Abstract
Previous studies using non-hydrolysable ATP analogues and hydrolysis-deficient cystic fibrosis transmembrane conductance regulator (CFTR) mutants have indicated that ATP hydrolysis precedes channel closing. Our recent data suggest that ATP binding is also important in modulating the closing rate. This latter hypothesis predicts that ATP analogues with higher binding affinities should stabilize the open state more than ATP. Here we explore the possibility of using N6-modified ATP/ADP analogues as high-affinity ligands for CFTR gating, since these analogues have been shown to be more potent than native ATP/ADP in other ATP-binding proteins. Among the three N6-modified ATP analogues tested, N6-(2-phenylethyl)-ATP (P-ATP) was the most potent, with a K½ of 1.6 ± 0.4 μm (>50-fold more potent than ATP). The maximal open probability (Po) in the presence of P-ATP was ∼30% higher than that of ATP, indicating that P-ATP also has a higher efficacy than ATP. Single-channel kinetic analysis showed that as [P-ATP] was increased, the opening rate increased, whereas the closing rate decreased. The fact that these two kinetic parameters have different sensitivities to changes of [P-ATP] suggests an involvement of two different ATP-binding sites, a high-affinity site modulating channel closing and a low affinity site controlling channel opening. The effect of P-ATP on the stability of open states was more evident when ATP hydrolysis was abolished, either by mutating the nucleotide-binding domain 2 (NBD2) Walker B glutamate (i.e. E1371) or by using the non-hydrolysable ATP analogue AMP-PNP. Similar strategies to develop nucleotide analogues with a modified adenine ring could be valuable for future studies of CFTR gating.
Cystic fibrosis transmembrane conductance regulator (CFTR), a member of the ATP-binding cassette (ABC) transporter superfamily, is a protein kinase A (PKA)-activated and ATP-gated chloride channel (reviewed by Gadsby & Nairn, 1999). Proteins in this family share many characteristic structural features, including two membrane-spanning domains and two nucleotide-binding domains (NBD1 and NBD2), each containing a Walker A motif, a Walker B motif and a signature sequence, which are important for ATP binding. Crystallographic and biochemical studies of several members of the ABC superfamily (e.g. Chen et al. 2003) indicate that the two NBDs form a head-to-tail dimer with two ATP molecules buried at the dimer interface. The bound nucleotides, as well as several amino acid residues from each NBD participating in nucleotide interactions, are intimately involved in forming a stable dimer. Because each binding pocket in a dimeric structure consists of molecular components from both NBDs, we define the NBD1 ATP-binding site (or NBD1 site) as the binding pocket containing Walker A and Walker B motifs in the NBD1 sequence and the signature sequence of the NBD2. An equivalent definition is applied to the NBD2 site.
Although there is no structural evidence for dimerization of the NBDs of CFTR, a recent functional study by Vergani et al. (2005) suggests that dimerization of the NBDs of CFTR is associated with the open channel conformation; their data also provide evidence that the NBD dimer of CFTR may assume a similar structure as seen in other members of the ABC superfamily. Since the ligand, ATP, becomes part of the open channel conformation as this new structure/kinetic model implies, the binding energy of ATP should be part of the free energy that determines the open-state stability. Indeed, our recent study provides functional data to support this idea. By examining macroscopic and microscopic kinetics of a hydrolysis-deficient mutant CFTR (i.e. E1371S), we demonstrate an [ATP]-dependent distribution of the open time constants, indicating that ATP binding can affect the life time of the open state (Bompadre et al. 2005a, b). In theory, the importance of ATP binding for the open channel stability can be tested by examining how changes of the nucleotide binding affinity affect the stability of the open state. One would expect that ATP analogues with higher binding affinity should stabilize the open state more than ATP.
Many ATP analogues have been used to study CFTR gating. Most commonly used ATP analogues, such as AMP-PNP and ATP-γ-S, are modified at the phosphate groups so that they become poorly hydrolysable (e.g. Gunderson & Kopito, 1994; Hwang et al. 1994). These analogues are useful in studying effects of ATP hydrolysis on gating, but they do not offer distinct advantages of a higher binding affinity. We chose to examine analogues with added hydrophobic moieties at the N6 position of the adenine ring of ATP or ADP for several reasons. First, it is known that a large hydrophobic pocket exists immediately adjacent to the adenine ring in the crystal structures of ATPase family members, including CFTR (Lewis et al. 2004, 2005). Therefore, analogues with hydrophobic moieties added to the adenine ring are more likely to assume higher affinities than ATP. Second, the N6 position of the adenine ring can be easily modified (Shah et al. 1997), and the resulting nucleotide analogues, with higher affinities than the native nucleotides, have been successfully used to study the structure/function relationship of other ATPase proteins, such as myosin isozymes (Gillespie et al. 1999). Third, analogues with modifications of the adenine ring, instead of the phosphate groups, may have less effect on ATP hydrolysis. Since multiple ATP-binding sites may be involved in CFTR gating (e.g. Hwang et al. 1994; Carson et al. 1995; Gunderson & Kopito, 1995; Zeltwanger et al. 1999; Ikuma & Welsh, 2000; Bompadre et al. 2005b; Randak & Welsh, 2005; Vergani et al. 2005), a high-affinity ATP analogue could also help resolve the functional roles of these sites.
We have tested three N6-modified ATP analogues; all exhibit higher potency than ATP for CFTR gating. The most potent one, N6-(2-phenylethyl)-ATP (P-ATP), shows a >50-fold higher apparent affinity than ATP. Single-channel kinetic analysis for channels opened by P-ATP demonstrates that both the opening rate and the closing rate of the channel are dependent on the concentration of the analogue, but with different sensitivities, suggesting a possible involvement of two different binding sites. Consistent with our hypothesis that nucleotide-binding energy is important for the stability of the open state, P-ATP-opened channels stay in the open state longer than ATP-opened channels.
