Abstract
Focal adhesions composed of integrins provide an important structural basis for anchoring the endothelial lining to its surrounding matrices in the vascular wall. Complex molecular reactions occur at the endothelial cell–matrix contact sites in response to physical and chemical stress present in the circulatory system. Recent experimental evidence points to the importance of focal adhesions in the regulation of microvascular barrier function. On one hand, the adhesive interaction between integrins and their extracellular ligands is essential to the maintenance of endothelial barrier properties, and interruption of integrin–matrix binding leads to leaky microvessels. On the other hand, focal adhesion assembly and activation serve as important signalling events in modulating endothelial permeability under stimulatory conditions in the presence of angiogenic factors, inflammatory mediators, or physical forces. The focal responses show distinctive patterns with different temporal characteristics, whereas focal adhesion kinase (FAK) plays a central role in initiating and integrating various signalling pathways that ultimately affect the barrier function. The molecular basis of focal adhesion-dependent microvascular permeability is currently under investigation, and advances in the technologies of computerized quantitative microscopy and intact microvessel imaging should aid the establishment of a functional significance for focal adhesions in the physiological regulation of microvascular permeability.
The semi-permeable barrier property of microvascular endothelium is maintained by a layer of endothelial cells that are attached to their underlying matrices through complex transmembrane structures termed focal adhesions. Focal adhesions are mainly composed of integrins, transmembrane receptors that tether the cytoskeleton to the extracellular matrix via cytoplasmic linker proteins. These adhesive complexes not only provide an anchor point for endothelial cells to adhere to the substratum, but they also transmit forces and biochemical signals between the cells and matrix. Over the past two decades, tremendous research efforts have been devoted to the characterization of cell–matrix interactions, whereby the central role of focal adhesion kinase (FAK) in the dynamic control of focal complex assembly and distribution has been revealed (Schaller, 2001; Parson, 2003; Schlaepfer et al. 2004). Signal transduction through focal adhesions has been implicated in many physiological and pathophysiological processes, including angiogenesis, wound healing, and vascular remodelling in response to physical or chemical stress (Petit & Thiery, 2000; Ingber, 2002; Wozniak et al. 2004; Chien et al. 2005). Despite these exciting advances, the specific contribution of focal adhesions to the regulatory mechanism of microvascular barrier function remains a mystery, and considerable controversy exists regarding how focal adhesion molecules affect endothelial permeability. Among the reasons given for the lack of such important knowledge is that there are few experimental models that enable direct and quantitative assessments of permeability in live microvessels under a native environment while the focal adhesion components can be specifically manipulated. Although studies using cultured endothelial cell monolayers have provided valuable insights into the molecular reactions occurring at the focal contact, the artificial environment of cell culture experiments, along with the remarkable heterogeneity among endothelial cells from different vascular origins, renders it difficult to determine the causal effect of focal adhesions on microvascular permeability.
In this review, we provide a brief introduction to the recent studies that characterize the molecular structure and function of focal adhesions, focusing on integrins and FAK with respect to their regulatory effects on microvascular endothelial permeability. In addition, some recent advances in methodologies and techniques used for studying focal adhesions and endothelial barrier function are discussed.
Integrins
Integrins represent a family of transmembrane glycoproteins expressed as α/β heterodimers. The cytoplasmic domains of integrins interact with the cytoskeleton either directly or indirectly through intracellular linker proteins, such as paxillin, talin, vinculin and α-actinin, and their extracellular domains bind to respective matrix components (Petit & Thiery, 2000; Hodivala-Dilke et al. 2003). Vascular endothelial cells express multiple integrins with distinct combinations of α/β subunits, including α1β1 and α1β2 (bind to collagen), α3β1, α6β1 and α6β4 (bind to laminin), α5β1 (binds to fibronectin), as well as αvβ3 and αvβ5 that bind to vitronectin (Albelda et al. 1989; Luscinskas & Lawler, 1994). Many integrins recognize the Arg-Gly-Asp (RGD) sequence in matrix proteins and thereby are able to interact with more than one extracellular ligand. The molecular organization of integrins varies depending on the chemical and physical states of extracellular matrices (Dejana et al. 1990).
