Abstract
We tested the hypothesis that skeletal muscle ubiquitin–proteasome-dependent proteolysis is dysregulated in ageing in response to feeding. In Experiment 1 we measured rates of proteasome-dependent proteolysis in incubated muscles from 8- and 22-month-old rats, proteasome activities, and rates of ubiquitination, in the postprandial and postabsorptive states. Peptidase activities of the proteasome decreased in the postabsorptive state in 22-month-old rats compared with 8-month-old animals, while the rate of ubiquitination was not altered. Furthermore, the down-regulation of in vitro proteasome-dependent proteolysis that prevailed in the postprandial state in 8-month-old rats was defective in 22-month-old rats. Next, we tested the hypothesis that the ingestion of a 5% leucine-supplemented diet may correct this defect. Leucine supplementation restored the postprandial inhibition of in vitro proteasome-dependent proteolysis in 22-month-old animals, by down-regulating both rates of ubiquitination and proteasome activities. In Experiment 2, we verified that dietary leucine supplementation had long-lasting effects by comparing 8- and 22-month-old rats that were fed either a leucine-supplemented diet or an alanine-supplemented diet for 10 days. The inhibited in vitro proteolysis was maintained in the postprandial state in the 22-month-old rats fed the leucine-supplemented diet. Moreover, elevated mRNA levels for ubiquitin, 14-kDa ubiquitin-conjugating enzyme E2, and C2 and X subunits of the 20S proteasome that were characteristic of aged muscle were totally suppressed in 22-month-old animals chronically fed the leucine-supplemented diet, demonstrating an in vivo effect. Thus the defective postprandial down-regulation of in vitro proteasome-dependent proteolysis in 22-month-old rats was restored in animals chronically fed a leucine-supplemented diet.
Ageing is characterized by a gradual loss of muscle proteins (sarcopenia), which is ultimately responsible for decreased mobility and autonomy. Sarcopenia also reduces the ability of the elderly to cope with nutritional, infectious or traumatic stresses, whose incidence increases in ageing (Young et al. 1989). Proteins in skeletal muscle, as in other mammalian tissues, undergo a continuous process of synthesis and degradation (Waterlow et al. 1978). Thus, sarcopenia should be due to an imbalance between rates of protein turnover. Some studies demonstrated an age-related decline in synthesis rate of mixed muscle proteins, myosin heavy chain and mitochondrial proteins (Rooyackers et al. 1996; Greenlund & Nair, 2003) although these findings have been challenged (Volpi et al. 2001).
Skeletal muscle protein synthesis decreases in the postabsorptive (PA) state and increases in the postprandial (PP) state, while protein breakdown follows the inverse pattern. In adults, net positive protein balance in the PP state and net negative protein balance in the PA state cancel each other. In humans protein mass increases in daytime and decreases overnight so that muscle protein mass does not change throughout the day and night cycle. There is strong evidence that the stimulatory effect of amino acids on protein synthesis is blunted in old muscles from both animals (Mosoni et al. 1995; Dardevet et al. 2000; Arnal et al. 2002) and humans (Guillet et al. 2004; Cuthbertson et al. 2005). A limited number of studies also suggest that there are alterations in muscle proteolysis in ageing in either the PA state in humans (Volpi et al. 2001) or the PP state in rats (Arnal et al. 2002). Similar observations have been reported in humans at the whole-body level in the PP state (Arnal et al. 1999). Changes in protein turnover rates in response to feeding are mediated by both hormones and nutrients. Among them leucine stimulates protein synthesis in incubated skeletal muscles from adult rats as all (Dardevet et al. 2000) or branched-chain (Anthony et al. 2000a) amino acids do, by enhancing translation initiation (Dardevet et al. 2000; Anthony et al. 2000a, b; Greiwe et al. 2001) via a rapamycin-sensitive pathway (Dardevet et al. 2000; Anthony et al. 2000a). Essential amino acids are primarily responsible for the meal-induced stimulation of muscle protein anabolism in the elderly (Volpi et al. 2003). Accordingly, in vivo a leucine-supplemented meal corrects the defective postprandial stimulation of muscle protein synthesis in old rats (Dardevet et al. 2002). Leucine also inhibits whole-body protein degradation in vivo (Frexes-Steed et al. 1992) and skeletal muscle proteolysis in vitro (Buse & Reid, 1975; Fulks et al. 1975; Tischler et al. 1982). However, to our knowledge, whether leucine has a possible inhibitory role on muscle proteolysis in the PP state is unknown.
Multiple proteolytic pathways, including the lysosomal, Ca2+-dependent, ubiquitin (Ub)–proteasome-dependent processes, and proteases (e.g. caspases and matrix metallo-proteases) are responsible for skeletal muscle proteolysis (Attaix et al. 2003). The Ub–proteasome-dependent pathway degrades the bulk of muscle proteins including myofibrillar components (Jagoe & Goldberg, 2001; Hasselgren & Fischer, 2001; Attaix et al. 2003). In this pathway, Ub targets specific intracellular proteins for degradation. Briefly, Ub conjugation proceeds via a thiol ester reaction cascade involving the Ub-activating enzyme (E1), Ub-conjugating enzymes (E2), and Ub-protein ligases (E3), which possess substrate recognition sites (for recent reviews see Pickart, 2001; Glickman & Ciechanover, 2002). Rapid degradation by the 26S proteasome requires the formation of a polyUb degradation signal that comprises at least four Ub moieties (Pickart, 2001).
Information on the regulation of muscle Ub–proteasome-dependent proteolysis in ageing and in response to feeding is very limited. The aim of the present study was to investigate whether (i) the muscle Ub–proteasome system is regulated in the PP state in both adult and old rats, and (ii) leucine supplementation in the diet affects this response. We report that an inhibition of Ub–proteasome-dependent proteolysis prevails in the PP state in 8-month-old rats, but is lacking in 22-month-old animals. We also demonstrate that leucine supplementation in the diet completely restores this defect in 22-month-old rats by regulating both proteasome activities and rates of substrate ubiquitination. Finally, we provide evidence that chronic leucine supplementation in the diet has long-lasting effects on the muscle Ub–proteasome system from 22-month-old rats.