Methods
Cell preparation and transient expression
Chinese hamster ovary (CHO) cells were used for transient expression of wild-type (WT) or mutant CFTR. CHO cells were kindly provided by Dr Karen Cone (University of Missouri-Columbia). These CHO cells (A23 genotype thymidine kinase minus) were originally obtained from Stanford Human Genome Center. The cDNA constructs are cotransfected with pEGFP-C3 (Clontech, Palo Alto, CA, USA), encoding the green fluorescent protein, using SuperFect transfection reagent (Qiagen, Valencia, CA, USA) according to manufacturer's instruction. The duration of the transfection reaction is 4 h. Cells were then trypsinized and seeded onto glass chips in fresh 35 mm Petri dishes. Electrophysiological recordings were performed 2–7 days after the transfection.
Electrophysiological recordings
Details of the recording methods have been previously described (e.g. Zhou et al. 2002). Briefly, electrophysiological recordings were performed on inside-out patches excised from CHO cells transiently expressing WT or mutant CFTR channels. The pipette solution (i.e. extracellular solution) contained (mm): 140 NMDG-Cl, 2 MgCl2, 5 CaCl2 and 10 Hepes (pH 7.4 with NMDG). Cells were perfused with a bath solution containing (mm): 145 NaCl, 5 KCl, 2 MgCl2, 1 CaCl2, 5 glucose, 5 Hepes and 20 sucrose (pH 7.4 with NaOH). After the establishment of an inside-out configuration, the patch was perfused with a standard perfusion solution (i.e. intracellular solution) containing (mm): 150 NMDG-Cl, 2 MgCl2, 10 EGTA and 8 Tris (pH 7.4 with NMDG). The membrane potential was held at −50 mV for all the experiments. Downward deflections of the current trace represent channel opening. Currents were recorded at room temperature using an EPC10 patch-clamp amplifier (Heka Electronic, Lambrecht, Germany). Current traces were filtered at 100 Hz with a built-in four-pole Bessel filter and digitized online at 500 Hz.
Data analysis
Estimations of the steady-state mean current amplitude and all curve fittings were performed with Igor Pro program (Version 3.11; Wavemetrics, Oswego, OR, USA). For single-channel kinetic analysis, data were further filtered at 50 Hz and analysed using software written by Dr Csanády (Csanády, 2000). Only recordings from patches containing no more than five channels (estimated from maximal number of simultaneous openings) were selected for kinetic analysis, except for the experiments with submicromolar P-ATP concentrations where patches with up to eight channels were also included because of the low channel activities at very low [P-ATP]. We routinely applied 30 μm P-ATP before and after the application of submicromolar P-ATP in the same patch. The maximum number of simultaneous openings at 30 μm P-ATP was used to estimate the number of channels at submicromolar P-ATP. A three-state kinetic model, C ⇌ O ⇌ B, was adopted to extract kinetic parameters as previously described (Csanády et al., 2000; Vergani et al. 2003; Bompadre et al. 2005a). This kinetic model was also used to extract single-channel kinetic parameters in the presence of P-ADP. This method is apparently an oversimplification since our previous studies indicate that ADP, by competing with ATP for channel opening, induces another closed state (Bompadre et al. 2005a). However, this oversimplified model, which lumps multiple closed states together, is sufficient for a crude estimation of the closed time. The purpose of our kinetic analysis with P-ADP is primarily to verify the effect of P-ADP on the closed time.
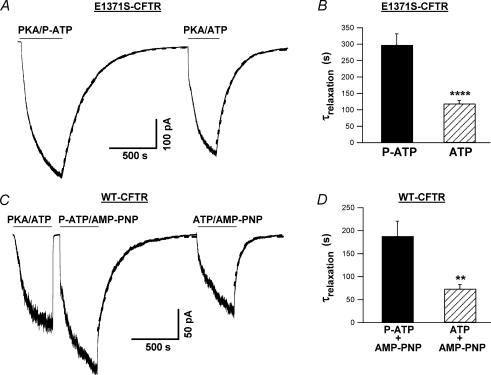
For the current relaxation experiments shown in Fig. 7, we designed different protocols to minimize potential time-dependent effects on current relaxation. These protocols also take into account a potential difference between ATP and P-ATP as substrates for PKA. For E1371S-CFTR current relaxation experiments (Fig. 7A), initially channels were activated by PKA plus P-ATP in some patches, while by PKA plus ATP in others. For WT-CFTR current relaxation experiments (Fig. 7C), channels were first activated by PKA plus ATP. Subsequently, ATP/AMP-PNP or P-ATP/AMP-PNP was applied alternately in the same patch. Since we did not find time-dependent changes in E1371S-CFTR experiments or in WT-CFTR experiments, data from each set of experiments were pooled for statistical analysis. To estimate the relaxation time constant, we fitted the time course of current decay with a single exponential function. The first 5 s of the data were routinely excluded from fitting because the current decay during this time period cannot be reliably measured due to the limited speed of our solution exchange system.
Figure 7. Effect of P-ATP on CFTR locked open state.
