Abstract
Reflex actions of muscle afferents in hindlimb flexor nerves were examined on ipsilateral motoneurone activity recorded in peripheral nerves during midbrain stimulation-evoked fictive locomotion and during fictive scratch in decerebrate cats. Trains of stimuli (15–30 shocks at 200 Hz) were delivered during the flexion phase at intensities sufficient to activate both group I and II afferents (5 times threshold, T). In many preparations tibialis anterior (TA) nerve stimulation terminated ongoing flexion and reset the locomotor cycle to extension (19/31 experiments) while extensor digitorum longus (EDL) stimulation increased and prolonged the ongoing flexor phase activity (20/33 preparations). The effects of sartorius, iliopsoas and peroneus longus muscle afferent stimulation were qualitatively similar to those of EDL nerve. Resetting to extension was seen only with higher intensity stimulation (5T) while ongoing flexor activity was often enhanced at group I intensity (2T) stimulation. The effects of flexor nerve stimulation were qualitatively similar during fictive scratch. Reflex reversals were consistently observed in some fictive locomotor preparations. In those cases, EDL stimulation produced a resetting to extension and TA stimulation prolonged the ongoing flexion phase. Occasionally reflex reversals occurred spontaneously during only one of several stimulus presentations. The variable and opposite actions of flexor afferents on the locomotor step cycle indicate the existence of parallel spinal reflex pathways. A hypothetical organization of reflex pathways from flexor muscle afferents to the spinal pattern generator networks with competing actions of group I and group II afferents on the flexor and extensor portions of this central circuitry is proposed.
It is now widely accepted that sensory input from muscle and cutaneous afferents can control motoneurone activity during locomotion by actions on the spinal neuronal circuitry that produces the rhythmic excitation and inhibition of motoneurones, the central pattern generator (CPG). Perhaps the best understood segmental sensory system regulating mammalian locomotion is that from hindlimb extensor group Ia (primary muscle spindle) and group Ib (tendon organ) afferents, collectively referred to as extensor group I afferents (reviewed in McCrea, 2001; Pearson, 2004; Donelan & Pearson, 2004b). Activation of extensor group I afferents during the stance phase of locomotion increases the activity of extensor motoneurones in man (e.g. Sinkjaer et al. 2000) and also during treadmill locomotion in decerebrate cats, where proprioceptive feedback from extensor group I afferents evokes reflexes that may be responsible for as much as 30–70% of the muscle activity occurring during the stance phase (Hiebert & Pearson, 1999; Stein et al. 2000; Donelan & Pearson, 2004a).
During fictive or treadmill locomotion in decerebrate cats, activation of ankle extensor group I afferents delays transition to the flexion phase (Duysens & Pearson, 1980) and prolongs the ongoing extension phase in both spinalized (Conway et al. 1987) and cord intact preparations (Guertin et al. 1995). During fictive locomotion stimulation of the same afferents in the flexion phase terminates flexion and initiates extension, i.e. produces a resetting to extension (Conway et al. 1987; Guertin et al. 1995). There is general agreement that much of the reflex control by extensor group I afferents of locomotor phase duration and phase transitions is produced by actions on the extensor part of the CPG (Conway et al. 1987; Gossard et al. 1994; Hultborn et al. 1998; McCrea, 2001; Lam & Pearson, 2002b; Pearson, 2004; Donelan & Pearson, 2004b). In addition there are locomotor-dependent reflex pathways that produce a group I disynaptic excitation of extensor motoneurones during the extensor phase (see McCrea et al. 1995; Angel et al. 1996, 2005; McCrea, 2001).
Flexor group I muscle afferents also evoke strong actions on the locomotor cycle. During treadmill locomotion in decerebrate cats, activation of group I afferents in nerves from hip (iliopsoas (Psoas) and sartorius (Sart)) and ankle (extensor digitorium longus (EDL)) flexors enhances ongoing flexor motoneurone activity (Hiebert et al. 1996; Lam & Pearson 2001, 2002 a) while the unloading of hip flexors decreases flexor burst duration (Lam & Pearson, 2001; McVea et al. 2005). During fictive locomotion evoked by midbrain locomotor region (MLR) stimulation, activation of Sart or posterior biceps and semitendinusis (PBSt) group I afferents also prolongs the ongoing flexion phase (Perreault et al. 1995). The enhancement of flexion phase activity by group I flexor afferents appears analogous to the facilitation of extensor motoneurone activity by extensor group I afferents and includes a flexor nerve-evoked disynaptic excitation of flexor motoneurones (Degtyarenko et al. 1998; Quevedo et al. 2000).
Group II afferents from secondary muscle spindle receptors are another source of proprioceptive feedback that may control motoneurone activity and cycle-period timing during locomotion. In acute spinal cats during l-DOPA-induced fictive locomotion, group II muscle afferents act in concert with high threshold cutaneous afferents to promote the ongoing flexion phase or to terminate extension and initiate flexion, i.e. reset to flexion (Schomburg et al. 1998). During MLR-evoked fictive locomotion, however, the reflex actions of extensor group II afferents appear weak (Guertin et al. 1995; Donelan & Pearson, 2004a) while flexor group II afferents can evoke powerful actions on step cycle activity that may be preparation dependent (Perreault et al. 1995). Thus activation of TA or EDL group II afferents promotes flexor motoneurone activity during treadmill walking (Hiebert et al. 1996) while during MLR-evoked fictive locomotion group II intensity TA nerve stimulation produces the opposite effect: an inhibition of flexor motoneurones and excitation of extensor motoneurones, i.e. a resetting to extension (Perreault et al. 1995).
Since the TA nerve-evoked resetting to extension was also seen with Sart and PBSt stimulation (Perreault et al. 1995), it originally appeared to us that flexor group II afferents might have common actions during MLR-evoked fictive locomotion. Subsequent experiments using the same preparation in which TA stimulation caused a resetting to extension, however, showed that stimulation of another ankle flexor nerve, EDL, prolonged ongoing flexor activity (McCrea et al. 2000). Further evidence for a complex organization of reflexes evoked by flexor muscle afferents came from the observation that during treadmill locomotion in decerebrate cats, Sart stimulation initially inhibited flexor bursts but after a few stimulus presentations, it increased the duration of the flexion phase (Lam & Pearson, 2002a). This variability is in contrast to the consistent extension-enhancing actions of extensor group I afferents during locomotion under several experimental conditions.
The present experiments were undertaken to further assess the variety and variability of effects evoked by flexor nerve stimulation during fictive locomotion. To this end, we examined the reflex actions from a number of hip and ankle flexor nerves including, EDL, Psoas, Sart, and peroneus longus (PerL) during fictive locomotion evoked by MLR stimulation and during fictive scratch. Comparisons were made between the effects of these and other flexor nerves during fictive locomotion and scratch and with results obtained earlier during fictive locomotion under similar experimental conditions (Perreault et al. 1995). In particular we wished to determine: (1) whether stimulation of afferents from the ankle flexor synergists, TA and EDL, evokes opposite reflex actions on the locomotor cycle in the same preparations, (2) whether group I or group II afferents are responsible for these effects, (3) the variability of flexor nerve-evoked actions on the CPG, and (4) whether flexor nerve-evoked actions are similar during fictive locomotion and scratch. The present report highlights variations in reflex effects produced under essentially identical experimental conditions. These include reversed effects between preparations as well as spontaneous reflex reversals in which reflex actions were opposite after adjacent stimulus presentations. Preliminary results have been reported (McCrea et al. 2000).
Methods
Data were collected from 43 cats (2–4 kg) some of which were also used for other studies (Gosgnach et al. 2000, Quevedo et al. 2000, 2005a, b). Surgical and experimental protocols were in compliance with the guidelines set out by the Canadian Council on Animal Care and the University of Manitoba. Surgery was performed on cats anaesthetized with 1–1.6% halothane delivered in a mixture of 70% nitrous oxide and 30% O2. A surgical plane of anaesthesia was confirmed by continuous monitoring of the arterial blood pressure via a carotid artery cannula and by repeatedly testing for the lack of pedal withdrawal and muscle tone. Surgical preparations and brainstem stimulation were performed as described in Quevedo et al. (2000, 2005a, b). Briefly, the sequence was to induce anaesthesia with halothane and nitrous oxide, dissect the peripheral nerves and make the laminectomy. A decerebration was performed in which the corticies and rostral brainstem structures were removed before withdrawal of anaesthetic agents and induction of neuromuscular block with mechanical ventilation. Neuromuscular block was induced with gallamine triethiodide or pancuronium bromide (with supplemental administration every 45 min) for the remainder of the experiment. At the end of the experiment, a lethal injection of pentobarbital anaesthetic was administered.
