Abstract
When the intended foot placement changes during a step, either due to an obstacle appearing in our path or the sudden shift of a target, visual input can rapidly alter foot trajectory. However, previous studies suggest that when intended foot placement does not change, the path of the foot is fixed after it leaves the floor and vision has no further influence. Here we ask whether visual feedback can be used to improve the accuracy of foot placement during a normal, unperturbed step. To investigate this we measured foot trajectory when subjects made accurate steps, at fast and slow speeds, to stationary floor-mounted targets. Vision was randomly occluded in 50% of trials at the point of foot-off. This caused an increase in foot placement error, reflecting lower accuracy and higher variability. This effect was greatest for slow steps. Trajectory heading analysis revealed that visually guided corrections occurred as the foot neared the target (on average 64 mm away). They occurred closer to the target for the faster movements thus allowing less time and space to execute corrections. However, allowing for a fixed reaction time of 120 ms, movement errors were detected when the foot was approximately halfway to the target. These results suggest that visual information can be used to adjust foot trajectory during the swing phase of a step when stepping onto a stationary target, even for fast movements. Such fine control would be advantageous when environmental constraints place limitations on foot placement, for example when hiking over rough terrain.
Perturbation experiments have shown that visual information can be used to alter foot trajectory rapidly during a step. After the presentation of an obstacle, or the movement of a floor-mounted target, appropriate changes in foot trajectory are seen within ∼120 ms of the visual stimulus (Patla et al. 1991; Weerdesteyn et al. 2004; Reynolds & Day, 2005). In these circumstances, the visually triggered mid-step adjustment of foot trajectory is determined by an externally imposed change in the intended placement of the foot. It is surprising that not much is known about whether vision can be used to guide the foot during the swing phase of a normal, unperturbed step. In that case the role of vision would not be to redefine the final position of the foot but to improve its placement accuracy. Usually, gaze is directed far ahead of the feet during locomotion, but when environmental constraints place limitations upon foot placement, gaze becomes directed downward towards the area of foot-fall for each step (Hollands et al. 1995). This raises the possibility that vision can be used to guide the foot mid-step, although the current weight of evidence suggests otherwise.
During a visually guided step, intended future foot placement and body motion are coupled and pre-planned before foot-off (Lyon & Day, 1997). This suggests that once a step is initiated, visual information might not be used on-line to control foot trajectory. In support of this, experiments that have occluded visual feedback during the swing phase of the locomotor cycle have not found any impairment of target-directed locomotion (Hollands & Marple-Horvat, 1996; Patla et al. 1996). However, continuous visual occlusion during walking has been found to impair foot placement accuracy (Thomson, 1983). Therefore, visual feedback can result in corrective step adjustments, but they typically occur up to four steps in advance of the step that places the foot onto the target (Lee et al. 1982; Laurent & Thomson, 1988). Taken together, these findings imply that during the swing phase of a step there is minimal or no role for on-line visual guidance of the foot to improve its terminal placement accuracy.
Theoretically, it is possible to use vision to adjust the foot during the swing phase of a normal step. This is because typical swing durations during self-paced steps (∼400–450 ms; Blanc et al. 1999; Mills & Barrett, 2001) are greatly in excess of 120 ms, the minimum time required to respond with the foot to a visual stimulus (Patla et al. 1991; Weerdesteyn et al. 2004; Reynolds & Day, 2005). So there should be enough time to detect and correct any errors in foot trajectory even for a relatively fast step. However, correcting for perturbations may be fundamentally different from guiding the foot to a stationary target. A target perturbation requires a change in movement planning, and does not require vision of the limb (Goodale et al. 1986). In contrast, if the target does not move, the original movement plan need not be altered, and vision of both limb and target may be necessary to detect movement error. There may also be other differences. Movement errors may be smaller, and accrue more gradually during continuous guidance of the limb towards a stationary target, as compared with a sudden discrete target jump. The necessary motor response may therefore be smaller, involving fine-tuning of an ongoing movement rather than the generation of a new one.