Methods
Animals and experimental design
The experiments were conducted in accordance with the French National Research Council's Guidelines for the Care and Use of Laboratory Animals. Eight- and 22-month-old Male Wistar rats (Iffa Credo, Lyon, France) were used. The experimental design has been previously described in detail (Dardevet et al. 2002; Rieu et al. 2003). In brief, rats were fed a standard diet during the 8-h dark period for 1 month. In Experiment 1, the effect of a unique meal supplemented with 5% of leucine was tested against a meal supplemented with 5% alanine (controls), so that the rats were fed the same amount of protein and energy. Over the 1-h feeding period, food intake was not affected by the meal consumed (Dardevet et al. 2002). In Experiment 2, diets were tested for 10 days. Rats daily received either the leucine-supplemented or the alanine-supplemented meal for the first hour of feeding followed by the standard diet for the next 7 h. Experimental and control diet daily intakes were similar in both 8- and 22-month-old rats during the first 9 days of the experimental period. On the day of the experiment, rats in the PA state were overnight fasted, whereas rats in the PP state consumed the same amount of food as on the previous days and were studied 2 h after the first meal was given (Rieu et al. 2003). In both experiments rats were anaesthetised with sodium pentobarbital (45 μg/g body weight) and killed by overdose of the anaesthetic.
Measurements of in vitro rates of proteolysis
Epitrochlearis muscles from rats in Experiment 1 or 2 were quickly excised and rinsed in Krebs-Henselheit bicarbonate buffer ((mm) NaCl 120, KCl 4.8, NaHCO3 25, KH2PO4 1.2 and MgSO4 1.2, pH 7.4), supplemented with 5 mm Hepes, 5 mm glucose and 0.1% BSA. Muscles were then transferred to plastic tubes containing 1.5 ml of fresh buffer saturated with a 95% O2–5% CO2 gas mixture. After 30 min of preincubation, muscles were transferred to a fresh medium of identical composition and further incubated for 1 h.
Rates of protein breakdown were measured by following the rates of tyrosine release into the medium in the presence of 0.5 mm cycloheximide, which blocks protein synthesis. Non-lysosomal and Ca2+-independent proteolysis was measured in a Ca2+-deprived medium supplemented with 50 μm leupeptin and 10 mm methylamine (Combaret et al. 2004). Proteasome-dependent proteolysis was calculated as the difference between the rates of non-lysosomal and Ca2+-independent proteolysis (measured in one muscle) and the rates of non-lysosomal, Ca2+-independent and proteasome-independent proteolysis (measured in the contralateral muscle) following incubation with 50 μm leupeptin, 10 mm methylamine and 40 μm MG132 in a Ca2+-deprived medium (Combaret et al. 2004). Protein degradation was expressed in nanomoles of tyrosine released in the medium per milligram protein per hour. Muscle protein content was measured according to the bicinchoninic acid procedure.
Peptidase activities of the proteasome
Proteins from pooled extensor digitorum longus (EDL) muscles of 8- and 22-month-old animals in the PA or PP states (Experiment 1) were homogenized in ice-cold buffer (pH 7.5) containing 50 mm Tris, 250 mm sucrose, 10 mm ATP, 5 mm MgCl2, 1 mm DTT, and protease inhibitors (10 μg ml−1 of antipain, aprotinin, leupeptin and pepstatin A, and 20 μm PMSF). The proteasomes were isolated by three sequential centrifugations as described previously (Hobler et al. 1999; Fang et al. 2000). The final pellet was resuspended in buffer containing 50 mm Tris (pH 7.5), 5 mm MgCl2 and 20% glycerol. The protein content of the proteasome preparation was determined according to Lowry et al. (1951). Peptidase activities of the proteasome were determined by measuring the hydrolysis of the fluorogenic substrates succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (LLVY), Boc-Leu-Arg-Arg-7-amido-4-methylcoumarin (LRR) and the Cbz-Leu-Leu-Glu-β-naphtylamide (LLE) (Sigma). These substrates are preferentially hydrolysed by the chymotrypsin-like, the trypsin-like and the peptidyl glutamyl peptide hydrolase (PGPH) activities of the proteasome, respectively (Craiu et al. 1997; Hobler et al. 1999). To measure peptidase activity, 10 μl of the proteasome extract was added to 40 μl of medium containing 50 mm Tris (pH 8.0), 10 mm MgCl2, 1 mm DTT, 2 U apyrase and 300 μm of LLVY, or 800 μm of LRR or LLE. The peptidase activity was determined by measuring the accumulation of the fluorogenic cleavage product (methylcoumaryl-amide, AMC or β-naphtylamide, β-Na) using a luminescence spectrometer LS50B (Perkin Elmer). Fluorescence was measured every 5 min at 37°C during 45 min at 380 nm (AMC) or 333 nm (β-Na) excitation wavelength, and 440 nm (AMC) or 450 nm (β-Na) emission wavelength. The difference between arbitrary fluorescence units recorded with or without 40 μm of the proteasome inhibitor MG132 (Affiniti) in the reaction medium was calculated, and the final data were corrected by the amount of protein in the reaction. The time course for the accumulation of AMC or β-Na after hydrolysis of the substrate was analysed by linear regression to calculate peptidase activities, e.g. the slopes of best fit of accumulated AMC or β-Na versus time. Different kinetics were performed to measure individually the three proteasome peptidase activities.