Dissected hindlimb nerves were placed on bipolar hook electrodes for either stimulation or recording. These nerves included posterior biceps and semitendinosus (PbSt), semimembrinosus and anterior biceps (SmAB), medial gastrocnemius (MG), lateral gastrocnemius and soleus (LGS), plantaris (PL), flexor digitorum longus (FDL), flexor hallucis longus (FHL), tibial (Tib), peroneus longus (PerL), tibialis anterior (TA), extensor digitorum longus (EDL), and superficial peroneal (SP) nerves. Cuff electrodes were used to mount iliopsoas (Psoas), sartorius (Sart), the vasti (Vas) and rectus femoris (RF) nerves and often the Vas and RF nerves mounted together as quadriceps (Q). Psoas nerve dissection was limited to some of the more caudal branches of the nerve. In a few experiments the branches to the medial and lateral sartorius (mSart and lSart) were mounted separately. Remaining ventral and dorsal nerve branches on the left side and all hindlimb nerves on the right side were cut and tendons around the hip were sectioned bilaterally.
Fictive locomotion was evoked by electrical stimulation (30–400 μA, 0.5–1 ms pulses, 10–30 Hz) of the MLR. Fictive scratch was evoked by topical application of curare (0.01–0.1%) to the ipsilateral dorsal root of the first cervical segment and rubbing the pinna or the skin of the face and neck area. In two experiments 4-aminopyridine (100 μg kg−1) was administered intravenously to improve locomotor behaviour.
Rectified, integrated and digitized electroneurogram (ENG) activity was used to monitor fictive motor output. Programs developed by the Spinal Cord Research Centre were used to capture the data on line and for subsequent analysis using Pentium PCs running Linux. The sampling rates of cord dorsum and rectified-integrated ENG recordings were 2500 Hz and 500 Hz, respectively. Latencies reported for averaged ENG records are given to the nearest millisecond. Using a software-based window discriminator, stimulus delivery to peripheral nerves was triggered in the early to mid flexion or extension phases of the step and/or scratch cycles. Peripheral nerves were stimulated with trains of stimuli (12–53 shocks, usually 200 Hz) at intensities expressed in multiples of threshold (T) for the most excitable fibres in the nerve as measured at the cord dorsum. Trains were usually delivered every two or three steps with the intervening, non-stimulated steps serving as control step cycles. Marker pulses generated by the window discriminator during control and stimulated steps were captured and used for aligning traces during subsequent averaging of ENG data. Unless stated otherwise, latencies for ENG amplitude changes were measured from the afferent volley recorded from the surface of the spinal cord in L6 or L7.
Cycle period was measured as the interval between the onset of consecutive bursts of activity in a selected nerve. Cycle period and onset values for the control and the stimulated steps were compared with Student's two tailed t test (assuming equal variances; α = 0.05). Runs failing the normality test were also analysed by using a non-parametric test (Wilcoxon's Rank Sum/Mann-Whitney U test). All means of cycle durations are reported ± standard deviation.
The effects of hindlimb flexor nerve stimulation delivered during the flexor phase were examined in 34 cats during fictive locomotion, in six animals only during fictive scratch and in three during both scratch and locomotion. Generally the procedure was to begin the experiment by determining if 5T electrical stimulation of the hindlimb flexor nerve under study altered the step cycle period or changed motoneurone activity as measured in the duration or amplitude of rectified–integrated ENG records. Nerve stimulation was usually repeated in several runs.
Results
TA and EDL stimulation evokes opposite effects on the locomotor cycle
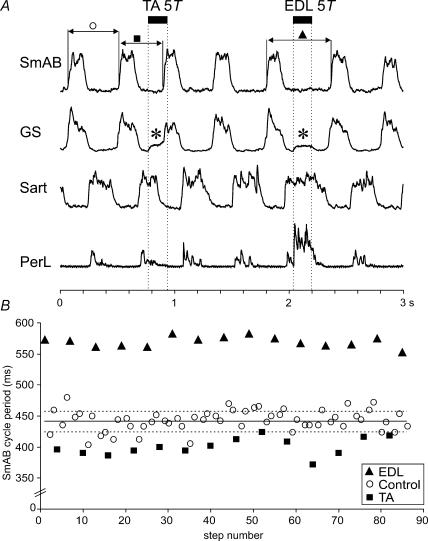
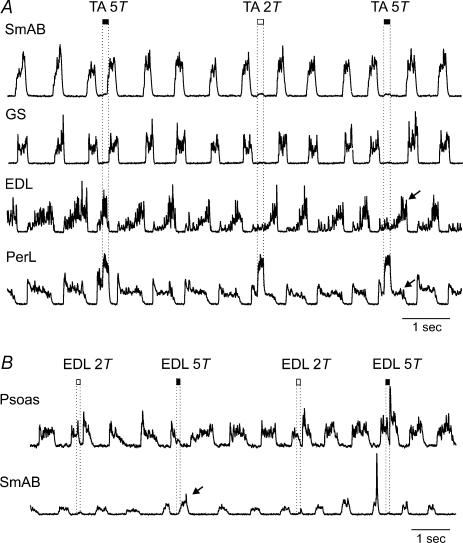
Figure 1A illustrates 3 s from a bout of fictive locomotion in which the effects of TA and EDL nerve stimulation were compared. In this run, stimulation was triggered about 100 ms after the onset of activity in the Sart nerve and delivered every third step cycle to TA and EDL nerves in an alternating fashion.
Figure 1. Step cycle shortening and prolongation evoked by TA and EDL nerve stimulation.
A, rectified–integrated ENGs recorded during MLR stimulation with alternating activity in two extensor (SmAB, GS) and flexor nerves (Sart, PerL). The dotted vertical lines mark the duration of the peripheral nerve stimulation (33 shocks, 200 Hz) at 5T strength delivered every third step, alternately to the TA and EDL nerves. The stimulus train was triggered from activity in the Sart ENG. The horizontal continuous lines indicate the cycle period of the SmAB nerve during control steps with no stimulation to peripheral nerves (○), steps with TA stimulation (▪), and steps with EDL stimulation (▴). In the GS ENG there is an artefact (asterisk) during stimulation. B, changes to locomotor cycle period of the SmAB nerve from TA stimulation and EDL nerve stimulation. Cycle period was measured from the onset of activity in the SmAB nerve for 86 steps in the 40-s period of locomotion from which panel A was taken. The mean control cycle period is shown by the continuous line. Dashed lines indicate the standard deviation.
Stimulation of the TA nerve (5T, 33 shocks) shortened the duration of hip (Sart) flexor ENG activity and initiated an extension phase as seen by the bursts of activity in hip (SmAB) and ankle (GS) extensors. In this example, the delay between the start of the 165 ms duration TA stimulus train and the onset of SmAB activity was about 130 ms. The new extension phase occurred sooner than expected, i.e. there was a phase advance. This can be seen in Fig. 1A by comparing the length of the preceding control cycle (open circle) with that during TA nerve stimulation (filled square). In Fig. 1B, the cycle periods (as measured by the time between the onset of adjacent SmAB bursts) are plotted for 86 steps in the 40 s long bout of fictive locomotion. Control cycle periods (no stimulation delivered, open circles) were almost always longer than the duration of step cycles in which the TA nerve was stimulated (filled squares). The control step cycle periods were on average 441 ± 16 ms, falling to 400 ± 14 ms with TA nerve stimulation (P < 0.01).
During the same bout of fictive locomotion, EDL nerve stimulation increased the duration of the Sart and PerL ENG bursts thereby prolonging the ongoing flexor phase (Fig. 1A) and delaying the initiation of the next extensor phase (filled triangles). The large increase in PerL ENG activity in Fig. 1A likely results in part from the monosynaptic excitation of PerL motoneurones by group Ia afferents in the EDL nerve (Eccles et al. 1957). Figure 1B shows the prolongation of cycle periods (from 441 ms to 568 ± 8 ms, P < 0.01) when the EDL nerve was stimulated (filled triangles). The opposite effects of stimulation evoked by the two ankle flexor nerves, TA and EDL, during the same run of fictive locomotion, show that pathways producing flexion-phase shortening (resetting to extension) and prolongation were both operational in this preparation.