It is well established that vision can be used to correct the hand trajectory when reaching towards stationary targets (Woodworth, 1899). The minimum movement duration for such corrections to occur may be as little as 135 ms (Carlton, 1981). If the stepping foot is like the reaching hand then visual control should be useful in guiding it to a stationary target, even for fast stepping movements. However, differences between the hand and foot may mean this is not necessarily so. Visual guidance of the hand may be more precise than that of the foot (Hoffmann, 1991). There is also the added complexity that, unlike the reaching hand, the stepping foot is concurrently engaged in maintaining balance which may impose limitations on the ability to adjust it after a step has been initiated (Lyon & Day, 2005; Reynolds & Day, 2005). Hence, without empirical evidence it cannot be assumed that visual guidance of the stepping foot is similar to that of the reaching hand.
Here we provide evidence that establishes a role for visual guidance of the foot during the swing phase of an unperturbed step. We do this by showing a detrimental effect of visual occlusion, at the point of foot-lift, on the accuracy and precision of terminal foot placement. By measuring the timing and location of corrective changes in foot trajectory, we also determine when and where vision is used during the step. Our results show that the stepping foot is similar to the reaching hand, in that visually guided corrections occur when the limb is close to its target.
Methods
Protocol
Ten subjects (seven male, three female; mean age, 31 years) gave informed consent to participate. The experiments were approved by the local ethics committee and carried out in accordance with the Declaration of Helsinki. Subjects made forward steps onto one of four targets. Multiple targets were used to avoid stereotypical or abnormal behaviour that might result from continuously stepping to the same target. These were custom-made for each person, being an exact copy of his or her footprint for the left and right feet. This was done by placing a cardboard stencil of the footprint over a sheet of electroluminescent paper. Prior to the experiment, each subject was asked to place his/her foot as accurately as possible on each of the four targets (with no time or movement constraints), and the position recorded. This represented perfect foot placement in terms of subject perception, defining the goal that they were trying to achieve during the stepping task. This was therefore defined as the target of the movement, and so error and heading were calculated relative to this foot placement. During the experiment, subjects were asked to place the foot on the target as accurately as possible during a forward step, making the goal of the step unequivocal. It was emphasized that a natural stepping movement was required. Two targets were placed with their centres 30 cm directly in front of the centres of both footprints in the starting position, and two were placed ahead by the same amount and also laterally by 21 cm. The starting positions of the feet were marked with chalk, the stance width being 28 cm between footprint centres. A beep signalled the onset of a trial, after which one of the four targets was lit. The instruction was to step onto the target as accurately as possible with the leading foot. The position of the trailing foot was not specified, except to request that stance should be roughly the same as that of the start position. Step speed was regulated on each trial by sounding a beep of 50 ms duration, triggered either 300 or 600 ms after the foot left the floor, for fast and slow steps, respectively. The subject was asked to make his/her foot-strike coincide with the beep. Some practice trials were performed to become acquainted with this task. It was strongly emphasized that foot placement accuracy was the primary goal of the task, and that timing was secondary. A total of 160 trials were performed for each of the fast and slow conditions. These were split into blocks of 80 trials, with alternate fast and slow blocks, balanced across subjects. In 50% of randomly selected trials, vision was occluded at the point of foot-off until at least 1 s after the step was completed. Subjects were told to attempt to maintain accuracy regardless of the visual condition. Visual feedback of foot placement was allowed at the end of all trials.