Ubiquitination rates
EDL muscles (Experiment 1) from the same group of rats were pooled and homogenized at 4°C with a Polytron in 50 mm Tris-HCl, pH 7.5, 1 mm dithiothreitol (DTT), 1 mm EDTA, 1 mm PMSF, 10 μg ml−1 pepstatin A and 10 μg ml−1 leupeptin (5 ml buffer (g muscle)−1). The homogenates were centrifuged (10 000 g, 10 min, 4°C). The resulting supernatants were centrifuged at 100 000 g for 60 min at 4°C. The final supernatants were stored at −80°C until use. Rates of ubiquitination were determined by incubation at 37°C of muscle extracts containing 50 μg of protein in 50 mm Tris-HCl (pH 7.5), 1 mm DTT, 2 mm MgCl2, 2 mm 5′-adenylylimidodiphosphate and 5 μm[125I]labelled Ub (∼3000 cpm (pmol)−1) in a total volume of 20 μl. The reaction was stopped at 0, 3, 6, 9 and 12 min by the addition of Laemmli buffer 1×. Conjugation rates were linear for this period of time and the concentration of exogenously labelled Ub was in significant excess of any endogenous Ub (e.g. addition of larger amounts of labelled Ub did not increase rates of ubiquitination) (Kee et al. 2003; Combaret et al. 2004). After incubation, Ub conjugates were resolved from free Ub by SDS–PAGE on 12% gels. After drying of the gel, high molecular weight radiolabelled conjugates were visualized with a Phosphofluoro-Imager (Molecular Dynamics), excised and the [125I]Ub bound to protein substrates was determined by measuring the radioactivity in a Cobra II auto-gamma counter (Packard). The time course for the accumulation of high molecular weight conjugates was analysed by linear regression to calculate ubiquitination rates, e.g. the slopes of best fit of cpm bound to Ub conjugates versus time.
Northern blots
EDL muscles of animals from Experiment 2 were rapidly excised, frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted (Chomczynski & Sacchi, 1987), and Northern blots were performed as previously described (Combaret et al. 2004). The membranes were hybridized with cDNA probes encoding polyUb, the 14-kDa E2, and the C2 and X subunits of the 20S proteasome (Mansoor et al. 1996; Tilignac et al. 2002; Combaret et al. 2004). The hybridizations were performed with [32P] cDNA fragments labelled by random priming. After stripping of the different probes, the filters were reprobed with a cDNA fragment encoding the 18S rRNA. The filters were autoradiographed, and the signals were quantified as previously described (Deval et al. 2001). Autoradiographic signals were normalized using the corresponding 18S rRNA signals to correct for variations in RNA loading.
Statistical analysis
All data are expressed as means ± s.e.m. Statistical analyses were performed using ANOVA or the unpaired Student's t test, as appropriate. If statistically significant differences were detected by ANOVA, post hoc comparisons between groups were made using the Fisher's PLSD test. Differences in proteasome activities and ubiquitination rates were assessed by comparing the slopes of best fit obtained by regression analysis (Kee et al. 2003). Significance was defined at the 0.05 level.
Results
Body weights and muscle masses
Body weights of 8- and 22-month-old rats were similar in Experiment 1 (Table 1). In Experiment 2 the body weights of the 22-month-old-rats were slightly higher than in their 8-month-old respective controls (Table 1). In both Experiments 1 and 2, an atrophy of the tibialis anterior muscle (and of the gastrocnemius, data not shown) prevailed in 22-month-old rats, compared with the respective 8-month-old animals, but was not observed in the EDL and epitrochlearis muscles (Table 1).
Table 1.
Body weights and muscle masses of 8- and 22-month-old rats in Experiments 1 and 2
| Experiment 1 | 8-month-old | 22-month-old | ||
|---|---|---|---|---|
| n | 29 | 31 | ||
| Body weight (g) | 598 ± 6A | 608 ± 10A | ||
| Muscle mass (g (100 g BW)−1) | ||||
| Tibialis anterior | 165 ± 3A | 150 ± 3B | ||
| EDL | 40 ± 1A | 40 ± 1A | ||
| Epitrochlearis | 23 ± 1A | 23 ± 1A | ||
| 8-month-old | 22-month-old | |||
| Experiment 2 | Ala | Leu | Ala | Leu |
| n | 20 | 20 | 22 | 22 |
| Final body weight (g) | 523 ± 8A | 533 ± 7AB | 567 ± 14BC | 572 ± 17C |
| Muscle mass (g (100 g BW)-1) | ||||
| Tibialis anterior | 178 ± 3A | 173 ± 3A | 164 ± 4B | 161 ± 3B |
| EDL | 42 ± 1A | 41 ± 1A | 41 ± 1A | 41 ± 1A |
| Epitrochlearis | 26 ± 1A | 25 ± 1A | 24 ± 1A | 26 ± 1A |
Values are means ± s.e.m. for the number of rats indicated (n). Different superscript letters within a line indicated significant differences by ANOVA (P < 0.05).
Ubiquitin–proteasome-dependent proteolysis in the postprandial and postabsorptive states
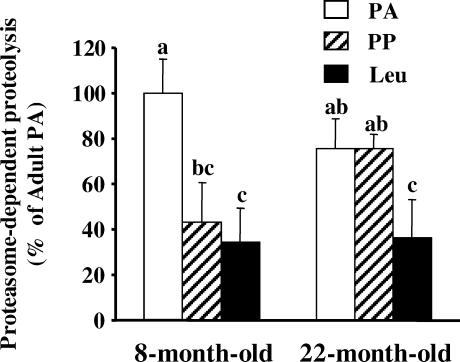
Figure 1 shows that in vitro proteasome-dependent proteolysis measured in the absence and presence of the proteasome inhibitor MG132 decreased by 56% (P < 0.05) after feeding 8-month-old rats a normal diet, but was not down-regulated in 22-month-old animals.
