediately after exercise even though there is an immediate fall in calcium and presumably a decrease in the Ca2+–calmodulin activity as sarcoplasmic calcium concentration falls? What about gene transcription? We are in the middle of a very exciting period of dissection of the control mechanisms of muscle protein metabolism which ought to lead us to a better understanding of alterations in composition (myosin type variation, mitochondrial biogenesis, anabolic hypertrophy, etc.). This work, although of major interest to athletes and sports scientists, is likely to have its pay-off in identifying therapeutic targets for drugs which could revolutionize treatment of muscle wasting in a variety of acute and chronic diseases.
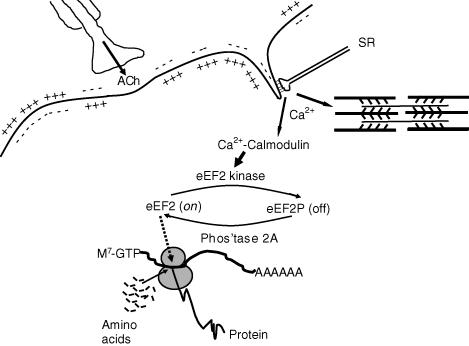
Figure 1.
Scheme showing events after depolarization of muscle during excitation, with Ca2+ activation, contraction and inhibition of protein synthesis via phosphorylation of eEF2.
References
- Bylund-Fellenius A-C, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Am J Physiol. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Am J Physiol. 1990;259:E470–E476. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJR, Smith K, Babraj J, Waddell T, Watt PW, Meier-Augenstein W, Rennie MJ, Hinsch M, Esser K. J Physiol. 2002;539P:S160. [Google Scholar]
- Rennie MJ, Krywawych S, Davies CTM, Halliday D, Waterlow JC, Millward DJ. Clin Sci London. 1981;61:627–639. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider M, Richter EA, Kiens B. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]