Abstract
Proprioceptive information from paraspinal tissues including muscle contributes to neuromuscular control of the vertebral column. We investigated whether the history of a vertebra's position can affect signalling from paraspinal muscle spindles. Single unit recordings were obtained from muscle spindle afferents in the L6 dorsal roots of 30 Nembutal-anaesthetized cats. Each afferent's receptive field was in the intact muscles of the low back. The L6 vertebra was controlled using a displacement-controlled feedback motor and was held in each of three different conditioning positions for durations of 0, 2, 4, 6 and 8 s. Conditioning positions (1.0–2.2 mm dorsal and ventral relative to an intermediate position) were based upon the displacement that loaded the L6 vertebra to 50–60% of the cat's body weight. Following conditioning positions that stretched (hold-long) and shortened (hold-short) the spindle, the vertebra was repositioned identically and muscle spindle discharge at rest and to movement was compared with conditioning at the intermediate position. Hold-short conditioning augmented mean resting spindle discharge by +4.1 to +6.2 impulses s−1; however, the duration of hold-short did not significantly affect this increase (F4,145 = 0.49, P = 0.74). The increase was maintained at the beginning of vertebral movement but quickly returned to baseline. Conversely, hold-long conditioning significantly diminished mean resting spindle discharge by −2.0 to −16.1 impulses s−1 (F4,145 = 11.23, P < 0.001). The relationship between conditioning duration and the diminished resting discharge could be described by a quadratic (F1,145 = 9.28, P = 0.003) revealing that the effects of positioning history were fully developed within 2 s of conditioning. In addition, 2 s or greater of hold-long conditioning significantly diminished spindle discharge to vertebral movement by −5.7 to −10.0 impulses s−1 (F4,145 = 11.0, P < 0.001). These effects of vertebral positioning history may be a mechanism whereby spinal biomechanics interacts with the spine's proprioceptive system to produce acute effects on neuromuscular control of the vertebral column.
The vertebral column is a multijoint complex and is inherently unstable when devoid of musculature (Wilke et al. 1999; Crisco et al. 1992). Many studies demonstrate the importance of the neuromuscular system for generating spinal stability and for controlling posture and movement of individual motion segments as well as spinal regions (Bergmark, 1989; Crisco & Panjabi, 1991; Crisco et al. 1992; Wilke et al. 1995; Cholewicki & McGill, 1996; Kong et al. 1996). Even small changes in muscle force can induce large impacts on the behaviour of individual motion segments. For example, low paraspinal muscle forces (20 N compared with 40 N and 60 N) increase segmental stabilization by producing the largest decrease in intersegmental range of motion and neutral zone (Panjabi et al. 1989). Lumbar multifidus, longissimus and iliocostalis muscles have the strongest influence on these biomechanical characteristics (Wilke et al. 1995). In addition, very small increases in muscle activity (1–3% of maximal voluntary contraction) from lumbar multifidus, iliocostalis and thoracic longissimus at L2–L4 restore spinal stability at the segmental level even when loading moments are increased up to 75% of body weight (Cholewicki & McGill, 1996).
An important contribution from these studies is the idea that the risk of injury to spinal structures is greatest during easy, non-demanding tasks (Cholewicki & McGill, 1996). Non-demanding tasks do not require high muscle forces and spinal segments appear most unstable at low levels of muscle activity. This would explain why leaning over to pick up a seemingly benign object, such as a pencil may lead to one's back giving out. Changes in neuromuscular control may allow disproportionately more movement at a single intersegmental level compared with the regional movement of the spine. Such a phenomenon has been documented by videofluoroscopy (Cholewicki & McGill, 1992). The anomalous movement evoked both pain and discomfort.
We are interested in factors that affect the responsiveness of paraspinal muscle spindles because this sensory input appears to play an important feedback role for position sense (Brumagne et al. 1999; Brumagne et al. 2000) and likely makes reflex contributions to muscle loading of the vertebral joints (Swinkels & Dolan, 2000; Leinonen et al. 2003; O'Sullivan et al. 2003; Skotte et al. 2005). Previous studies in the limbs of humans and cats demonstrate that muscle history affects muscle spindle discharge and causes errors in limb repositioning (Enoka et al. 1980; Morgan et al. 1984; Gregory et al. 1986, 1987, 1988, 1990; Wood et al. 1996; Hagbarth & Nordin, 1998). In particular, muscle history can affect the magnitude of the stretch reflex and, at the same time, produce converse effects on the H reflex arising from changes in resting spindle discharge (Gregory et al. 1990; Wood et al. 1996). These proprioceptive effects led us to investigate a biomechanical mechanism in the lumbar vertebral column that might alter the responsiveness of paraspinal muscle spindles. Small, sustained changes in segmental position might influence spindle signalling.
In the lumbar spine of the cat our preliminary data from a small sample of muscle spindles suggested that the history of vertebral positioning does affect paraspinal muscle spindle discharge (Pickar & Kang, 2001). A vertebral position that stretched lumbar paraspinal muscles for 5 s decreased muscle spindle responsiveness by up to 12 impulses s−1 (imp s−1) compared with a shortening position. However, due to the study's design only relative changes in responsiveness could be determined because two vertebral positions were compared. We now wanted to know if the history and duration of vertebral positioning could both increase and decrease spindle responsiveness. Therefore, we have used an intermediate vertebral position for comparison. In this study we have determined (1) if vertebral positioning can both increase and decrease spindle responsiveness, (2) how the duration of vertebral positioning affects spindle responsiveness, and (3) how an afferent's dynamic index affects the history-dependent responsive.