A, macroscopic E1371S-CFTR current relaxations upon removal of 50 μm P-ATP or 1 mm ATP. For this particular experiment, the relaxation time constants are 255.1 s (P-ATP) and 150.8 s (ATP), respectively. B, mean data of the current relaxation experiments of E1371S-CFTR. The current relaxation time constants (τrelaxation) for E1371S-CFTR channels are 297.6 ± 34.0 s (n = 5) upon the removal of 50 μm P-ATP, and 118.8 ± 9.4 s (n = 5) upon the removal of 1 mm ATP. ****P < 0.001. C, macroscopic WT-CFTR current relaxations upon washout of 10 μm P-ATP plus 2 mm AMP-PNP, or 0.5 mm ATP plus 2 mm AMP-PNP. Note that > 95% of the current decays 5 s after an initial washout of PKA and ATP. For this particular experiment, the relaxation time constants are 178.6 s (P-ATP plus AMP-PNP) and 66.7 s (ATP plus AMP-PNP), respectively. D, mean data of the current relaxation experiments of WT-CFTR. τrelaxation values are 188.1 ± 32.7 s (n = 12) upon removal of 10 μm P-ATP plus 2 mm AMP-PNP, and 73.5 ± 9.3 s (n = 9) upon removal of 0.5 mm ATP plus 2 mm AMP-PNP. **P < 0.01. Dashed lines in A and C are single exponential fits to the data. PKA is present in all nucleotide-containing solutions to minimize potential effects of dephosphorylation by membrane-associated protein phosphatases.
All averaged data are presented as means ± s.e.m.; n represents the number of experiments. Student's t tests were performed using SigmaPlot (Version 8.0) and results were considered significant if P < 0.05.
DNA constructs
We have previously described in detail the subcloning of wild-type CFTR cDNA into the mammalian expression vector pcDNA 3.1 Zeo(+) (Invitrogen, Carlsbad, CA, USA) (Powe et al. 2002). Point mutation E1371S was introduced into the pcDNA3.1 wild-type CFTR by QuikChange XL method (Stratagene, La Jolla, CA, USA). To obtain plasmid pBudCE4.1/split ΔR-CFTR for expressing ΔR-CFTR channels, cDNAs encoding CFTR residues 1–633 and residues 837–1480 were subcloned into pBudCE4.1 (Invitrogen) under the control of the CMV and EF1-α promoters, respectively (see Ai et al. 2004 for details).
Reagents
N6-(2-phenylethyl)-ATP (P-ATP) (purity, 99.63%), N6- benzyl-ATP (B-ATP) (99.73%), N6-(2-methylbutyl)-ATP (M-ATP) (98.04%) and N6-(2-phenylethyl)-ADP (P-ADP) (98.12%) were purchased from Biolog Life Science Institute (Bremen, Germany). PKA was purchased from Promega (Madison, WI, USA). Mg-ATP (∼95%) was purchased from Sigma (St Louis, MO, USA). AMP-PNP was purchased from Roche (Indianapolis, IN, USA).
Results
Characterization of N6-modified ATP analogues
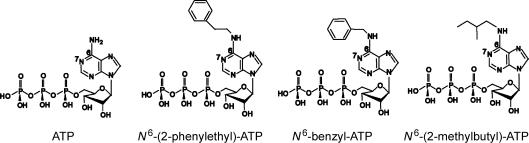
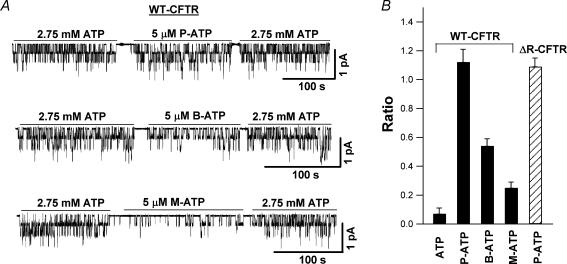
We tested three ATP analogues with added hydrophobic moieties at the N6 position of the adenine ring (Fig. 1) on WT-CFTR. All three such ATP analogues, including P-ATP, B-ATP and M-ATP, were more potent than ATP in activating the channel (Fig. 2). Figure 2A shows representative current traces of WT-CFTR in the presence of ATP and the three ATP analogues examined. Inside-out membrane patches were first exposed to PKA (40 U ml−1) and 1 mm ATP for channel activation. ATP (2.75 mm), applied before and after the application of 5 μm of each ATP analogue, served as a control to ensure minimal time-dependent channel rundown. The ratios of the mean current induced by each ATP analogue (5 μm) to that induced by 2.75 mm ATP were calculated, and the mean values are shown in Fig. 2B. The ratios for the three ATP analogues were much higher than that of 5 μm ATP (data from Zeltwanger et al. 1999), indicating that all three ATP analogues are more potent than ATP, with P-ATP being the most potent analogue tested. It is interesting to note that the mean current in the presence of 5 μm P-ATP is larger than that with 2.75 mm ATP, a saturating [ATP] for WT-CFTR, suggesting that P-ATP is not only more potent but also more effective than ATP. We therefore chose P-ATP for the rest of the study.
Figure 1. Chemical structures of ATP and N6-modified ATP analogues.
The three ATP analogues tested were N6-(2-phenylethyl)-ATP (P-ATP), N6-benzyl-ATP (B-ATP) and N6-(2-methylbutyl)-ATP (M-ATP).
Figure 2. N6-modified ATP analogues are more potent than ATP.
A, after patch excision, wild-type cystic fibrosis transmembrane conductance regulator (WT-CFTR) channels were activated by protein kinase A (PKA) and 1 mm ATP. ATP (2.75 mm) was then applied before and after the application of each ATP analogue (5 μm). B, mean current responses to 2.75 mm ATP and 5 μm ATP analogues of WT-CFTR channels were compared. The ratios of the mean current induced by ATP analogue (5 μm) to that by 2.75 mm ATP for WT-CFTR channels are 1.12 ± 0.09 (n = 10), 0.54 ± 0.05 (n = 10) and 0.25 ± 0.04 (n = 8) for P-ATP, B-ATP and M-ATP, respectively. This ratio is 0.07 ± 0.04 (n = 8) for 5 μm ATP (data from Zeltwanger et al. 1999). For ΔR-CFTR channels, this ratio is 1.09 ± 0.06 (n = 9) for 5 μm P-ATP.