Apparatus
Targets were made from electroluminescent paper whose luminance could be controlled electronically (Pacel Electronics, Poole, UK). Foot-timing was measured by placing a small current (∼20 μA) through the subject, completing a circuit with the floor which was broken when a foot was raised. In this way, the timing of foot-lift, foot-strike, and therefore swing duration, could be accurately measured. Visual occlusion was achieved with Plato LCD spectacles (Translucent Technologies, Toronto, Canada). The foot-lift signal was used as a trigger to make the spectacles opaque. Hence, vision could be occluded precisely at the onset of the swing phase of a step. This signal was also used to trigger the onset of the timing beep, after a delay of 300 or 600 ms. Infra-red markers were placed on the hallux (big toe) and heel of each foot. Foot trajectories were recorded at 200 Hz using three Codamotion mpx30 cameras (Charnwood Dynamics, Leicestershire, UK). At the beginning of the experiment, footprints were chalk-marked and digitized by tracing around them with an infra-red marker. The location of the footprint was coregistered with the positions of the lights on the foot. The footprint could be subsequently reconstructed wherever the foot went, given the coordinates of the two lights.
Analysis
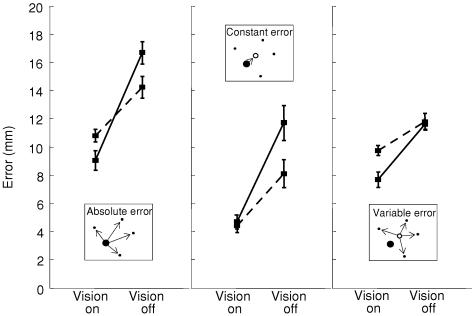
Foot placement error was measured separately for each speed (fast/slow) and visual condition (vision on/off). When questioned, all subjects reported a preference for the right foot (as determined using questionnaire from Bell & Gabbard, 2000). However, paired t tests revealed no significant effect of the foot used upon foot placement error. Therefore, data from all targets were combined. The centre of mass (centroid) of the footprint at the end of each trial was calculated (see Fig. 1). Errors in foot placement were measured in three ways (see inset graphics in Fig. 2). Absolute error was measured as the distance of each centroid from the target centroid, reflecting overall accuracy. Constant error was the distance of the mean centroid position from the target, reflecting bias. Variable error was the distance of each centroid from the mean centroid position, reflecting consistency of movement (for elaboration see chapter 2 in Schmidt & Lee, 1999).
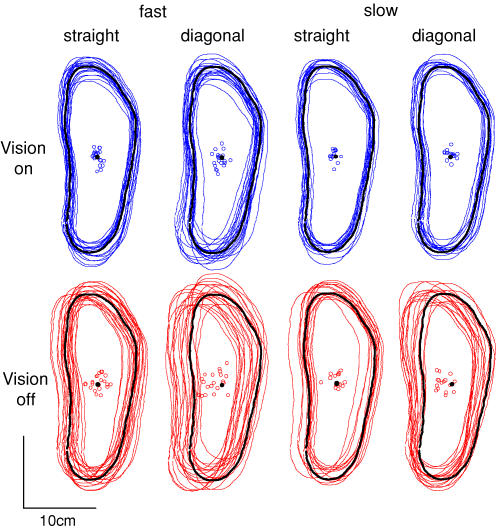
Figure 1. Foot placement.
Locations of footprints for individual trials from a representative subject. Black footprints show target locations. Open circles show footprint centroids, with target centroid shown as black circle.
Figure 2. Foot placement error.
The inset graphics show the three methods of error measurement. The filled circle shows the target, dots show example endpoints of footprint centroids, and the open circle shows the mean endpoint position. Dashed lines show fast steps, continuous lines show slow steps.
Foot speed was calculated as the magnitude of the instantaneous velocity vector of the hallux marker. A two-factor repeated-measures ANOVA was used to determine the effect of each factor upon swing duration, maximum foot speed, trajectory length and errors (General Linear Model, SPSS 11.01 SPSS Inc., Chicago, Illinois, USA). The factors were vision (vision on/off) and speed (fast/slow). Newman–Keuls tests were used for post hoc comparisons.
