Abstract
Purkinje cells, the sole output of the cerebellar cortex, encode the timing signals required for motor coordination in their firing rate and activity pattern. Dendrites of Purkinje cells express a high density of P/Q-type voltage-gated calcium channels and fire dendritic calcium spikes. Here we show that dendritic subthreshold Kv1.2 subunit-containing Kv1 potassium channels prevent generation of random spontaneous calcium spikes. With Kv1 channels blocked, dendritic calcium spikes drive bursts of somatic sodium spikes and prevent the cell from faithfully encoding motor timing signals. The selective dendritic function of Kv1 channels in Purkinje cells allows them to effectively suppress dendritic hyperexcitability without hindering the generation of somatic action potentials. Further, we show that Kv1 channels also contribute to dendritic integration of parallel fibre synaptic input. Kv1 channels are often targeted to soma and axon and the data presented support a major dendritic function for these channels.
While dendrites of cerebellar Purkinje cells lack voltage-gated sodium channels and do not fire fast sodium-dependent action potentials (Llinas & Sugimori, 1980b; Stuart & Hausser, 1994), they are far from non-excitable membranes. They express a high density of P/Q-type voltage-gated calcium channels (Usowicz et al. 1992) and can fire dendritic calcium-dependent action potentials (Llinas & Sugimori, 1980b; Womack & Khodakhah, 2004). These dendritic calcium-dependent action potentials play an important physiological role as part of the complex spike caused by the activation of the cell's climbing fibre synaptic input (Eccles et al. 1966; Martinez et al. 1971). Since the firing rate of Purkinje cells encodes the timing signals required for motor coordination, it is important that dendritic calcium spikes are only generated when intended (Ito, 1984). The random occurrence of spontaneous dendritic calcium spikes will drive bursts of somatic sodium spikes and prevent Purkinje cells from faithfully encoding timing signals in their pattern and rate of activity. Such failure in the transfer of information would be devastating for motor coordination. Thus, there are likely to be mechanisms that prevent dendritic hyperexcitability in Purkinje cells such as the expression of subthreshold or low-threshold voltage-gated potassium channels.
One class of subthreshold potassium channels is the Kv1 family. Malfunction of these channels has been associated with episodic ataxia type-1 (EA-1), a dominant human neurological disorder characterized by cerebellar ataxia (Browne et al. 1994), although the cellular mechanism by which mutations of these channels cause ataxia is not clear. Kv1-type potassium channels are expressed in the cerebellum at both the mRNA and protein levels (Wang et al. 1993, 1994; McNamara et al. 1993, 1996; Sheng et al. 1994; Veh et al. 1995; Laube et al. 1996; Koch et al. 1997; Chung et al. 2001a, b). There is some evidence that in Purkinje cells subthreshold potassium channels contribute to regulation of dendritic excitability. It is postulated that block of Kv1-type potassium channels lowers the threshold for generating calcium spikes (Midtgaard et al. 1993; Etzion & Grossman, 1998; Etzion & Grossman, 2001; McKay et al. 2005). This effect can be modelled with computer simulations (Miyasho et al. 2001). Also, in organotypic cerebellar slice cultures, block of 4-aminopyridine (4-AP)-sensitive potassium channels controls the dendro-somatic propagation of low-threshold calcium spikes (Cavelier et al. 2002, 2003). It has also been suggested that low-threshold potassium channels contribute to synaptic integration in Purkinje cells (Midtgaard, 1995; Takagi, 2000; McKay et al. 2005).
We used Kv1-selective toxins to evaluate the role of Kv1-type potassium channels in regulating Purkinje cell excitability. We found that block of α-dendrotoxin (α-DTX)-sensitive Kv1 channels caused random transient increases in firing. Using dual somatic and dendritic recordings from single neurones, we showed that these bursts of increased somatic firing were driven by dendritic calcium spikes. Block of α-DTX-sensitive potassium channels also increased dendritic synaptic gain allowing for greater responses to the same synaptic input. Our data demonstrate a prominent role for the Kv1 class of potassium channels in regulating the excitability of Purkinje cells.
Methods
Sagittal or transverse slices (300 μm) were prepared from the cerebellum of Wistar rats and CDI mice (14–25 days old) with a vibratome. Slices were visualized using a 40 × water immersion objective on an upright Zeiss microscope, and were superfused continuously at a rate of 1.5 ml min−1 with (mm): NaCl 125, KCl 2.5, NaHCO3 26, NaH2PO4 1.25, MgCl2 1, CaCl2 2 and glucose 10; pH 7.4 with 5% CO2–95% O2. The solution also contained kynurenic acid (5 mm) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 100 μm), picrotoxin (100 μm) and TTX (1 μm) where indicated. The slice temperature was maintained at 35 ± 0.5°C. The whole-cell pipette solution contained (mm): potassium gluconate 122, KCl 7, MgCl2 1.8, EGTA 0.9, Hepes 9, creatine phosphate 14, MgATP 4, Tris GTP 0.3 (pH 7.4; 293 mosmol kg−1). All toxins were purchased from Alomone Laboratories Ltd (Jerusalem, Israel), and to ensure specificity were used at 10 times their KD values as determined by bioassay by Alomone Laboratories Ltd. Margotoxin was purchased from both Alomone Laboratories and Sigma. Magnesium green was obtained from Molecular Probes and 4-Methoxy-7-nitroindolincyl-caged glutamate from Taris. Local application of toxins was performed as described previously (Womack & Khodakhah 2002, 2003). Briefly, a glass pipette connected to a reservoir containing perfusate was positioned just above the surface of the slice. Fast green (0.4%) or Phenol Red (0.4%) was included in the perfusate in order to monitor the location of the perfusate. At these concentrations, neither of the dyes affected the firing of Purkinje neurones. A suction pipette was placed downstream from the perfusion pipette in order to limit the spread of the perfusate. The extent to which the perfusion was truly localized was assessed by monitoring the DC offset recorded by the differential amplifier when the perfusate was devoid of any ions (isotonic sucrose). Given our experimental setup and the position of perfusion and suction pipettes, the visible dye front was determined to be a reliable measure of the extent of localized perfusion.
Extracellular field potential recordings were made from individual Purkinje neurones using a home-made differential amplifier as previously described (Womack & Khodakhah, 2003). For dual dendritic and somatic recordings, a second recording pipette was positioned within the molecular layer, typically two to three cell diameters away from the soma (Womack & Khodakhah, 2004). Parallel fibre (PF) stimulation was achieved by a glass pipette positioned within the molecular layer using single 200-μs electrical pulses of ∼50 μA every 30 s. Whole-cell current-clamp recordings were made with 3- to 5-MΩ patch pipettes using an Optopatch amplifier (Cairn, UK).
Data were sampled at 10 kHz using a National Instruments A/D-D/A card (MIO-16XE-10) and a Dell computer. Data acquisition and analyses were done with LabView software written in-house. To analyse firing rate, the number of spikes crossing a set threshold was counted every 500 ms.