Gating of CFTR by P-ATP
We next examined the single-channel kinetics of CFTR channels in the presence of P-ATP. Our recent studies (Bompadre et al. 2005a) indicate that ΔR-CFTR, a mutant CFTR with the regulatory (R) domain (residues 634–836) deleted (see Methods for details), can be a useful tool for single-channel kinetic analysis because of its resistance to dephosphorylation-dependent rundown. Moreover, the relatively low expression level of ΔR-CFTR channels greatly improves the chance of obtaining patches containing fewer channels for data analysis. Most importantly, the single-channel kinetics of ΔR-CFTR is similar to that of the WT. To test if P-ATP had a similar effect on ΔR-CFTR channels as observed with WT-CFTR, we performed the same experiment as shown in Fig. 2A on ΔR-CFTR channels, and obtained a ratio for 5 μm P-ATP similar to that of WT-CFTR channels (Fig. 2B).
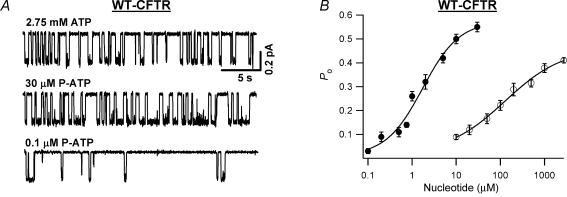
Figure 3A shows representative single-channel current traces of ΔR-CFTR in the presence of 2.75 mm ATP, 30 μm P-ATP and 0.1 μm P-ATP. Even by eye inspection, the open time in the presence of 30 μm P-ATP appears to be longer than that with 2.75 mm ATP, a saturating [ATP] for ΔR-CFTR (Bompadre et al. 2005a), or 0.1 μm P-ATP. The measured open probability (Po) at various [P-ATP] was used to construct the P-ATP dose–response relationship (Fig. 3B). The dose–response curve of P-ATP shifts drastically to the left, compared with that of ATP, with a K½ of 1.6 ± 0.4 μm, indicating that P-ATP is >50-fold more potent than ATP (K½ = 127.9 ± 43.1 μm, data from Bompadre et al. 2005a). Since the ATP preparation used in the current study may contain a higher percentage of impurity (see Methods), the observed differences between ATP and P-ATP could result from the higher contamination of ADP in the ATP preparation. Based on our previous data on ADP-dependent inhibition (Bompadre et al. 2005a) and a 2.5% ADP contamination in the ATP preparation (data from Sigma), we estimated that the Po of ΔR-CFTR in the presence of ATP was underestimated by ∼10%. The corrected K½ for ATP is now 118.0 ± 34.6 μm, which is not significantly different from that before correction. Therefore, P-ATP is indeed more potent than ATP. In addition, consistent with the macroscopic results shown in Fig. 2A, the corrected Po in the presence of 2.75 mm ATP (Po = 0.45 ± 0.01, n = 12) is still lower than that with 30 μm P-ATP (Po = 0.55 ± 0.02, n = 6), suggesting the P-ATP is more effective than ATP as well.
Figure 3. Concentration dependence of ΔR-CFTR activity in response to P-ATP or ATP.
A, ΔR-CFTR single-channel current traces in the presence of 2.75 mm ATP, 30 μm P-ATP and 0.1 μm P-ATP. B, open probability (Po) dose–response relationships of P-ATP (•) and that of ATP (○; from Bompadre et al. 2005a). Continuous lines are Hill equation fits to the data. The n values for the fits are 1.05 ± 0.30 for P-ATP and 0.66 ± 0.23 for ATP.
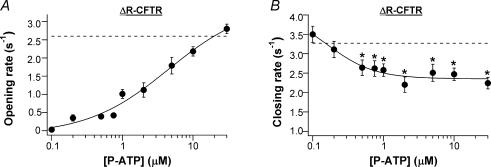
Detailed kinetic analysis shows that both the opening rate and the closing rate are [P-ATP] dependent (Fig. 4). However, these two kinetic parameters exhibit different sensitivities to changes of [P-ATP]. The opening rate (Fig. 4A) appears less sensitive to [P-ATP] (K½ = 4.5 ± 2.7 μm) and reaches a maximal value that is similar to that in the presence of saturating [ATP] (2.6 ± 0.2 s−1, n = 20, data from Bompadre et al. 2005a; dashed line in Fig. 4A). On the other hand, the closing rate (Fig. 4B) decreases with [P-ATP] (K½ = 0.16 ± 0.24 μm), and reaches a minimal value that is ∼50% smaller than that in the presence of ATP (3.3 ± 0.1 s−1, n = 8, data from Bompadre et al. 2005a; dashed line in Fig. 4B). The different sensitivity of the opening and closing rates to changes of [P-ATP] suggests that at least two different ATP-binding sites are involved: a low-affinity site for its effect on the opening rate, and a high-affinity site for its effect on the closing rate.
Figure 4. Single-channel kinetic parameters of ΔR-CFTR in the presence of P-ATP.
The dose–response relationship between P-ATP and the opening rate (A) or the closing rate (B). Continuous lines are Hill equation fits to the data. The n values for the fits are 0.75 ± 0.30 for the opening rates, and 1.30 ± 0.90 for the closing rates. The dashed line in A shows the maximal opening rate in the presence of saturating [ATP] (2.6 ± 0.2 s−1, n = 20). The dashed line in B shows the mean closing rate at various [ATP] (3.3 ± 0.1 s−1, n = 8) (data from Bompadre et al. 2005a). *P < 0.05 compared with the closing rate at 0.1 μm P-ATP.