Methods
Preparation
Experiments were performed on 30 deeply anaesthetized adult cats. All cats were treated in accordance with the ethical standards of the Institutional Animal Use and Care Committee of Palmer College of Chiropractic. A preparation was developed to keep as intact as possible the tissues and innervation of the L6–7 lumbar spine and, at the same time, expose the L6 dorsal roots. The preparation was originally described by Pickar (1999). Briefly, surgical anaesthesia was initially maintained using a mixture of O2 (2 l min−1) and halothane (3%). Deep anaesthesia was maintained using Nembutal (i.v. 35 mg kg−1) and the cat was mechanically ventilated (model 681, Harvard Apparatus Company, Inc., Millis, MA, USA). Additional doses (5 mg kg−1) were administered when the cat demonstrated a withdrawal reflex to noxious pinching of the toe pad, when mean arterial pressure increased above 120 mmHg, or when the cat exhibited a pressor response to surgical manipulation. Arterial pH, PCO2 and PO2 were monitored (i-STAT System, i-STAT Corporation, East Windsor, NJ, USA). Arterial blood gas values were maintained within the normal range (pH: 7.32–7.43; PCO2: 32–35 mmHg; and PO2: > 85 mmHg).
A surgical approach was used that kept the vertebral column completely intact bilaterally from the L6 vertebra caudalwards. In addition, all soft paraspinal tissue on the right side at L4 and L5 remained intact except for their attachments to the posterior elements of the vertebrae. This was accomplished by opening only the lumbodorsal fascia from L4 to L5 using a paramedial incision 3–4 mm to the left of midline. Multifidus, longissimus and lumbococcygeus muscles at L4 and L5 on the left were removed. An L4–L5 hemi-laminectomy was performed on the left side, and a sublaminar hemi-laminectomy was used to remove the right half of L4 and L5 thus exposing the spinal cord entry level of the L6 roots. The lumbar spine was anchored at L4 and the pelvis by fixing the L4 spinous process and the iliac crests in a Kopf spinal unit. The paraspinal tissues were bathed in warm mineral oil (37°C) to prevent desiccation.
Recording nerve impulse activity from the dorsal roots
Standard electrophysiological techniques were used to record single unit activity from teased L6 dorsal root filaments. The L6 root innervates the L6–L7 facet joint and muscles attaching to the L6 vertebra (Bogduk, 1976). All receptive fields were in the L6–L7 paraspinal muscles. Due to the relatively small laminectomy, it was not possible to access the L6 ventral roots without potentially injuring the spinal cord. Therefore the ventral roots were not cut. Because Nembutal anaesthesia was maintained at a deep level, γ-motoneurone discharge was considered depressed and not labile (Collins et al. 1995). Nerve activity was acquired at 22 kHz and single units were recognized using a PC based data acquisition system (Spike2, v4, Cambridge Electronic Design Limited, Cambridge, UK). Analysis was performed off-line using Spike2.
Mechanical loading of the L6 vertebra
Mechanical loads were applied to the L6 vertebra in a dorsal–ventral direction using a feedback motor system (model 300B, Aurora Scientific Inc., Ontario, Canada). The system was operated in displacement mode and measured the applied length and force. The motor was coupled to the L6 spinous process via a pair of adjustable tissue forceps (1 × 2 teeth). The forceps were clamped tightly onto the lateral surfaces of the L6 spinous process through a thin slit (approximately 2 mm long) along either side of the L6 spinous process. Little of the multifidus muscle was detached from the vertebra using this method because most of the muscle fibres attach onto the caudal edge of the spinous process via a tendon insertion. Only a small portion of the multifidus muscle inserts onto the lateral surface of the lumbar spinous processes (Bogduk, 1980).
Identification of muscle spindles
Putative paraspinal muscle spindle afferents were first identified by their high frequency discharge in response to gentle probing of the lumbar paraspinal muscles and to manual movement of the L6 vertebra. Dynamic sensitivity was assessed using ramp and hold movement of the L6 vertebra. The vertebra was slowly translated (1 mm s−1) in either a ventral- or dorsalward direction, whichever direction stimulated the afferent. When the movement produced a force load of 50–60% of the cat's body weight (BW), the vertebra was then held stationary for 20 s. Larger loads often stretched the dorsal root filament and tore it from the electrode. Afferent activity was resolved into phasic and static components represented by the peak instantaneous discharge frequency during the ramp, and the mean discharge frequency 0.5–1.0 s after the end of the ramp, respectively. A dynamic index was calculated by subtracting the static from the phasic component. Afferents were grouped based upon having a dynamic index greater than or less than 20 imp s−1. The complexity of the lumbar paraspinal muscles made it impossible to isolate individual muscle tendons. While loading to 50–60% BW provided a standardized reference, controlling vertebral position along the dorsal–ventral axis could not ensure the muscles or muscle spindles were necessarily stretched parallel to their long axis. Nonetheless, the dynamic index is thought to be independent of the stretch magnitude (Matthews, 1972).
After the end of the experiment we opened the intact lumbodorsal fascia and used a variety of approaches to confirm that the single unit innervated a lumbar paraspinal muscle spindle. We removed the sacrocaudalis dorsalis lateralis (lumbococcygeus) muscle lying between the lumbar multifidus and longissimus muscles to improve the mechanical isolation of the latter two muscles. First and second sacral nerves innervate the lumbococcygeus (Bogduk, 1983) muscle so that none of the afferent recordings were lost by its removal. That the source of neural activity was from a receptive ending in the lumbar longissimus or multifidus muscles was confirmed by using calibrated nylon filaments (von Frey-like hairs; Stoelting, IL, USA) to determine the most sensitive area for mechanically activating the afferent was in the low back.
Two methods were used to confirm that neural activity was from a muscle spindle: decreased discharge to a muscle twitch and increased discharge to succinylcholine (100–400 μg kg−1, i.a.). Because lumbar paraspinal muscle nerves could not be isolated for direct electrical stimulation the afferent's response to muscle contraction was determined by direct muscle stimulation (0.1–10 mA, 0.05–0.2 ms) using two needle electrodes inserted into the muscle. Typically the electrodes were inserted into either side of the most sensitive portion of the afferent's receptive field.
Conduction velocity was obtained by inserting two stimulating needle electrodes in the vicinity of the L6–7 intervertebral foramen. Conduction velocity was determined by dividing the conduction distance by the time for an impulse to reach the recording electrode in response to stimulation. Conduction distance was approximate and was determined by measuring the length of a thin thread extending from the recording electrode along the dorsal root and spinal nerve to its entrance at the intervertebral foramen. Typically conduction times were ≤ 1 ms and conduction distances 45 mm. Conduction distances in error by 10 mm would over- or underestimate conduction velocity by about 10–13 m s−1. Using the L6–7 facet as landmark, we quite consistently were able to position the tip of the stimulating electrodes in the vicinity of the L6 spinal nerve and repeatedly evoke identically shaped action potentials with identical conduction times.