For calcium measurements, PFs in transverse cerebellar slices were labelled by local application of a solution containing membrane-permeable form of magnesium green following the procedure described by Regehr & Atluri (1995). We used a 480 ± 15-nm excitation filter, a 505-nm dichroic, and a 520-nm long-pass emission filter. The emitted fluorescence was restricted to the region of interest, and was measured using a Hamamatsu photomultiplier (H7421) with custom-written software.
For localized photolysis of caged glutamate, light (100 mW) from a continuous multiline ultraviolet (UV) krypton ion laser (I 302C, Coherent) was gated, and its intensity modulated, with an acusto-optical modulator (AOM 35085-3-350, Neos) and launched into the epifluorescence port of the microscope via a fibre optic. A set of lenses and a pair of galvo-driven aluminium mirrors were used to form and position a 50-μm spot of UV light in the specimen plane. Pulses of light (0.5–1 ms) were used to photo-release glutamate from 200 μm caged glutamate pre-equilibrated with the slice.
Data are reported as means ± s.e.m., and statistical significance was determined using one-way ANOVA.
Results
α-DTX causes transient bursts of increased somatic activity
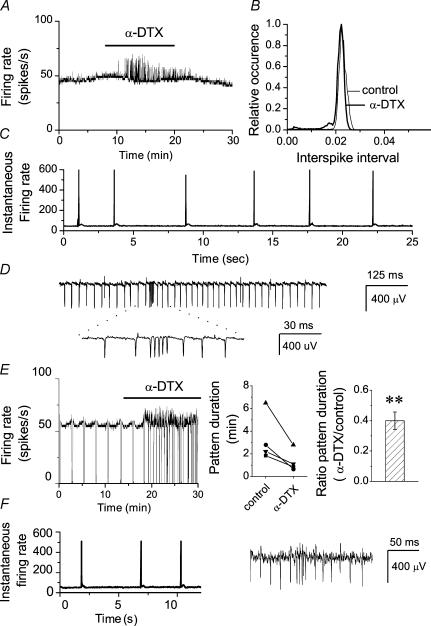
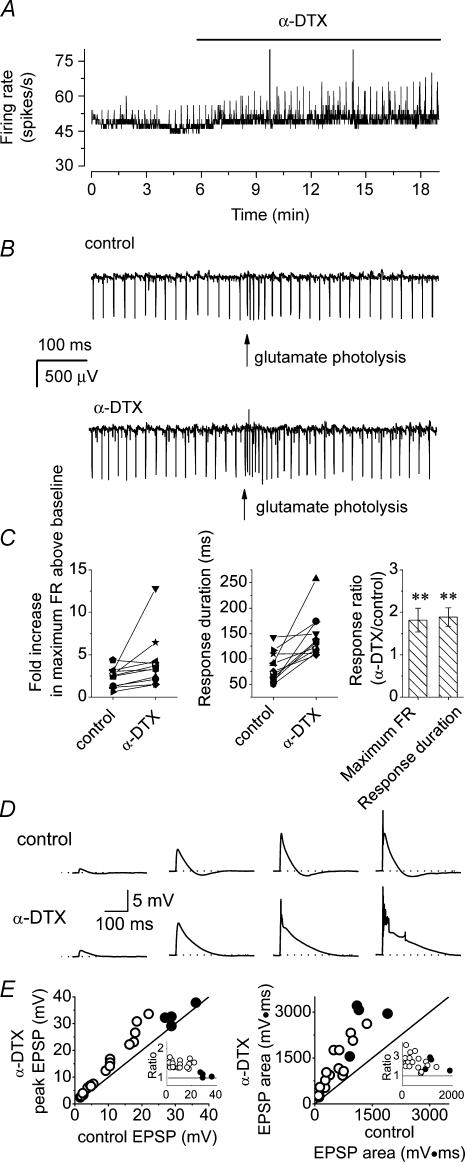
Because the spontaneous firing of Purkinje cells is perturbed with whole-cell recordings (Womack & Khodakhah, 2003), we used single-cell extracellular recordings. Fast glutamatergic and GABAergic synaptic transmission were blocked to ensure that effects seen following block of Kv1 channels were not due to a presynaptic effect. Two types of spontaneous activity, namely tonic firing and the trimodal pattern of activity (regular sequential periods of tonic firing, bursting and quiescence), were observed as reported earlier (Womack & Khodakhah, 2002). The effects of blocking Kv1-type potassium channels on the rate and pattern of activity of each of these firing behaviours were examined. To block Kv1-type potassium channels we added 142 nm α-DTX to the recording solution. α-DTX selectively blocks channels containing Kv1.1, Kv1.2 and Kv1.6 subunits (Harvey, 2001). The average firing rate of a tonic cell, estimated every 500 ms, is shown in Fig. 1A. Application of α-DTX did not significantly increase the baseline firing rate, but resulted in brief transient increases in the activity. Spontaneous bursts occurred randomly and relatively infrequently, such that the interspike interval histogram showed little change in the presence of α-DTX (Fig. 1B). Examination of the instantaneous firing rate, defined as the reciprocal of each interspike interval, showed that the α-DTX-induced transients in the average firing rate corresponded to brief periods of very large increase in the firing rate (corresponding to ∼600 Hz in this cell, Fig. 1C). These were the consequence of short bursts of increased activity (Fig. 1D). Qualitatively similar results were seen in all cells examined (n > 35). α-DTX did not affect the baseline firing rate in any of the cells (control, 50.6 ± 5.2 spikes s−1; α-DTX, 53.8 ± 7.9 spikes s−1; P > 0.2, n = 34). Quantitative analysis of bursts in seven cells showed that on average the α-DTX-induced spontaneous bursts occurred at a rate of 14.9 ± 3.8 bursts min−1, with a duration of 193.1 ± 43.9 ms, and included 12.4 ± 2.2 spikes per burst with each burst having a maximum firing rate of 401.2 ± 46.6 Hz.
Figure 1. Block of Kv1 channels results in the generation of random bursts of increased somatic activity without altering the baseline firing rate.
The firing rate of Purkinje neurones were monitored in cerebellar slices using a differential amplifier. A, average firing rate of a tonically firing Purkinje cell (calculated every 500 ms) before and after bath application of 142 nm α-DTX. Brief transient increases in the average firing rate are apparent in the presence of α-DTX although the baseline firing rate remains the same. B, interspike interval histogram of the cell described in A in control solution and in the presence of α-DTX. The average firing rate did not significantly alter following α-DTX application. C, instantaneous firing rate (1/interspike interval) of the Purkinje cell shown in A in the presence of α-DTX. Note the large transient increases in the firing rate. D, sample raw data of the spontaneous firing of the same Purkinje cell as in A in the presence of α-DTX. The transient increases in the firing rate correspond to the bursts of increased activity. E, effects of bath perfusion of 142 nm α-DTX on the pattern and rate of activity of a Purkinje cell with the trimodal pattern of activity. α-DTX significantly reduced the pattern duration (**P < 0.01, one-way ANOVA). F, in addition to reducing the pattern duration, α-DTX also resulted in the spontaneous bursts of increased somatic activity during the tonic phase of the trimodal pattern. Instantaneous firing rate and raw data from the tonic phase of the cell shown in E in the presence of α-DTX.