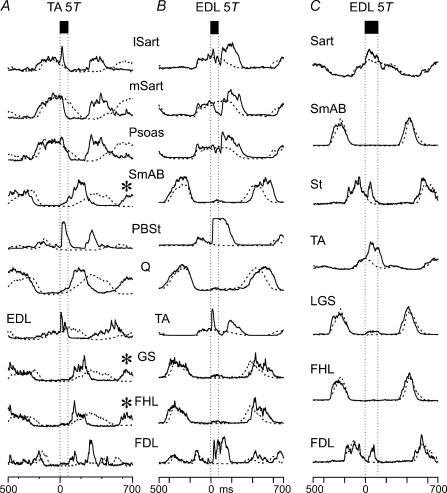
Figure 2A and B compares the effects of TA and EDL nerve stimulation in another experiment. Averages of the ENG activity occurring with (continuous lines) and without (dashed lines) nerve stimulation are superimposed and aligned to the window discriminator output pulse (see Methods). In this example the stimulation was delivered later in the flexor phase than in the experiment shown in Fig. 1. Stimulation of the TA nerve during flexion (Fig. 2A) terminated flexor activity (hip, mSart and Psoas; and ankle, EDL) and advanced the onset of extensor activity (hip, SmAB; knee, Q; and ankle, GS). Consistent with its largely extensor activity during fictive locomotion (Fleshman et al. 1984), FHL is recruited along with the other hindlimb extensors. Extensor activity began during the stimulus train (bounded by the vertical lines) and increased after the end of stimulation. The amplitude of these phase-advanced extensor bursts was larger than that during control steps without TA nerve stimulation (dashed traces).
Figure 2. Opposing actions of TA and EDL affect motoneurone pools throughout the ipsilateral hindlimb.
Averaged, rectified–integrated ENG records during control (no stimulation) step cycles (dashed traces) and during nerve stimulation (continuous traces). Electrical stimulation at 200 Hz of TA (A: 15 shocks, n = 44) and EDL nerves (B: 15 shocks, n = 53; C: 25 shocks, n = 57) at 5T was triggered from flexor nerve activity. A, TA nerve stimulation truncated flexor nerve activity (mSart, Psoas and EDL) and phase advanced extensor activity (Q, SmAB, GS, FHL, FDL). TA stimulation resulted in a resetting of the locomotor period such that activity in the next step is also phase advanced (asterisks). B, in the same experiment, EDL nerve stimulation prolonged ongoing flexor activity and delayed the onset of the next extensor burst. C, in another experiment EDL stimulation affects the amplitude of flexor activity but not step cycle timing.
In this example of TA-evoked resetting to extension and as illustrated by Perreault et al. (1995), the excitation of ankle extensors occurred later than that of hip extensors. In 6/10 experiments in which measurements were made, the onset of ankle extensor activity was delayed from that of SmAB. In those cases, the mean onset of extensor activity as measured from the artefact from the first shock was 54 ± 18 ms in SmAB and 128 ± 69 in GS motoneurone pools (P < 0.05). The example in Fig. 1A shows similar onsets of hip and ankle extensor activity although stimulus artifact obscures the onset of GS excitation. In addition to the effects on step cycle timing, TA stimulation during flexion produced a short-latency excitation of the lSart, PBSt and EDL motoneurone pools with approximate latencies of 7, 15 and 5 ms (as measured from the shock artefact), respectively.
The effects of EDL nerve stimulation in the same animal are shown in Fig. 2B with records obtained about 2 min after those presented in Fig. 2A. Flexor phase activity in lSart, mSart, Psoas and TA was enhanced and prolonged. In the hip flexors, this enhancement was preceded by a brief inhibition during the stimulus train (latency from artefact of the first shock of 21 ms in mSart and 32 ms in Psoas). Note the large increase in flexor motoneurone activity occurring after the stimulus train. Following the increased flexor-phase activity, the onset of the next extensor phase (see Q, SmAB and GS nerves) was delayed by about 100 ms compared to control cycles (dashed lines). Such actions after the end of the stimulus train suggest that the predominant effects of EDL and TA nerve stimulation are not simply stimulus-locked reflexes but are evoked through the central locomotor circuitry. As for the increase of PerL ENG activity in Fig. 1A, the short latency (5 ms) excitation in the TA nerve most likely included a monosynaptic excitation from EDL (Eccles et al. 1957) that preceded the longer latency (112 ms), presumably CPG-mediated, excitation.
Data illustrated in Fig. 2C from a different experiment, show an EDL nerve-evoked enhancement of flexor motoneurone activity unaccompanied by changes in step cycle period or phase timing. Although the amplitudes of Sart and TA ENGs are increased with EDL stimulation (continuous lines), the onset of subsequent extensor bursts occurred close to the time of control step cycles (dashed lines). In this case, the stimulus (5T, 25 shocks at 200 Hz) produced a short latency excitation of hip (Sart) and ankle flexor (TA) motoneurones (2 and 5 ms, respectively, measured from the afferent volley at the cord dorsum) during the stimulus train. The increased activity of St and FDL motoneurones occurred with a latency of about 25 ms.
A change in timing of the step following the one in which TA and EDL nerve stimulation was delivered was seen in four and in one experiments, respectively. Note in Fig. 2A that the onset of the extensor activity in the subsequent burst is visible in the averaged record when the TA nerve is stimulated (continuous traces) but not in control traces (i.e. occurs after the end of the illustrated segment, see stars on SmAB and GS traces). This occurs because the flexor burst immediately following the stimulated step is shorter than in control steps. These effects of stimulation on the timing of the subsequent step cycle strengthen the argument that TA and EDL afferents evoke their reflex actions mainly through the CPG circuitry.
Effects of other flexor nerves
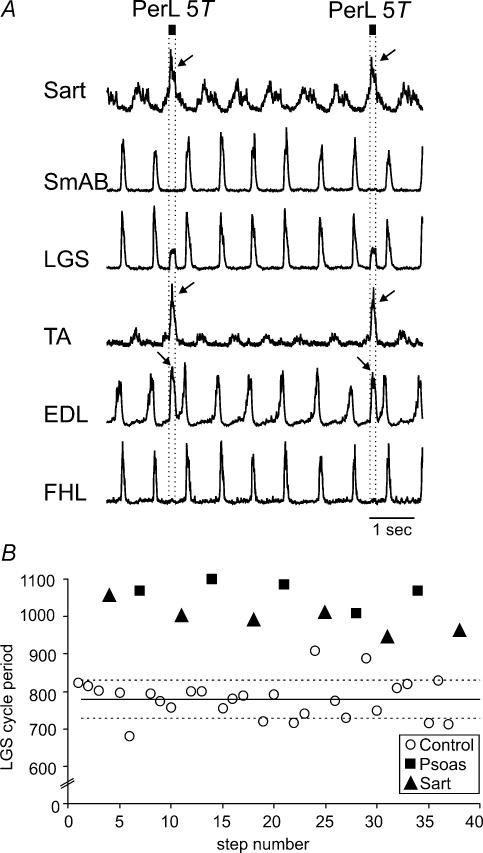
In all four experiments in which the PerL nerve was stimulated there was an excitation of other flexor motoneurones but little effect on the duration of the flexion phase. Figure 3A shows 7 s of a bout of locomotion in which 5T PerL nerve stimulation was delivered every sixth step during the flexion phase of the fictive step cycle. PerL nerve stimulation increased flexor nerve activity (Sart, TA and EDL, arrows) without prolonging the ongoing flexor phase. In the EDL motoneurone pool, a second peak of activity was evoked about 100 ms after the end of the PerL stimulus train.
Figure 3. Flexion enhancement by stimulation of flexor nerves.
A, stimulation of PerL (25 shocks at 200 Hz, dotted lines) was triggered by Sart ENG activity. PerL stimulation increased amplitude but not burst duration of other flexor nerves. The activity in LGS during the stimulus delivery is stimulus artefact. Arrows indicate the increased amplitude of hip (Sart) and ankle (TA and EDL) flexor ENGs during stimulation. B, the effects of stimulation of the Psoas and Sart nerve (trains of 30 pulses, 200 Hz) at 5T delivered during flexion over 38 steps from a 70 s long bout of MLR-evoked fictive locomotion are shown. Similar format as Figure 1B. ○, control cycle periods without peripheral nerve stimulation; ▪, steps with Psoas 5T stimulation; ▴, steps with Sart 5T stimulation.