Cats were killed at the completion of the experiment by the intravenous administration of Nembutal (i.v., 60 mg kg−1) followed by saturated potassium chloride solution (i.v.) to fibrillate the heart.
Experimental protocol
At the start of each protocol the L6 vertebra was held for 5 s at an intermediate position defined by the absence of a force load. The motion segment was deconditioned by rapidly alternating the vertebra's position about the intermediate position (10 mm s−1). The magnitude of these displacements loaded the spine by 50–60% BW. The motion segment was then conditioned by holding the L6 vertebra in one of three positions at a displacement that also loaded the spine by 50–60% BW. Two of the three hold-positions represented symmetrical vertebral displacements about the third position, hold-intermediate. The vertebral position that loaded the muscle spindle was called hold-long and conversely the vertebral position that unloaded the muscle spindle was called hold-short. Conditioning displacement was maintained for 0, 2, 4, 6 and 8 s, where 0 s of conditioning served as a control for the hold position by evoking only the slow ramp up to and ramp down from the hold position. At the end of hold-short and hold-long, the L6 vertebra was returned to the intermediate position (10 mm s−1). Thus, 15 loading protocols were applied (3 conditioning positions × 5 conditioning durations). The presentation order of the three conditioning positions and the five conditioning durations was randomized to minimize ordering effects produced by the biomechanical non-linearity of paraspinal soft tissues (Panjabi et al. 1994; Wilke et al. 1995; Ogon et al. 1997). Changes in the response measures could be ascribed to the conditioning position and duration and not to the conditioning order.
The effects of conditioning direction and duration on subsequent muscle spindle activity were identified using two measures. The L6 vertebra was returned to the intermediate position and its resting discharge recorded for 0.5 s. This response measure was termed the static test (ST). The vertebra was then slowly displaced at a constant velocity (0.2 mm s−1) in the direction that loaded the spindle and to a displacement magnitude that loaded the spine to 50–60% BW. This response measure was termed the dynamic test (DT).
Data analysis
The effect of conditioning on ST was quantified as mean instantaneous frequency (MIF). The effect of conditioning on DT was quantified as mean frequency (MF) because each dynamic test lasted at least 5 s. If large rapid changes occurred over this 5 s interval, the value of MIF would be inappropriately skewed toward higher values. Therefore we were conservative in the metric we used for DT. The effect of conditioning history was always determined with reference to hold-intermediate by subtracting ST or DT for the hold-intermediate protocol from the ST or DT for the hold-short (ΔSTshort and ΔDTshort) or hold-long protocol (ΔSTlong and ΔDTlong). Consequently, when ΔST or ΔDT was positive, hold-long or hold-short conditioning had increased muscle spindle responsiveness relative to hold-intermediate conditioning. Conversely when ΔST or ΔDT was negative conditioning history had decreased muscle spindle responsiveness. When ΔST or ΔDT equalled 0, the effect of hold-long or hold-short conditioning was the same as the effect of hold-intermediate conditioning.
Primary analyses used one-way analysis of variance (ANOVA) to compare each response across the five conditioning durations. Based upon standard deviations obtained from our preliminary data (Pickar & Kang, 2001) a sample size of 30 provided 80% power to detect a 5 imp s−1 difference between the conditioning durations at the 0.05 level of significance. Position sensitivity of the passive spindle is considered ∼3.5–5 imp s−1 mm−1 (Granit, 1958; Matthews, 1972). Normality and equal variance assumptions were not violated. Measurements taken during each of the 15 protocols were considered independent of one another because deconditioning initiated all protocols and established identical motion segment histories across protocols in each cat.
Secondary analyses used two-way ANOVA to investigate how the groupings based upon dynamic index affected responses across the five conditioning durations and included an interaction term if it was significant. Using linear regression we also explored whether the range of conditioning vertebral displacements needed to load the cats' spines to 50–60% BW affected either ΔST or ΔDT. The secondary analyses were not necessarily powered adequately because we did not control the selection of spindles for their dynamic index or the magnitude of conditioning displacement across preparations.
Data are reported as means (lower 95% confidence limit, upper 95% confidence limit) unless otherwise indicated. Statistical analyses were conducted using SAS (version 8, SAS Institute, Cary, NC, USA). Significance was determined at P≤ 0.05.
Results
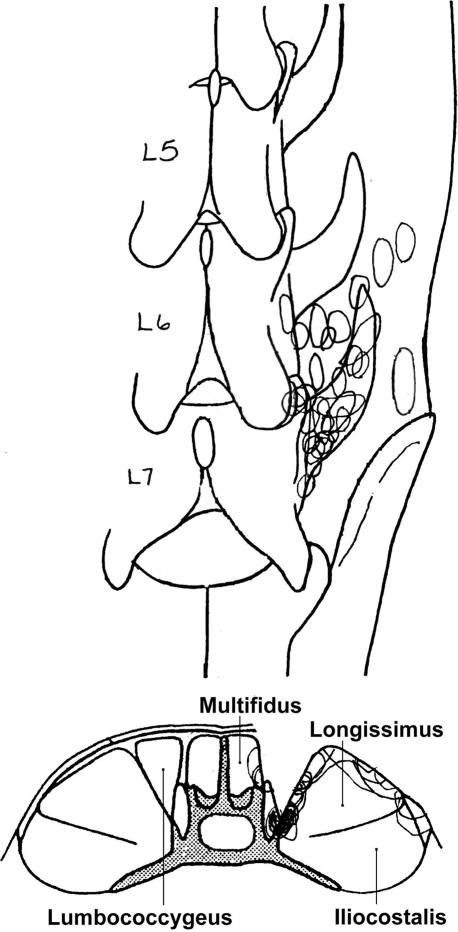
Single unit recordings were obtained from 30 muscle spindle afferents. Receptive fields were located in the lumbar multifidus or longissimus muscles (Fig. 1). Longissimus muscle contained the receptive field of 27 afferents and the multifidus muscle contained the receptive field of the remaining three afferents. Most of the fields were located near the L6–7 facet joint or on the medial surface of the longissimus muscle. Mechanical threshold obtained using nylon monofilaments ranged between 0.2 and 75.9 g (7.0 (19.0) g; mean (s.d.)). Generally, the afferents with low mechanical thresholds were found closer to the facet suggesting the receptive endings were located superficially. The receptive endings of afferents with high mechanical thresholds were likely located deeper in the paraspinal muscles.