Individual stepping trajectories of the feet were examined to determine where and when vision was being used. Heading of the hallux marker was calculated during the swing phase. This was done by calculating the angle, in three dimensions, between the instantaneous velocity vector and the vector defined by the position of the marker with respect to the target location. In this case, the target location was the projection of the hallux marker onto the target footprint. This measure of heading angle gave an impression of how ‘on-target’ the movement was throughout its extent. A heading of 0 deg indicates that the foot is perfectly on track for the target, whereas 180 deg indicates that it is moving directly away from the target. A reduction in heading when vision is present is indicative of a visually guided correction. Heading was averaged separately with respect to the beginning and end of the trajectory (foot-off and foot strike). The time at which vision-on and vision-off traces separated was determined visually for each subject and speed. To determine what spatial point this time corresponded to, space–time graphs were plotted. A paired two-tailed Student's t test was used to detect any differences between the separation points for fast and slow steps.
P < 0.05 was considered statistically significant for all tests. Errors reported in the text are standard deviations. All errors in the figures and tables are standard errors of the mean.
Results
Step duration, speed and length
As expected, swing duration and maximum foot speed were significantly different for fast and slow steps (see Table 1; F1,9 ≥ 92.38, P ≤ 0.001). Mean swing duration was 328 ms and 525 ms for fast and slow steps, respectively. Visual occlusion had no effect on either duration or speed (F1,9≤ 4.907, P≥ 0.054). However, it did cause trajectories to be marginally longer, by up to 4 mm (Table 1; F1,9 = 10.97, P = 0.009). Slow steps were also longer than fast steps by up to 12 mm (F1,9 = 25.19, P < 0.001).
Table 1.
Mean step parameters
| Vision on | Vision off | ||
|---|---|---|---|
| Swing duration (ms) | Fast | 329 ± 8 | 327 ± 8 |
| Slow | 529 ± 16 | 520 ± 15 | |
| Maximum speed (m s−1) | Fast | 1.43 ± 3 | 1.42 ± 3 |
| Slow | 1.04 ± 5 | 1.02 ± 5 | |
| Trajectory length (mm) | Fast | 321 ± 3 | 324 ± 3 |
| Slow | 332 ± 3 | 336 ± 4 |
Accuracy and precision of foot placement
Figure 1 shows for a representative subject that foot placement was more variable when vision was occluded. For this subject, the effect was particularly clear for fast diagonal steps. This can also be seen in the spread of the footprint centroids, which were used to calculate foot placement error.
For the group, all three types of measured error increased when vision was occluded (Fig. 2, F1,9 ≥ 26.01, P ≤ 0.001). The effect was greatest for slow steps, resulting in significant speed–vision interactions in all cases (F1,9 ≥ 7.48, P ≤ 0.023). Nevertheless, post hoc comparison shows that even for fast steps there was a significant effect of vision on all types of error (P < 0.05). The effect of speed was different for the two visual conditions. When vision was available, fast steps showed greater absolute and variable error than slow steps (P < 0.05). When vision was occluded, however, fast steps showed less absolute and constant error (P < 0.05). Hence, the nature of the vision–speed interaction was different for all three types of error.
Trajectory heading
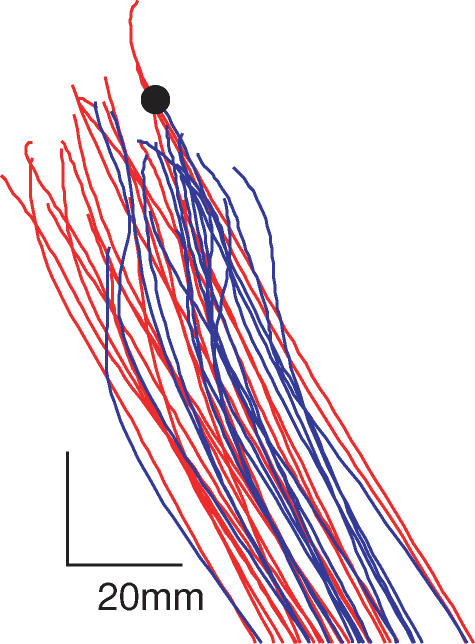
Figure 3 shows typical trajectories of the hallux marker with and without vision. As the target was approached, the presence of visual feedback caused the foot to be steered towards the target. To quantify these corrections, we analysed the 3-dimensional heading angle of the hallux marker to determine how ‘on-target’ the foot was throughout the course of the movement. Figure 4A shows heading averaged with respect to foot-off. An angle of 0 deg would indicate that the foot is perfectly on-target, whereas 180 deg would indicate it is headed directly away from the target. All the traces form U-shaped curves due to the shape of the trajectory. At the start of the step, the foot was lifted vertically from the floor, and so heading was constrained not to be directed towards the target. In the middle of the trajectory, heading was reduced as the foot moved through space more directly towards the target. As the foot got close to the target, heading increased again. This is because the geometry is such that a given spatial error accentuates the heading angle the closer the foot is to the target.