P-ADP is more potent than ADP in inhibiting ATP-induced currents
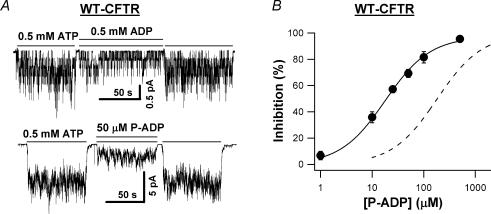
Since P-ATP is more potent than ATP in opening the channel, we expect that P-ADP should be more potent than ADP in inhibiting the channel. This is indeed the case. Figure 5A shows representative WT-CFTR current traces in the presence of 0.5 mm ADP or 50 μm P-ADP. When 50 μm P-ADP was applied in the presence of 0.5 mm ATP, 74.1 ± 2.6% (n = 4) of the current was inhibited (versus 58.9 ± 3.0%, n = 6, for 0.5 mm ADP). The K½ value of P-ADP in the presence of 0.5 mm ATP was 17.9 ± 1.3 μm (Fig. 5B), which is ∼10-fold smaller than that of ADP under the same experimental conditions (dashed line in Fig. 5B; data from Bompadre et al. 2005a).
Figure 5. P-ADP is more potent than ADP in inhibiting CFTR.
A, WT-CFTR current traces in the presence of 0.5 mm ADP or 50 μm P-ADP, both with 0.5 mm ATP. B, concentration dependence of P-ADP inhibition of WT-CFTR currents in the presence of 0.5 mm ATP. The continuous line is the Michaelis-Menten fit to the data for 0.5 mm P-ADP plus 0.5 mm ATP. The r2 value for the fit is 0.998. The dashed line is the Michaelis-Menten fit to the data for 0.5 mm ADP plus 0.5 mm ATP (data from Bompadre et al. 2005a). The r2 value for the fit is 0.993. K½ of ADP in the presence of 0.5 mm ATP is 180.1 ± 38.6 μm.
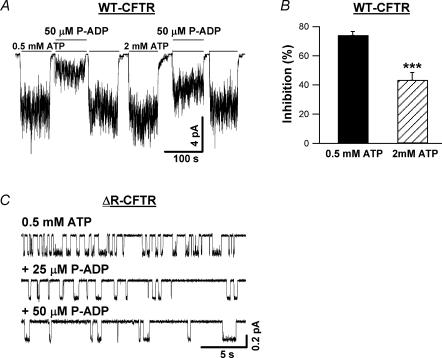
Previous studies indicate that ADP may inhibit CFTR channel opening by competing with ATP for the same binding site (Anderson & Welsh, 1992; Gunderson & Kopito, 1994; Schultz et al. 1995; Bompadre et al. 2005a). We performed the following experiments to test if P-ADP also competes with ATP. First, we examined the effect of [ATP] on P-ADP inhibition. In the same membrane patches, when [ATP] was increased from 0.5 to 2 mm (Fig. 6A), the inhibition of 50 μm P-ADP decreased significantly (Fig. 6B), consistent with the idea that P-ADP competes with ATP.
Figure 6. Effects of [ATP] and [P-ADP] on P-ADP-dependent inhibition.
A, inhibition of WT-CFTR currents by 50 μm P-ADP in the presence of two different [ATP]: 0.5 and 2 mm. B, mean data from four experiments. When [ATP] is increased from 0.5 to 2 mm, inhibition of the current by 50 μm P-ADP decreases from 74.1 ± 2.6 to 43.4 ± 5.1% (n = 4). ***P < 0.005. C, single-channel traces of ΔR-CFTR in the presence of 0.5 mm ATP alone, 0.5 mm ATP plus 25 μm P-ADP, and 0.5 mm ATP plus 50 μm P-ADP.
Our previous studies show that ADP, by competing with ATP for channel opening, induces another closed state (Bompadre et al. 2005a). To verify that the effect of P-ADP is also on the opening rate, we performed single-channel kinetic analysis. Again, we resorted to ΔR-CFTR channels for reasons described above. Figure 6C shows representative single-channel traces of ΔR-CFTR channels in the presence of 0.5 mm ATP alone, 0.5 mm ATP plus 25 μm P-ADP, and 0.5 mm ATP plus 50 μm P-ADP. While P-ADP has little effect on the open time, it prolongs the closed time significantly in a concentration-dependent manner. Since the P-ADP-induced closed state is expected to be much more stable than the one induced by ADP, it is technically very difficult to collect single-channel recordings that are long enough for dwell-time analysis. We therefore analysed the data from patches containing up to three channels using a simplified C ⇌ O ⇌ B model for multichannel kinetic analysis (Csanády, 2000; also see Methods). Although this method lumps together multiple closed states, it allows us to quantify the effect of P-ADP on the closed time. The closed times in the presence of 0.5 mm ATP, 0.5 mm ATP plus 25 μm P-ADP, and 0.5 mm ATP plus 50 μm P-ADP are 0.49 ± 0.06 s (n = 13), 0.92 ± 0.09 s (n = 11) and 1.32 ± 0.14 s (n = 10), respectively. These results are thus consistent with a simple competitive inhibition of channel opening by P-ADP, although other mechanisms (e.g. Randak & Welsh, 2005) cannot be ruled out. Nevertheless, P-ADP, as an ADP analogue with a higher affinity, may be useful to further differentiate between different mechanisms of ADP-dependent inhibition of CFTR gating.
P-ATP stabilizes the locked open state of CFTR channels
We demonstrated above that the closing rate is lower at saturating [P-ATP] compared with that with ATP (Fig. 4B). Since previous studies suggest that channel closing is controlled mainly by ATP hydrolysis (e.g. Gunderson & Kopito, 1994; Hwang et al. 1994; Carson et al. 1995; Zeltwanger et al. 1999; Vergani et al. 2003), one possible explanation for this effect of P-ATP on the closing rate is that P-ATP may be hydrolysed more slowly than ATP. This mechanism predicts that the closing rate should be independent of [P-ATP]. Our data showing a [P-ATP]-dependent decrease of the closing rate argue against this mechanism. Alternatively, this effect of P-ATP on the closing rate could be due to tight binding of P-ATP. Our recent studies support this latter hypothesis (Bompadre et al. 2005a, b). First, there exist multiple open states, and the distribution of the open time constants is dependent on [ATP]. Second, ADP, a smaller molecule than ATP, shortens the open time. These results suggest that nucleotide binding does affect the stability of the open state.