Figure 1. The most mechanically sensitive area of a spindle afferent's receptive field is indicated by black perimeters.
A, dorsal view; B, cross-sectional view collapsing across all vertebrae. Note the sulcus between the multifidus and longissimus muscles after removing the lumbococcygeus muscle which enabled us to probe deeper in the back muscles.
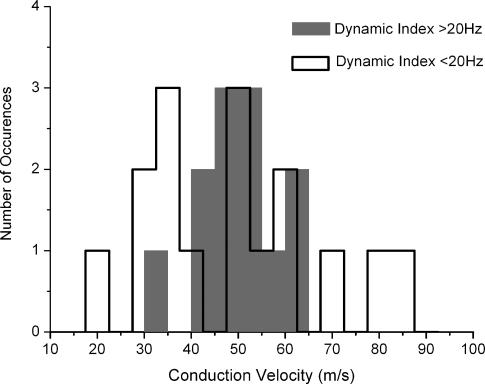
Twenty-seven of the 30 afferents had a resting discharge with the L6 vertebra positioned at the intermediate position. Mean discharge was 25.6 (17.2; s.d.) imp s−1 (range: 0.0–92.9 imp s−1). Three afferents had either no or a very low resting discharge at the intermediate position (< 3 imp s−1). Most of the afferents (25 of 30) were loaded when the L6 vertebra was translated ventralward; five were loaded during dorsalward translation. Across cats, displacement of the L6 vertebra ranged between 1.0 and 2.2 mm (1.6 (0.6) mm; mean (s.d.)) during the 50–60% BW load. Thirteen spindle afferents had a dynamic index > 20 imp s−1 and 17 had a dynamic index < 20 imp s−1. Conduction velocities were obtained for 28 of the 30 afferents (Fig. 2). Their distribution was unimodal and ranged from 21.2 to 85.0 m s−1. There was no correlation between conduction velocity and dynamic index.
Figure 2. Distribution of conduction velocities based upon dynamic index of muscle spindles in the lumbar paraspinal muscles.
DI, dynamic index. For DI > 20 imp s−1, n = 13; for DI < 20 imp s−1, n = 17.
Primary analysis: effect of conditioning duration – all afferents
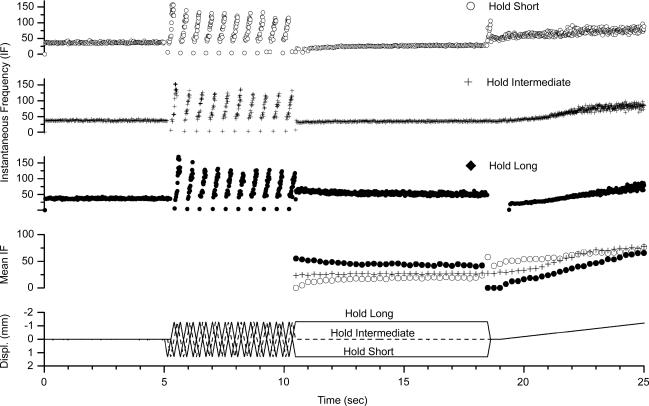
The history of vertebral position differentially affected the discharge of lumbar paraspinal muscle spindles. Figure 3 is an example from one afferent showing its discharge after holding the L6 vertebra at an intermediate position for 8 s versus holding it in positions that lengthened or shortened the paraspinal muscles. Hold-long diminished spindle responsiveness and, conversely, hold-short augmented spindle responsiveness.
Figure 3. Representative response from a paraspinal muscle spindle afferent showing the effect of 8 s conditioning on the test protocols.
The spindle's sensitivity to the static and dynamic test was diminished in response to holding the L6 vertebra in a position that lengthened the paraspinal muscle, and conversely was augmented in response to holding the vertebra in a position that shortened the muscle when compared to conditioning at the intermediate position. Symbols in the 4th panel (mean IF) represent the average from 250 ms time bins. IF given in imp s−1.
Static test
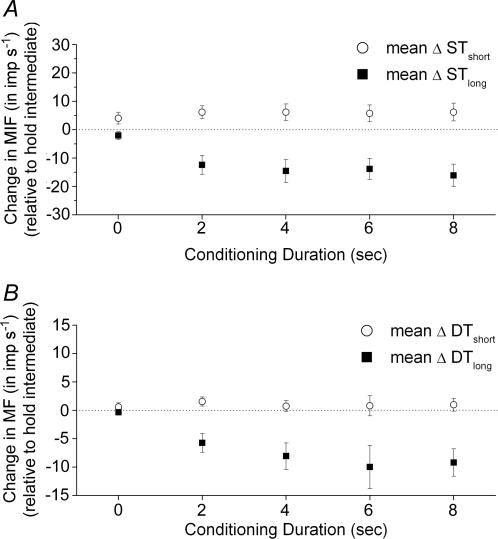
Hold-short conditioning increased mean ΔSTshort by +4.1 (2.0, 6.2), +6.2 (+4.0, +8.4), +6.2 (+3.3, +9.1), +5.8 (+2.8, +8.7) and +6.2 (+3.1, +9.4) imp s−1 at 0, 2, 4, 6 and 8 s, respectively (Fig. 4A). None of the 95% confidence intervals crossed 0 imp s−1. The duration of hold-short conditioning did not significantly affect the increase in mean ΔSTshort (F4,145 = 0.49, P = 0.74).
Figure 4. Effect of conditioning direction and conditioning duration on muscle spindle responses to the static (A) and dynamic (B) test.