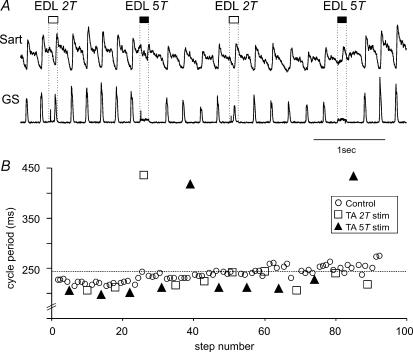
An example of the effects of Psoas nerve stimulation at 5T on the cycle period from another experiment is illustrated in Fig. 3B. Each stimulus train to the Psoas nerve (filled squares) increased the duration of the ongoing flexion phase and delayed the onset of the subsequent extension phase (measured as interval between the onset of adjacent LGS ENG bursts). Cycle period was prolonged by 20% (P < 0.01). These effects are similar to those of EDL illustrated in Fig. 1B. In two other experiments, Psoas nerve stimulation was marginally effective, increasing the ongoing flexion phase duration by only 3% and 4%.
During the same bout of locomotion, Sart nerve stimulation at 5T (filled squares in Fig. 3B) also prolonged the step cycle period (28%, from 779 ± 51 ms, open circles, to 993 ± 35 ms, filled triangles; P < 0.01). Sart nerve stimulation significantly prolonged flexion and delayed the onset of the extension phase in two other experiments (by 11 and 30%). These flexion-promoting effects of 5T Sart nerve stimulation are in contrast to the resetting to extension reported earlier (Perreault et al. 1995).
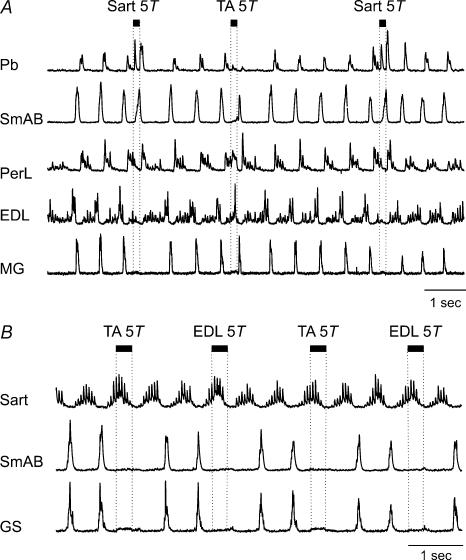
Resetting to extension by 5T Sart stimulation was seen in 2/6 experiments in the present series. An example is shown in Fig. 4A. Sart stimulation at 5T (25 shocks, 200 Hz) terminated flexor phase activity (PerL and EDL traces) and initiated a premature onset of hip extensor motoneurone activity (SmAB ENG). In the 70 s bout of locomotion from which Fig. 4A was taken, Sart nerve stimulation evoked similar effects in all 10 trials, shortening the step cycle period by 21% (P < 0.01). In the other experiment, all six Sart 5T stimulus presentations shortened the step cycle (by 70%, P < 0.01). Unlike the resetting to extension produced by TA nerve stimulation in Fig. 4A, no activity was evoked in ankle extensors when the step cycle was reset to extension by Sart nerve stimulation. In one experiment in which Sart stimulation reset the step cycle to extension, EDL stimulation also reset the cycle to extension. In the other experiment EDL stimulation prolonged the ongoing flexion phase. Sart 5T stimulation had no significant effects on the cycle period in one experiment. The effects evoked by 5T stimulation of flexor nerves during the flexion phase of fictive locomotion are summarized in Table 1.
Figure 4. Omissions of extensor activity following flexor nerve stimulation.
A, rectified–integrated ENGs from hip (SmAB) and ankle (MG) extensor, and ankle flexor (PerL, EDL) and bifunctional (hip extensor, knee flexor, Pb) nerves. Stimulation of the Sart nerve during the flexion phase (5T, 25 shocks, 200 Hz) stopped ongoing flexor phase activity and evoked activity in the SmAB nerve but not in the ankle extensor, MG, nerve. TA nerve stimulation (5T, 25 shocks, 200 Hz) evoked a resetting to extension that included activity in the ankle extensor nerve in the illustrated step and the other 6 stimulus presentations in this trial. B, rectified–integrated ENGs recorded during MLR-evoked fictive locomotion in two extensor (SmAB, GS), and one flexor (Sart) nerve. The effects evoked by TA and EDL stimulation during the flexion phase are similar, and both resulted in the absence of expected extensor phase activity in the SmAB and GS nerves. These actions were observed for all 7 stimulus presentations to the TA and all 7 to the EDL nerves during this run of fictive locomotion.
Table 1.
Effects evoked by nerve stimulation at 5T during the flexion phase of fictive locomotion. Numbers in brackets indicate the number of experiments in which a nerve was tested
| Nerve stimulated | Enhance and/or prolong flexion | Reset to extension | No effects on cycle period |
|---|---|---|---|
| TA (31) | 1 | 19 | 11 |
| EDL (33) | 20 | 3 | 10 |
| PerL (4) | 4 | — | — |
| Psoas (3) | 3 | — | — |
| Sart (6) | 3 | 2 | 1 |
Variability of TA and EDL effects
The effects evoked by TA and EDL nerve stimulation during the flexion phase were compared in 30 preparations. In 13 the effects were opposite; TA nerve stimulation reset the step cycle to extension (i.e. significantly shortening the cycle period, P < 0.05) and EDL stimulation prolonged the cycle period. In four experiments, EDL stimulation prolonged the cycle period while TA stimulation had no effect. In three preparations, TA stimulation reset the step cycle to extension while EDL stimulation had no significant effect on cycle timing. In one experiment, both TA and EDL stimulation prolonged the flexion phase (P < 0.01, data not illustrated). In three experiments, 5T EDL stimulation consistently reset the step cycle to extension and shortened the cycle durations by 18%, 55% and 69%, respectively (P < 0.01). TA stimulation reset to extension in two of these three preparations and was without effect in the third. In six cases neither nerve evoked significant actions on the cycle period or on flexor ENG activity. In two of these six experiments, the effects of 2T stimulation of extensor group I afferents were examined. In both cases extensor nerve stimulation during the flexion phase reset the step cycle to extension and during the extension phase, produced an enhancement of ongoing extensor activity (not illustrated, see Guertin et al. 1995).
In one experiment in which neither TA nor EDL nerve stimulation during flexion affected step cycle timing, stimulation of both nerves resulted in a suppression of hip (SmAB) and ankle (GS) extensor motoneurone activity in the following extensor phase (Fig. 4B) every time (n = 12) the TA and EDL nerves were stimulated (52 shocks, 200 Hz).
In addition to differences in the reflex effects between preparations, there were also spontaneous variations in TA and EDL-evoked reflexes within some preparations. Figure 5A shows a 9.5-s period of locomotion in which trains of TA nerve stimulation at 2 and 5T were alternated during flexion. In Fig. 5A, the 5T stimulus train on the left stopped ongoing flexor activity (EDL) and evoked a burst in extensors (SmAB and GS), i.e. reset the locomotor cycle to extension. The 5T stimulus presentation on the right, however, prolonged ongoing flexor phase activity (arrows) and delayed the onset of extension. In keeping with the mainly group II afferent source of the resetting actions of the TA nerve (Perreault et al. 1995), twice threshold TA stimulation (middle) had little effect on cycle phase timing. All three stimulus presentations produced short latency excitation of PerL for the duration of the train, presumably by monosynaptic excitation of PerL motoneurones by TA group Ia afferents (Eccles et al. 1957). During this locomotor run, TA nerve stimulation caused a resetting to extension four times and a flexion phase prolongation 10 times. These reflex reversals occurred spontaneously and without any obvious change in experimental conditions. A similar spontaneous variation in TA reflexes was observed in another experiment in which TA stimulation reset the step cycle to extension three times and prolonged flexion twice.
Figure 5. Spontaneous reversal of TA- and EDL-evoked reflexes.