Figure 1. Skeletal muscle proteasome-dependent proteolysis after feeding 8- and 22-month-old animals.
Rats from Experiment 1 were overnight starved (PA), or fed during 1 h either a alanine-supplemented (PP) or a leucine-supplemented meal (Leu). Proteasome-dependent proteolysis was measured as indicated in Methods in incubated epitrochlearis muscles harvested in the PA state or 2 h after meal ingestion. Values are means ± s.e.m. (vertical bars) for 9–10 animals and are expressed as a percentage of 8-month-old PA. Columns with different letters are significantly different from each other as assessed by ANOVA (P < 0.05).
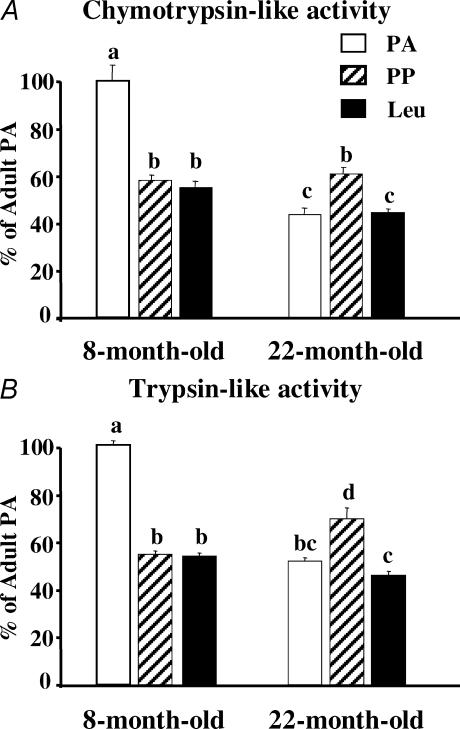
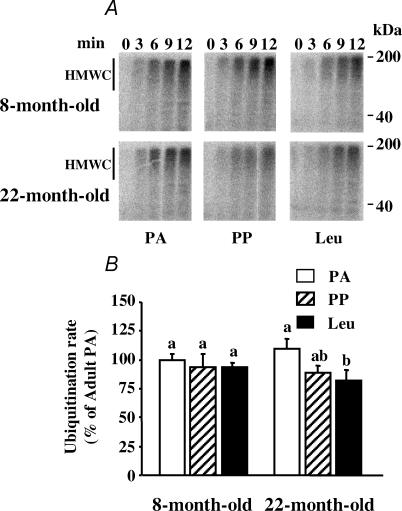
There are two major steps in the Ub–proteasome system: (i) the labelling of substrates by a poly Ub degradation signal, and (ii) the subsequent breakdown of the targeted protein by the proteasome (Pickart, 2001; Glickman & Ciechanover, 2002). Thus, we measured ubiquitination rates and major proteasome peptidase activities. The PP inhibition of the Ub–proteasome system in the muscle of the 8-month-old rats was due to a 41–45% decrease in chymotrypsin-, trypsin-like and PGPH proteasome activities (Fig. 2, and data not shown for the PGPH activity), without any change in rates of ubiquitination (Fig. 3). By surprising contrast, muscle proteasome activities slightly increased following meal ingestion in the 22-month-old rats (Fig. 2).
Figure 2. Chymotrypsin-like (A), and trypsin-like (B) peptidase activities of the proteasome in pooled extensor digitorum longus muscles (n = 7–10 rats) from 8- and 22-month-old rats.
Animals from Experiment 1 were overnight starved (PA) or fed during 1 h (PP or Leu) as described in Fig. 1 legend. Data represent the slopes of best fit of arbitrary fluorescence units released from Suc-LLVY-AMC (chymotrypsin-like activity) or Boc-LRR-AMC (trypsin-like activity) versus time. Data are expressed as percentage of 8-month-old PA and bars denote standard errors of the slopes. Columns with different letters are significantly different by comparing the slopes of best fit (P < 0.05).
Figure 3. Ubiquitination rates in pooled extensor digitorum longus muscles (n = 9–11 rats) from 8- and 22-month-old rats.
The formation of high molecular weight [125I]Ub conjugates (HMWC) in extensor digitorum longus muscle extracts was followed by autoradiography (A). Animals from Experiment 1 were overnight starved (PA) or fed during 1 h (PP or Leu) as described in Fig. 1 legend. B, the comparison of ubiquitination rates (e.g. the slopes of best fit of cpm bound to HMWC following gel excision versus time). Data are expressed as percentage of 8-month-old PA and bars denote standard errors of the slopes. Columns with different letters are significantly different by comparing the slopes of best fit (P < 0.05).
Effects of ageing on ubiquitin–proteasome-dependent proteolysis
Rates of proteasome-dependent proteolysis with ageing tended to decrease in the PA state and to increase in the PP state, but these variations were not significant (Fig. 1). By contrast, there were significant decreases in both chymotrypsin-like and trypsin-like proteasome activities in the PA state with ageing (Fig. 2). Only the trypsin-like proteasome activity increased with ageing in the PP state (Fig. 2), and the rate of ubiquitination was unaffected by ageing in both the PA and PP states (Fig. 3).
Effects of a single leucine-supplemented meal on ubiquitin–proteasome-dependent proteolysis
A full stimulation of skeletal muscle protein synthesis in the PP state was restored in 22-month-old rats fed a leucine-supplemented diet (Dardevet et al. 2002). This prompted us to investigate whether leucine may also inhibit muscle proteolysis. Figure 1 shows that a leucine-supplemented diet had no additive effect with feeding in 8-month-old rats. Accordingly, neither proteasome activities (Fig. 2) nor rates of ubiquitination (Fig. 3) were affected in such conditions. By contrast, the leucine-supplemented diet fully restored the defective postprandial inhibition of in vitro proteasome-dependent proteolysis in 22-month-old rats (Fig. 1). Increased proteasomal activities observed after feeding 22-month-old rats a normal diet were completely prevented by leucine supplementation (Fig. 2), and rates of ubiquitination also decreased (−25%, P < 0.05; Fig. 3).