MIF, mean instantaneous frequency; MF, mean frequency; ST, static test; DT, dynamic test. Subscripts long and short refer to whether the vertebral position loaded or unloaded the muscle spindles, respectively, during the conditioning protocol. Each symbol represents the mean ± 95% confidence interval of 30 observations.
By contrast hold-long conditioning decreased mean ΔSTlong, by −2.0 (−3.4, −0.7) −12.4 (−15.7, −9.1), −14.5 (−18.6, −10.5), −13.8 (−17.5, −10.1) and −16.1 (−20.0, −12.1) imp s−1 at 0, 2, 4, 6 and 8 s, respectively, and none of the 95% confidence intervals crossed 0 imp s−1. The duration of hold-long conditioning significantly affected the decrease in mean ΔSTlong (F4,145 = 11.23, P < 0.001). Preplanned comparisons demonstrated the decrease evoked by the 8 s hold-long duration was greater than that evoked by the 0 s hold-long duration (F1,145 = 35.27, P < 0.001). In addition, the relationship between the decrease in ΔSTlong and conditioning duration was quadratic (F1,145 = 9.28, P = 0.003) as evidenced by the sharp inflection near the 2 s conditioning duration (Fig. 4A). These comparisons suggested that the conditioning effect of a vertebral position which lengthened the lumbar paraspinal muscles was fully developed by 2 s of conditioning.
Dynamic test
Figure 4B shows that at least 2 s of hold-long positioning altered the dynamic response paraspinal muscle spindles. Mean ΔDTlong was diminished by −5.7 (−7.4, −4.1), −8.1 (−10.4, −5.7), −10.0 (−13.8, −6.2) and −9.2 (−11.6, −6.8) imp s−1 at 2, 4, 6 and 8 s, respectively, but not at 0 s (−0.4 (−0.8, 0.1) imp s−1). Conditioning duration had a significant effect on mean ΔDTlong (F4,145 = 11.0, P < 0.001). Preplanned comparisons confirmed that the mean decrease was greater after 8 s compared with 0 s hold-long conditioning (F1,145 = 28.61, P < 0.001) and that a quadratic described the relationship (F1,145 = 8.55, P = 0.004) between the decrease in spindle responsiveness and conditioning duration.
On the other hand, hold-short conditioning had little, if any, effect on mean ΔDTshort (Fig. 4B). Spindle discharge increased by +0.6 (−0.1, +0.7), +1.6 (+0.7, +0.8), +0.7 (−0.3, +1.0), +0.8 (−1.0, +1.8) and +1.0 (−0.1, +1.1) imp s−1 at 0, 2, 4, 6 and 8 s, respectively. Conditioning duration had no effect on these small changes in mean frequency (F4,145 = 0.44, P = 0.78).
Secondary analysis: effect of conditioning magnitude – all afferents
Because conditioning was based upon loading the spine by 50–60% BW, the L6 vertebra translated between 1.0 and 2.2 mm across cats. We were concerned that such a range of displacements might influence the effect of conditioning history. Therefore, we regressed displacement against ΔSTshort, ΔDTshort, ΔSTlong, and ΔDTlong. Mean slopes were not significantly different from zero (P =0.05) indicating the range of displacements we used did not systematically affect the responses during the static or dynamic tests.
Secondary analysis: effect of conditioning duration – based upon dynamic index
Static test
Hold-short vertebral conditioning similarly affected spindles with dynamic indices greater and less than 20 imp s−1 (F1,144 = 0.71, P = 0.40). Examples of 0, 2 and 4 s conditioning durations are shown in Table 1, columns 1 and 2 and the mean change in spindle responsiveness for all conditioning durations is shown graphically in Fig. 5 (filled symbols at ‘Static Test’ on the x-axis).
Table 1.
Difference in responsiveness of muscle spindles based upon dynamic index (DI)
| Static test mean ΔST | Dynamic test mean ΔDT | |||||||
|---|---|---|---|---|---|---|---|---|
| Hold-short | Hold-long | Hold-short | Hold-long | |||||
| Conditioning duration | DI > 20 (n = 13) | DI < 20 (n = 17) | DI > 20 (n = 13) | DI < 20 (n = 17) | DI > 20 (n = 13) | DI < 20 s (n = 17) | DI > 20 (n = 13) | DI < 20 (n = 17) |
| 0 s | 7.0 | 1.8 | −2.6 | −1.6 | 1.3 | 0.1 | −0.7 | 0 |
| (5.7) | (4.4) | (4.6) | (2.9) | (2.4) | (1.4) | (1.4) | (0.9) | |
| 2 s | 6.8 | 5.7 | −18.3 | −7.8 | 2.0 | 1.2 | −9.7 | −2.7 |
| (8.6) | (2.9) | (9.2) | (5.3) | (2.9) | (1.4) | (3.7) | (1.9) | |
| 4 s | 5.4 | 6.7 | −21.9 | −8.9 | 0.4 | 1.0 | −13.6 | −3.8 |
| (10.8) | (4.7) | (10.8) | (7.0) | (3.6) | (1.6) | (4.8) | (2.9) | |
| Overall | 6.3 | 5.3 | −16.9 | −7.8 | 1.0 | 0.9 | −11.4 | −3.0 |
| (9.8) | (4.2) | (11.7) | (6.9) | (9.8) | (4.2) | (11.7) | (6.9) | |
All values given in impulses s−1 as mean (s.d.); n = number of afferents. ΔST change in spindle discharge during the static test relative to the effect of hold-intermediate conditioning; ΔDT, change in spindle discharge during the dynamic test relative to the effect of hold-intermediate conditioning.