A, alternating stimulation to the TA nerve at 2 and 5T (25 shocks, 200 Hz) triggered from PerL ENG activity. The 5T stimulus on the left evoked a resetting to extension by inhibiting ongoing flexor bursts and initiating premature extensor activity. The second 5T train (right) increased the flexor ENG amplitude and prolonged the ongoing flexor burst duration (arrows in EDL and PerL). Note that TA 2T stimulation also evoked some enhancement of flexion phase activity. B, alternating stimulation of the EDL nerve (15 shocks, 200 Hz) at 2 and 5T was triggered from TA ENG activity. The first 5T stimulus train terminated ongoing flexor activity and evoked a premature extensor burst (SmAB ENG, arrow). The second train of 5T stimuli enhanced and prolonged ongoing flexor activity. EDL 2T stimulation was also effective in evoking flexion enhancement.
Figure 5B shows a portion of a locomotor run from another experiment in which 5T EDL stimulation usually prolonged the ongoing flexor phase. Selected stimulus presentations from this run were used to construct the averages shown in Fig. 2B. In the segment illustrated in Fig. 5B, however, the 5T EDL nerve stimulation on the left evoked a resetting to extension. Note the ‘premature’ extensor burst in SmAB (arrow) and the cessation of ongoing activity in Psoas after the 5T stimulus train on the left. The next 5T stimulus presentation produced the opposite effect and prolonged ongoing flexor activity. The two 2T stimulus presentations illustrated also produced some flexion phase prolongation. During this 2 min long locomotor trial, 5T EDL stimulation reset the rhythm to extension in four of 16 stimulus presentations. There were two other experiments (out of 33) in which EDL stimulation resulted in an occasional resetting to extension. In one experiment, EDL stimulation evoked a resetting to extension during one stimulus presentation and prolonged flexion seven times. In the other experiment, EDL stimulation evoked a resetting to extension in six consecutive stimulus trials in one run but prolonged flexion during all other runs in this experiment. These spontaneous changes in the effects of TA and EDL nerve stimulation support the idea of parallel reflex pathways available to flexor afferents (Lam & Pearson, 2002a).
Stimulus intensity and effects of flexor nerve stimulation
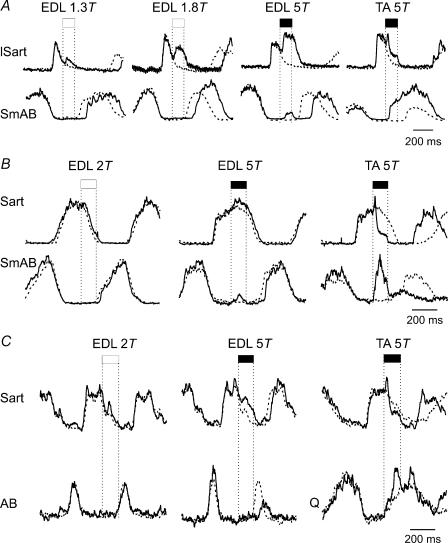
Since the primary purpose of this investigation was to examine the effects of 5T stimulation of flexor nerves in a large number of preparations, stimulus intensity was not systematically varied in each preparation. By comparing the effectiveness of lower and higher intensity EDL nerve stimulation in four experiments, we could nevertheless demonstrate that stimulation at less than 5T strengths is sufficient for evoking the prolongation of flexion. In the experiment illustrated in Fig. 6A, 1.3T EDL stimulation prolonged the flexion phase and delayed the transition to extension (SmAB). Increasing the intensity to 1.8T and then 5T further prolonged the flexion phase (Sart) and further delayed the onset of extension. In the same experiment, TA 5T stimulation evoked a premature onset of extension (resetting). In the experiment illustrated in Fig. 6B, 2T EDL stimulation had no effect while 5T intensity produced a small increase in the amplitude of the hip flexor ENG and a small delay in the onset of hip extensor activity (P < 0.01). Again, 5T EDL stimulation evoked a small burst of activity in SmAB during the stimulus train. In the same run, 5T TA stimulation terminated ongoing flexion (lSart) and reset the cycle to extension (SmAB, Fig. 6A, right). In the third experiment illustrated in Fig. 6C, stimulation of EDL at 2T did not alter ongoing flexor activity (Sart) or the timing of the following extensor burst (AB). Increasing the stimulus strength to 5T delayed the onset of extension (P < 0.01). In the same experiment, TA 5T stimulation reset the cycle to extension. In one experiment, both 2T and 5T EDL nerve stimulation were without effect.
Figure 6. Effects of EDL nerve stimulation at different intensities.
Averaged ENG activity from selected flexor and extensor nerves during fictive locomotion (500 ms before and 700 ms after stimulus delivery) is shown from 3 experiments. Vertical dotted lines mark the stimulus train (25 shocks at 200 Hz) triggered by flexor ENG activity. In each panel, the right-most traces show the effects of TA nerve stimulation. A, flexion enhancement is evoked by low strength EDL nerve stimulation. B, EDL nerve stimulation at strengths lower than 2T had no actions on the ongoing flexor activity and stimulation at 5T had marginal effects. C, EDL nerve stimulation at 5T prolonged ongoing flexor ENG activity and delayed the onset of extension.
Twice threshold Psoas nerve stimulation in 1 of 2 experiments and 2T Sart stimulation in 2 of 4 experiments significantly prolonged ongoing flexor phase activity. In both experiments in which 2T and 5T PerL stimulation was compared, stimulation at 5T but not 2T excited flexor motoneurones. These results are broadly in agreement with those of Perreault et al. (1995) showing that flexor group I afferents can produce prolongation of cycle periods by enhancing the duration of flexor phase activity in decerebrate fictive locomotor preparations. In some preparations, an additional contribution from group II afferents or higher threshold group I afferents is needed to affect step cycle timing. In contrast to flexion phase prolongation, the resetting to extension produced by TA and Sart nerve stimulation shows a clear dependence on recruitment of higher threshold, presumably group II afferents (Fig. 5A; discussed in Perreault et al. 1995).
Effects of flexor afferent stimulation during extension
The effects of TA nerve stimulation delivered during the extensor phase of fictive locomotion were tested in seven preparations. In two experiments the cycle was reset to flexion; in one the ongoing extension phase was prolonged (see also Perreault et al. 1995) and in the others there was no effect. The EDL nerve was stimulated during extension in six experiments. In two there was no effect and in four the ongoing extension phase was terminated and the cycle reset to flexion. Psoas stimulation (5T) during extension reset the step cycle to flexion in two experiments and was ineffective in a third. PerL (5T) stimulation during extension was ineffective in the three experiments tested. Sart stimulation prolonged extension in one and had no effect in the other experiment in which it was tested.
Effects of flexor afferent stimulation during fictive scratch
Stimulation of TA and EDL afferents at 5T during the flexor phase of fictive scratch was examined in nine preparations (3 of which were also used to study flexor nerve effects during fictive locomotion). The short scratch cycle period often necessitated using fewer shocks in the train to ensure that stimulation remained within the phase in which it was initiated. This complicated comparisons with effects obtained during fictive locomotion. Figure 7A shows a short portion of a bout of fictive scratch evoked by pinna manipulation following topical curare application to the dorsal surface of the ipsilateral cervical spinal cord. EDL nerve stimulation at 5T resulted in a prolongation of the ongoing flexor phase (see Sart activity) while delaying the onset of extension (GS). The length of the flexion phase increased to nearly twice its control value (from 227 ± 16 ms to 481 ± 40 ms, P < 0.01). The effects of 5T TA nerve stimulation in another bout of scratch in the same experiment are shown in Fig. 7B (open squares). Stimulation of the TA nerve at 5T moderately shortened the cycle period (by 10%, in 4 stimulated steps, P < 0.01).
Figure 7. Contrasting actions of TA and EDL nerve stimulation during fictive scratch.