Effects of feeding a leucine-supplemented diet for 10 days on ubiquitin–proteasome-dependent proteolysis
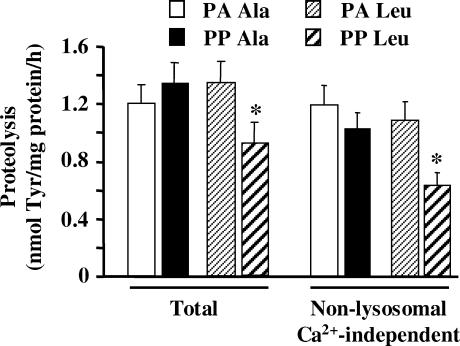
Since leucine-supplemented meal feeding for 10 days had beneficial effects on muscle protein synthesis in 22-month-old rats (Rieu et al. 2003), we next investigated if proteolysis was also regulated long-term by leucine. Figure 4 shows that overall in vitro muscle proteolysis was inhibited in the PP state in 22-month-old animals chronically fed a leucine-supplemented diet compared with animals chronically fed an alanine-enriched diet. The leucine-supplemented diet inhibited a non-lysosomal Ca2+-independent proteolytic process since the PP inhibition of in vitro muscle proteolysis was still detected in the presence of inhibitors of both cathepsins and calpains (Fig. 4).
Figure 4. Total and non-lysosomal Ca2+-independent proteolysis in 22-month-old rats chronically fed an alanine- or leucine-supplemented diet.
Rats from Experiment 2 received a diet supplemented with either alanine or leucine for 10 days. The day of the experiment they were overnight starved (PA Ala or PA Leu) or fed during 1 h either diet (PP Ala or PP Leu). Rates of total proteolysis and non-lysosomal Ca2+-independent proteolysis were measured in incubated epitrochlearis muscles as described in Methods. Values are means ± s.e.m. (vertical bars) for 9–10 animals and are expressed in nmol of Tyr (mg protein)−1 h−1. *P < 0.05 versus PA Leu as assessed by ANOVA.
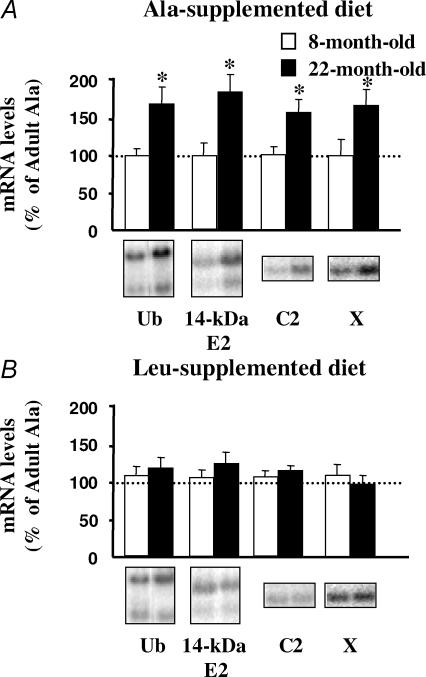
As these experiments were performed with incubated muscles and may not reflect the in vivo situation, we next measured mRNA levels for components of the Ub–proteasome system in both 8- and 22-month-old rats. The mRNA levels for Ub, the 14-kDa E2, and the non-catalytic C2 or catalytic X subunit of the 20S proteasome increased in 22-month-old rats compared with 8-month-old animals (Fig. 5A).
Figure 5. mRNA levels for genes encoding components of the Ub–proteasome pathway in 8- and 22-month-old rats chronically fed an alanine- or leucine-supplemented diet.
Eight- and 22-month-old rats from Experiment 2 received for 10 days a diet supplemented with either alanine (A) or leucine (B). The day of the experiment animals were overnight starved, extensor digitorum longus muscles were harvested, and Northern blots for Ub, the 14-kDa E2, and subunits C2 and X of the 20S proteasome were performed as described in Methods. Hybridization signals were quantified and normalized using the corresponding 18S rRNA signals to correct for uneven unloading. Both transcripts for Ub and the lower transcript 14-kDa E2 were quantified. Values are means ± s.e.m. (vertical bars) for n = 5–7 rats, and are expressed as percentage of 8-month-old rats fed the Ala-supplemented diet. Representative Northern blots are also shown. *P < 0.05 versus 8-month-old rats by the unpaired Student's t test.
Supplementation of the diet with leucine for 10 days completely suppressed the increased muscle mRNA levels for Ub, the 14-kDa E2, and C2 or X proteasome subunits in 22-month-old rats (Fig. 5B). These data demonstrate that chronic administration of a leucine-supplemented diet has long-lasting effects on the Ub-proteasome system in vivo, and clearly support that leucine inhibited a non-lysosomal and Ca2+-independent proteolytic process in ageing (Fig. 4).