Figure 3. Visual influence upon foot trajectory.
Trajectories of the hallux marker during the swing phase are shown for a subject stepping slowly to the left diagonal target. Target position is shown by the black circle. Vision-on traces are shown in blue, vision-off in red.
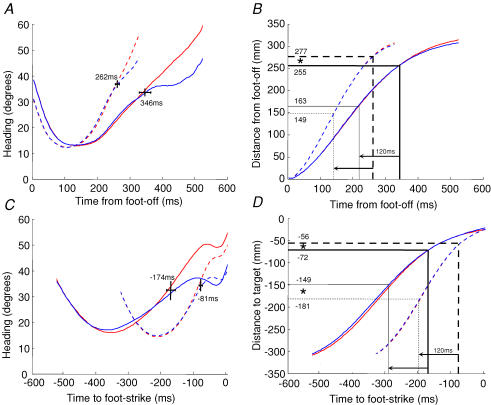
Figure 4. Heading of the foot towards the target.
Heading of the hallux marker with respect to the target was averaged with respect to foot-off (A). Dashed lines show fast steps, continuous lines show slow steps. Thick lines show vision-on traces and thin lines show vision-off traces. The temporal point at which these traces deviate is shown by the short vertical line, with horizontal lines representing standard errors. Thick grid lines in the corresponding distance–time plot (B) show the equivalent separation points in space. Thin grid lines show the spatial points 120 ms earlier in time, to account for reaction time. C and D show the equivalent plots for heading averaged with respect to foot-strike. *Significant differences between distances (P < 0.05).
When vision was available, heading was reduced towards the end of the movement compared with the vision-off condition. This means that the foot veered more towards the target. During fast movements, visually guided corrections occurred at 262 ± 23 ms (mean ±s.d.) after foot-off. During slow movements, this point occurred significantly later in time at 346 ± 56 ms (t = 3.18, P = 0.016). The space–time graph reveals that these times correspond to significantly different distances of 277 ± 12 mm and 255 ± 12 mm from foot-off, for fast and slow steps, respectively (thick grid lines in Fig. 4B; t = 3.17, P = 0.015). Heading was also averaged with respect to the end of the movement, as shown in Fig. 4C. The times at which visually guided corrections were made were −81 ± 20 ms (fast) and −174 ± 44 ms (slow) from foot-strike (t = 6.75, P < 0.001). Figure 4D shows that this corresponds to −56 ± 12 mm (fast) and −72 ± 23 mm (slow) from the target (t = 2.62, P < 0.034).
When correcting a movement in response to visual input, there is a neural lag (reaction time) between the visual event and the motor correction. To account for this, we recalculated the spatial points where movement errors may have been first detected. Assuming a fixed reaction time of 120 ms (Patla et al. 1991; Weerdesteyn et al. 2004; Reynolds & Day, 2005), this results in distances of 149 ± 30 mm (fast) and 163 ± 27 mm (slow) from foot-off, which are not significantly different (t = 0.730, P = 0.489; see thin grid lines in Fig. 4B). For heading averaged with respect to foot-strike, it gave significantly different distances of −181 ± 16 mm (fast) and −149 ± 36 mm (slow) from foot-strike (t = 2.39, P = 0.048; Fig. 4D).