Similarly, we examined the consequences of blocking Kv1-type potassium channels with α-DTX in the cells that exhibited the trimodal pattern of activity. As shown in Fig. 1E, α-DTX decreased the cycle duration of the trimodal pattern of activity in all cells tested. The ratio of the pattern duration in the presence of α-DTX to that of control was 0.4 ± 0.6 (n = 4). In addition to shortening the duration of the trimodal pattern of activity, α-DTX also induced the spontaneous bursts of increased activity seen in tonic cells (Fig. 1F). These bursts appeared at random, were most noticeable during the tonic phase of the trimodal pattern, and were absent during the silent phase. The bursts of increased activity in the cells with the trimodal pattern of activity were indistinguishable from those in the tonically firing cells. Further, as noted for the tonically firing cells, α-DTX did not increase the baseline rate of spontaneous firing (control, 53.3 ± 8.2 spikes s−1; α-DTX, 57.4 ± 10.5 spikes s−1; P > 0.2, n = 4).
Blockade of Kv1.2 subunit-containing potassium channels mimics the effects of α-DTX
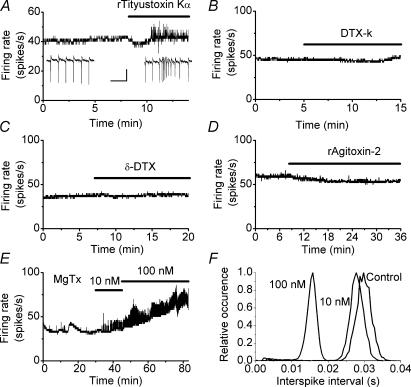
To date, seven Kv1 subunits have been identified and these co-assemble in tetramers to form homomeric and heteromeric channels. We used toxins with known selectivity for specific Kv1 subunits to explore the identity of the Kv1 channel subunits that contribute to the regulation of the excitability of Purkinje cells. α-DTX blocks Kv1.1, Kv1.2 and Kv1.6 subunit-containing channels (Harvey, 2001). Application of 50 nm rTityustoxin K, a blocker of Kv1.2 and 1.3 subunit-containing channels (Werkman et al. 1993; Rodrigues et al. 2003), replicated the effects of α-DTX in all 15 cells examined (Fig. 2A). Immunoprecipitation experiments suggest that in the cerebellum over 80% of channels that contain a Kv1.2 subunit also contain a Kv1.1 subunit (Koch et al. 1997). The same study also demonstrated that in about one-third of channels, Kv1.2 subunits are co-assembled with either Kv1.3 or Kv1.6 subunits. Surprisingly, however, 100 nm α-DTX, a potent blocker of Kv1.1 and Kv1.6 channels (Imredy et al. 1998), had no effect on the spontaneous firing rate of the seven cells examined (Fig. 2C). Further, 100 nm DTX-K, a potent and reportedly selective blocker of Kv1.1 channels (Wang et al. 1999; Akhtar et al. 2002), had no effect on the Purkinje cell firing rate in any of the six cells studied (Fig. 2B). The bioassay provided by Alomone Laboratories Ltd, the supplier of the toxins used here, suggests that in addition to blocking Kv1.1 channels, DTX-K also blocks cloned Kv1.2 channels expressed in Xenopus oocytes, although with lower potency. In two cells in which 100 nm DTX-K did not affect the firing of Purkinje cells, increasing the concentration of the toxin to 300 nm resulted in the generation of random somatic bursts as seen with α-DTX (data not shown). Lastly we tested the effects of rAgitoxin-2, a potent blocker of Kv1.1, Kv1.3 and Kv1.6 channels (Garcia et al. 1994). Perfusion of 20–200 nm rAgitoxin-2 had no effect on the firing of the Purkinje cells in any of the nine cells tested (Fig. 2D).
Figure 2. Pharmacology of Purkinje cell Kv1 channels.
The effects of toxins with selectivity for different Kv1 subunits were examined by monitoring spontaneous firing rate of Purkinje cells. The figure summarizes the findings by presenting sample experiments. A, bath perfusion of 50 nm rTityustoxin K affects the firing of Purkinje cells by inducing random bursts of increased somatic activity without altering the baseline firing rate. The transient bursts were identical to those seen with α-DTX. Similar results were seen in all 15 cells examined. Insets show raw data before and after application of the toxin. Scale bars correspond to 300 μV and 50 ms. Bath perfusion of 100 nm DTX-K in six cells (B), 100 nm α-DTX in seven cells (C) and 20–200 nm rAgitoxin-2 in nine cells (D) did not affect the firing of Purkinje cells. E, application of MgTx (10 or 100 nm) increased the baseline somatic firing rate and induced random transient bursts in all five cells examined. F, the interspike interval histograms of the cell shown in E. MgTx clearly increased the baseline firing rate in a dose-dependent manner as shown by the leftward shift in the interspike interval histograms.
Thus, toxins that block Kv1.2 subunit-containing channels induce brief transient increases in the rate of spontaneous activity of Purkinje cells without affecting their baseline tonic firing rate. The finding that these toxins do not affect the baseline firing rate suggests that Kv1.2 subunit-containing channels do not make a significant contribution to pacemaking in Purkinje cells because if they did the baseline rate of activity would have changed. Since none of the toxins used affected the baseline firing rate, this conclusion can be applied to all Kv1 channels. The soma and axon of Purkinje cells are the compartments involved in pacemaking (Llinas & Sugimori, 1980a; Stuart & Hausser, 1994). Our data therefore suggest that the density of functional Kv1-type potassium channels in these compartments is too low to affect their role in pace-making under physiological conditions. Such an interpretation is consistent with the published studies of Southan & Robertson (2000) where Kv1-type potassium channels were not found in patches obtained from Purkinje cell somata. Similarly, application of 500 nm α-DTX does not affect potassium currents in outside-out patches obtained from the soma of Purkinje cells, but significantly reduces the potassium currents in dendritic patches (a reduction of 13 ± 3% in the potassium current of dendritic patches versus 1.4 ± 1.19% in somatic patches; n = 5 and 4, respectively; M. Martina, personal communication).
It has recently been suggested that the Purkinje cell soma contain Kv1 channels and that blockade of these channels increases the rate of sodium spiking (McKay et al. 2005). This report is in contrast to our findings that blockade of Kv1 channels does not affect the baseline rate of spontaneous firing in Purkinje cells, and also the data of of Southan & Robertson (2000) and M. Martina (personal communication), which demonstrate the absence of α-DTX-sensitive Kv1 channels in Purkinje cell somatic patches. In their study, McKay et al. (2005) pooled data from experiments using relatively high concentrations (up to 100 nm) of margatoxin (MgTx) with data obtained using other Kv1 blockers such as α-DTX. While MgTx is a potent blocker of Kv1.3 (KD, 30 pm) and Kv1.6 (KD, 5 nm) channels, data are lacking on its specificity or selectivity for Kv1 channels versus other potassium channel families although it is known that it blocks at least one Shaker (KD, 150 nm) potassium channel (Garcia-Calvo et al. 1993). Further, the concentrations of MgTx used by McKay et al. (2005) were quite high compared with that required to block Kv1 channels (several orders of magnitude higher than the reported KD value). Thus, we examined the consequences of application of comparable concentrations of MgTx on spontaneous activity of Purkinje cells. As shown in Fig. 2E and F, we found that perfusion of 10 and 100 nm MgTx significantly increased the rate of baseline firing of Purkinje cells (control, 43.9 ± 5.4; 10 nm MgTx, 49.6 ± 4.3, n = 5, P < 0.05; 100 nm MgTx, 73.7 ± 10.6, n = 5, P < 0.01). This contrasts markedly with the data presented earlier which showed that none of the other Kv1 blockers used affected the rate of baseline firing of Purkinje cells. Thus the effects of MgTx on sodium spiking, and on the somatic potassium currents is most probably the consequence of blockade of non-Kv1 potassium channels.
