Abstract
Exogenous cannabinoids have been shown to significantly alter neuroendocrine output, presaging the emergence of endogenous cannabinoids as important signalling molecules in the neuroendocrine control of homeostatic and reproductive functions, including the stress response, energy metabolism and gonadal regulation. We showed recently that magnocellular and parvocellular neuroendocrine cells of the hypothalamic paraventricular nucleus and supraoptic nucleus (SON) respond to glucocorticoids by releasing endocannabinoids as retrograde messengers to modulate the synaptic release of glutamate. Here we show directly for the first time that both of the main endocannabinoids, anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), are released in an activity-dependent fashion from the soma/dendrites of SON magnocellular neurones and suppress synaptic glutamate release and postsynaptic spiking. Cannabinoid reuptake blockade increases activity-dependent endocannabinoid levels in the region of the SON, and results in the inhibition of synaptically driven spiking activity in magnocellular neurones. Together, these findings demonstrate an activity-dependent release of AEA and 2-AG that leads to the suppression of glutamate release and that is capable of shaping spiking activity in magnocellular neurones. This activity-dependent regulation of excitatory synaptic input by endocannabinoids may play a role in determining spiking patterns characteristic of magnocellular neurones under stimulated conditions.
Cannabinoids have robust effects on hormone secretion from the pituitary gland, and a major site of cannabinoid action responsible for these effects appears to be at the level of the hypophysiotropic cells in the hypothalamus (Tyrey & Murphy, 1984). The main effect of cannabinoids on anterior pituitary hormone secretion is inhibitory, suppressing, for example, thyroid-stimulating hormone, gonadotropin, growth hormone and prolactin secretion (Murphy et al. 1998; Wenger & Moldrich, 2002). An exception to this is the excitatory effect on the hypothalamic–pituitary–adrenal (HPA) axis, where exogenous cannabinoids cause an increase in adrenocorticotropin and corticosteroid secretion (Puder et al. 1982; Wenger et al. 1997; Brown & Dobs, 2002), although this appears to be caused by excitatory cannabinoid actions upstream from the hypothalamus (Puder et al. 1982) and is not mediated by CB1 receptor activation (Wenger et al. 2003). Indeed, we have recently shown that cannabinoids inhibit glutamate release onto hypothalamic paraventricular nucleus parvocellular neuroendocrine cells via CB1 receptor activation (Di et al. 2003), suggesting a broad inhibitory influence of cannabinoids on PVN hypophysiotropic cell function.
Exogenous cannabinoids have also been shown to exert a robust inhibitory effect on posterior pituitary hormone secretion. Marijuana consumption in humans or application of the active cannabinoid in marijuana, Δ9-tetrahydrocannabinol (THC), in rats results in increased diuresis, which is thought to be mediated by central inhibitory cannabinoid actions on vasopressin release (Ames, 1958; Sofia et al. 1977). THC in lactating rats blocked suckling-induced milk ejection mediated by the secretion of oxytocin (Tyrey & Murphy, 1988). It was shown recently that endogenous cannabinoids are released as retrograde messengers in the hypothalamic supraoptic nucleus (SON) by magnocellular neurones and that they suppress synaptic glutamate release (Hirasawa et al. 2004; Di et al. 2005).
Endocannabinoids regulate synaptic transmission by serving as retrograde messengers at diverse synapses in the central nervous system (Kreitzer & Regehr, 2001; Wilson & Nicoll, 2001; Alger, 2002). Endocannabinoids bind to CB1 receptors on presynaptic axon terminals, where they lead to the suppression of GABA and glutamate release (Davies et al. 2002; Freund et al. 2003; Iversen, 2003). Interestingly, the retrograde release of endocannabinoids can be stimulated by a variety of different signalling mechanisms, including via G protein-coupled receptor activation (e.g. metabotropic glutamate and muscarinic receptors; Maejima et al. 2001a; Varma et al. 2001; Kim et al. 2002), by rapid steroid actions (Di et al. 2003, 2005), and by activity-dependent mechanisms (Maejima et al. 2001b; Wilson & Nicoll, 2001; Brown et al. 2003). Although it has not been shown directly, the activity-dependent release of endocannabinoids has been inferred from in vitro pharmacological studies in which the stimulation of postsynaptic neurones leads to a reduction of the release of either GABA or glutamate from presynaptic terminals, referred to, respectively, as depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE), and this is blocked by cannabinoid receptor antagonists (Alger, 2002).