Cell culture studies reveal that integrins are essential to the establishment and stabilization of endothelial monolayers, because interruption of integrin–matrix binding causes cell detachment from the substratum (Dejana et al. 1990; Cheng et al. 1991). Synthetic peptides that compete the RGD-binding sequence or antibodies directed against α5β1 produce a dramatic increase in transendothelial flux of water and large solutes (Wheatley et al. 1993; Curtis et al. 1995; Qiao et al. 1995). Direct evidence that underscores the physiological significance of integrin–matrix interactions comes from our recent study showing that inhibition of integrin binding to either fibronectin or vitronectin with specific RGD peptides dose-dependently increased venular permeability by 2- to 3-fold (Wu et al. 2001). The RGD-induced hyperpermeability was time dependent and reversible upon clearance of the peptides, indicating that the effect was not merely due to a permanent disruption of the endothelium. The rapid onset and magnitude of hyperpermeability is consistent with the modulation of cell–matrix tethering forces as an important mechanism in vascular permeability response to physical or chemical factors (Yuan 2000, 2003). In other words, integrins provide an important structural support to dynamic changes that occur at the endothelial barrier. This structural support is multidirectional and may not be limited to the basal lateral site of endothelial cells. Indeed, some members of the integrins family have been identified as located at endothelial cell–cell borders (Lampugnani et al. 1991). It is suggested that these integrins collaborate with other intercellular molecules to form lateral junctions. Thus, blocking integrin function could disrupt the junctional connection leading to permeation of macromolecules across endothelial monolayers.
Not only do integrins maintain the endothelial barrier property, but they also act as signalling molecules that mediate endothelial responses to growth factors or inflammatory mediators under certain physiological or pathophysiological conditions. For example, β5 integrin has been identified as a key molecule involved in the recruitment of FAK to focal adhesions in endothelial cells upon vascular endothelial growth factor (VEGF) stimulation (Avraham et al. 2003). Mice deficient in integrin β5 display a reduced vascular permeability response to the growth factor (Eliceiri et al. 2002). Another well-characterized hyperpermeability factor similar to VEGF is thrombin. It has been shown that thrombin contains an RGD-binding sequence that can interact with αvβ3 on endothelial cells leading to enhanced angiogenesis (Byzova & Plow, 1998; Hodivala-Dilke et al. 2003). Furthermore, emerging evidence supports a role for integrins in the mechanotransduction of endothelial cells in response to shear stress, a well-known modulator of vascular endothelial permeability (Davies et al. 1994; Ingber, 2002; Chien et al. 2005). Putting these findings together, it appears that various physical and chemical signals can be sensed and coordinated at the focal contact sites where integrins may play a central role in transmembrane cross-talk between the cells and extracellular matrix.
Focal adhesion kinase
Focal adhesion kinase is composed of a central catalytic domain flanked by large N- and C-terminal domains. The N-terminal region contains the FERM homology that can bind integrins and growth factor receptors. The non-catalytic domain in the C-terminal, also referred to as FRNK (FAK-related non-kinase), carries the FAT sequence which not only directs FAK to adhesion complexes for signalling, but also provides binding sites for other docking molecules to interact with the cytoplasmic proteins (Schaller, 2001; Parson, 2003; Schlaepfer et al. 2004). To date, at least five tyrosine residues have been identified in FAK as important sites for focal adhesion assembly and signalling (Parson, 2003). The major site for tyrosine phosphorylation is Tyr-397 located in the kinase domain, which undergoes rapid phosphorylation in response to integrin clustering. This creates a binding site for the SH2 domain-containing molecules, such as Src, to be recruited and activated, leading to further phosphorylation on Tyr-861 or Tyr-925 at the C-terminal. In addition, Tyr-576/577 positioned in the catalytic loop of FAK can be phosphorylated during Src–FAK interactions. Phosphorylation of these tyrosine residues directly correlates with the kinase activity and is a critical step in integrin engagement-induced FAK activation (Parson, 2003). In addition, FAK activation can occur independent of integrin clustering. It has been reported that thrombin phosphorylates FAK on Tyr-397, Tyr-576, and Tyr-925 (Shikata et al. 2003; van Nieuw Amerongen et al. 2004), whereas VEGF-induced FAK phosphorylation occurs predominantly at Tyr-861 (Eliceiri et al. 2002). It has been shown that VEGF induces a rapid translocation of FAK from its diffusive cytoplasmic distribution to focal contacts; the kinetics of this subcellular redistribution correlates with a transient phosphorylation of FAK (Eliceiri et al. 2002). The phosphorylation-coupled FAK activation and translocation represents an important mechanism for the coordination of various cellular responses to VEGF during angiogenesis.