Dendritic calcium spikes drive the α-DTX-induced spontaneous bursts of activity
Spontaneous bursts in Purkinje cells are controlled by dendritic calcium spikes (Womack & Khodakhah, 2004). It is possible that block of dendritic Kv1-type potassium channels facilitates the generation of dendritic calcium spikes, and that these dendritic calcium spikes drive the somatic bursts seen in the presence of α-DTX. The notion that spontaneous bursts of increased activity in the presence of α-DTX are the consequences of dendritic calcium spikes is consistent with the noticeable expression of Kv1-type potassium channels in the molecular layer and Purkinje cell dendrites (Wang et al. 1993, 1994; Sheng et al. 1994; Veh et al. 1995; Laube et al. 1996; Chung et al. 2001a, b), and with the presence of α-DTX-sensitive potassium currents in patches pulled from dendrites of Purkinje cells (M. Martina, personal communication) or in whole-cell voltage-clamp recordings from young Purkinje cells where dendritic conductances can be clamped (Sacco & Tempia, 2002). To examine this possibility, we first selectively blocked somatic and axonal Kv1 channels by local perfusion of α-DTX (142 nm) onto the soma and axons of three Purkinje cells (Womack & Khodakhah 2002, 2003). Local perfusion of α-DTX on the soma and axon did not in any way affect spontaneous activity in any of the cells (data not shown). We next examined the consequences of selectively blocking the dendritic Kv1 channels with local dendritic perfusion of α-DTX in three different cells. In all cells, selective blockade of dendritic α-DTX-sensitive channels resulted in random transient increases in the spontaneous activity as seen with bath perfusion of the toxins (data not shown). The responses obtained with local dendritic application of α-DTX were indistinguishable from those obtained with bath perfusion of the toxins.
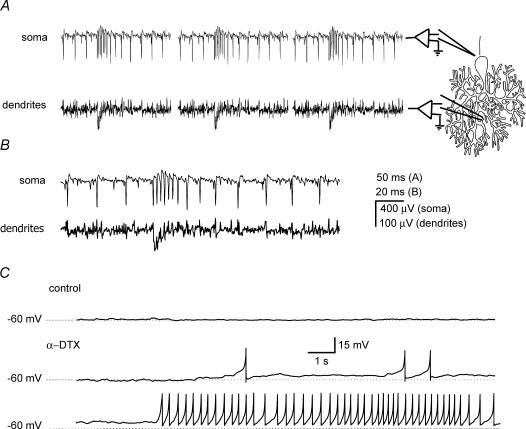
To directly demonstrate the presence of dendritic calcium spikes, we performed dual extracellular somatic and dendritic recordings as previously described (Womack & Khodakhah, 2004) in six tonically firing cells. As shown in Fig. 3, there was a negative field in the molecular layer coincident with each α-DTX-induced burst of increased somatic activity. These fields correspond with dendritic calcium spikes (Womack & Khodakhah, 2004). In all cases the dendritic calcium spike started before or concurrently with the first sodium spike in the burst (Fig. 3B), in marked contrast to calcium spikes that occur at the end of a spontaneous burst in Purkinje cells (Womack & Khodakhah, 2004). In the six cells tested, every burst of somatic activity after application of α-DTX was accompanied with a dendritic calcium spike. Dendritic spikes were not seen in any of the tonically firing cells in the absence of α-DTX.
Figure 3. The α-DTX-induced somatic bursts of activity are concurrent with dendritic calcium spikes.
Simultaneous somatic and dendritic recordings of α-DTX-induced spontaneous bursts in a Purkinje cell. A, two differential amplifiers were used to simultaneously record from the soma and dendrites of the same cell. The approximate location of the recording electrodes is schematically demonstrated. The somatic bursts of increased activity in the presence of 142 nm α-DTX were concurrent with dendritic calcium spikes. B, a single burst from the cell described in A shown on an expanded time scale. The dendritic spike starts at the same time as the increase in the somatic firing rate. C, whole-cell current-clamp recording form a Purkinje cell in the presence of TTX under control conditions, and in the presence of 142 nm α-DTX. Application of α-DTX resulted in the generation of spontaneous calcium spikes.
Spontaneous calcium spikes could also be seen in whole-cell current-clamp recordings (Fig. 3C). In the presence of TTX to block voltage-gated sodium channels, the holding current was adjusted to maintain the membrane potential of the cell at −60 mV. In all four cells examined, when 142 nm α-DTX was applied, spontaneous calcium spikes were seen. The fact that after application of α-DTX spontaneous calcium spikes occur in the absence of voltage-gated sodium channels suggests that sodium channels are not needed for triggering calcium spikes. On occasions, long trains of spontaneous calcium spikes occurred at frequencies of several Hertz (Fig. 3C). High-frequency long trains of calcium spikes were never seen with extracellular recordings. McKay et al. (2005), who used whole-cell recordings in their study, also found that α-DTX resulted in the appearance of long trains of high-frequency calcium spikes. Washout of intracellular constituents and alterations in phosphorylation state of ion channels in whole-cell recordings is likely to alter the extent to which the channels participate in regulation of excitability. Thus, the high-frequency calcium spikes seen with whole-cell recordings are most probably artifacts of alterations in the excitability of Purkinje cells brought about by dialysis of the cell.
Collectively, the data presented so far suggest that under physiological conditions Kv1-type potassium channels selectively prevent dendritic hyperexcitability by preventing the generation of spontaneous dendritic calcium spikes in Purkinje cells.