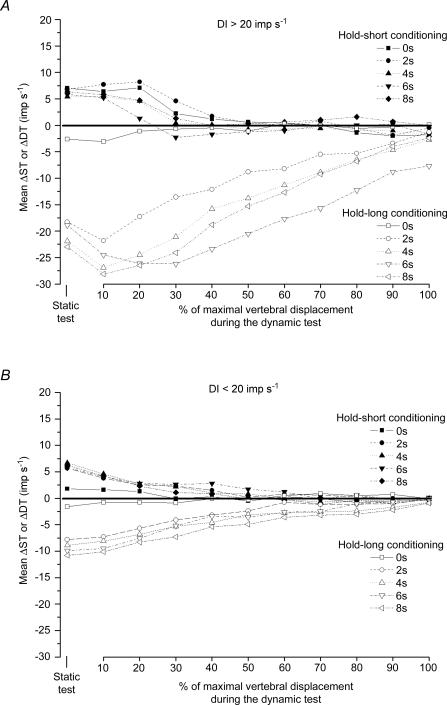
Figure 5. Effect of conditioning direction and conditioning duration on muscle spindle responses over the time course of the dynamic test in 10% increments.
The x-axis is not in units of time because maximal displacement was unique for each spindle (based upon the displacement that loaded the spine 50–60% of body weight). The rate of displacement during the dynamic test was constant (0.2 mm s−1) and displacements ranged from 1 to 2.2 mm; therefore the duration of the dynamic test ranged from 5 to 11 s. The static test is included for comparison. ΔST, change in discharge frequency during the static test; ΔDT, change in discharge frequency during the dynamic test; DI, dynamic index. Each symbol represents the mean of 30 observations.
In contrast, conditioning duration and dynamic index interacted significantly when vertebral conditioning lengthened the paraspinal muscles (F4,140 = 2.81, P = 0.03). The decreased responsiveness by spindles with a DI > 20 imp s−1 was more than twice that of spindles with a DI < 20 imp s−1 at the 2, 4, 6 and 8 s conditioning durations but was similar at the 0 s duration (Table 1 columns 3 and 4 and Fig. 5, open symbols at ‘Static test’). The group of spindles with DI > 20 imp s−1 contributed largely to the decrease of mean ΔSTlong shown in Fig. 4A.
Dynamic test
Similar to the static test, hold-short vertebral conditioning affected the two groups of muscle spindles similarly at all conditioning durations for the dynamic test (F5,144 = 0.13, P = 0.72; see Table 1, columns 5 and 6). On the other hand, hold-long vertebral conditioning had a larger effect on muscle spindles with a dynamic index > 20 imp s−1 compared to < 20 imp s−1 across the five conditioning durations (interaction effect: F4,140 = 10.47, P < 0.001, see Table 1, columns 7 and 8). The decreased responsiveness of primary spindle endings was nearly four times greater than that of secondary endings.
Figure 5 shows the trend in ΔDT over the course of the dynamic test for muscle spindles with dynamic indices > 20 imp s−1 and < 20 imp s−1, respectively. In addition the figure enables a contrast with the static test. Due to hold-long vertebral conditioning, spindles with a DI > 20 imp s−1 were even less responsive during the first 20% of the dynamic test than they had been during the static test (Fig. 5A, open circles). This did not appear to occur in spindles with a dynamic index < 20 imp s−1 (Fig. 5B, open circles). Compared to the static test, responsiveness during the first 10% of the dynamic test for spindles with a DI > 20 imp s−1 decreased by an additional −4.8 imp s−1 compared to a 0.6 imp s−1 increase for spindles with a DI < 20 imp s−1, when averaged over conditioning durations 2, 4, 6 and 8 s. As the L6 vertebra was moved toward its maximal displacement, the effects of the hold-long vertebral history persisted longer for spindles with a DI > 20 imp s−1 relative to a DI < 20 imp s−1.
Hold-short conditioning had similar effects on both groups of muscle spindles (Fig. 5, filled symbols). The increased responsiveness of spindles with a DI > 20 imp s−1 during the static test was generally maintained during the first 10–20% of the dynamic test whereas the discharge of spindles with a DI < 20 imp s−1 slowly declined toward the discharge caused by hold-intermediate conditioning. However, these trends represent small differences in discharge rates (< 3 imp s−1). At approximately 30–40% of maximal vertebral displacement the responsiveness of both groups of spindles had returned close to that caused by hold-intermediate conditioning.
Discussion
The purpose of the present study was to investigate whether passive changes in vertebral position can affect signalling from muscle spindles, a consequence which could impact neuromuscular control of the vertebral column. The responsiveness of spindles in the lumbar longissimus and multifidus muscles was altered by positional changes in a lumbar vertebra ∼1–2 mm in magnitude. Two seconds of vertebral positioning that lengthened the lumbar paraspinal muscles relative to the same duration at an intermediate position significantly decreased both resting spindle discharge (static test) and the spindles response to vertebral movement (dynamic test). The magnitude of the decrease (up to ∼16 imp s−1) was similar at longer conditioning durations of 4, 6 and 8 s. The decrease was maintained during vertebral movement but became diminished as the movement approached the hold-long conditioning position. The magnitude of the decreased responsiveness was greater for muscle spindles with a dynamic index > 20 imp s−1. Conversely, conditioning that shortened the lumbar paraspinal muscles increased spindle discharge both at rest and to movement. These increases were smaller in absolute magnitude compared to the decrease caused by the hold-long vertebral position and, unlike hold-long, were abolished with small vertebral movements. Spindles regardless of their dynamic index responded similarly to a history of hold-short conditioning.
There were two reasons for grouping afferents relative to a dynamic index of 20 imp s−1. The data of Matthews (1963) suggest that secondary endings have a dynamic index less than 25 imp s−1 when stretch velocity is 10 mm s−1 or slower. Secondly, Richmond & Abrahams (1979) used a dynamic index of 20 imp s−1 to differentiate primary from secondary endings in the cervical spine of cats. Thus 20 imp s−1 provided a reference point for comparison. However, we did not classify the sensory endings as primary or secondary for two reasons. In the cat hindlimb, the dynamic index for these endings strongly correlates with afferent conduction velocity (Matthews, 1963). The distribution of dynamic indexes for lumbar spindles did not segregate with conduction velocity in our experiments. In addition, muscle spindle morphology is different from that in the hindlimb (Richmond & Abrahams, 1975), and to our knowledge the morphology of lumbar paraspinal muscle spindles is not yet known. How spindle morphology affects the relationship between dynamic index and the type of ending has also not been studied.