Although FAK-dependent endothelial cell growth and motility are subjects of extensive investigation, not until recently has it been recognized that FAK is also an endothelial barrier modulator. The involvement of this signalling molecule is evidenced by spatial and temporal correlations between FAK activation and cell morphology indicative of changes in barrier properties. Most studies are performed in vitro using different endothelial cell lines, resulting in discrepant data and controversial interpretations regarding the specific effect of FAK on permeability. For example, several groups of investigators suggest that activation of FAK enhances endothelial barrier function. This is supported by a reciprocal relationship between FAK activity and monolayer permeability (Garcia et al. 2000; Harrington et al. 2005), and that expressing kinase-deficient mutant FAK blunts the barrier-strengthening effect of hyperosmolarity (Quadri et al. 2003). In contrast, activated FAK has been shown to contribute to endothelial barrier dysfunction caused by various pathological stimuli, including bacteria (Reddy et al. 2000), virus (Avraham et al. 2004), reactive oxygen species (Alexander et al. 2001), thrombin (Shikata et al. 2003; van Nieuw Amerogen et al. 2004), and the serine phosphatase inhibitor calyculin (Mucha et al. 2002). Consistent with these findings, studies combining the isolated venules and cultured endothelial cells from the same vessel have demonstrated a temporal correlation between FAK phosphorylation and hyperpermeability during stimulation by histamine and phorbol esters (Yuan et al. 1998). More importantly, transfection of FRNK into venular endothelium as an effective means of specifically competing with endogenous FAK for tyrosine kinase activity, attenuates the increases in permeability caused by VEGF and activated neutrophils (Wu et al. 2003; Guo et al. 2005). Because the studies are done in intact venules with a specific FAK inhibitor, the data clearly indicate the involvement of focal adhesions in the microvascular hyperpermeability response.
Possible explanations for the discrepant effects of FAK on barrier function include the heterogeneity of cell types, different approaches used to measure endothelial permeability, and different experimental conditions. Interestingly, both barrier enhancing and attenuating effects of FAK have been seen in the same experimental setting (Mehta et al. 2002). Subsequent studies show that FAK activation promotes barrier recovery after transient hyperpermeability responses (Mehta et al. 2002; Shikata et al. 2003; van Nieuw Amerogen et al. 2004). Given the dynamic nature of FAK activity, it is reasonable to believe that its barrier modulatory effect involves complex mechanisms varied by time and the chemical/physical states of the endothelium. Within this context, the constitutive activity of FAK and its associated integrin–matrix interactions may be considered an essential component of the barrier structure under the physiological conditions. Upon stimulation by pathological or inflammatory agents, the focal adhesion proteins can be further activated, and these may act in concert with other cellular responses, such as cell contraction and intercellular gap formation, in mediating the paracellular transport of fluid and macromolecules.
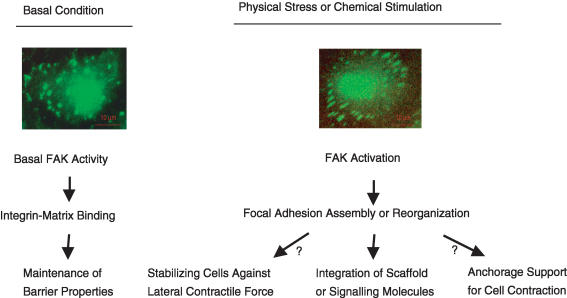
The molecular mechanisms by which focal adhesions contribute to the stimulated permeability response represent an area of active investigation. Several models have been proposed to explain the effect of focal adhesion formation on the barrier structure. Alexander et al. suggest that the increased adhesion of endothelial cells to the extracellular matrix may help to stabilize monolayers against detachment due to the lateral contractile forces produced by inflammatory mediators (Alexander & Elrod, 2002). Thus, focal adhesion activation may occur in parallel with cell contraction to compensate for the diminished cell–cell binding during inflammatory stimulation. Another hypothesis proposes that FAK activation and focal adhesion reorganization actively contribute to the opening of endothelial cell–cell junctions by providing a mechanical basis for endothelial cells to contract or change shape (Yuan, 2000). The last, but not the least, possibility is that the focal complex serves as a point of convergence for multiple scaffold proteins or signalling molecules to be integrated, which in turn affect the barrier function (Fig. 1).