A, rectified, integrated ENGs collected during fictive scratch activity evoked by mechanical stimulation of the pinna following topical application of curare to the ipsilateral first cervical dorsal roots. EDL nerve stimulation (25 shocks at 200 Hz) at 2T (open bars) and 5T (filled bars) was delivered during flexion throughout the 20 s-long bout of fictive scratch. The 5T stimulus trains strongly inhibited ankle extensor (GS) activity and prolonged the ongoing flexor activity (Sart). Cycle period increased from 227 ± 15 ms (n = 22) during control to 481 ± 40 ms (n = 3, P > 0.01) during stimulated steps. Note the continued activity in the Sart ENG following the stimulus trains (vertical dotted lines). The 2T stimulus trains had less effects on motoneurone activity. B, cycle period measurements during another (22 s) bout of fictive scratch from the same experiment. In this run, the TA nerve was stimulated (15 shocks at 200 Hz) during flexion every fourth or fifth scratch cycle at 2T and 5T strengths. In most stimulus presentations, TA 5T shortened the cycle period (P < 0.05, measured as the onset between subsequent Sart ENG activity). Mean values were 223 ± 7 ms during control and 205 ± 5 ms during stimulated steps. Note that 2T stimulation was often less effective in shortening the cycle period than 5T stimulation. During one 2T and two 5T TA stimulus presentations, the step cycle was prolonged. These variable actions within preparations are similar to those seen during locomotion. Not shown on this graph are values from two control steps, in which there was a prolonged flexor burst and no extensor activity (i.e. deletion of extensor activity).
In 3 of 8 experiments, TA and EDL nerve stimulation evoked opposing actions similar to those in Fig. 7. In two animals, neither TA nor EDL evoked significant effects during scratch. In two preparations, stimulation of both TA and EDL nerves prolonged ongoing flexion. In the eighth experiment EDL prolonged flexion but TA had no effect. In a ninth animal only EDL was tested and it prolonged flexion. During fictive scratch, 2T EDL stimulation also enhanced ongoing flexor activity but 5T stimulation was more effective in the two experiments in which both 2T and 5T stimulation were tested. Psoas and Sart 5T stimulation prolonged ongoing flexor activity during scratch in 2/2 and 2/3 experiments, respectively. Sart 5T stimulation evoked a resetting to extension in one case. In qualitative terms, the effects evoked by flexor afferent stimulation during fictive locomotion can also be evoked during fictive scratch.
Discussion
The main finding of the present study is that stimulation of flexor group II afferents during the flexion phase of fictive locomotion resulted in two distinct and contrasting actions on rhythmic motor activity. A prolongation of the ongoing flexor phase and a delay in the onset of subsequent extensor activity were routinely seen following stimulation of the EDL, Psoas, PerL and Sart nerves. In contrast, stimulation of TA almost always terminated ongoing flexion and reset the locomotor cycle to extension. Similar actions were also evoked during fictive scratch. An important observation is that the common actions of 5T stimulation of the TA nerve to reset to extension and of EDL stimulation to prolong flexion were reversed in some preparations and during some stimulus presentations in the same run of fictive locomotion.
Combining the present and earlier results (Perreault et al. 1995), stimulation of TA or PBSt at 5T during the flexion phase routinely produces a resetting to extension while EDL or Psoas stimulation enhances ongoing flexion phase activity during MLR-evoked fictive locomotion. In the present series, the effects of Sart nerve stimulation were variable with 5T stimulation intensity evoking a resetting to extension in some preparations and a flexion phase prolongation in others. This observation is in apparent conflict with the resetting actions of Sart nerve stimulation reported by Perreault et al. (1995). Upon re-examination, however, we found two examples in that series where 5T Sart nerve stimulation resulted in a substantial prolongation of the ongoing flexion phase. Thus in both the present and earlier experiments, the actions of 5T Sart nerve stimulation were variable between preparations. Of the nerves tested during locomotion, the weakest actions were those evoked by PerL afferents. The effects of PerL stimulation were not tested during scratch but it is interesting to note that the flexor phase activity of PerL motoneurones during fictive locomotion changes to mainly extensor phase activity during fictive scratch (see Lafreniere-Roula & McCrea, 2005).
Group I intensity stimulation of Psoas, Sart, PBSt and EDL nerves can promote ongoing flexor activity during MLR-evoked fictive locomotion. This is in agreement with the results obtained during treadmill locomotion in decerebrate cats upon stimulation of the EDL and Psoas nerves (Hiebert et al. 1996). The ability of TA afferents to increase flexor phase activity during treadmill locomotion (Hiebert et al. 1996) differs, however, from the more commonly observed resetting to extension seen in the present and previous (Perreault et al. 1995) experiments. During fictive locomotion raising the intensity to 5T increases the response to flexor nerve stimulation. This suggests that group II afferents in flexor nerves may also contribute to prolonging flexion phase activity but a contribution of higher threshold group I afferents cannot be excluded. A discussion of the afferent types recruited at different intensities of stimulation of flexor nerves is included in Perreault et al. (1995).
In both the present and the earlier study (Perreault et al. 1995), a resetting to extension was evoked only when higher intensity stimulation was employed. This suggests that recruitment of group II muscle spindle afferents in flexor nerves is necessary to affect the extensor portion of the CPG. The strong actions of flexor group II afferents on the extensor half-centre are in contrast to the apparent lack of actions from extensor group II afferents during extension, as they do not contribute to the group I afferent-evoked extension enhancement (Guertin et al. 1995; Donelan & Pearson, 2004a). The present results show, however, that 5T stimulation of TA and EDL often evokes opposite actions on the step cycle.
Central pathways mediating the effects of flexor nerve stimulation
The effects of flexor group II afferent stimulation show some similarities to the reflex actions of the cutaneous, superficial peroneal (SP), nerve. In several of the preparations examined here, low threshold (2T) electrical stimulation of the SP nerve evokes the stumbling correction reflex when stimulated during the flexion phase of fictive locomotion (Quevedo et al. 2005a,b). The characteristic pattern of motoneurone activation during stumbling correction includes: (1) brief inhibition and then excitation and prolongation of ankle flexor (TA) activity, (2) strong, short-latency excitation of knee flexor (PBSt) activity, (3) prolonged excitation of hip flexor (Sart, Psoas) activity, and (4) a brief and large excitation of the ankle extensors (LGS, MG and Pl) (Quevedo et al. 2005a,b). Figure 2B illustrates that EDL nerve stimulation evokes several of the these features. However, the lack of a brief burst of ankle extensor activity during the stimulus train clearly differentiates the effects of EDL group II afferents from those of cutaneous SP afferents (for further discussion see Quevedo et al. 2005a).
Some responses to flexor nerve stimulation began soon after the onset, and terminated immediately following the end, of the stimulus train. TA stimulation also produced a short-latency excitation of the lSart, PBSt and EDL motoneurone pools before their activity was terminated during the resetting to extension (Fig. 2A). EDL stimulation produced a brief period of inhibition in the hip flexors before enhancing flexor motoneurone activity in the limb (Fig. 2B). PerL nerve stimulation evoked a second peak of activity in the EDL nerve soon after the stimulus train (Fig. 3A). Finally, 5T EDL stimulation evoked a small burst of activity in SmAB during the stimulus train (Figs 2B, 5B, and 6A–C). Because of the more limited distribution of these effects to select motoneurone pools, we assume that these actions were evoked by reflex pathways outside of those affecting the locomotor timing and flexion or extension phase recruitment of motoneurones. The earlier onset of activity in hip than in ankle extensors during TA-evoked resetting in some stimulus presentations (Fig. 2A) was also seen by Perreault et al. (1995). Intracellular recording would be needed to shed further light on the nature and latencies of these actions.
As discussed in the Introduction, stimulation of a single nerve that simultaneously affects all ipsilateral hindlimb flexors or extensors and changes the timing of the cycle, is evidence that the reflex actions are evoked through locomotor (or scratch) CPG circuitry (McCrea, 2001; Lam & Pearson, 2001; Pearson, 2004). To this evidence, we can add the ability of flexor nerve stimulation to affect motoneurone activity in the locomotor phase following the stimulus presentation (e.g. TA effects on extensor activity in Fig. 2A). Based on the present observations, the most common actions of TA and EDL afferents are evoked through access to reflex pathways exciting the extensor and flexor portions of the CPG, respectively. Spontaneous variations in the effects of flexor nerve stimulation during MLR-evoked fictive locomotion, suggest the existence of parallel reflex pathways from these afferents to the CPG networks that control step cycle timing and phase transition.