A recent study reported a form of DSE in magnocellular neurones of the supraoptic nucleus that was facilitated by oxytocin release (Hirasawa et al. 2004). Here we provide direct biochemical evidence for the activity-dependent release of the endogenous cannabinoids anandamide (AEA) and arachidonoylglycerol (2-AG) from hypothalamic neurones, and demonstrate that endo-genously released endocannabinoids can modulate postsynaptic spiking activity in hypothalamic magno-cellular neurones by suppressing presynaptic glutamate release. We suggest that this activity-dependent release of endocannabinoids may represent a mechanism by which characteristic spiking patterns are determined in oxytocin- and vasopressin-secreting magnocellular neurones.
Methods
Slice preparation
Male Sprague-Dawley rats (3–5 weeks, Charles River, Wilmington, MA, USA) were used according to a protocol approved by the Tulane University Institutional Animal Care and Use Committee. Rats were decapitated under pentobarbital sodium anaesthesia (50 mg (kg bw)−1). The brain was immersed in a 1–2°C, oxygenated artificial cerebral spinal fluid (aCSF) composed of (mm): 140 NaCl, 3 KCl, 1.3 MgSO4, 1.4 NaH2PO4, 2.4 CaCl2, 11 glucose, 5 Hepes; the pH was adjusted to 7.2–7.3 with NaOH. The hypothalamus was blocked and two coronal hypothalamic slices (400 μm) containing the SON were sectioned, bisected along the midline and submerged in a holding chamber in oxygenated aCSF at room temperature, where they were allowed to equilibrate for 1–2 h before the first slice was transferred to the recording chamber.
Electrophysiological methods
Patch pipettes were pulled from borosilicate glass (1.65 mm o.d., 1.2 mm i.d.; KG33; Garner Glass, Claremont, CA, USA) with a Flaming/Brown P-97 micropipette puller (Sutter Instruments, Novato, CA, USA) to a resistance of 3–6 MΩ. The pipette solution contained (mm): 120 potassium gluconate, 10 KCl, 1 NaCl, 1 MgCl2, 1 EGTA, 2 Mg-ATP, 0.3 Na-GTP, 10 Hepes; the pH was adjusted to 7.3 with KOH and the osmolarity was adjusted to 290–300 mosmol l−1 with 20 mmd-sorbitol.
Hemi-slices were transferred one at a time to either a submerged or an interface recording chamber and allowed to equilibrate for at least 15 min prior to recordings. In some experiments, SON magnocellular neurones were visualized directly in submerged slices and targeted for recordings using a cooled CCD camera, infrared illumination and differential interference contrast optics; in other experiments, the magnocellular neurones were recorded using the ‘blind’ technique in an interface recording chamber. Series resistance and whole-cell capacitance were adjusted and monitored continuously during experiments. One cell from each slice was recorded in voltage clamp and/or current clamp using an Axopatch 1-D or a Multiclamp 700A amplifier (Molecular Probes, Sunnyvale, CA, USA) and the recording was monitored continuously on a digital storage oscilloscope (Hitachi, Tokyo, Japan). Data were low-pass filtered at 2 kHz, converted to digital video format at 22 kHz (Neuro-Corder, NeuroData Instruments, New York), and stored on videotape for off-line analysis. Selected data were subsequently digitized at 4 kHz and recorded on a personal computer using the Digidata 1200 interface and pCLAMP 9.0 software (Molecular Probes). The electrode liquid junction potential was 11 mV and was compensated for following recordings (Neher, 1992).