Figure 1. A hypothetical model for the focal adhesion-dependent modulation of vascular endothelial barrier function.
Under basal conditions, the constitutive activity of focal adhesion kinase (FAK) and associated adhesive interactions of integrins with their matrix ligands provide a tethering force for the endothelial lining to be anchored to the extracellular matrix, which plays an essential role in the maintenance of microvascular barrier function. Upon stimulation by physical forces or chemical agents, FAK is activated, leading to focal adhesion assembly and/or reorganization. The resulting increase in cell–matrix adhesion may help stabilize the endothelial lining against detachment due to the lateral contractile forces produced by the stimuli. On the other hand, such a focal adhesion response may actively contribute to the opening of endothelial cell–cell junctions by providing a mechanical basis for cells to contract or change shape. More importantly, activated focal adhesions serve as a point of convergence for multiple scaffold proteins or signalling molecules to be integrated, which in turn affect the barrier function.
The signalling importance of focal adhesions is exemplified in the case of the VEGF-induced barrier response. This growth factor induces a rapid and transient increase in microvascular permeability, and the effect is initiated by its tyrosine kinase receptor KDR and culminated with activation of phospholipase C (PLC), protein kinase C (PKC), endothelial nitric oxide synthase (eNOS), mitogen-activated protein kinases (MAPKs), and the phosphoinositide 3-kinase (PI3K)-dependent PKB (protein kinase B) pathway (Wu et al. 1996, 1999; Bates et al. 2002; Breslin et al. 2002, Aramoto et al. 2004). All of these signalling molecules have been shown to directly or indirectly alter microvascular permeability (Kubes & Granger, 1992; He et al. 1997, Duran et al. 2000; Verma et al. 2002). Interestingly, molecular characterization of the KDR receptor reveals its direct association with focal adhesion complexes via integrin (αvβ3) binding (Soldi et al. 1999). Upon VEGF stimulation, FAK is quickly recruited to the αvβ3-coupled cell–matrix contact site, and the resulting focal adhesion assembly promotes a series of intracellular signalling reactions (Avraham et al. 2003). In addition to the well-characterized activation of MAPK, PI3K, and eNOS (Zachary & Gliki, 2001), potential signalling events downstream from FAK include the myosin light chain phosphorylation-triggered actin–myosin contraction and Rho-dependent stress fibre formation (Carragher & Frame, 2004), which are characteristic features of paracellular permeability.
Methodological aspects
Recent advances in imaging technologies enable direct visualization of focal adhesions at multiple dimensions with high temporal and spatial resolution (Kam et al. 2001). The development of microscopic techniques, such as fluorescence resonance energy transfer (FRET), fluorescence recovery after photo bleaching (FRAP), and total internal reflection fluorescence (TIRF), along with the availability of various fluorescently tagged biosensors, allow investigators to perform real-time analyses of focal adhesion dynamics and associated changes in cytoarchitecture (Webb et al. 2003). For quantitative assessment of the adhesive interaction between endothelial cells and matrix proteins, traction force microscopy (TFM) provides values that indicate the forces exerted by cells during spreading on RGD-polyacrylamide gel (Bershadsky et al. 2003; Reinhart-King et al. 2005), whereas atomic force microscopy (AFM) measures the binding strength between endothelial integrins and matrix protein-coated surface (Trache et al. 2005). More recently, Tsien, Newton and Chien's groups have developed several novel FRET-based, genetically encoded fluorescence reporters to monitor the spatio-temporal dynamics of cytoskeleton- or focal adhesion-associated molecules in living cells (Violin et al. 2003; Y Wang et al. 2005). For example, Src is known to be an important signalling molecule involved in various endothelial responses, including hyperpermeability, to growth factors and physical stress (Eliceiri et al. 2002; Yuan, 2003; Chien et al. 2005). In order to visualize the Src-mediated mechanotransduction, a genetically encoded reporter has been designed that consists of a cyan fluorescent protein (CFP), the Src substrate peptide, the SH2 domain, and a yellow fluorescent protein (YFP), with the CFP and YFP in a juxtaposition to yield a high FRET. Upon Src phosphorylation, the substrate peptide binds to the SH2 domain and separates YFP from CFP, thus decreasing the FRET. The specific application of this probe was tested in human umbilical vein endothelial cells subjected to mechanical stimulation via laser-tweezer traction on fibronectin-coated beads adhering to the cells. The focal pulling force caused a directional and long-range activation of Src which was abolished by disrupting actin filaments or microtubules. The study provides direct evidence that Src-mediated mechanotransduction is a dynamic process that directs signals via the cytoskeleton to spatial destinations.