Parallel reflex pathways available to flexor muscle group I and II afferents
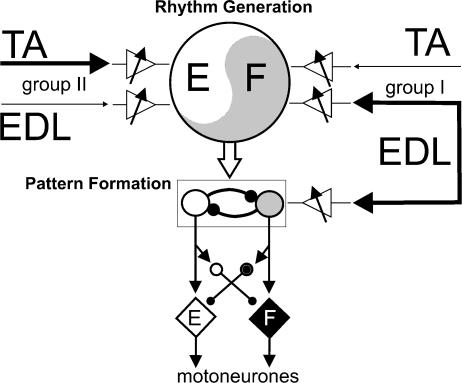
There is growing evidence (see Lafreniere-Roula & McCrea, 2005) that the organization of the locomotor CPG acting within a limb can be divided into two networks: one for rhythm generation (i.e. cycle period and phase) and the other for distributing excitation and inhibition to motoneurones, or pattern formation. These divisions are shown schematically in Fig. 8, which was developed with suggestions from Dr Ilya Rybak. This scheme assumes a bipartite organization of the mammalian CPG with alternating activity in flexors and extensors; the complex locomotor activity of some bifunctional motoneurone pools is not considered. In order to interpret the present results, we hypothesize that flexor muscle group I afferents have access to both the rhythm generation (RG) and pattern formation (PF) networks on the flexor side of the CPG (right side of Fig. 8). For simplicity only inputs from EDL and TA nerves are shown in Fig. 8 but the scheme could be expanded to include other afferents. We suggest that group II afferents in the TA and EDL nerves primarily contact extensor-related portions of the RG network. Variable gain elements (triangles) control the effectiveness of afferent input to the CPG. These variable gain elements could be interneurones interposed in the pathways between flexor afferents and the CPG, or systems that presynaptically regulate the efficacy of transmitter release from afferents in flexor nerves (Perreault et al. 1999). The most common actions following stimulation of TA (resetting to extension) and EDL nerves (enhancing flexion) are indicated by the thick lines in Fig. 8. Because of their sometimes relatively weak effects (e.g. Fig. 2C), connections from flexor group II afferents to the flexor side of the RG network have been omitted for clarity in Fig. 8.
Figure 8. Schematic representation of how flexor muscle afferents might evoke their actions through CPG circuitry.
The locomotor CPG is divided into separate networks for rhythm generation and pattern formation. The rhythm generation network is represented by the large circle with flexor (F) and extensor (E) timing elements. It controls the timing of the step cycle (period) and phase transitions. The pattern formation network is represented by groups of reciprocally coupled neurones exciting flexor (diamond, F) and extensor motoneurones (diamond, E). The pattern formation network also excites interneurones (small circles) responsible for motoneurone hyperpolarization during the inactive phase of locomotion (and scratch). The triangles with arrows represent the mechanisms that allow for selection of specific reflex pathways accessed by flexor muscle afferents from TA and EDL. Group I afferents are hypothesized to access the flexor side of the CPG, from EDL at both the pattern formation and the rhythm generation levels but only at the rhythm generation level from TA. Group II afferents are shown to access only the extensor portion of the CPG and only at the rhythm generation layers. The thick lines represent the actions most commonly observed in the present experiments, i.e. the resetting to extension by TA stimulation and the flexion prolongation by EDL stimulation. For simplicity, connections from EDL group II afferents to the flexor portion of the CPG are not shown as they evoke weaker actions than EDL group I afferents. Filled arrows represent excitatory, and filled circles represent inhibitory connections.
We suggest that the present observations on the common and less frequently encountered actions of flexor nerves can be explained by controlling the effectiveness or weighting of afferent input to portions of the CPG. For example, an increase in the weighting of input from flexor group I muscle afferents to the PF networks would explain how stimulation can augment motoneurone activity without affecting cycle or phase transition timing (e.g. PerL actions in Fig. 3A). On the other hand, an increase in the effectiveness of activating the RG networks, would alter phase transition and cycle timing (e.g. Psoas actions in Fig. 3B).
Electrical stimulation at 5T will activate both group I (Ia and Ib) and group II afferents. A coactivation of both static (group II) and dynamic (group I) spindle afferents is also to be expected during many movements. Tendon organ (Ib) and primary muscle spindle afferents (Ia) would be coactivated during eccentric muscle contractions or by increased gamma drive during isometric or shortening contractions. It is important therefore that a discussion of CPG-mediated reflexes addresses what happens when both group I and II afferents are activated. In our scheme, activation of flexor group I and II afferents results in usually opposing actions on the extensor and flexor sides of the CPG. The resulting reflex action then depends upon which of the inputs has a stronger effect on the CPG. Relatively small excitability changes (controlled by the variable gain elements) would tip the balance of opposing reflex effects to either a resetting to extension, or a flexion-phase enhancement, i.e. produce a reflex reversal. In the case of coactivation of group I and II fibres, the resulting effect on motoneurones evoked through the CPG would be determined by changes in weighting of inputs to particular portions of the CPG network(s). One feature of this scheme is that there is no active inhibition (switching off) of one reflex pathway. Instead, by controlling the inputs to the network, network interactions result in expression of one of the competing actions through the CPG circuitry. In other words, it is the CPG circuitry that switches the reflex actions. At the level of motoneurones, the net result would be one reflex effect and not a mixture of excitation and inhibition.
The variations in flexor nerve reflex actions observed within and between preparations could result from changes in the weighting of sensory input to the extensor and flexor portions of the CPG. These weightings could produce consistent reflexes throughout an experiment or could change spontaneously to produce the occasional reflex reversals illustrated in Fig. 5. The differing actions of 5T Sart nerve stimulation reported here (flexion phase prolongation and resetting to extension) might also be explained by a reduction in the weighting of the Sart group II input to the extensor portion of the RG in some of the present experiments. An alternative explanation could be an increased effect from the usually weak actions of group II afferents to the flexor side of the RG (not indicated in Fig. 8). Presumably in more intact preparations, there would be better control of these opposing reflex pathways and reflexes would be produced that were consistent and appropriate for the ongoing motor task.
Activation of TA group Ia muscle afferents enhances flexor phase activity during treadmill locomotion in decerebrate cats (Hiebert et al. 1996). This is in contrast to the resetting to extension produced by 5T stimulation of the TA nerve seen in most experiments during fictive locomotion. In two of the present experiments, however, 5T TA stimulation prolonged the flexion phase in a few stimulus trials. According to the hypothetical scheme in Fig. 8, the results reported by Hiebert et al. (1996) would be produced by a stronger weighting of TA group I input to flexor portions of the CPG in those preparations. In most of the fictive locomotion preparations examined, this weighting is shifted to allow TA group II afferents to produce actions through extensor portions of the CPG. If the scheme in Fig. 8 is correct, the apparently conflicting results of TA and Sart nerve stimulation reported during treadmill and fictive locomotion can be viewed as quantitative differences in the effectiveness of access to parallel reflex pathways in different preparations.
Separate variable gain elements controlling access to the CPG from different nerves and afferent fibre groups are needed to explain observations such as in Fig. 6 where the effects of EDL stimulation were weak, while those from TA reset the locomotor cycle to extension. They are also needed to explain how extensor group I afferents can reset the step cycle to extension in preparations where flexor nerves are ineffective. The lack of ankle extensor activity produced by Sart stimulation during flexion (Fig. 4A) is particularly intriguing. This observation may be in keeping with the recent suggestion that the hindlimb CPG consists of multiple PF networks (Lafreniere-Roula & McCrea, 2005). Accordingly, Sart stimulation may both reset the step cycle at the RG network level and inhibit activity in the PF network controlling ankle extensor excitation.
The possibility of a presynaptic locus for regulating synaptic transmission from flexor afferents to control synaptic weighting and hence reflex reversals has experimental support. There is a differential presynaptic depression of synaptic transmission from terminals of the same group II afferents ending in intermediate but not dorsal areas of the spinal cord during MLR-evoked fictive locomotion (Perreault et al. 1999). Furthermore, when monosynaptic group II field potentials from the TA and EDL nerves are recorded simultaneously at the same location, there is often an unequal presynaptic depression of transmission from one of the nerves (Stecina et al. 2002). A selective central presynaptic control of transmission at the first synapse in the reflex pathway from particular afferents would result in a reduced activation of selected neurone populations responsible for some CPG-mediated reflexes. The existence of presynaptic mechanisms affecting transmission from selected terminals of flexor nerves does not preclude the possibility that interneurones also control flexor nerve-evoked reflexes during locomotion. An attractive possibility is that flexor activity in the contralateral leg regulates ipsilateral reflexes evoked from flexor nerves (Hiebert et al. 1996).