Changes in muscle spindle responsiveness induced by a vertebra's positional history were similar to changes caused by passive length changes applied directly to a fusiform leg muscle via its tendon (Morgan et al. 1984). It was suggested the hold-long position decreases muscle spindle responsiveness as opposed to the hold-short position augmenting it. In the lumbar spine, Pickar & Kang (2001) could not determine if the responses were augmented and/or decreased. The present study's design enabled us to determine that vertebral positions that conditioned in hold-short augmented and in hold-long decreased muscle spindle responsiveness. However, two differences between hold-long and hold-short in the present study bear emphasis. First, the absolute magnitude of the increased responsiveness was less than that of the decrease regardless of the spindle's dynamic index (see Fig. 4 and Table 1). For hold-long conditioning, the effect of muscle history during both the static and dynamic tests was greater for spindles with a dynamic index > 20 imp s−1. Second, the augmentation occurred even when the hold-short conditioning was simply ramped up without the vertebra being held in position (i.e. 0 s conditioning duration). By comparison, the decrease in spindle discharge occurred only when the hold-long conditioning had been held for at least 2 s. This response contrasts with muscle spindles in the leg (Proske et al. 1992) where a simple ramp stretch decreases spindle discharge. The conditioning effects of lengthening compared with shortening of the lumbar paraspinal muscles may be more dependent on stretch velocity because the displacement rate we used to condition the paraspinal muscles was substantially faster than that used by Proske et al. (1992) to condition the leg muscles (10.0 versus 0.2 mm s−1, respectively).
In the cat soleus muscle, length history altered muscle spindle responsiveness by up to 20 imp s−1 (Morgan et al. 1984; Gregory et al. 1986). Muscle spindle afferents from this fusiform muscle discharge at 3.5–5 imp s−1 for every 1 mm of linear change in resting muscle length (Granit, 1958; Matthews, 1972). Soleus muscle cells are up to 26 mm long in cat (Walmsley & Proske, 1981) and thus a 20 imp s−1 decrease in soleus spindle responsiveness could provide the central nervous system with an incorrect estimate (up to 20%) of muscle fibre length. In the present study, hold-long conditioning depressed paraspinal spindle responsiveness by up to 17 imp s−1 and hold-short conditioning increased it by up to 6 imp s−1. The length of multifidus and longissimus muscle fibres is not known. We recorded from the L6 dorsal root which innervates the L6 multifidus and longissimus fascicles. These muscles cross two vertebral segments at most and the distance between two segments is substantially shorter compared to the distance between soleus muscle's calcaneal and tibial attachments. The effects of vertebral position history, especially a position that lengthened the paraspinal muscles, could lead to a substantial underestimation of paraspinal muscle fibre length.
In the appendicular skeleton, the effects of muscle history on spindle responsiveness appear to have proprioceptive consequences, affecting both position sense and spindle-mediated muscle reflexes. In humans, actively contracting a shortened biceps brachii muscle leads to errors in forearm position (Gregory et al. 1988). In animal experiments, passive shortening combined with contraction of the soleus muscle increases muscle spindle discharge when soleus is stretched. Changes in muscle history produced by ankle joint positioning in both humans and cats alter deep tendon and H reflexes but in opposite directions. The size of the Achilles tendon jerk reflex is larger after triceps surae contraction with the foot plantarflexed (i.e. calf muscles held-short) than with the foot dorsiflexed (Gregory et al. 1987, 1990; Wood et al. 1996). Conversely, the H reflex is smaller after triceps surae contraction with the foot plantarflexed than with the foot dorsiflexed (Gregory et al. 1990; Wood et al. 1996). These contrasting effects represent excitatory influences on homonymous α-motoneurones and presynaptic inhibition to homonymous muscle spindle afferents, respectively (Wood et al. 1996; Gregory et al. 1998; Pinniger et al. 2001).
Functional perspective
Several investigators (Hutton & Atwater, 1992; Proske et al. 1993) have suggested that the significance of intrafusal fibre thixotropy for motor control lies in the introduction of unpredictability for the timing and magnitude of central neural responses. In the spinal column there also appears to be biomechanical unpredictability in vertebral position because intervertebral motion contains a neutral zone, a region of the force–displacement curve where the facet joints and intervertebral disc produce little resistance to motion (Panjabi, 1992; Oxland & Panjabi, 1992; Thompson et al. 2003). The appropriateness of a spindle's response to changes in posture or movement could depend on the direction of movement relative to the vertebra's positional history in the neutral zone. Moreover, static postures that lengthen the paraspinal muscles may reduce or delay spindle activity during subsequent movement. Two seconds was sufficient to condition the spindles into decreased responsiveness. Although speculative, the effects of vertebral position, and consequently muscle history, shown in this study may be a mechanism whereby spinal biomechanics interacts with the spine's proprioceptive system to produce acute effects on neuromuscular control of axial muscles. Spinal manipulation as practiced by chiropractors, osteopaths and physiotherapists may alter spindle sensitivity and help avoid or resolve reflex action that contributes to muscle spasm in the low back.
Acknowledgments
The authors thank Mr Tom Cobb for technical assistance. This publication was made possible by National Institutes of Health grant number NS46818. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR15433 from the National Center for Research Resources, National Institutes of Health.