We further differentiated between these two possibilities by using the hydrolysis-deficient mutant CFTR, E1371S (Vergani et al. 2003; Bompadre et al. 2005b). This mutant channel can open, in response to millimolar ATP concentrations, for minutes. The mean open time for this mutant can be quantified by measuring the relaxation time constant upon removal of the nucleotide. Figure 7A shows an example of such an experiment. Interestingly, macroscopic E1371S-CFTR channel currents can be activated by 50 μm P-ATP and PKA, indicating that P-ATP not only can support ATP-dependent gating, but also can be used as a substrate for PKA-dependent phosphorylation of the R domain. The relaxation time constant is estimated from single exponential fit to the data. The current decay upon removal of P-ATP is significantly slower than that of ATP (Fig. 7B) (P < 0.001). These results suggest that P-ATP stabilizes the locked open state of E1371S-CFTR due to tight binding.
Another way to circumvent the issue of ATP hydrolysis and to isolate the effect of P-ATP binding on the closing rate is to use AMP-PNP to lock open WT-CFTR channels. It has been shown previously that AMP-PNP, a non-hydrolysable ATP analogue, can lock open CFTR channels for minutes in the presence of ATP (e.g. Gunderson & Kopito, 1994; Hwang et al. 1994). Under this condition, WT-CFTR channels probably close through a non-hydrolytic pathway. We set out to examine the relaxation time constant in the presence of AMP-PNP and P-ATP, and in the presence of AMP-PNP and ATP. In excised inside-out patches, WT-CFTR channels were first activated by 0.5 mm ATP plus PKA (Fig. 7C). Subsequently, the channels were exposed to either P-ATP (10 μm) plus AMP-PNP (2 mm), or ATP (0.5 mm) plus AMP-PNP (2 mm). At least two components of the current relaxation time course were seen upon washout of nucleotides. The fast component represents the current relaxation of the unlocked channels, and the slow one represents the current relaxation of the AMP-PNP locked open channels. Since the fast component is beyond the resolution of our solution change system and is already excluded by our fitting routine (see Methods), the time constant of the slow component can be estimated from a single exponential fit. The relaxation time constant for channels locked open by P-ATP plus AMP-PNP is significantly longer, compared with that of channels locked open by ATP plus AMP-PNP (Fig. 7D) (P < 0.01).
Discussion
In this work, we demonstrate that high-affinity N6-modified ATP/ADP analogues can serve as new tools for CFTR gating studies. P-ATP is not only >50-fold more potent than ATP, but also ∼30% more effective in activating the channel. At a single-channel level, as [P-ATP] is increased, the opening rate is increased, while the closing rate is decreased. These two kinetic parameters exhibit different sensitivities to changes of [P-ATP], suggesting an involvement of at least two P-ATP-binding sites. The effect of P-ATP on the stability of the open state is likely to be due to tight binding because this effect is also present in conditions where hydrolysis is abolished.
The current study establishes that addition of a hydrophobic moiety at the N6 position of the adenine ring indeed increases the apparent affinity of the resulting nucleotides for CFTR gating. Whether this strategy affects the hydrolysability of these analogues awaits biochemical confirmation, but we argue that N6-modified ATP analogues can be readily hydrolysed by CFTR for the following reasons. First, P-ATP can be used as a substrate by PKA for phosphorylation-dependent activation of CFTR (Fig. 7A). Second, while non-hydrolysable ATP analogues are poor CFTR openers, P-ATP can readily open CFTR even with a higher apparent affinity. Indeed, other hydrolysable ATP analogues, such as 2′- and 3′-deoxy-ATP (Aleksandrov et al. 2002) and 8-azido-ATP (e.g. Aleksandrov et al. 2001; Basso et al. 2003), also readily open CFTR. Third, unlike non-hydrolysable ATP analogues whose γ-phosphates are modified, the triphosphate backbone in P-ATP is preserved. In addition, while non-hydrolysable ATP analogues lock open CFTR for minutes in the presence of ATP, P-ATP only slightly prolongs the open time of CFTR.
If P-ATP indeed can be readily hydrolysed by CFTR, the mechanism underlying the prolongation of the open time by P-ATP may be different from that by non-hydrolysable ATP analogues. It should be pointed out first that the prolongation of the open time by P-ATP is not limited to ΔR-CFTR, since a similar effect on the open time was also observed in WT-CFTR. Although it is difficult to obtain enough single-channel data for WT-CFTR channels over a wide range of [P-ATP], we nevertheless managed to acquire some single-channel data at 30 μm P-ATP for WT-CFTR channels (see Fig. S1 in Supplemental materials). Compared to 2.75 mm ATP, 30 μm P-ATP prolonged the open time by a similar degree for WT-CFTR (∼30%) and ΔR-CFTR (∼40%). It is, however, puzzling that a concentration dependence of the open time is not regularly seen with ATP. Previous studies, with the exception of Zeltwanger et al. (1999), show that the closing rate is hardly affected by changes of [ATP]. Although the exact reason is unknown, we speculate that the difference in binding affinity between P-ATP and ATP may be responsible. If the hypothesis that the open state of CFTR corresponds to an nucleotide-binding-domain dimer with ATP sandwiched at the dimer interface (Vergani et al. 2003, 2005; Bompadre et al. 2005a, b) is correct, the binding energy provided by the bound ATP molecules should contribute to the overall energetics of the open state. Because of its tight binding, P-ATP is expected to stabilize the open state more than ATP. Since closing rates for low and high [P-ATP] differ by only ∼50% (Fig. 4B), an even smaller effect of ATP on the closing rate may escape detection. To make the matter worse, it is known that the gating patterns of the CFTR channels can change with time even under steady experimental condition (i.e. mode shift) so that the closing rate of CFTR varies notably even in the same expression system (see Bompadre et al. 2005a for details).