Despite these exciting technological innovations, most of the studies relating to focal adhesions have been carried out in vitro using cultured cells grown on flat culture substrates or beads recovered by matrix proteins. Results derived from such simplified cell models may lead to misinterpretations of the dynamic properties of focal adhesion responses in the multidimensional environment as occurring under physiological conditions. Recently, in an attempt to mimic the in vivo situation more accurately, three-dimensional matrices and cultured tissue slices have been employed to analyse cell migration and focal adhesion responses (Yamada et al. 2003). For example, cryostat sections of embryo tissues from which cellular contents are extracted with alkaline detergent are used to provide a natural 3-D matrix for culturing fibroblasts. Not surprisingly, cells grown in such environment display morphological and motile characteristics significantly different from those seen in 2-D gels. It is suggested that both the matrix composition (collagen versus multiple matrix proteins) and configuration (3-D versus 2-D) affect the cell growth and behaviour. Interestingly, FAK in 3-D matrix adhesions is poorly phosphorylated at Tyr-397, indicating that the cells may have different signalling responses when in a 3-D environment. These initial findings signal the need for extensive work to validate the 3-D models in studying focal adhesion signalling.
With regard to the functional significance of focal adhesions in regulating endothelial barrier properties, commonly used approaches involve pharmacological antagonists and molecular manipulation or gene ablation. In many cases, the barrier property is evaluated by measuring the macromolecular flux across endothelial cell monolayers grown on the Transwell membrane, or by measuring transendothelial electrical resistance using an electric cell impedance sensor (ECIS). In parallel, the conformational characteristics of focal adhesions are revealed in separate dishes of cells under the same experimental conditions. Conclusions are often drawn based on a temporal correlation between changes in focal adhesion function and permeability response to a particular stimulation. Although a recent modification to the ECIS technique provides the capability to evaluate the relative contribution of cell–cell adhesions versus cell–matrix adhesions to the transendothelial resistance (Moy et al. 2000), most of the in vitro permeability assays are not able to discern these two processes. Furthermore, because the expression and organization of focal contacts are highly variable depending on the specific substrates present in culture, endothelial monolayers grown on artificial substratum containing limited matrix proteins may not represent the intact microvascular wall. Thus, large research efforts shall be directed to the development of more physiologically relevant models for in vitro analyses. The aforementioned 3-D matrix system may be of particular use for the quantification of endothelial cell–cell and cell–matrix interactions.
Currently used in vivo models of permeability include tissue clearance of colorimetric or radioactive tracers, microdialysis assay for tissue uptake of plasma proteins, as well as intravital microscopic quantification of macromolecular extravasation (van der Heyde, 2001; Clough & Church, 2002; Mayhan, 2003; Kim et al., 2000). The in vivo studies (van der Heyde, 2001) have been much appreciated because they yield physiologically relevant information on microvascular transport. On the other hand, the transvascular movement of tracer molecules is subject to the moment-to-moment changes in haemodynamics and hormonal or humoral activities, which may confound the effect of a factor on endothelial permeability. Furthermore, the lack of focal adhesion-specific antagonists, along with the technical difficulty involved in site-specific delivery of large molecular inhibitors into microvascular endothelium, imposes a great challenge to the investigation of focal adhesion-dependent microvascular permeability.