Discussion
These findings indicate that the PP inhibition of skeletal muscle ubiquitin–proteasome-dependent proteolysis is defective in 22-month-old animals and can be corrected by a dietary leucine supplementation. Rates of proteasome-dependent proteolysis were measured in vitro as there is no alternative technique for such measurements in vivo. Although such measurements are not quantitative they usually reflect qualitative changes observed in vivo in a number of catabolic or anabolic conditions (for example see Kee et al. 2003). In large 8- and 22-month-old rats in vitro rates of proteolysis can only be measured in a thin muscle (i.e. the epitrochlearis) to avoid the development of hypoxic central cores (Maltin & Harris, 1985). Proteasome activities and ubiquitination rates were measured in the EDL in Experiment 1, as well as mRNA levels in Experiment 2. Both the epitrochlearis and the EDL comprise mostly type II fibres. We have previously reported comparable measurements for these and other type II muscles (i.e. the tibialis anterior and the gastrocnemius) for various parameters of ubiquitin–proteasome-dependent proteolysis (Combaret et al. 2004). However, we used 22-month-old rats that do not exhibit atrophy of all studied muscles. The lack of atrophy of the EDL muscle in 22-month-old animals is consistent with previous observations in Wistar rats. A reduction in the mass of this muscle was only detected at 25 months of age (Mosoni et al. 2004). Since defects in proteasome-dependent proteolysis did not correlate with a high degree of atrophy of the EDL (or epithrochlearis) muscle, one may hypothesize that the defects reported here in 22-month-old animals were early events that will lead to subsequent muscle atrophy. In addition, mortality increases dramatically (by about 50%) between 22 and 25 months of age in this strain of rats. Thus, we used 22-month-old rats to avoid the selection of survivors that may not reflect an average aged population.
Eight-month-old rats but not 22-month-old animals exhibit decreased in vitro proteasome-dependent proteolysis in the postprandial state
Previous studies have detected abnormalities in the regulation of muscle proteolysis in aged muscles (Volpi et al. 2001; Arnal et al. 2002). Since the Ub–proteasome-dependent proteolytic pathway plays a major role in skeletal muscle (Jagoe & Goldberg, 2001; Hasselgren & Fischer, 2001; Attaix et al. 2003), we investigated whether this process was down-regulated in the PP state in 8- and 22-month-old rats. We show that in vitro proteasome-dependent proteolysis was down-regulated in the PP state in 8-month-old rats but not in 22-month-old animals (Fig. 1). These findings clearly support the lack of inhibition of overall muscle proteolysis in the PP state previously reported in old rats (Arnal et al. 2002), and further show that in standard nutritional conditions the skeletal muscle Ub–proteasome system from old rats was insensitive to meal-induced anabolic stimuli.
The lack of regulation of the ubiquitin–proteasome system in ageing may impair skeletal muscle protein deposition
The lack of responsiveness of the Ub–proteasome system that prevailed in the PP state is not unique in old muscles. Indeed, the Ub–proteasome pathway is not activated in muscles from old rats treated in vivo with the synthetic glucocorticoid analogue dexamethasone (Dardevet et al. 1995). By contrast, glucocorticoids stimulate muscle Ub–proteasome-dependent proteolysis in adult rats by various mechanisms (Combaret et al. 2004). Thus, this major proteolytic machinery became insensitive to both anabolic and catabolic stimuli in ageing. This may have negative consequences on muscle protein deposition, because the efficiency of this process is greatly enhanced with simultaneous changes, even of limited amplitude, in rates of both protein synthesis and breakdown (Waterlow et al. 1978). Old rats are only able to slightly increase muscle protein synthesis in response to feeding (Mosoni et al. 1995). Thus, our observations may contribute to the explanation of why muscle recovery is strongly impaired in ageing following negative nitrogen balance conditions (e.g. catabolic treatments (Dardevet et al. 1995) or starvation (Mosoni et al. 1999)). Moreover, the incidence of pathologies, which results in such catabolic conditions (Mitch & Goldberg, 1996; Attaix et al. 2003), increases with ageing. Thus, the inability of old animals to alter muscle rates of proteolysis may also contribute to the explanation of the progressive establishment of sarcopenia. Finally, the Ub–proteasome pathway degrades major contractile proteins such as actin and type II myosins (Mitch & Goldberg, 1996; Jagoe & Goldberg, 2001; Hasselgren & Fischer, 2001; Attaix et al. 2003), and protein synthesis of myosin heavy chain decreased in ageing (Greenlund & Nair, 2003). Overall, this should impair the ability of the organism to maintain the pool of this very abundant contractile protein in ageing.
The postprandial inhibition of in vitro skeletal muscle proteolysis in 8-month-old rats reflects decreased proteasome activities
In 8-month-old rats the decreased proteasome peptidase activities in the PP state (Fig. 2) are consistent with the inhibition of in vitro proteasome-dependent rates of proteolysis (Fig. 1). By contrast, muscle proteasome activities slightly increased following meal ingestion in the 22-month-old rats (Fig. 2). These data were repeatedly obtained in two different experiments and were confirmed by measuring proteasome activities using another technique (i.e. partially purified proteasomes on a glycerol gradient (Tilignac et al. 2002; Combaret et al. 2004; data not shown)). However, rates of ubiquitination also tended to decrease (P < 0.06) in the PP state in the 22-month-old animals (Fig. 3). Overall, the opposite regulation of rates of ubiquitination and of proteasome activities in muscles from 22-month-old animals is presumably responsible for the lack of significant variation in in vitro proteasome-dependent proteolysis following meal ingestion (Fig. 1). Alternatively, the increased measured proteasome peptidase activities in the PP state in 22-month-old rats were detected with exogenous fluorogenic substrates. We cannot rule out that these measurements did not reflect proteasome-dependent proteolysis of the actual endogenous muscle substrates.
Ageing decreases muscle proteasome activities in the postabsorptive state
A strong decrease in proteasome activities characterized ageing in the PA state (Fig. 2). Previous studies in ageing reported no change in activity (Radak et al. 2002a) or decreased activity in either all catalytic sites (Bardag-Gorce et al. 1999; Husom et al. 2004) or limited to the trypsin-like activity (Radak et al. 2002b). These discrepancies are not unexpected since proteasome activities are largely influenced by the nutritional status of the animals (Fig. 2), which was not indicated in all these studies. Our findings further show that changes in the rate of ubiquitination in ageing are unlikely to play a major role in age-related disturbances of the Ub–proteasome system (Fig. 3). Taken together, our data support the down-regulation of the pathway in aged muscle as already reported in a variety of tissues (Carney et al. 1991; Keller et al. 2000; Bulteau et al. 2002; Husom et al. 2004).