Discussion
A speed–accuracy trade-off for stepping
When vision was available there was a speed–accuracy trade-off in that fast steps showed greater absolute and variable error than slow steps. This is compatible with the findings of Drury & Woolley (1995) who showed that visually guided stepping is subject to the same kind of speed–accuracy trade-off as reaching. Rather than measuring step accuracy as in our experiment, they measured step duration when walking on targets of variable width and separation. They found that movement times were well accounted for by Fitts' law, which implies an inverse relationship between the difficulty of a movement and the speed with which it can be performed (Fitts, 1954).
Upper-limb reaching studies have shown that the speed–accuracy trade-off does not apply when moving without vision; constant and variable errors are unaffected by movement speed (Adamovich et al. 1994). Here, we found this to be true for variable error (see Fig. 2, vision off), but for constant error we found that the speed–accuracy relationship was actually reversed; fast steps were more accurate than slow steps (Fig. 2, vision off). This suggests a time-dependent drift of the foot in the absence of vision. It is uncertain whether this reflects a fundamental difference between hand and foot motor control, or is simply due to methodological differences.
Visual feedback increases accuracy and precision of foot placement
When vision was removed throughout the swing phase of the step, all types of foot-placement error became greater. The foot ended up further away from the target, as revealed by increases in absolute and constant error. Foot placement was also more variable, as shown by an increase in variable error. Hence, visual feedback improved both accuracy and precision.
Visual occlusion had the greatest effect on foot placement when movement speed was slow. Nevertheless, there was still a clear effect during the fast steps in which the mean swing duration of 326 ms was considerably less than during typical self-paced locomotion (∼400–450 ms; Blanc et al. 1999; Mills & Barrett, 2001). The effect also occurred despite the fact that subjects repeated the same stepping movements many times during the course of the experiment. So even for fast, well-practiced steps, where one might expect predictive control to dominate, visual feedback can still be used to improve step accuracy.
A recent study showed that during an unplanned forward step, induced by a loss of balance, shifting gaze downwards improved foot placement accuracy (Zettel et al. 2005). However, for visually guided steps which are predictable and preplanned, such as in our experimental task, previous studies suggest no role for vision during the swing phase of a step. Hollands & Marple-Horvat (1996) found that visual denial during the swing phase did not affect the ability to step over a series of stepping stones. However, subjects were allowed a leeway of approximately 17.5 cm in foot placement in the medio-lateral direction, meaning that changes in accuracy smaller than this went undetected. Nevertheless, the results of that study and others (Laurent & Thomson, 1988; Patla et al. 1996) suggest that vision plays a major role in determining foot placement before the stepping foot leaves the floor. Of course, the results presented here do not contradict these findings. We have simply shown that when precision is demanded, additional fine-tuning of foot placement can occur after the foot has left the floor. This results in the foot being closer to the target by up to 8 mm on average (constant error, Fig. 2). Although this may seem small, such fine visuomotor control of the foot may be utilized in situations where environmental constraints place strict limitations upon foot placement. For example, when hiking over rough terrain small adjustments may be necessary to avoid slipping or twisting an ankle. Similarly, when a gymnast performs on the beam or an acrobat walks the tightrope, accurate foot placement becomes crucial.
Mid-trajectory corrections steer the foot towards its target
We analysed heading of the foot with respect to the target to estimate when and where vision was being used to correct the foot trajectory. Perhaps surprisingly, the heading angle was reduced slightly towards the end of the movement even in the absence of vision (continuous lines, Fig. 4C). This raises the possibility that non-visual cues, such as efferent information (Desmurget & Grafton, 2000) or proprioception, may have been used to correct movements. Nevertheless, in all conditions we observed that the presence of vision caused alterations in heading which steered the foot more towards the target compared with the no-vision condition. For all movements, these corrections started to occur as the foot neared (56–72 mm) the target. They occurred closer to the target for the faster movements thus allowing less time and space to execute corrections. This goes some way towards explaining the speed–accuracy trade-off described above in which endpoint accuracy and precision were worse for fast than for slow steps when vision was present.