A few groups of investigators have used in situ models for measuring hydraulic conductivity, reflection coefficient, and solute permeability coefficient, parameters indicative of the convective and diffusive permeability. Huxley et al. have described a microscope densitometric technique for measuring the apparent permeability coefficient of fluorescently labelled solutes in perfused capillaries of frog mesentery (Huxley et al. 1987). Subsequently, Yuan et al. have developed a single isolated porcine coronary venule model for quantification of albumin permeability under defined intraluminal pressure and flow rate (Yuan et al. 1993). This technique has now been modified to assess microvascular permeability in different types of vessels from different species, such as the porcine coronary arteriole and rat mesenteric or skeletal muscle venules (Huang et al. 2003; J Wang et al. 2005). For measuring hydraulic conductivity, a modified Landis technique has been used in association with the intact cannulated mesenteric capillary preparation (He et al. 1997; Michel & Curry, 1999; Neal & Bates, 2002). Although these cannulated microvessel models have their own inherent limitations (e.g. potential damage of the endothelium during cannulation), they provide a unique means of quantitatively studying the barrier property of intact endothelium embedded in its native matrix. The utility of such in situ permeability assays has been further tested in an effort to examine focal adhesion-mediated microvascular permeability (Wu et al. 2003; Guo et al. 2005). Using the microvascular transfection technique developed in Yuan's laboratory (Tinsley et al. 2001), recombinant FRNK was delivered directly into the endothelium of isolated venules. The transfection resulted in a specific inhibition of FAK activation coupled with an attenuated venular permeability response to angiogenic and inflammatory factors. These studies establish the first line of evidence that directly points to the functional significance of focal adhesion signalling in the pathophysiological regulation of microvascular barrier properties.
In summary, integrin-linked endothelial cell–matrix adhesions are an essential component of the microvascular barrier structure. Under stimulated conditions, focal adhesions contribute to altered barrier function by acting as signal transducers and/or structural modulators. The effect, either barrier strengthening or weakening, varies depending on the nature of stimuli and the physical/chemical states of surrounding matrices, and the mechanism of action involves dynamic interactions with the cytoskeleton and cell–cell junctions. The precise molecular basis of focal adhesion-dependent changes in barrier function remains to be established. A successful identification of the underlying mechanisms will largely rely on the advancement of methodologies. In this regard, live cell imaging in combination with molecular manipulation via DNA/protein transfection, gene silencing or the transgene approaches will continue to provide excellent tools for the study of focal adhesions. Quantitative microscopic technologies that allow simultaneous measurements of focal adhesion dynamics and permeability in intact exchange microvessels represent a unique direction of future development.
Acknowledgments
This work was supported by National Institutes of Health grant R01 HL-73324.
References
- Albelda MS, Daise M, Levine EM, Buck CA. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989;83:1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat. 2002;200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JS, Zhu Y, Elrod JW, Alexander B, Coe L, Kalogeris TJ, Fuseler J. Reciprocal regulation of endothelial substrate adhesion and barrier function. Microcirculation. 2001;8:389–401. doi: 10.1038/sj/mn/7800111. [DOI] [PubMed] [Google Scholar]
- Aramoto H, Breslin JW, Pappas PJ, Hobson RW, Duran WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol Heart Circ Physiol. 2004;287:H1590–H1598. doi: 10.1152/ajpheart.00767.2003. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Lee TH, Prakash O, Avraham S. HIV-1 Tat-mediated effects on focal adhesion assembly and permeability in brain microvascular endothelial cells. J Immunol. 2004;173:6228–6233. doi: 10.4049/jimmunol.173.10.6228. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Lee TH, Koh Y, Kim TA, Jiang S, Sussman M, Samarel AM, Avraham S. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J Biol Chem. 2003;278:36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–597. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2002;284:H92–H100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Plow EF. Activation of avb3 on vascular cells controls recognition of prothrombin. J Cell Biol. 1998;143:2081–2092. doi: 10.1083/jcb.143.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher NO, Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Cluman RI, Enenstein J, Waleh H, Pytela R, Kramer RH. The integrin complex αvβ3 participates in the adhesion of microvascular endothelial cells to fibronectin. Exp Cell Res. 1991;194:69–77. doi: 10.1016/0014-4827(91)90131-d. [DOI] [PubMed] [Google Scholar]