Function
The results presented in this paper are further evidence that reflexes evoked by proprioceptive activity in afferents arising from hindlimb flexor nerves may be an integral part of motoneurone activity during the swing phase of locomotion and scratch. The flexion enhancement produced from hip (Psoas and Sart) and ankle (EDL and PerL) flexor nerves during fictive locomotion may be analogous to the control of extensor motoneurones by extensor group I afferents (McCrea, 2001; Donelan & Pearson, 2004b). During real locomotion, activation of these reflexes would automatically augment flexor activity during swing and might provide a significant contribution to ongoing flexor phase activity (Lam & Pearson, 2002b). When increased loading or unexpected increases in muscle length occurred, the same systems could act to automatically adjust flexor phase duration and increase flexor motoneurone activity (see Quevedo et al. 2000). It is likely that these systems compliment, and in the case of hip flexors contribute to, the ability of hip displacement to control swing and stance phase transitions during fictive locomotion (Andersson & Grillner, 1983; Kriellaars et al. 1994) and treadmill locomotion in cats (Pearson et al. 2003) as well as in human infants (Pang & Yang, 2000). Compared to the reflexes evoked by extensor muscle afferents during fictive locomotion, those from flexor afferents are more diversified and exhibit considerable variability. This in turn may reflect the need for complex reflex systems to control the complex patterns of flexor motoneurone activity observed during different locomotor tasks such as the activation of ankle and hip flexor muscles during the stance phase of downslope walking (Smith et al. 1998).
The functional implications of the often opposing actions of TA and EDL afferents observed during fictive locomotion are not yet clear. It is unknown whether they would be expressed during treadmill locomotion (e.g. Hiebert et al. 1996) or in more intact preparations. The EDL muscle affects both the ankle and the toes and it is unclear whether the activation of TA and EDL muscle receptors would occur at the same time since TA and EDL motoneurone pools can display different activity profiles during both fictive (Quevedo et al. 2005a) and real locomotion (Smith et al. 1998). Furthermore, secondary spindle afferent firing will be strongly influenced by gamma-motoneurone activity. During treadmill locomotion in cats, the peak rate and the depth of modulation of group II afferent firing can exceed that of Ia fibres (Prochazka & Gorassini, 1998). During fictive locomotion, a complex drive to gamma motoneurones can result in continued activity of group II afferents throughout both phases of the step cycle with peaks of activity at times when the muscles are at shortened length (Bessou et al. 1990; Taylor et al. 2000). A future challenge will be to determine under which conditions reflexes evoked from flexor group I and II afferents play a role in regulating locomotion, and the control mechanisms that adjust the balance between expression of parallel reflex pathways.
The present data show that unlike the common actions of several afferent types in evoking flexion reflexes in spinal preparations (Schomburg et al. 1998), the actions of flexor nerves in spinal cord intact preparations are not homogeneous during fictive locomotion. The concept of parallel spinal reflexes available for group II afferents is not a novel idea. Eccles & Lundberg (1959) suggested that alternative pathways (one excitatory and one inhibitory) exist from group II afferents to flexor motoneurones and that these pathways were reciprocally linked. An organization conceptually similar to that in Fig. 8 could select between alternative reflex pathways. Lam & Pearson (2002a) have also suggested the existence of parallel reflex pathways available to Sart afferents during locomotion. The scheme in Fig. 8 offers suggestions for how parallel pathway selection might be accomplished and in the absence of proper control mechanisms, allow for the expression of spontaneous reflex reversals.
Acknowledgments
This study was supported by the Canadian Institutes for Health Research and from the National Institutes of Health. The skilful assistance of Sharon McCartney is gratefully appreciated. The authors thank Dr Simon Gosgnach, S. Chakrabarty and M. Lafreniere-Roula for participating in some of the experiments. J.Q. was supported by the Rick Hansen Man in Motion Legacy Fund and by the Manitoba Neurotrauma Initiative.
References
- Andersson O, Grillner S. Peripheral control of the cat's step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during fictive locomotion. Acta Physiol Scand. 1983;118:229–239. doi: 10.1111/j.1748-1716.1983.tb07267.x. [DOI] [PubMed] [Google Scholar]
- Angel MJ, Jankowska E, McCrea DA. Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J Physiol. 2005;563:597–610. doi: 10.1113/jphysiol.2004.076034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel MJ, Guertin P, Jiménez I, McCrea DA. Group I. extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol. 1996;494:851–861. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Joffroy M, Montoya R, Pages B. Evidence of the co-activation of α-motoneurones and static ɛ-motoneurones of the sartorius medialis muscle during locomotion in the thalamic cat. Exp Brain Res. 1990;82:191–198. doi: 10.1007/BF00230851. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cord. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Norden-Krichmar T, Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol. 1998;79:447–463. doi: 10.1152/jn.1998.79.1.447. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol. 2004a;92:2093–2104. doi: 10.1152/jn.00325.2004. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of sensory feedback to ongoing ankle extensor activity during the stance phase of walking. Can J Physiol Pharmacol. 2004b;82:589–598. doi: 10.1139/y04-043. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generator by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Synaptic action in motoneurones by afferents which may evoke the flexion reflex. Arch Ital Biol. 1959;97:199–221. [Google Scholar]
- Fleshman JW, Lev-Tov A, Burke RE. Peripheral and central control of flexor digitorum longus and flexor hallucis longus motoneurons: the synaptic basis of functional diversity. Exp Brain Res. 1984;54:133–149. doi: 10.1007/BF00235825. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol. 2000;526:639–652. doi: 10.1111/j.1469-7793.2000.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during MLR-evoked fictive locomotion in the cat. J Physiol. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. J Neurophysiol. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan P, Prochazka A, Pearson KG. Contribution of hindlimb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1–12. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard J-P, Brownstone R, Schomburg ED, Enriquez-Denton M, Perreault M-C. Neuronal mechanisms for generating locomotor activity. Ann NY Acad Sci. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol. 2005;94:1120–1132. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lam T, Pearson K. Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J Neurophysiol. 2001;86:1321–1332. doi: 10.1152/jn.2001.86.3.1321. [DOI] [PubMed] [Google Scholar]
- Lam T, Pearson KG. Sartorius muscle afferents influence the amplitude and timing of flexor activity in walking decerebrate cats. Exp Brain Res. 2002a;147:175–185. doi: 10.1007/s00221-002-1236-0. [DOI] [PubMed] [Google Scholar]
- Lam T, Pearson KG. Adv Exp Med Biol. Vol. 508. 2002b. The role of proprioceptive feedback in the regulation and adaptation of locomotor activity; pp. 343–355. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea D, Stecina K, Quevedo J, Gosgnach S. Flexor group ii muscle afferents can enhance flexor activity during fictive locomotion. Soc Neurosci Abs. 2000 460.2. [Google Scholar]
- Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol. 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II muscle afferents during fictive locomotion. J Physiol. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M-C, Shefchyk SJ, Jimenez I, McCrea DA. Depression of muscle and cutaneous afferent-evoked monosynaptic field potentials during fictive locomotion in the cat. J Physiol. 1999;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Fedirchuk B, Gosgnach S, McCrea D. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol. 2000;525:549–564. doi: 10.1111/j.1469-7793.2000.t01-1-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Stecina K, McCrea DA. Stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol. 2005a;94:2045–2052. doi: 10.1152/jn.00175.2005. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Stecina K, McCrea DA. Intracellular analysis of the stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol. 2005b;94:2053–2062. doi: 10.1152/jn.00176.2005. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB. Major role of sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol. 1998;79:1702–1716. doi: 10.1152/jn.1998.79.4.1702. [DOI] [PubMed] [Google Scholar]
- Stecina K, Ridell J, Chakrabarty S, Gosgnach S, Lafreniere-Roula M, McCrea D. Differential depression of group II muscle and cutaneous afferent input during fictive locomotion and scratch. FENS Abs. 2002 (in press) [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol. 2000;525:781–791. doi: 10.1111/j.1469-7793.2000.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Ellaway PH, Durbaba R, Rawlinson S. Distinctive patterns of static and dynamic gamma motor activity during locomotion in the decerebrate cat. J Physiol. 2000;529:825–836. doi: 10.1111/j.1469-7793.2000.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]