Corrections in the vicinity of the target have also been observed when reaching with the hand. It has been proposed that reaching involves an initial pre-programmed ballistic transport phase followed by a visually guided homing-in phase when the limb is close to its target (Woodworth, 1899; Paillard, 1996). However, the neural lags involved in the visuomotor process would mean that vision is first used when the limb is at a greater distance from the target. When we assumed a fixed lag of 120 ms, based on the minimal reaction time to a visual perturbation (Patla et al. 1991; Weerdesteyn et al. 2004; Reynolds & Day, 2005), we estimated that vision was first used for error detection when the foot was approximately half-way along its path.
What determines the point at which visual feedback first becomes useful?
To correct the trajectory it is necessary for the CNS to predict the foot's final error. Sensory feedback provides valuable information for this prediction from the moment the foot leaves the floor. Why then is visual information not used at this very early stage? One possibility could be that it is prevented from being used because of a refractory period after step initiation. This seems unlikely because corrections did not occur at a fixed time after foot-lift for the two speeds of step (84 ms difference; Fig. 4A). A second possibility is that the foot's final position relative to the target can only be estimated after it has travelled a minimum distance. Our results are compatible with this suggestion as there was no significant difference in the path travelled prior to correction (around 155 mm) for the two speeds of step. A third possibility is that the foot needs to be within a maximum distance from the target to predict the final position error. This could arise, for example, through a process that requires both target and foot to be seen simultaneously to assess the foot's motion relative to the target. If this were the explanation, the foot–target distance could not be fixed. We estimate that the corrections of fast steps used vision when the foot was 32 mm further from the target compared with slow steps. However, the basic principle may still hold because when the foot has greater speed, a prediction of final error may be made with the same accuracy at a greater foot–target distance.
Are corrections based on vision of the foot, target or both?
In our protocol, subjects were allowed simultaneous vision of both the foot and target. Studies of upper-limb movements in which vision of the limb is removed when reaching for stationary targets have given mixed results. Some results suggest that vision of the limb does improve reach accuracy, particularly for the final phase of the movement (Carlton, 1981; Berthier et al. 1996), and others not (Connolly & Goodale, 1999). Given this lack of agreement, it is presently unclear whether vision of both the foot and the target is likely to be necessary throughout the step to produce the corrective movements we observed.
In conclusion, we have shown that vision can be used midway through the swing phase of a step to guide the foot towards its target and improve its accuracy. This occurs even for fast, well-practiced movements. This visuomotor process may be particularly useful in situations where environmental constraints impose strict limits upon foot placement.
Acknowledgments
The Medical Research Council funded this work. We would like to thank Mr E. Bye for technical assistance.