Block of α-DTX-sensitive channels potentiates PF-evoked responses
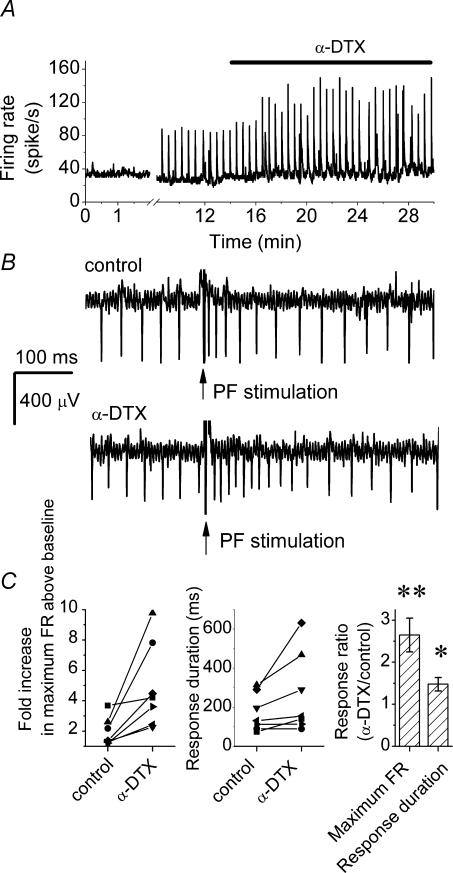
The dendritic low-threshold potassium channels might also contribute to dendritic integration of synaptic inputs. We tested this prediction by monitoring PF-evoked responses in the presence and absence of α-DTX. Figure 4 shows such an experiment. After obtaining a baseline recording, PFs were electrically stimulated every 30 s. The transient increases in the average firing rate shown in Fig. 4A correspond to post-stimulus increases in the firing rate of the cell (Fig. 4B). α-DTX potentiated the PF-evoked responses (Fig. 4A), by increasing both the maximum post-stimulus firing rate and the duration of the response (Fig. 4B). Similar results were seen in all seven cells examined with an average 2.64 ± 0.4-fold increase in PF-evoked firing rate and 1.47 ± 0.14-fold increase in the response duration after application of α-DTX (mean ±s.e.m, n = 7; Fig. 4C).
Figure 4. α-DTX increases the response of Purkinje cells to PF synaptic input.
Parallel fibres were electrically stimulated by a glass pipette positioned in the molecular layer every 30 s with a 50-μA, 200-μs pulse while the firing of the Purkinje cell was monitored. A, the average firing of a Purkinje cell. The transient increases in the average firing rate after the baseline correspond to electrical stimulation of the PFs. Bath perfusion of 142 nm α-DTX increased the response of the Purkinje cell to PF stimulation. B, sample raw data showing the response of a Purkinje cell to electrical stimulation of PFs in the absence and presence of α-DTX. C, scatter plots of increase in the maximum firing rate response and the response duration in seven Purkinje cells with electrical stimulation of PFs in the control and α-DTX-containing solutions. The average of individually normalized responses is shown in the right-hand plot (mean ± s.e.m., n = 7). *P < 0.01, **P < 0.001, determined by one-way ANOVA.
α-DTX does not affect PF presynaptic calcium transients
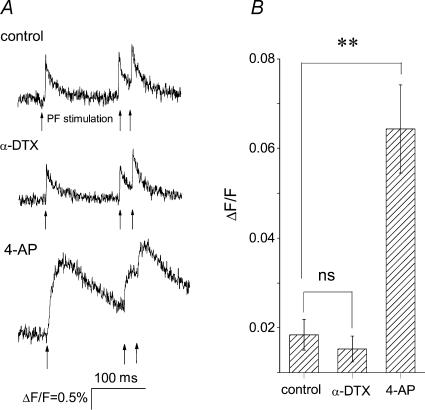
The potentiating effects of α-DTX on PF-evoked responses could be the consequence of increased synaptic transmission from PFs, changes in dendritic integration by Purkinje cells, or both. Blocking low-threshold potassium channels may allow more PFs to reach the threshold for action potential generation, or it may prolong the action potential duration at the PF nerve terminals. Both of these alterations will result in greater glutamate release. To test whether block of α-DTX-sensitive potassium channels affects synaptic transmission, we monitored stimulus-evoked calcium transients in PFs before and after application of α-DTX. If the increase in the PF-evoked responses seen after block of α-DTX-sensitive potassium channels is due to a presynaptic mechanism, then block of these channels should increase the magnitude or duration of calcium transients recorded from the PF nerve terminals (Regehr & Atluri, 1995; Sabatini & Regehr 1997, 1998). In transverse cerebellar slices, PFs were loaded with the calcium indicator magnesium green. The indicator was allowed to diffuse along the PFs, and changes in the fluorescence emitted by the indicator when the fibres were electrically stimulated were measured at a site at least 250 μm away from the loading site (Fig. 5A). This arrangement ensured that the fluorescence signal originated from PFs and not from Purkinje cells or interneurones that would have also been loaded with the dye at the site of dye loading. It is also important to note that given the large size of the PF nerve endings compared with that of their axons, the recorded calcium transients provide a good and highly sensitive measure of calcium signals at the PF nerve endings and correlate well with glutamate release at this synapse (Regehr & Atluri, 1995; Sabatini & Regehr 1997, 1998). An example of one experiment is shown in Fig. 5A. Electrical stimulation of PFs resulted in a fast calcium transient that monotonically decayed to prestimulus levels within 100 ms. Bath perfusion of α-DTX did not affect the magnitude or time course of the calcium transient. As a positive control, 4-AP (100 μm) was bath applied. 4-AP significantly increased both the amplitude and time course of the calcium transient (Fig. 5A), affirming the adequacy of our experimental resolution. Similar results were seen in all nine experiments (Fig. 5B). Our finding that α-DTX does not affect neurotransmitter release from PFs is in agreement with the fact that PF-evoked postsynaptic currents measured in voltage-clamped Purkinje cells are not altered by block of α-DTX-sensitive Kv1 channels (McKay et al. 2005).
Figure 5. α-DTX does not affect PF presynaptic calcium transients.
PFs were locally loaded with the low-affinity calcium indicator magnesium green. The change in the calcium concentration following electrical stimulation of PFs was monitored by measuring the fluorescence emitted by the indicator. A, a representative calcium signal measurement from PFs in control condition and following bath perfusion of 140 nm α-DTX or 100 μm 4-AP. PFs were stimulated at the times shown by the arrows. Electrical stimulation of parallel fibres increased calcium concentration (measured as change in fluorescence divided by fluorescence; ΔF/F). Bath application of α-DTX did not alter the magnitude or time course of the calcium transients, whereas 4-AP significantly increased both the peak ΔF/F and the duration of the response. B, average peak ΔF/F of PF calcium transients in control conditions, and in the presence of 142 nm α-DTX and 100 μm 4-AP. Data are presented as means ± s.e.m., n = 9 for control and in the presence of α-DTX, and n = 5 in the presence of 4-AP. **P < 0.001, determined by one-way ANOVA.
Block of α-DTX-sensitive channels directly affects dendritic synaptic integration
The results described above suggest that effects of α-DTX on PF-evoked responses are postsynaptic. To directly test this hypothesis, we examined the consequences of blocking α-DTX-sensitive channels on the response of Purkinje cells to photolytic application of glutamate to dendrites. Caged glutamate (200 μm) was bath applied and allowed to equilibrate with the slice. Short pulses of UV light (0.5 or 1 ms) were focused onto a 50-μm spot in the molecular layer and their intensity adjusted to obtain rapid and transient increases in the Purkinje cell firing (Fig. 6B). Once a baseline recording was obtained, the effect of block of Kv1 channels with α-DTX was examined. An example of one such experiment is shown in Fig. 6A. The response of the cell to photo-release of glutamate can be seen as a transient increase in the average firing rate of the cell (Fig. 6A). Application of α-DTX increased the amplitude of these transients. The effects of α-DTX are best appreciated by examination of the raw data (Fig. 6B). α-DTX increased both the maximum firing rate and the duration of the glutamate-evoked responses. Similar effects were seen in all 11 cells studied (Fig. 6C). On average, in the presence of α-DTX the maximum firing rate increased by 1.81 ± 0.27-fold, and the response duration increased by 1.89 ± 0.21-fold (mean ± s.e.m., n = 11).