Whole-cell recordings of miniature excitatory postsynaptic currents (mEPSCs) were performed in voltage clamp mode at a holding potential of −60 mV in the presence of tetrodotoxin (TTX, 1 μm) at room temperature or at 32–34°C. Miniature EPSCs were confirmed to be mediated by glutamate release by blocking them with the ionotropic glutamate receptor antagonists, AP-5 (50 μm) and DNQX (30 μm, n = 4), but not with the GABAA receptor antagonist bicuculline methiodide (30 μm, n = 5). Whole-cell recordings of action potential firing were performed in current clamp mode. Data were collected after a minimum of 10 min of recording during which a stable baseline amplitude and frequency of mEPSCs, or frequency of continuous spiking activity, were established in control conditions. Spiking activity and mEPSCs were analysed by comparing the data during a 3 min control period just prior to drug application with the data during the last 3 min of a 10-min drug application using Minianalysis 5.0 (Synaptosoft Inc., Decatur, GA, USA).
Evoked synaptic responses were elicited using a bipolar stimulating electrode (75 μm diameter) placed dorso-medial to the SON (0.2 mA, 0.1–0.5 ms, 0.33 Hz). To isolate evoked EPSCs, stimulation experiments were performed in the presence of bicuculline methiodide (30 μm) to eliminate GABAA receptor-mediated events. Evoked EPSCs were also confirmed as glutamatergic synaptic responses by their blockade with the ionotropic glutamate receptor antagonists AP-5 (50 μm) and DNQX (30 μm) (n = 3).
In order to study the effect of activity-dependent endocannabinoid release on action potential firing, it was necessary to trigger repetitive spiking activity by glutamatergic synaptic inputs. We accomplished this (1) by depolarizing the cells to a just-subthreshold membrane potential with direct current injection and (2) by increasing synaptic glutamate release by one or more of three methods: increase of extra-cellular potassium concentration to 6–8 mm, increase of extracellular osmolarity to ∼340 mosmol l−1 (Bourque, 1998), and/or application of 10 μm noradrenaline or 5 μm phenylephrine in the bath perfusion (Boudaba et al. 2003). Because of the predominantly presynaptic action of endo-cannabinoids (Hirasawa et al. 2004; Di et al. 2005), it was determined empirically in these experiments that the threshold for spike generation was reached not by direct depolarization of the magnocellular neurones via a postsynaptic mechanism, but by glutamatergic synaptic inputs.
Drug application
The cannabinoid agonist WIN 55,212–2, antagonist AM 251, and transporter blockers AM 404 and OMDM-2 (Tocris) were stored as 10-mm stock solutions in DMSO at −20°C and were dissolved to their final concentrations in aCSF before bath application. The endogenous cannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (Tocris) were prepared and applied under low-light conditions due to their photosensitivity. The DMSO and the standard plain emulsion (Tocris) used to dissolve AEA were tested and had no effect on mEPSCs at the concentrations used.
Quantitative analysis of endocannabinoids
Hypothalamic hemi-slices containing the SON were prepared identically to those described above for whole cell recordings, except that the hemi-slices were trimmed ∼1 mm dorsal to the SON and ∼1 mm lateral to the optic chiasm. Following equilibration in an oxygenated holding chamber (see above), two hemi-slices from each rat were placed in an interface chamber and perfused with oxygenated, heated aCSF (32–34°C). High frequency stimulation (HFS) was delivered by a bipolar electrode dorsal–lateral to the SON, and consisted of two 1-s trains (100Hz, 0.6 mA) applied 20 s apart (Stella et al. 1997). The two hemi-slices were treated consecutively. The control hemi-slices were sham stimulated, with the electrode placed dorso-lateral to the SON without passing current. Immediately following stimulation, the slices were collected and homogenized in 1 ml of ice-cold methanol in preparation for liquid chromatography–tanden mass spectrometry (LC-MS-MS) analysis. Two hemi-slices and two sham-stimulated hemi-slices from each animal were pooled separately, providing ∼0.7–0.8 mg of total protein in each of the two samples, and each pool of two hemi-slices served as a single data point such that paired control and experimental samples were obtained from the same brains.