References
- Adamovich S, Berkinblit M, Smetanin B, Fookson O, Poizner H. Influence of movement speed on accuracy of pointing to memorized targets in 3D space. Neurosci Lett. 1994;172:171–174. doi: 10.1016/0304-3940(94)90689-0. [DOI] [PubMed] [Google Scholar]
- Bell J, Gabbard C. Foot preference changes through adulthood. Laterality. 2000;5:63–68. doi: 10.1080/713754351. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Clifton RK, Gullapalli V, McCall DD, Robin DJ. Visual information and object size in the control of reaching. J Mot Behav. 1996;28:187–197. doi: 10.1080/00222895.1996.9941744. [DOI] [PubMed] [Google Scholar]
- Blanc Y, Balmer C, Landis T, Vingerhoets F. Temporal parameters and patterns of the foot roll over during walking: normative data for healthy adults. Gait Posture. 1999;10:97–108. doi: 10.1016/s0966-6362(99)00019-3. [DOI] [PubMed] [Google Scholar]
- Carlton LG. Processing visual feedback information for movement control. J Exp Psychol Hum Percept Perform. 1981;7:1019–1030. doi: 10.1037//0096-1523.7.5.1019. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA. The role of visual feedback of hand position in the control of manual prehension. Exp Brain Res. 1999;125:281–286. doi: 10.1007/s002210050684. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Drury CG, Woolley SM. Visually-controlled leg movements embedded in a walking task. Ergonomics. 1995;38:714–722. doi: 10.1080/00140139508925143. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–391. [PubMed] [Google Scholar]
- Goodale MA, Pelisson D, Prablanc C. Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature. 1986;320:748–750. doi: 10.1038/320748a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann ER. A comparison of hand and foot movement times. Ergonomics. 1991;34:397–406. doi: 10.1080/00140139108967324. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE. Visually guided stepping under conditions of step cycle-related denial of visual information. Exp Brain Res. 1996;109:343–356. doi: 10.1007/BF00231792. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE, Henkes S, Rowan AK. Human eye movements during visually guided stepping. J Mot Behav. 1995;27:155–163. doi: 10.1080/00222895.1995.9941707. [DOI] [PubMed] [Google Scholar]
- Laurent M, Thomson JA. The role of visual information in control of a constrained locomotor task. J Mot Behav. 1988;20:17–37. doi: 10.1080/00222895.1988.10735430. [DOI] [PubMed] [Google Scholar]
- Lee DN, Lishman JR, Thomson JA. Regulation of gait in long jumping. J Exp Psychol Hum Percept Perform. 1982;8:448–459. [Google Scholar]
- Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res. 1997;115:345–356. doi: 10.1007/pl00005703. [DOI] [PubMed] [Google Scholar]
- Lyon IN, Day BL. Predictive control of body mass trajectory in a two-step sequence. Exp Brain Res. 2005;161:193–200. doi: 10.1007/s00221-004-2058-z. [DOI] [PubMed] [Google Scholar]
- Mills PM, Barrett RS. Swing phase mechanics of healthy young and elderly men. Hum Mov Sci. 2001;20:427–446. doi: 10.1016/s0167-9457(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Paillard J. Fast and slow feedback loops for the visual correction of spatial errors in a pointing task: a reappraisal. Can J Physiol Pharmacol. 1996;74:401–417. [PubMed] [Google Scholar]
- Patla AE, Adkin A, Martin C, Holden R, Prentice S. Characteristics of voluntary visual sampling of the environment for safe locomotion over different terrains. Exp Brain Res. 1996;112:513–522. doi: 10.1007/BF00227957. [DOI] [PubMed] [Google Scholar]
- Patla AE, Beuter A, Prentice S. A two stage correction of limb trajectory to avoid obstacles during stepping. Neurosci Res Commun. 1991;8:153–159. [Google Scholar]
- Reynolds RF, Day BL. Rapid visuo-motor processes drive the leg regardless of balance constraints. Curr Biol. 2005;15:R48–R49. doi: 10.1016/j.cub.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and Learning: a Behavioral Emphasis. 3. Champaign, Illinois, USA: Human Kinetics; 1999. [Google Scholar]
- Thomson JA. Is continuous visual monitoring necessary in visually guided locomotion? J Exp Psychol Hum Percept Perform. 1983;9:427–443. doi: 10.1037//0096-1523.9.3.427. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Hampsink B, Duysens J. Gait adjustments in response to an obstacle are faster than voluntary reactions. Hum Mov Sci. 2004;23:351–363. doi: 10.1016/j.humov.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. The accuracy of voluntary movement. Psychol Rev. 1899;3:1–119. [Google Scholar]
- Zettel JL, Holbeche A, McIlroy WE, Maki BE. Redirection of gaze and switching of attention during rapid stepping reactions evoked by unpredictable postural perturbation. Exp Brain Res. 2005;165:392–401. doi: 10.1007/s00221-005-2310-1. [DOI] [PubMed] [Google Scholar]