Figure 6. α-DTX potentiates responses of Purkinje cells to dendritic photolytic release of glutamate.
Glutamate was locally released by photolysis on the dendrite of a Purkinje cell and the response of the cell monitored. A, average firing rate of a Purkinje cell in the presence and absence of 142 nm α-DTX. The transient increases in the firing rate correspond to photolytic pulses of glutamate. α-DTX increased the amplitude of responses. B, raw data showing the response of the Purkinje cell shown in A to photolytic release of glutamate in the presence and absence of α-DTX. C, scatter plots of the response of 11 Purkinje cells to dendritic photolytic release of glutamate in the presence and absence of α-DTX. The right-hand plot shows the average of individually normalized responses (mean ± s.e.m., n = 11). **P < 0.01, one-way ANOVA. D, the intensity of the UV light required for photolysis was adjusted to release different concentrations of glutamate on the dendrites of a Purkinje cell to produce EPSPs of varying amplitude. The traces of raw data show these EPSPs under control conditions and in the presence of 142 nm α-DTX. Application of α-DTX increased both the peak amplitude and duration of responses. The dotted lines mark −60 mV. E, the scatter plots show EPSP peak amplitude and area in four different experiments before and after application of α-DTX. Filled circles show EPSPs that triggered a calcium spike. The inset in each graph shows the ratio of the peak amplitude or EPSP area before and after application of α-DTX.
Using whole-cell current-clamp recordings, we also examined the consequences of blocking Kv1 channels on photolysis-evoked excitatory postsynaptic potentials (EPSPs). In the presence of TTX and 200 μm caged glutamate, sufficient current was injected to maintain the membrane potential of the cell at −60 mV. The intensity of the UV light for photolysis was adjusted to produce EPSPs with amplitudes of 2–20 mV (Fig. 6D). After recording baseline EPSPs, 142 nm α-DTX was applied to the cell and the effects on amplitude and shape of the EPSPs examined. α-DTX increased the peak amplitude of EPSPs and was as effective in increasing the amplitude of small EPSPs as it was for larger ones (Fig. 6D and E). The only exceptions were EPSPs in which a calcium spike was triggered in the absence of α-DTX. For these EPSPs, α-DTX did not increase the amplitude of the calcium spike. α-DTX also prolonged the duration of EPSPs (Fig. 6D and E). The effects of α-DTX on EPSPs are consistent with its effects on PF- or glutamate-evoked changes in the spontaneous firing of Purkinje cells. In a recent study, blockade of Kv1 channels did not increase the amplitude of EPSPs generated by injection of synaptic-like currents into the soma of Purkinje cells (McKay et al. 2005). This finding, taken together with the data presented here, supports the notion that the effects of α-DTX on PF-evoked responses are mediated by blockade of dendritic Kv1 channels.
Discussion
The data presented show that under physiological conditions dendritic Kv1.2-containing, low-threshold, voltage-gated potassium channels selectively prevent dendritic hyperexcitability in cerebellar Purkinje cells. In their absence, the high density of P/Q-type voltage-gated calcium channels results in the random generation of spontaneous dendritic calcium spikes. The selective functional dendritic expression of Kv1 channels in Purkinje cells allows them to effectively suppress dendritic hyperexcitability without hindering the generation of somatic action potentials.
Kv1 channels prevent generation of random spontaneous dendritic calcium spikes. By preventing generation of random dendritic calcium spikes, Kv1 channels prevent degradation of the synaptic information encoded within the rate and pattern of activity of Purkinje cells. Errors in transmission by Purkinje cells will probably have devastating consequences for motor coordination.
In addition to preventing dendritic hyperexcitability, Kv1 channels may also help maintain specificity of synaptic plasticity within the cerebellar cortex. The large dendritic calcium influx mediated by the calcium-dependent action potential during the climbing fibre-generated complex spike (Miyakawa et al. 1992; Callaway et al. 1995) plays an essential role in the induction of long-term depression (LTD) of PF synaptic inputs (Ito, 1989; Sakurai, 1990). This form of cerebellar synaptic plasticity is thought to be the underlying mechanism for several forms of associative motor learning (Marr, 1969; Albus, 1971; Ito & Kano, 1982; Ito, 1989; Mauk et al. 1998). LTD is spatially confined to PFs that synapse onto the branchlet that is activated by conjunctive stimulation (Wang et al. 2000). To avoid random depression of synapses and to maintain this synapse specificity, it is important that dendritic calcium spikes are generated only in response to climbing fibre synaptic input. As demonstrated here, dendritic Kv1 channels ensure that the dendrites of Purkinje cells do not randomly generate calcium-dependent action potentials, thus ensuring maintenance of LTD synapse specificity.
Kv1 channels contribute to dendritic integration of synaptic inputs
The extensive dendritic tree of Purkinje cells integrates sensory and cortical information from over 150 000 excitatory synaptic inputs supplied by PFs (Palay & Chan-Palay, 1974). The work presented here suggests that by lessening the impact of each PF, dendritic Kv1 channels may increase the dynamic range of synaptic input, necessitating activation of a greater fraction of a cell's 150 000 afferent population before it is driven to its maximum firing rate.
Purkinje cell subthreshold potassium channels contain Kv1.2 subunits
It is clear on the basis of the pharmacology presented that the Kv1 channels that affect dendritic excitability in Purkinje cells contain Kv1.2 subunits. However, interpretation of the lack of potency of toxins selective for Kv1.1 subunits is more ambiguous. By tandem linkage of subunits it has been demonstrated that even a single Kv1.1 subunit in a tetramer confers DTX-K sensitivity to the resulting channel (Akhtar et al. 2002). The fact that DTX-K and other Kv1.1-preferring toxins did not affect the excitability of Purkinje cells suggests that the Kv1.2 subunit-containing channels do not have Kv1.1 subunits. Kv1.1-containing channels might be present at a low density, not associated with Kv1.2 subunits, and have little or no effect on dendritic or somatic membrane excitability. A precedent for such segregation in function and expression has been reported (Dodson et al. 2003). Paradoxically, however, the cerebellar molecular layer stains more intensely with anti-Kv1.1 antibody than it does with anti-Kv1.2 antibody (Koch et al. 1997). Further, it has been demonstrated that > 80% of cerebellar Kv1.2-containing channels are heterotetramers that express at least one Kv1.1 subunit (Koch et al. 1997). We did not find any evidence for the presence of functional α-DTX-sensitive channels in PFs, suggesting that Kv1.1-containing channels are located on the dendrites of Purkinje cells. It is difficult to reconcile this conclusion, however, with the ineffectiveness of the three Kv1.1-selective toxins used in altering the excitability of Purkinje cells. It should be noted that even in basket cell terminals, which show the highest staining for Kv1.1 channels within the cerebellum (Wang et al. 1993, 1994; Sheng et al. 1994; Veh et al. 1995; Laube et al. 1996; Koch et al. 1997; Rhodes et al. 1997; Chung et al. 2001a, b), Agitoxin-2 has relatively little potency in blocking the channels (Southan & Robertson, 1998).