It is also interesting to note that our single-channel kinetic analysis reveals that the opening and closing rates of CFTR channels are both [P-ATP]-dependent, but with different sensitivities to changes of [P-ATP]. These observations, together with our previous findings (Bompadre et al. 2005a, b), suggest the involvement of at least two different binding sites, with one site controlling channel opening and the other site modulating channel closing. Although we cannot rule out the possibility that P-ATP exerts its effects through site(s) other than the NBD1 and NBD2 sites (e.g. Randak & Welsh, 2005) or on other ATP-binding proteins that may be involved in regulating CFTR gating (e.g. AMP-activated protein kinase; Hallows et al. 2003), the simplest explanation for our observation is that these two sites for the action of P-ATP actions correspond to the NBD1 and NBD2 sites.
Our earlier studies show that a mutation (i.e. K464A) that causes a decrease of ATP binding affinity at the NBD1 site (Basso et al. 2003) does not affect the opening rate or the ATP dose–response relationship (Powe et al. 2002; cf. Vergani et al. 2003), suggesting ATP binding at the NBD1 site may not be absolutely required for channel opening (also see Bompadre et al. 2005b). We therefore propose that P-ATP, like ATP, may act on the NBD2 site to open the channel.
The proposition that P-ATP acts on the NBD1 site to modulate channel closing is also based on our earlier studies with the K464A mutant. Although this mutation does not affect channel opening, K464A-CFTR exhibits a shorter open time (Powe et al. 2002). In addition, K464A mutation decreases the locked open time of hydrolysis-deficient mutants K464A/K1250A and K464A/E1371S (Powe et al. 2002; Vergani et al. 2003; Bompadre et al. 2005b), supporting the idea that the strength of ligand binding at the NBD1 site affects the stability of the open state. Furthermore, data from experiments using different nucleotides as ligands on CFTR channels are consistent with this notion as well. For example, a smaller ADP, a nucleotide presumably with lower binding strength than ATP, shortens the open time of the channel (Bompadre et al. 2005a, b). On the other hand, our current results show that P-ATP, with a higher apparent affinity than ATP, can prolong the open time.
This effect of P-ATP on the open time is more prominent when hydrolysis is abolished in WT-CFTR with AMP-PNP (Fig. 7C and D). AMP-PNP induced locked open state is more stable with P-ATP than that with ATP. Previous research suggests that the long-lived open state in the presence of ATP and AMP-PNP reflects tight binding of the non-hydrolysable AMP-PNP (e.g. Gunderson & Kopito, 1994; Hwang et al. 1994). The slow closing of AMP-PNP locked open channel represents a slow dissociation of the bound AMP-PNP. Since ATP alone can lock open mutant channels where ATP hydrolysis at NBD2 is abolished (e.g. Carson et al. 1995; Zeltwanger et al. 1999; Vergani et al. 2003), it is generally believed that AMP-PNP locks open the channel by binding to the NBD2 site (Gadsby & Nairn, 1999; Zou & Hwang, 2001). If this current view of the action of AMP-PNP is correct, the simplest explanation for our results would be that when the NBD2 site is occupied by AMP-PNP, and the NBD1 site is occupied by P-ATP, the locked open state is more stable than that with ATP and AMP-PNP bound at the NBD1 and NBD2 sites, respectively.
Using P-ATP as a new tool, we are able to reveal that the closing rate is more sensitive to changes of [P-ATP] than the opening rate (Fig. 4). If we accept the argument that P-ATP binds to the NBD1 site to modulate the closing rate (see above), our results suggest that the NBD1 site assumes a higher affinity to P-ATP than the NBD2 site. This assignment is consistent with what we know about CFTR's two ATP-binding sites, biochemically and structurally. Biochemical studies using 8-azido-ATP labelling (Aleksandrov et al. 2001; Basso et al. 2003) indicate that the NBD1 site has a higher nucleotide-binding affinity than the NBD2 site. Moreover, recently solved crystal structures of CFTR's NBD1 (Lewis et al. 2004; cf. Lewis et al. 2005) show an involvement of multiple aromatic residues in interacting with the adenine ring of ATP, a feature shared by many proteins with high affinity to nucleotides (e.g. phosphodiesterases in Huai et al. 2003, 2004; and myosin isozymes in Gillespie et al. 1999). On the other hand, there is only one such aromatic residue in the NBD2 of CFTR according to sequence alignment and homology model (Zou & Hwang, 2001; Moran et al. 2005).
It should be noted that the primary goal of the current work is not to definitely identify the functional role of CFTR's two ATP-binding sites. Nevertheless, in conjunction with the crystallographic information about CFTR's NBDs, the high affinity ATP/ADP analogues reported here will be useful tools to further elucidate functional roles of the two ATP-binding pockets at the molecular level. As the first attempt to search for high-affinity adenine-ring-modified nucleotide analogues as potential tools to study CFTR gating, we focused on those modified at the N6 position. Given the chemical nature of the adenine ring, we contemplate with the idea that modifications at positions other than N6 may yield interesting nucleotide analogues as novel tools to study the structure–function relationships of CFTR, as well as other ABC transporters.
Acknowledgments
We thank Drs Kevin Gillis and Silvia Bompadre for their comments on the paper. We are grateful to Cindy Chu and Shenghui Hu for technical assistance. Support from Biolog Life Science Institute (Germany) is gratefully acknowledged. This work is supported by NIHR01DK55835 (T.-C. Hwang), NIHR01HL53445 (T.-C. Hwang) and NIHDK61529 (X. Zou). Y. Sohma is supported by the Japan Society for the Promotion of Science (15590196). Z. Zhou is a recipient of a Postdoctoral Fellowship from the Cystic Fibrosis Foundation.