References
- Bergmark A. Stability of the lumbar spine: a study in mechanical engineering. Acta Orthop Scand. 1989;60:2–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Bogduk N. The lumbosacral dorsal rami of the cat. J Anat. 1976;122:653–662. [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The dorsal lumbar muscles of the cat. Acta Anz, Jena. 1980;148:55–67. [PubMed] [Google Scholar]
- Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286–293. doi: 10.1097/00007632-198304000-00009. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Lysens R, Swinnen S, Verschueren S. Effect of paraspinal muscle vibration on position sense of the lumbosacral spine. Spine. 1999;24:1328–1331. doi: 10.1097/00007632-199907010-00010. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Lumbar posterior ligament involvement during extremely heavy lifts estimated from fluoroscopic measurements. J Biomech. 1992;25:17–28. doi: 10.1016/0021-9290(92)90242-s. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord: contributions to the state of general anesthesia. Trends Neurosci. 1995;18:549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Panjabi MM. The intersegmental and multisegmental muscles of the lumbar spine: a biomechanical model comparing lateral stabilizing potential. Spine. 1991;16:793–799. doi: 10.1097/00007632-199107000-00018. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Panjabi MM, Yamamoto I, Oxland TR. Euler stability of the human ligamentous lumbar spine. Part II Experiment Clin Biomech. 1992;7:27–32. doi: 10.1016/0268-0033(92)90004-N. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Hutton RS, Eldred E. Changes in excitability of tendon tap and hoffmann reflexes following voluntary contractions. Electroenceph Clin Neurophysiol. 1980;48:664–672. doi: 10.1016/0013-4694(80)90423-x. [DOI] [PubMed] [Google Scholar]
- Granit R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958;143:387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Mark RF, Morgan DL, Patak A, Polus B, Proske U. Effects of muscle history on the stretch reflex in cat and man. J Neurophysiol. 1990;424:93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol. 1986;56:451–461. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Changes in size of the stretch reflex of cat and man attributed to aftereffects in muscle spindles. J Neurophysiol. 1987;58:628–640. doi: 10.1152/jn.1987.58.3.628. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol. 1988;59:1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Wise AK, Wood SA, Prochazka A, Proske U. Muscle history, fusimotor activity and the human stretch reflex. J Physiol. 1998;513:927–934. doi: 10.1111/j.1469-7793.1998.927ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K-E, Nordin M. Postural after-contractions in man attributed to muscle spindle thixotropy. J Physiol. 1998;506:875–883. doi: 10.1111/j.1469-7793.1998.875bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton RS, Atwater SW. Acute and chronic adaptations of muscle proprioceptors in response to increased use. Sports Med. 1992;14:406–421. doi: 10.2165/00007256-199214060-00007. [DOI] [PubMed] [Google Scholar]
- Kong WZ, Goel VK, Gilbertson LG, Weinstein JN. Effects of muscle dysfunction on lumbar spine mechanics. Spine. 1996;21:2197–2207. doi: 10.1097/00007632-199610010-00004. [DOI] [PubMed] [Google Scholar]
- Leinonen V, Kankaanpaa M, Luukkonen M, Kansanen M, Hanninen O, Airaksinen O, Taimela S. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in disc herniation-related back pain. Spine. 2003;28:842–848. [PubMed] [Google Scholar]
- Matthews PBC. The response of de-efferented muscle spindle receptors to stretching at different velocities. J Physiol. 1963;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. Baltimore: Williams & Wilkin Co; 1972. [Google Scholar]
- Morgan DL, Prochazka A, Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan PB, Burnett A, Floyd AN, Gadsdon K, Logiudice J, Miller D, Quirke H. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28:1074–1079. doi: 10.1097/01.BRS.0000061990.56113.6F. [DOI] [PubMed] [Google Scholar]
- Ogon M, Bender BR, Hooper DM, Spratt KF, Goel VK, Wilder DG, et al. A dynamic approach to spinal instability. Part I: Sensitization of intersegmental motion profiles to motion direction and load condition by instability. Spine. 1997;22:2841–2858. doi: 10.1097/00007632-199712150-00007. [DOI] [PubMed] [Google Scholar]
- Oxland TR, Panjabi MM. The onset and progression of spinal injury: a demonstration of neutral zone sensitivity. J Biomech. 1992;25:1165–1172. doi: 10.1016/0021-9290(92)90072-9. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–397. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Kuniyoshi A, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces: a biomechanical model. Spine. 1989;14:194–199. doi: 10.1097/00007632-198902000-00008. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Oxland TR, Yamamoto I, Crisco JJ. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J Bone Joint Surg Amer. 1994;76:413–424. doi: 10.2106/00004623-199403000-00012. [DOI] [PubMed] [Google Scholar]
- Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Meth. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- Pickar JG, Kang YM. Short-lasting stretch of lumbar paraspinal muscle decreases muscle spindle sensitivity to subsequent muscle stretch. J Neuromusculoskel Sys. 2001;9:88–96. [Google Scholar]
- Pinniger GJ, Nordlund M, Steele JR, Cresswell AG. H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol. 2001;534:913–923. doi: 10.1111/j.1469-7793.2001.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Muscle history dependence of responses to stretch of primary and secondary endings of cat soleus muscle spindles. J Physiol. 1992;445:81–95. doi: 10.1113/jphysiol.1992.sp018913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Richmond FJR, Abrahams VC. Morphology and distribution of muscle spindles in dorsal muscles of cat neck. J Neurophysiol. 1975;38:1322–1339. doi: 10.1152/jn.1975.38.6.1322. [DOI] [PubMed] [Google Scholar]
- Richmond FJR, Abrahams VC. Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol. 1979;42:604–615. doi: 10.1152/jn.1979.42.2.604. [DOI] [PubMed] [Google Scholar]
- Skotte J, Hjortskov N, Essendrop M, Schibye B, Fallentin N. Short latency stretch reflex in human lumbar paraspinal muscles. J Neurosci Meth. 2005;145:145–150. doi: 10.1016/j.jneumeth.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Swinkels A, Dolan P. Spinal position sense is independent of the magnitude of movement. Spine. 2000;25:98–104. doi: 10.1097/00007632-200001010-00017. [DOI] [PubMed] [Google Scholar]
- Thompson RE, Barker TM, Pearcy MJ. Defining the Neutral Zone of sheep intervertebral joints during dynamic motions: an in vitro study. Clin Biomech. 2003;18:89–98. doi: 10.1016/s0268-0033(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Proske U. Comparison of stiffness of soleus and medial gastrocnemius muscles in cats. J Neurophysiol. 1981;46:250–259. doi: 10.1152/jn.1981.46.2.250. [DOI] [PubMed] [Google Scholar]
- Wilke H-J, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures int he intervertebral disc in daily life. Spine. 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Wolf S, Claes LE, Arand M, Weisand A. Stablility increase of the lumbar spine with different muscle groups: a biomechanical in vitro study. Spine. 1995;20:192–198. doi: 10.1097/00007632-199501150-00011. [DOI] [PubMed] [Google Scholar]
- Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol. 1996;497:279–290. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]