LC-MS-MS analysis of the endocannabinoids anandamide (AEA) and 2-arachadonoyl glycerol (2-AG) was performed on chloroform methanol (2: 1) lipid extracts, which were loaded with deuterated standard mixture (AEA-d8, 2-AG-d8), and purified by SPE extraction on C18 columns (Varian, Walnut Creek, CA, USA). Samples were eluted with 10 ml of 1% methanol in ethyl acetate (EM Science) and concentrated on a N2 stream evaporator prior to LC-MS-MS analysis. Samples were loaded on a Biobasic-AX column (100 mm × 2.1 mm, 5 μm particle size; Thermo-Hipersyl-Keystone, Bellefonte, PA, USA). The column was run with a 45-min gradient protocol starting with solvent solution A (40: 60: 0.01 methanol–water–acetic acid, pH 4.5) at a flow rate of 300 μl min−1, reached 100% of solvent B (99.99: 0.01 methanol–acetic acid) in 30 min, and run isocratic for 5 min, after which the system returned to 100% solvent solution A in 10 min. LC effluents were diverted to an electro-spray-ionization probe (ESI) on a TSQ Quantum triple quadropole mass spectrometer (Thermo-Finnigan, San Jose, CA, USA) running on negative ion detection mode. Electro-spray voltage was 3 kV; sheath gas was argon at 1.5 mTorr. The instrument runs on full scan mode to detect MS2-spectra and selected reaction mode for quantitative analysis to detect parent/daughter ion pairs simultaneously. The selected parent/daughter ion pairs were 346.3/259.3, 377.2/285.2, 353.2/266.3 and 385.4/310.2 m/z for AEA, 2-AG, AEA-d8, and 2-AG-d8, respectively.
Data analysis
All data are expressed as means ± standard error of the mean. Statistical comparisons of electrophysiological data were performed using Student's paired t test for within-cell comparisons and unpaired t test for between-group comparisons. For comparison of the LC-MS-MS, data were analysed statistically using a paired t test to compare endocannabinoid levels in pooled stimulated and unstimulated SON hemi-slices from the same animals; ‘n’ values are number of animals tested. Probability values of < 0.05 were considered significant for all comparisons.
Results
Whole-cell patch clamp recordings were performed in neurones located in the SON in acutely prepared hypothalamic slices. A total of 68 putative magnocellular neurones identified based on their location in the SON and on their electrical properties (Tasker & Dudek, 1991; Armstrong & Stern, 1997) were recorded in slices from 52 rats.
Cannabinoid inhibition of glutamate release
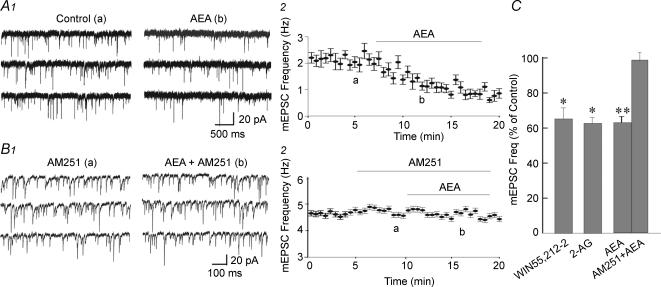
The cannabinoids AEA, 2-AG and WIN55,212-2 applied in the bath perfusion had no effect on the input resistance or holding current, but suppressed glutamatergic synaptic activity in magnocellular neurones. At a holding potential of −60 mV, the endocannabinoid AEA (0.5 μm) caused a 37.5% decrease in the frequency of mEPSCs (from 2.7 ± 0.6 to 1.8 ± 0.6 Hz; n = 5; P < 0.01) (Fig. 1A), without affecting either mEPSC amplitude (21.3 ± 2.6 versus 21.8 ± 2.7 pA; P = 0.66) or decay time (peak to 63% decay: 2.9 ± 0.4 versus 2.8 ± 0.6 ms; P = 0.83). This effect was blocked by prior bath application of the type I cannabinoid receptor (CB1) antagonist, AM 251 (1 μm, n = 4) (Fig. 1B); AM 251 alone had no effect on input resistance, holding current, or mEPSC frequency. AEA (0.5 μm) applied in the bath perfusion also abolished induced spiking activity (see Methods) in 4 of 5 magnocellular neurones tested (data not shown).