- Chien S, Li S, Shiu YT, Li YS. Molecular basis of mechanical modulation of endothelial cell migration. Front Biosci. 2005;10:1985–2000. doi: 10.2741/1673. [DOI] [PubMed] [Google Scholar]
- Clough GF, Church MK. Vascular responses in the skin: an accessible model of inflammation. News Physiol Sci. 2002;17:170–174. doi: 10.1152/nips.01378.2001. [DOI] [PubMed] [Google Scholar]
- Curtis TM, McKeown-Longo PJ, Vincent PA, Homan SM, Wheatley EM, Saba TM. Fibronectin attenuates increased endothelial monolayer permeability after RGD peptide, anti-α5β1, or TNF-α exposure. Am J Physiol. 1995;269:L248–L260. doi: 10.1152/ajplung.1995.269.2.L248. [DOI] [PubMed] [Google Scholar]
- Davies PF, Robotewskyj A, Griem ML. Quantitative studies of endothelial cell adhesion: directional remodeling of focal adhesion sites in response to flow forces. J Clin Invest. 1994;93:2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Lampugnani MG, Giorgi M, Gaboli M, Marchisio PC. Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor. Blood. 1990;75:1509–1517. [PubMed] [Google Scholar]
- Duran WN, Seyama A, Yoshimura K, Gonzalez DR, Jara PI, Figueroa XF, Boric MP. Stimulation of NO production and of eNOS phosphorylation in the microcirculation in vivo. Microvasc Res. 2000;60:104–111. doi: 10.1006/mvre.2000.2250. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin αvβ5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–159. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JGN, Schaphorst KL, Verin AD, Vepa S, Patterson CE, Natarajan V. Diperoxovanadate alters endothelial cell focal contacts and barrier function: role of tryrosine phosphorylation. J Appl Physiol. 2000;89:2333–2343. doi: 10.1152/jappl.2000.89.6.2333. [DOI] [PubMed] [Google Scholar]
- Guo M, Wu MH, Granger HJ, Yuan SY. Focal adhesion kinase in neutrophil-induced microvascular hyperpermeability. Microcirculation. 2005;12:223–232. doi: 10.1080/10739680590905251. [DOI] [PubMed] [Google Scholar]
- Harrington EO, Shannon CJ, Morin N, Rowlett H, Murphy C, Lu Q. PKCδ regulates endothelial basal barrier function through modulation of RhoA GTPase activity. Exp Cell Res. 2005;308:407–421. doi: 10.1016/j.yexcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- He P, Zeng M, Curry FE. Effect of nitric oxide inhibitors on basal microvessel permeability and endothelial cell [Ca2+]i. Am J Physiol. 1997;273:H747–H755. doi: 10.1152/ajpheart.1997.273.2.H747. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314:131–144. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- Huang Q, Xu W, Ustinova EE, Wu MH, Childs E, Hunter F, Yuan SY. Myosin light chain kinase-dependent microvascular hyperpermeability in thermal injury. Shock. 2003;20:363–368. doi: 10.1097/01.shk.0000079425.0000.db. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol. 1987;252:H188–H197. doi: 10.1152/ajpheart.1987.252.1.H188. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Kam Z, Zamir E, Geiger B. Probing molecular processes in live cells by quantitative multidimensional microscopy. Trends Cell Biol. 2001;11:329–334. doi: 10.1016/s0962-8924(01)02067-0. [DOI] [PubMed] [Google Scholar]
- Kim DD, Ramirez MM, Duran WN. Platlet-acturating factor modulates microvascular dynamics through phospholipase C in the hamster cheek pouch. Microvasc Res. 2000;59:7–13. doi: 10.1006/mvre.1999.2195. [DOI] [PubMed] [Google Scholar]
- Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas FW, Lawler J. Integrins as dynamic regulators of vascular function. FASEB J. 1994;8:929–938. doi: 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Pial vessel permeability to tracers using cranial windows. Meth Mol Med. 2003;89:121–131. doi: 10.1385/1-59259-419-0:121. [DOI] [PubMed] [Google Scholar]
- Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol. 2002;539:779–789. doi: 10.1113/jphysiol.2001.013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- Moy AB, Winter M, Kamath A, Blackwell K, Reyes G, Giaever I, Keese C, Shasby DM. Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am J Physiol Lung Cell Mol Physiol. 2000;278:L888–L898. doi: 10.1152/ajplung.2000.278.5.L888. [DOI] [PubMed] [Google Scholar]
- Mucha DR, Myers CL, Schaeffer RC. Endothelial contraction and monolayer hyperpermeability are regulated by Src kinase. Am J Physiol Heart Circ Physiol. 2002;284:H994–H1002. doi: 10.1152/ajpheart.00862.2002. [DOI] [PubMed] [Google Scholar]