One explanation may be that Kv1.1-selective toxins do not block Kv1.1 subunit-containing channels expressed in Purkinje cells. It has been shown that α-DTX blocks the same (cloned) channel with differing affinities depending on the amount of mRNA injected into the oocyte (Guillemare et al. 1992). α-DTX also blocks cloned channels with differing potency when they are expressed in different expression systems (Harvey, 2001). Further, expression of other Kv1 subunits might have hampered the effectiveness of Kv1.1 toxins in blocking the channels (Hopkins, 1998). Our attempts to address this possibility have yielded ambiguous data because at higher concentrations toxins lose their specificity and selectivity for Kv1 channels. Thus, whether dendrites of Purkinje cells express Kv1.1 subunit-containing channels remains an open question. If the Kv1.2-containing channels in Purkinje cells do indeed contain Kv1.1 subunits, then it is likely that malfunction of Purkinje cells contributes to episodic ataxia type1, a dominant human neurological disorder linked directly to mutations in the Kv1.1 gene (Browne et al. 1994).
Functional expression of Kv1 channels in the dendrites of Purkinje cells
A conserved T1 tetramerization domain in the subunit of Kv1 channels is required for association of the channel with Kv subunits and is responsible for axonal targeting of Kv1 channels (Gu et al. 2003). In accord with this polarized axonal/somatic expression of Kv1 channels, to date the functions associated with Kv1 channels have been mainly the regulation of spike timing and accommodation in the soma, and the tuning of the reliability of action potential propagation in axonal branch points, nerve endings and varicosities (Southan & Robertson, 1998; Golding et al. 1999; Zhang et al. 1999; Bekkers & Delaney, 2001; Glazebrook et al. 2002; Mo et al. 2002; Dodson et al. 2002, 2003; Herson et al. 2003; Brew et al. 2003; Shen et al. 2004; Faber & Sah, 2004). In CA1 pyramidal neurones, block of Kv1 channels lowers the threshold for generation of dendritic calcium spikes, but the mechanism of this action is mainly a change in the somatic excitability (Golding et al. 1999). While low-threshold potassium channels do directly regulate dendritic excitability in many neurones, these are of the Kv4 family (Hoffman et al. 1997; Song et al. 1998; Golding et al. 1999; Yuan et al. 2002; Lien et al. 2002; Rivera et al. 2003; Shibasaki et al. 2004). The work presented here shows for the first time that a neuronal Kv1 channel may regulate dendritic, but not somatic, excitability, and provides strong evidence in support of the hypothesis that Kv1 channels are expressed in the dendrites in Purkinje cells. It will be interesting to explore the mechanisms responsible for dendritic targeting of Purkinje cell Kv1 channels.
Acknowledgments
This work was supported by the New York City Council Speaker's Fund for Biomedical Research and the National Institutes of Health.
References
- Akhtar S, Shamotienko O, Papakosta M, Ali F, Dolly JO. Characteristics of brain Kv1 channels tailored to mimic native counterparts by tandem linkage of alpha subunits: implications for K+ channelopathies. J Biol Chem. 2002;277:16376–16382. doi: 10.1074/jbc.M109698200. [DOI] [PubMed] [Google Scholar]
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Bekkers JM, Delaney AJ. Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J Neurosci. 2001;21:6553–6560. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J Physiol. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15:2777–2787. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Desplantez T, Beekenkamp H, Bossu JL. K+ channel activation and low-threshold Ca2+ spike of rat cerebellar Purkinje cells in vitro. Neuroreport. 2003;14:167–171. doi: 10.1097/00001756-200302100-00001. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Pouille F, Desplantez T, Beekenkamp H, Bossu JL. Control of the propagation of dendritic low-threshold Ca2+ spikes in Purkinje cells from rat cerebellar slice cultures. J Physiol. 2002;540:57–72. doi: 10.1113/jphysiol.2001.013294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Shin CM, Kim MJ, Lee BK, Cha CI. Immunohistochemical study on the distribution of six members of the Kv1 channel subunits in the rat cerebellum. Brain Res. 2001a;895:173–177. doi: 10.1016/s0006-8993(01)02068-6. [DOI] [PubMed] [Google Scholar]
- Chung YH, Shin CM, Kim MJ, Lee BK, Cha CI. Age-related changes in the distribution of Kv1.1 and Kv1.2 channel subunits in the rat cerebellum. Brain Res. 2001b;897:193–198. doi: 10.1016/s0006-8993(01)02124-2. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Billups B, Rusznak Z, Szucs G, Barker MC, Forsythe ID. Presynaptic rat Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. J Physiol. 2003;550:27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the purinje cells of the cerebellum. J Physiol. 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzion Y, Grossman Y. Potassium currents modulation of calcium spike firing in dendrites of cerebellar Purkinje cells. Exp Brain Res. 1998;122:283–294. doi: 10.1007/s002210050516. [DOI] [PubMed] [Google Scholar]
- Etzion Y, Grossman Y. Highly 4-aminopyridine sensitive delayed rectifier current modulates the excitability of guinea pig cerebellar Purkinje cells. Exp Brain Res. 2001;139:419–425. doi: 10.1007/s002210100788. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Opioids inhibit lateral amygdala pyramidal neurons by enhancing a dendritic potassium current. J Neurosci. 2004;24:3031–3039. doi: 10.1523/JNEUROSCI.4496-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ML, Garcia-Calvo M, Hidalgo P, Lee A, MacKinnon R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry. 1994;33:6834–6839. doi: 10.1021/bi00188a012. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ, Garcia ML. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- Glazebrook PA, Ramirez AN, Schild JH, Shieh CC, Doan T, Wible BA, Kunze DL. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol. 2002;541:467–482. doi: 10.1113/jphysiol.2001.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–649. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- Guillemare E, Honore E, Pradier L, Lesage F, Schweitz H, Attali B, Barhanin J, Lazdunski M. Effects of the level of mRNA expression on biophysical properties, sensitivity to neurotoxins, and regulation of the brain delayed-rectifier K+ channels Kv1.2. Biochemistry. 1992;31:12463–12468. doi: 10.1021/bi00164a024. [DOI] [PubMed] [Google Scholar]
- Harvey AL. Twenty years of dendrotoxins. Toxicon. 2001;39:15–26. doi: 10.1016/s0041-0101(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, Maylie J. A mouse model of episodic ataxia type-1. Nat Neurosci. 2003;6:378–383. doi: 10.1038/nn1025. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hopkins WF. Toxin and subunit specificity of blocking affinity of three peptide toxins for heteromultimeric, voltage-gated potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;285:1051–1060. [PubMed] [Google Scholar]