Figure 1. Effect of cannabinoids on glutamate release.
A, sample traces (1) and a running average plot of mEPSC frequency (2) from a SON magnocellular neurone showing an AEA-mediated decrease in mEPSC frequency. B, sample traces (1) and a running average plot of mEPSC frequency (2) from another magnocellular neurone showing that the CB1 receptor antagonist AM251 (1 μm) blocked the AEA-induced suppression of mEPSCs. Letter designations a and b in the running average plots correspond to traces denoted by a and b; all data points in the running average plots represent a mean ± s.e.m. of 30 s of continuous events. C, mean changes in the average mEPSC frequencies caused by the synthetic cannabinoid WIN55,212-2 (1 μm, n = 8) and the endogenous cannabinoids 2-AG (0.5–1 μm, n = 5) and AEA (0.5 μm, n = 5). The AEA effect on mEPSC frequency was blocked by AM251 (1 μm, n = 4). *P < 0.05; *P < 0.01.
The other major endocannabinoid, 2-AG (0.5–1 μm), had a similar inhibitory effect on mEPSCs, causing a 40% decrease in the mEPSC frequency (from 2.0 ± 0.5 to 1.2 ± 0.4 Hz; P < 0.05; n = 5) (Fig. 1C), without altering either the mEPSC amplitude (26.2 ± 7.6 versus 25.1 ± 7.2 pA; P = 0.63; n = 5) or decay time (2.6 ± 0.4 versus 2.7 ± 0.4 ms; P = 0.48; n = 5). Similarly, the high-affinity synthetic cannabinoid agonist WIN55,212-2 (1 μm) also caused a 36% decrease in mEPSC frequency (2.1 ± 0.5 versus 1.5 ± 0.4 Hz; P < 0.05; n = 8) (Fig. 1C), but had no effect on mEPSC amplitude (20.0 ± 3.9 versus 20.1 ± 3.9 pA; P = 0.9; n = 8) or decay time (2.4 ± 0.03 versus 2.4 ± 0.2 ms; P = 0.8; n = 8). The inhibitory effect of the cannabinoids on mEPSC frequency did not reverse after 20–30 min washout of the drugs.
Activity-dependent endocannabinoid release
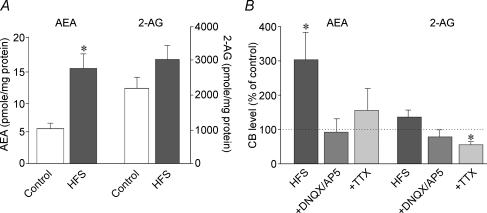
Mass spectrometric analysis of activity-dependent changes in the levels of the endocannabinoids AEA and 2-AG was undertaken in trimmed hypothalamic slices containing the SON (see Methods). High frequency extracellular stimulation (HFS: 2 × 100 Hz, 0.6 mA, 1 s at an interval of 20 s) elicited a significant 205% increase in AEA levels, from 6.8 ± 1.2 to 15.7 ± 2.9 pmol (mg protein)−1 (P < 0.05; n = 15) and a non-significant 35% increase in 2-AG levels, from 2287.9 ± 286.7 to 2925.8 ± 424.0 pmol (mg protein)−1 (P = 0.12; n = 15) (Fig. 2A). The increase in AEA in response to HFS was abolished in a perfusion medium containing the ionotropic glutamate receptor antagonists DNQX (20 μm) and AP5 (50 μm) (from 7.1 ± 1.8 to 5.0 ± 1.5 pmol (mg protein)−1; P = 0.38; n = 6) or the sodium channel blocker TTX (1 μm) (from 6.6 ± 1.7 to 7.5 ± 1.1 pmol (mg protein)−1; P = 0.74; n = 4) (Fig. 2B). The levels of 2-AG following HFS were also reduced in DNQX/AP5 and in TTX (Fig. 2B).