- Neal CR, Bates DO. Measurement of hydraulic conductivity of single perfused Rana mesenteric microvessels between periods of controlled shear stress. J Physiol. 2002;543:947–957. doi: 10.1113/jphysiol.2002.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Petit V, Thiery JP. Focal adhesion: structure and dynamics. Biol Cell. 2000;92:477–494. doi: 10.1016/s0248-4900(00)01101-1. [DOI] [PubMed] [Google Scholar]
- Qiao R, Yan W, Lum H, Malik AB. Arg-gly-asp peptide increases endothelial hydraulic conductivity: comparison with thrombin response. Am J Physiol. 1995;269:C110–C117. doi: 10.1152/ajpcell.1995.269.1.C110. [DOI] [PubMed] [Google Scholar]
- Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem. 2003;278:13342–13349. doi: 10.1074/jbc.M209922200. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Wass CA, Kim KS, Schlaepfer DD, Prasadarao NV. Involvement of focal adhesion kinase in Escherichia coli invasion of human brain microvascular endothelial cells. Infect Immun. 2000;68:6423–6430. doi: 10.1128/iai.68.11.6423-6430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89:676–689. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochem Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochem Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JGN. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17:2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JH, Zawieja DC, Ustinova EE, Wu MH, Xu W, Yuan SY. Protein transfection of intact microvessels specifically modulates vasoreactivity and permeability. J Vas Res. 2001;38:444–452. doi: 10.1159/000051077. [DOI] [PubMed] [Google Scholar]
- Trache A, Trzeciakowski JP, Reeves L, Sun Z, Muthuchamy M, Guo M, Yuan SY, Meininger GA. Histamine effects on endothelial cell fibronectin interaction studied by atomic force microscopy. Biophys J. 2005;89:2888–2898. doi: 10.1529/biophysj.104.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyde HC, Bauer P, Sun G, Chang WL, Yin L, Fuseler J, Granger DN. Assessing vascular permeability during experimental cerebral malaria by a radiolabeled monoclonal antibody technique. Infect Immun. 2001;69:3460–3465. doi: 10.1128/IAI.69.5.3460-3465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Natarajan K, Yin G, Hoefen RJ, Osawa M, Haendeler J, Ridley AJ, Fujiwara K, van Hinsbergh VW, Berk BC. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res. 2004;94:1041–1049. doi: 10.1161/01.RES.0000125627.77235.0C. [DOI] [PubMed] [Google Scholar]
- Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW, 2nd, Duran WN. p42/44 MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res. 2002;63:172–178. doi: 10.1006/mvre.2001.2381. [DOI] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Whitt SP, Rubin LJ, Huxley VH. Differential coronary microvascular exchange responses to adenosine: role of receptor and microvessel subtypes. Microcirculation. 2005;12:313–326. doi: 10.1080/10739680590934736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Brown CM, Horwitz AF. Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr Opin Cell Biol. 2003;15:614–620. doi: 10.1016/s0955-0674(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Wheatley EM, McKeown-Longo PJ, Vincent PA, Saba TM. Incorporation of fibronectin into matrix decreases TNF-induced increase in endothelial monolayer permeability. Am J Physiol. 1993;265:L148–L157. doi: 10.1152/ajplung.1993.265.2.L148. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wu H, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- Wu MH, Guo M, Yuan SY, Granger HJ. Focal adhesion kinase contributes to VEGF-elicited microvascular hyperpermeability. J Physiol. 2003;552:691–699. doi: 10.1113/jphysiol.2003.048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Ustinova E, Granger HJ. Integrins binding to fibronectin and vitronectin maintains the barrier function of isolated porcine coronary venules. J Physiol. 2001;532:785–791. doi: 10.1111/j.1469-7793.2001.0785e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Yuan Y, Zawieja DC, Tinsley JH, Granger HJ. Role of phospholipase C, protein kinase C and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Pankov R, Cukierman E. Dimensions and dynamics in integrin function. Braz J Med Biol Res. 2003;36:959–966. doi: 10.1590/s0100-879x2003000800001. [DOI] [PubMed] [Google Scholar]
- Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]
- Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vas Pharmacol. 2003;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Granger HJ, Chilian WM, Zawieja DC. Permeability to albumin in isolated coronary venules. Am J Physiol. 1993;265:H543. doi: 10.1152/ajpheart.1993.265.2.H543. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Meng F, Huang Q, Hawker J, Wu HM. Tyrosine phosphorylation of paxillin and pp125FAK and endothelial barrier function. Am J Physiol. 1998;275:H84–H93. doi: 10.1152/ajpheart.1998.275.1.H84. [DOI] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovas Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]