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2005.095083 http://jp.physoc.org/cgi/content/full/jphysiol.2005.095083/DC1 and contains supplemental material consisting of a figure entitled:
Figure S1. Comparison of the dwell times of WT-CFTR and ΔR-CFTR channels in the presence of 2.75 mm ATP or 30 μm P-ATP.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Ai T, Bompadre SG, Wang X, Hu S, Li M, Hwang TC. Capsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride-channel currents. Mol Pharmacol. 2004;65:1415–1426. doi: 10.1124/mol.65.6.1415. [DOI] [PubMed] [Google Scholar]
- Aleksandrov AA, Aleksandrov L, Riordan JR. Nucleotide triphosphate pentose ring impact on CFTR gating and hydrolysis. FEBS Lett. 2002;518:183–188. doi: 10.1016/s0014-5793(02)02698-4. [DOI] [PubMed] [Google Scholar]
- Aleksandrov L, Mengos A, Chang X, Aleksandrov A, Riordan JR. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2001;276:12918–12923. doi: 10.1074/jbc.M100515200. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide binding domains. Science. 1992;257:1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- Basso C, Vergani P, Nairn AC, Gadsby DC. Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating. J Gen Physiol. 2003;122:333–348. doi: 10.1085/jgp.200308798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompadre SG, Ai T, Cho JH, Wang X, Sohma Y, Li M, Hwang TC. CFTR gating I: Characterization of the ATP-dependent gating of a phosphorylation-independent CFTR channel (ΔR-CFTR) J Gen Physiol. 2005a;125:361–375. doi: 10.1085/jgp.200409227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompadre SG, Cho JH, Wang X, Zou X, Sohma Y, Li M, Hwang TC. CFTR gating II: Effects of nucleotide binding on the stability of open states. J Gen Physiol. 2005b;125:377–394. doi: 10.1085/jgp.200409228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transports cycle. Mol Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Csanády L. Rapid kinetic analysis of multichannel records by a simultaneous fit to all dwell-time histograms. Biophys J. 2000;78:785–799. doi: 10.1016/S0006-3495(00)76636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csanády L, Chan KW, Seto-Young D, Kopsco DC, Nairn AC, Gadsby DC. Severed channels probe regulation of gating of cystic fibrosis transmembrane conductance regulator by its cytoplasmic domains. J Gen Physiol. 2000;116:477–500. doi: 10.1085/jgp.116.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77–S107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Gillespie SK, Mercer JA, Shah K, Shokat KM. Engineering of the myosin-ibeta nucleotide-binding pocket to create selective sensitivity to N6-modified ADP analogues. J Biol Chem. 1999;274:31373–31381. doi: 10.1074/jbc.274.44.31373. [DOI] [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Effects of pyrophosphate and nucleotide analogues suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J Biol Chem. 1994;269:19349–19353. [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role of ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003;284:C1297–1308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- Huai Q, Colicelli J, Ke H. The crystal structure of AMP-bound PDE4 suggests a mechanism for phosphodiesterase catalysis. Biochemistry. 2003;42:13220–13226. doi: 10.1021/bi034653e. [DOI] [PubMed] [Google Scholar]
- Huai Q, Liu Y, Francis SH, Corbin JD, Ke H. Crystal structures of phosphodiesterases 4 and 5 in complex with inhibitor 3-isobutyl-1-methylxanthine suggest a conformation determinant of inhibitor selectivity. J Biol Chem. 2004;279:13095–13101. doi: 10.1074/jbc.M311556200. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator Cl− channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma M, Welsh MJ. Regulation of CFTR Cl− channel gating by ATP binding and hydrolysis. Proc Natl Acad Sci U S A. 2000;97:8675–8680. doi: 10.1073/pnas.140220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, et al. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HA, Zhao X, Wang C, Sauder JM, Rooney I, Noland BW, et al. Impact of the deltaF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–1353. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- Moran O, Galietta LJV, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe A, Al-Nakkash L, Li M, Hwang TC. Mutation of Walker-A lysine 464 in cystic fibrosis transmembrane conductance regulator reveals functional interaction between its nucleotide binding domains. J Physiol. 2002;539:333–346. doi: 10.1113/jphysiol.2001.013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randak CO, Welsh MJ. ADP inhibits function of the ABC transporter cystic fibrosis transmembrane conductance regulator via its adenylate kinase activity. Proc Natl Acad Sci U S A. 2005;102:2216–2220. doi: 10.1073/pnas.0409787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz BD, Venglarik CJ, Bridges RJ, Frizzell RA. Regulation of CFTR Cl− channel gating by ADP and ATP analogues. J Gen Physiol. 1995;105:329–361. doi: 10.1085/jgp.105.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Liu Y, Deirmengian C, Shokat KM. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc Natl Acad Sci U S A. 1997;94:3565–3570. doi: 10.1073/pnas.94.8.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Nairn AC, Gadsby DC. On the mechanism of MgATP-dependent gating of CFTR Cl− channels. J Gen Physiol. 2003;121:17–36. doi: 10.1085/jgp.20028673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltwanger S, Wang F, Wang GT, Gillis K, Hwang TC. Gating of CFTR by nucleoside triphosphates: quantitative analysis of a cyclic gating scheme. J Gen Physiol. 1999;113:541–554. doi: 10.1085/jgp.113.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hu S, Hwang TC. Probing an open CFTR pore with organic anion blockers. J Gen Physiol. 2002;120:647–662. doi: 10.1085/jgp.20028685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Hwang TC. Gating of CFTR by ATP hydrolysis: Structure and function. Biochemistry. 2001;40:5579–5786. doi: 10.1021/bi010133c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.