Figure 2. Activity-dependent increase in endocannabinoid levels.
A, high frequency stimulation (HFS) caused a significant increase in AEA (P < 0.05, n = 15) and a non-significant increase in 2-AG (n = 15) in a pairwise LC-MS-MS analysis of endocannabinoid levels in trimmed hypothalamic slices. B, the same analysis in the presence of the ionotropic glutamate receptor antagonists DNQX and AP5 (n = 6) and the sodium channel blocker TTX (n = 4) showed that blocking glutamate neurotransmission or action potentials reversed the HFS-induced increase in AEA and 2-AG.
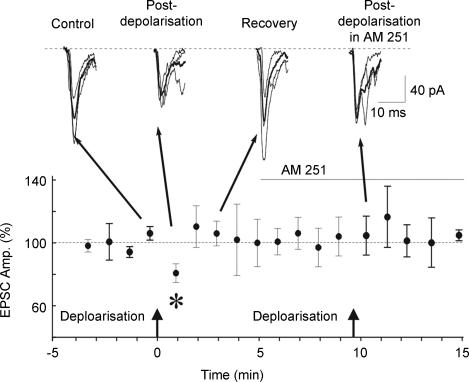
EPSCs were evoked in SON neurones by electrical stimulation (0.2 mA, 0.1 ms, 0.33 Hz) in the presence of the GABAA receptor antagonist bicuculline methiodide (30 μm) and averaged over 1 min epochs (n = 3 responses/epoch). After obtaining a stable baseline evoked response, a train of 100 ms voltage steps to 0 mV (2–4 Hz for 10 s) was delivered to the postsynaptic neurone. This caused a transient decrease in the amplitude of the evoked EPSCs, or DSE, in the first minute following stimulation in 5 of 5 magnocellular neurones tested, with reductions ranging from 7% to 35% (18.4 ± 5.1%; P < 0.05) (Fig. 3). The DSE reversed completely within 2–3 min following the postsynaptic depolarization, and could be elicited repeatedly during a given recording (data not shown). The DSE was completely abrogated by application during the depolarization protocol of the CB1 antagonist AM251 (1 μm) – the EPSC amplitude following postsynaptic stimulation was 105.0 ± 8.4% of its prestimulation control value (n = 4) (Fig. 3).
Figure 3. Depolarization-induced retrograde endocannabinoid release.
Depolarisation of a postsynaptic magnocellular neurone (arrows, 100 ms voltage steps to 0 mV at 3 Hz for 10 s) caused a transient 20% decrease in the amplitude of the EPSC evoked by electrical stimulation, or DSE, which was blocked by AM251 (1 μm). EPSCs are averages (black traces) of 3 evoked responses (grey traces) over 1 min; baseline (dashed line, 100%) represents the average of all responses over the 4-min period prior to depolarization (n = 12). *P < 0.05.