Leucine fully restored the defective postprandial inhibition of proteasome-dependent proteolysis in 22-month-old rats
The leucine-supplemented diet fully restored the defective postprandial inhibition of in vitro proteasome-dependent proteolysis in 22-month-old rats (Fig. 1), by normalizing proteasomal activities (Fig. 2) and by decreasing rates of ubiquitination (Fig. 3). These effects are presumably due to the increase in leucine availability since plasma leucine increased twofold in rats receiving the leucine-supplemented meal without significant modification in the levels of other amino acids or insulin (Dardevet et al. 2002; Rieu et al. 2003). In similar in vitro experiments, leucine stimulated protein synthesis (Dardevet et al. 2000). By contrast, incubating muscles with 200–600 μm leucine had no effect on proteolysis (data not shown). Furthermore, Mitch & Clark (1984) showed that the inhibitory effect of leucine on muscle proteolysis requires transamination. Taken together these observations suggested that the inhibitory effect of leucine on in vitro proteasome-dependent proteolysis in this study is likely to be indirect. Leucine enhances muscle protein synthesis by stimulating the mammalian target of the rapamycin pathway (mTOR), as well as the 70-kDa ribosomal protein S6 kinase activity, and by enhancing eIF4E-binding protein phosphorylation and the association of eukaryotic initiation factor eIF4E with eIF4G, both in vitro and in vivo (Dardevet et al. 2000; Anthony et al. 2000a, b; Greiwe et al. 2001). mTOR is the target of Akt, and Akt activation also blocks the up-regulation of atrogin-1/MAFbx (Lee et al. 2004), a muscle-specific E3 that is critical for enhanced Ub–proteasome-dependent proteolysis (Bodine et al. 2001; Gomes et al. 2001). Thus, one may hypothesize that leucine simultaneously stimulated muscle protein synthesis and inhibited the Ub–proteasome pathway by acting on Akt.
Chronic administration of a leucine-supplemented diet has long-lasting effects on the Ub–proteasome system
The elevated mRNA levels for components of the Ub–proteasome pathway in 22-month-old rats fed the control alanine-supplemented diet (Fig. 5A) are in accordance with observations in aged human muscle (Welle et al. 2003). Furthermore, the increased mRNA levels for the proteasome subunit C2 observed in ageing (Fig. 5A) also agrees with an increased protein content for this particular subunit in muscle tissue from old rats (Husom et al. 2004). Thus, proteasome activities decreased with ageing in the PA state (Fig. 2) although there was enhanced expression of proteasome subunits (Fig. 5A). It should be pointed out that an increased expression (Husom et al. 2004 and this experiment) or protein content (Husom et al. 2004) of 20S proteasome subunits does not imply high rates of proteasome-dependent proteolysis in ageing. Indeed, the content of the 19S complex is dramatically reduced in the aged muscle and seems inadequate for complete activation of the 20S proteasome catalytic properties (Husom et al. 2004; Ferrington et al. 2005).
Supplementation of the diet with leucine for 10 days completely suppressed the increased muscle mRNA levels for Ub, the 14-kDa E2, and C2 or X proteasome subunits in 22-month-old rats (Fig. 5B). Although such measurements may not necessarily correlate with rates of proteolysis, they strongly suggest that chronic administration of a leucine-supplemented diet has long-lasting effects on the Ub–proteasome system in vivo. Furthermore, they further support the observation that leucine inhibited a non-lysosomal and Ca2+-independent proteolytic process in the incubated muscles from 22-month-old rats (Fig. 4).
Since chronic leucine administration has also long-lasting effects on the restoration of enhanced muscle protein synthesis in the PP state in ageing (Rieu et al. 2003), our observations strongly suggest that leucine may contribute to the improvement of protein balance in sarcopenia. This is further supported by recent observations indicating that a leucine-supplemented diet stimulated protein synthesis and inhibited the activation of the ubiquitin–proteasome system in the muscles from cancer animals (Ventrucci et al. 2004).
Conclusion
The present findings provide evidence for decreased in vitro proteasome-dependent proteolysis in the aged muscle. We also demonstrate that the defect in PP anabolism observed in ageing results from a lack of inhibition of Ub–proteasome-dependent proteolysis that can be fully restored by leucine supplementation in the diet. Preventing sarcopenia is a major socioeconomic and public health issue. Our observations suggest that very simple nutritional supports (i.e. leucine supplementation or leucine-rich dietary components) may help the elderly to preserve muscle mass.
Acknowledgments
We thank Agnès Claustre and Claire Sornet for expert technical assistance. This research was supported by a grant to D.A. from the Institut National de la Recherche Agronomique. M.N.P. was supported by a postdoctoral fellowship from ESPEN. We thank Dr Catherine DiCostanzo for critically reading the manuscript, and Dr Simon S. Wing (McGill University, Montréal, Canada) and Dr Keiji Tanaka (The Tokyo Metropolitan Institute of Medical Science, Japan) for providing us with cDNAs encoding the 14-kDa E2 and proteasome subunits, respectively.