- Imredy JP, Chen C, MacKinnon R. A snake toxin inhibitor of inward rectifier potassium channel ROMK1. Biochemistry. 1998;37:14867–14874. doi: 10.1021/bi980929k. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- Koch RO, Wanner SG, Koschak A, Hanner M, Schwarzer C, Kaczorowski GJ, Slaughter RS, Garcia ML, Knaus HG. Complex subunit assembly of neuronal voltage-gated K+ channels. Basis for high-affinity toxin interactions and pharmacology. J Biol Chem. 1997;272:27577–27581. doi: 10.1074/jbc.272.44.27577. [DOI] [PubMed] [Google Scholar]
- Laube G, Roper J, Pitt JC, Sewing S, Kistner U, Garner CC, Pongs O, Veh RW. Ultrastructural localization of Shaker-related potassium channel subunits and synapse associated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Brain Res Mol Brain Res. 1996;42:51–61. doi: 10.1016/s0169-328x(96)00120-9. [DOI] [PubMed] [Google Scholar]
- Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K+ channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980a;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980b;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Molineux ML, Mehaffey WH, Turner RW. Kv1 K+ channels control Purkinje cell output to facilitate postsynaptic rebound discharge in deep cerebellar neurons. J Neurosci. 2005;25:1481–1492. doi: 10.1523/JNEUROSCI.3523-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara NM, Averill S, Wilkin GP, Dolly JO, Priestley JV. Ultrastructural localization of a voltage-gated K+ channel alpha subunit (KV 1.2) in the rat cerebellum. Eur J Neurosci. 1996;8:688–699. doi: 10.1111/j.1460-9568.1996.tb01254.x. [DOI] [PubMed] [Google Scholar]
- McNamara NM, Muniz ZM, Wilkin GP, Dolly JO. Prominent location of a K+ channel containing the alpha subunit Kv 1.2 in the basket cell nerve terminals of rat cerebellum. Neuroscience. 1993;57:1039–1045. doi: 10.1016/0306-4522(93)90047-j. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FE, Crill WE, Kennedy TT. Electrogenesis of cerebellar Purkinje cell responses in cats. J Neurophysiol. 1971;34:348–356. doi: 10.1152/jn.1971.34.3.348. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Garcia KS, Medina JF, Steele PM. Does cerebellar LTD mediate motor learning? Toward a resolution without a smoking gun. Neuron. 1998;20:359–362. doi: 10.1016/s0896-6273(00)80978-2. [DOI] [PubMed] [Google Scholar]
- Midtgaard J. Spatial synaptic integration in Purkinje cell dendrites. J Physiol (Paris) 1995;89:23–32. doi: 10.1016/0928-4257(96)80548-1. [DOI] [PubMed] [Google Scholar]
- Midtgaard J, Lasser-Ross N, Ross WN. Spatial distribution of Ca2+ influx in turtle Purkinje cell dendrites in vitro: role of a transient outward current. J Neurophysiol. 1993;70:2455–2469. doi: 10.1152/jn.1993.70.6.2455. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Lev-Ram V, Lasser-Ross N, Ross WN. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J Neurophysiol. 1992;68:1178–1189. doi: 10.1152/jn.1992.68.4.1178. [DOI] [PubMed] [Google Scholar]
- Miyasho T, Takagi H, Suzuki H, Watanabe S, Inoue M, Kudo Y, Miyakawa H. Low-threshold potassium channels and a low-threshold calcium channel regulate Ca2+ spike firing in the dendrites of cerebellar Purkinje neurons: a modeling study. Brain Res. 2001;891:106–115. doi: 10.1016/s0006-8993(00)03206-6. [DOI] [PubMed] [Google Scholar]
- Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K+ currents contribute to accommodation in murine spiral ganglion neurons. J Physiol. 2002;542:763–778. doi: 10.1113/jphysiol.2002.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. Berlin: Springer-Verlag; 1974. [Google Scholar]
- Regehr WG, Atluri PP. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995;68:2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci. 2003;6:243–250. doi: 10.1038/nn1020. [DOI] [PubMed] [Google Scholar]
- Rodrigues AR, Arantes EC, Monje F, Stuhmer W, Varanda WA. Tityustoxin-K (alpha) blockade of the voltage-gated potassium channel Kv1.3. Br J Pharmacol. 2003;139:1180–1186. doi: 10.1038/sj.bjp.0705343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Optical measurement of presynaptic calcium currents. Biophys J. 1998;74:1549–1563. doi: 10.1016/S0006-3495(98)77867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Tempia F. A-type potassium currents active at subthreshold potentials in mouse cerebellar Purkinje cells. J Physiol. 2002;543:505–520. doi: 10.1113/jphysiol.2002.022525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc Natl Acad Sci U S A. 1990;87:3383–3385. doi: 10.1073/pnas.87.9.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hernandez-Lopez S, Tkatch T, Held JE, Surmeier DJ. Kv1.2-containing K+ channels regulate subthreshold excitability of striatal medium spiny neurons. J Neurophysiol. 2004;91:1337–1349. doi: 10.1152/jn.00414.2003. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. J Neurosci. 1994;14:2408–2417. doi: 10.1523/JNEUROSCI.14-04-02408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Nakahira K, Trimmer JS, Shibata R, Akita M, Watanabe S, Ikenaka K. Mossy fibre contact triggers the targeting of Kv4.2 potassium channels to dendrites and synapses in developing cerebellar granule neurons. J Neurochem. 2004;89:897–907. doi: 10.1111/j.1471-4159.2004.02368.x. [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Modulation of inhibitory post-synaptic currents (IPSCs) in mouse cerebellar Purkinje and basket cells by snake and scorpion toxin K+ channel blockers. Br J Pharmacol. 1998;125:1375–1381. doi: 10.1038/sj.bjp.0702218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Hausser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron. 1994;13:703–712. doi: 10.1016/0896-6273(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Takagi H. Roles of ion channels in EPSP integration at neuronal dendrites. Neurosci Res. 2000;37:167–171. doi: 10.1016/s0168-0102(00)00120-6. [DOI] [PubMed] [Google Scholar]
- Usowicz MM, Sugimori M, Cherksey B, Llinas R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992;9:1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Wang SS, Khiroug L, Augustine GJ. Quantification of spread of cerebellar long-term depression with chemical two-photon uncaging of glutamate. Proc Natl Acad Sci U S A. 2000;97:8635–8640. doi: 10.1073/pnas.130414597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang FC, Parcej DN, Dolly JO. alpha subunit compositions of Kv1.1-containing K+ channel subtypes fractionated from rat brain using dendrotoxins. Eur J Biochem. 1999;263:230–237. doi: 10.1046/j.1432-1327.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkman TR, Gustafson TA, Rogowski RS, Blaustein MP, Rogawski MA. Tityustoxin-K alpha, a structurally novel and highly potent K+ channel peptide toxin, interacts with the alpha-dendrotoxin binding site on the cloned Kv1.2 K+ channel. Mol Pharmacol. 1993;44:430–436. [PubMed] [Google Scholar]
- Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–10612. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar purkinje neurons. J Neurosci. 2003;23:2600–2607. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24:3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Messing A, Chiu SY. Specific alteration of spontaneous GABAergic inhibition in cerebellar purkinje cells in mice lacking the potassium channel Kv1.1. J Neurosci. 1999;19:2852–2864. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]