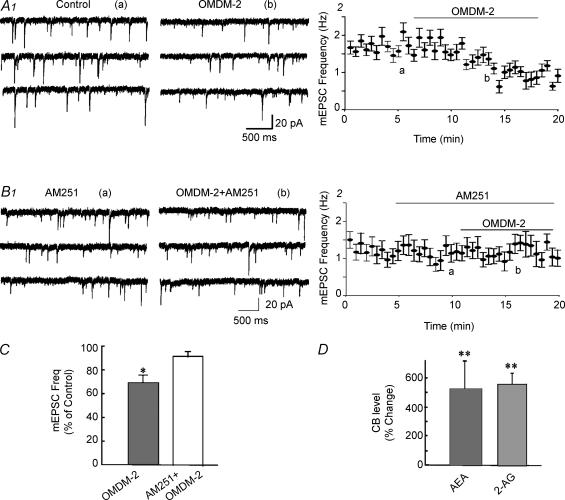
If endocannabinoids are released in an activity-dependent manner and if the extracellular endo-cannabinoid levels are controlled by transporter-mediated cannabinoid reuptake, then blocking cannabinoid transport should augment extracellular endocannabinoid levels and enhance the presynaptic endocannabinoid effects. Bath application of the cannabinoid transporter blocker OMDM-2 (5 μm) had no significant effect on the mean input resistance or the holding current of recorded neurones, but caused a 31% decrease in mEPSC frequency (0.9 ± 0.2 versus 0.6 ± 0.2 Hz; n = 5; P < 0.05), which was blocked by the CB1 receptor antagonist AM251 (1 μm; n = 4) (Fig. 4A, B and C). Extracellular HFS in the presence of the cannabinoid transporter blocker AM404 (1 μm) caused endocannabinoid levels measured by LC-MS-MS to increase approximately 500% compared to control, unstimulated levels (Fig. 4D), resulting in significant changes in both AEA and 2-AG. Thus, AEA rose from 4.8 ± 0.8 pmol (mg protein)−1 in control slices to 27.1 ± 7.3 pmol (mg protein)−1 in stimulated slices (P < 0.01, n = 5), and 2-AG levels rose from 1902.4 ± 419.6 to 11890.6 ± 2329.6 pmol (mg protein)−1 (P < 0.01, n = 5) (Fig. 4). The increase in endocannabinoids elicited by HFS was significantly higher in the presence than in the absence of AM 404 for both AEA (P < 0.05) and 2-AG (P < 0.01).
Figure 4. Blockade of cannabinoid reuptake increased endocannabinoid levels.
A, continuous recording of mEPSCs (1) and running average plot of mEPSC frequency (2) from the same SON magnocellular neurone showing that the cannabinoid transporter blocker OMDM-2 (5 μm) caused a decrease in mEPSC frequency. B, continuous recording of mEPSCs (1) and running average plot of mEPSC frequency (2) from another SON neurone showing blockade of the OMDM-2-induced suppression of mEPSCs by the CB1 antagonist AM251 (1 μm). C, average changes in the mean mEPSC frequency caused by OMDM-2 (n = 5) and OMDM-2 in AM251 (n = 5). D, HFS of trimmed SON slices in the presence of the cannabinoid transporter blocker AM404 (1 μm) elicited a robust increase in the levels of both AEA and 2-AG compared to controls, measured by LC-MS-MS (n = 5). *P < 0.05; **P < 0.01.
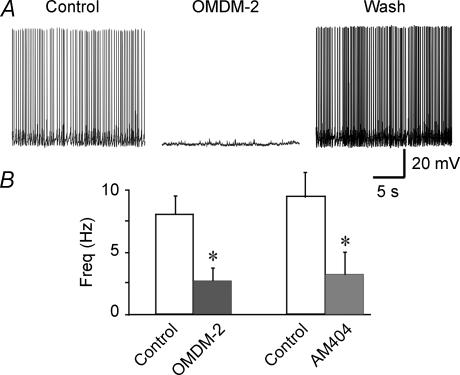
We also tested the effect of cannabinoid reuptake blockers on induced spiking activity in SON magnocellular neurones (see Methods). Consistent with the inhibitory effects of endocannabinoids on EPSCs and induced spiking activity, bath application of the cannabinoid transporter blockers AM404 (1–2 μm) and OMDM-2 (5 μm) abolished (n = 13) or reduced (n = 3) spiking activity in 16 of 20 magnocellular neurones tested (Fig. 5). The mean spike frequencies of all the neurones tested with AM404 diminished by 65%, from 9.5 ± 2.0 to 3.4 ± 1.7 Hz (n = 7; P < 0.05), and the mean frequencies of neurones tested with OMDM-2 diminished by 68%, from 8.0 ± 1.4 to 2.5 ± 1.1 Hz (n = 13; P < 0.05) (Fig. 5B). This effect was reversed in 7 of the 16 responsive neurones with washout of the inhibitors. These findings indicate a tonic release of endocannabinoids in the SON and suggest that endo-cannabinoid release is capable of altering spiking patterns in magnocellular neurones.