References
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000a;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000b;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Arnal M-A, Mosoni L, Boirie Y, Houlier M-L, Morin L, Verdier E, Ritz P, Antoine J-M, Prugnaud J, Beaufrère B, Patureau Mirand P. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- Arnal M-A, Mosoni L, Dardevet D, Ribeyre M-C, Bayle G, Prugnaud J, Patureau Mirand P. Pulse protein feeding pattern restores stimulation of muscle protein synthesis during the feeding period in old rats. J Nutr. 2002;132:1002–1008. doi: 10.1093/jn/132.5.1002. [DOI] [PubMed] [Google Scholar]
- Attaix D, Combaret L, Kee AJ, Taillandier D. Mechanisms of ubiquitination and proteasome-dependent proteolysis in skeletal muscle. In: Zempleni J, Daniel H, editors. Molecular Nutrition. Wallingford, Oxon, UK: CABI Publishing; 2003. pp. 219–235. [Google Scholar]
- Bardag-Gorce F, Farout L, Veyrat-Durebex C, Briand Y, Briand M. Changes in 20S proteasome activity during ageing of the LOU rat. Mol Biol Rep. 1999;26:89–93. doi: 10.1023/a:1006968208077. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Szweda LI, Friguet B. Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys. 2002;397:298–304. doi: 10.1006/abbi.2001.2663. [DOI] [PubMed] [Google Scholar]
- Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Combaret L, Taillandier D, Dardevet D, Béchet D, Rallière C, Claustre A, Grizard J, Attaix D. Glucocorticoids regulate mRNA levels for subunits of the 19 S regulatory complex of the 26 S proteasome in fast-twitch skeletal muscles. Biochem J. 2004;378:239–246. doi: 10.1042/BJ20031660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Taillandier D, Savary I, Attaix D, Grizard J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J Clin Invest. 1995;96:2113–2119. doi: 10.1172/JCI118264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval C, Mordier S, Obled C, Béchet D, Combaret L, Attaix D, Ferrara M. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J. 2001;360:143–150. doi: 10.1042/0264-6021:3600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CH, Li BG, Fischer DR, Wang JJ, Runnels HA, Monaco JJ, Hasselgren PO. Burn injury upregulates the activity and gene expression of the 20 S proteasome in rat skeletal muscle. Clin Sci. 2000;99:181–187. [PubMed] [Google Scholar]
- Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- Frexes-Steed M, Lacy DB, Collins J, Abumrad NN. Role of leucine and other amino acids in regulating protein metabolism in vivo. Am J Physiol. 1992;262:E925–E935. doi: 10.1152/ajpendo.1992.262.6.E925. [DOI] [PubMed] [Google Scholar]
- Fulks RM, Li JB, Goldberg AL. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975;250:290–298. [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund LJ, Nair KS. Sarcopenia-consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124:287–299. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70, S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E466–E471. doi: 10.1152/ajpendo.2001.281.3.E466. [DOI] [PubMed] [Google Scholar]
- Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobler SC, Williams A, Fischer D, Wang JJ, Sun XY, Fischer JE, Monaco JJ, Hasselgren PO. Activity and expression of the 20S proteasome are increased in skeletal muscle during sepsis. Am J Physiol Regul Integr Comp Physiol. 1999;277:R434–R440. doi: 10.1152/ajpregu.1999.277.2.R434. [DOI] [PubMed] [Google Scholar]
- Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Metab Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Kee AJ, Combaret L, Tilignac T, Souweine B, Aurousseau E, Dalle M, Taillandier D, Attaix D. Ubiquitin-proteasome-dependent muscle proteolysis responds slowly to insulin release and refeeding in starved rats. J Physiol. 2003;546:765–776. doi: 10.1113/jphysiol.2002.032367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Lee SW, Dai G, Hu Z, Du Wang XJ, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maltin CA, Harris CI. Morphological observations and rates of protein synthesis in rat muscles incubated in vitro. Biochem J. 1985;232:927–930. doi: 10.1042/bj2320927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor O, Beaufrère B, Boirie Y, Rallière C, Taillandier D, Aurousseau E, Schoeffler P, Arnal M, Attaix D. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A. 1996;93:2714–2718. doi: 10.1073/pnas.93.7.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch WE, Clark AS. Specificity of the effects of leucine and its metabolites on protein degradation in skeletal muscle. Biochem J. 1984;222:579–586. doi: 10.1042/bj2220579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Breuillé D, Buffière C, Obled C, Patureau Mirand P. Age-related changes in glutathione availability and skeletal muscle carbonyl content in healthy rats. Exp Gerontol. 2004;39:203–210. doi: 10.1016/j.exger.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Malmezat T, Valluy M-C, Houlier M-L, Attaix D, Patureau Mirand P. Lower recovery of muscle protein lost during starvation in old rats despite a stimulation of protein synthesis. Am J Physiol. 1999;277:E608–E616. doi: 10.1152/ajpendo.1999.277.4.E608. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Valluy M-C, Serrurier B, Prugnaud J, Obled C, Guezennec C-Y, Patureau Mirand P. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol. 1995;268:E328–E335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002a;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- Radak Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Goto S. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol. 2002b;37:1423–1430. doi: 10.1016/s0531-5565(02)00116-x. [DOI] [PubMed] [Google Scholar]
- Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, Papet I, Grizard J, Dardevet D. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133:1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilignac T, Temparis S, Combaret L, Taillandier D, Pouch M-N, Cervek M, Cardenas DM, Le Bricon T, Debiton E, Samuels SE, Madelmont J-C, Attaix D. Cancer Res. 2002;62:2771–2777. [PubMed] [Google Scholar]
- Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982;257:1613–1621. [PubMed] [Google Scholar]
- Ventrucci G, Mello MA, Gomes-Marcondes MC. Proteasome activity is altered in skeletal muscle tissue of tumour-bearing rats a leucine-rich diet. Endocr Relat Cancer. 2004;11:887–895. doi: 10.1677/erc.1.00828. [DOI] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow JC, Garlick PJ, Millward DJ. Protein Turnover in Mammalian Tissues and in the Whole Body. Amsterdam: Elsevier-North Holland; 1978. [Google Scholar]
- Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics. 2003;14:149–159. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- Young VR, Munro HN, Fukagawa N. Protein and functional consequences of deficiency. In: Horwitz A, MacFayden DM, Munro HN, Scrimshaw NS, Steen B, Williams TF, editors. Nutrition in the Elderly. New York: Oxford University Press; 1989. pp. 65–84. [Google Scholar]