Figure 5. Blockade of cannabinoid uptake modulates spiking activity in SON magnocellular neurones.
A, bath application of the cannabinoid transporter blocker OMDM-2 (5 μm) abolished the spiking activity induced in a SON neurone by DC injection (9 pA) and high-osmolarity aCSF (340 mosmol l−1). The spiking activity recovered after ∼30 min of washout of the OMDM-2. B, average changes in the mean firing frequency of SON neurones caused by the cannabinoid transporter blockers OMDM-2 (5 μm; n = 13) and AM404 (1–2 μm, n = 7). *P < 0.05.
Discussion
We present findings from electrophysiological and biochemical analyses that demonstrate an activity-dependent release of endocannabinoids from magno-cellular neuroendocrine cells in the hypothalamic SON. We demonstrate directly for the first time increased levels of the two main endocannabinoids, AEA and 2-AG, caused by an activity-dependent mechanism, and show that the activity-dependent retrograde release of both endocannabinoids causes activation of presynaptic CB1-type cannabinoid receptors and suppresses the synaptic release of glutamate onto magnocellular neurones. The activity-dependent suppression of excitatory synaptic inputs by retrograde endocannabinoids is sufficient to inhibit spiking activity in these neurones.
Several recent studies provided evidence for the activity-dependent retrograde release of endo-cannabinoids and resulting cannabinoid receptor-mediated suppression of glutamate and GABA release from presynaptic terminals (Kreitzer & Regehr, 2001; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001; Brown et al. 2003; Hirasawa et al. 2004). Presynaptic suppression of GABA release (DSI) appears to be the most prevalent of the activity-dependent retrograde actions of endocannabinoids; however, suppression of glutamate release (DSE), similar to that described here, has been reported in the cerebellum, hippocampus and ventral tegmentum (Kreitzer & Regehr, 2001; Ohno-Shosaku et al. 2002; Yoshida et al. 2002; Melis et al. 2004), as well as recently in the SON (Hirasawa et al. 2004). The actions of cannabinoids on GABA and glutamate release in the CNS are due mainly to the activation of CB1 receptors (Pertwee & Ross, 2002, although see Van Sickle et al. 2005) although the cannabinoid suppression of glutamate release in the hippocampus is conserved in CB1 receptor knockout mice (Hajos et al. 2001). Since CB2 receptors do not appear to be expressed in the brain (Pertwee & Ross, 2002), this suggests that a third cannabinoid receptor may be responsible for the endocannabinoid actions on glutamate release in the hippocampus. The effects of endogenous and exogenous cannabinoids on glutamate release in the SON in this study and in previous studies (Hirasawa et al. 2004; Di et al. 2005) were abolished by the selective blockade of CB1 receptors, suggesting that the cannabinoid modulation of glutamate release in the SON is mediated by CB1 receptors and therefore that it differs from that in the hippocampus.
A high frequency stimulation protocol similar to that used here to induce endocannabinoid release also has been shown to elicit a form of short-term facilitation of glutamatergic inputs to SON magnocellular neurones (Boudaba et al. 1997; Kombian et al. 2000), and thus have an effect opposite to that of retrograde endo-cannabinoids. High frequency stimulation of synaptic afferents to the SON leads to an increase in the frequency of glutamatergic EPSCs in magnocellular neurones that lasts for several minutes and that is capable of increasing spiking activity (Kombian et al. 2000). This synaptic facilitation involves a presynaptic mechanism that is independent of electrical activity in and peptide release from the postsynaptic magnocellular neurones, thus excluding as a possible mechanism the activity-dependent release of a peptide or other retrograde signal from the dendrites of the magnocellular neurones. This phenomenon differs therefore from the endocannabinoid actions described here and by Hirasawa et al. (2004) in that it represents a facilitation, rather than a suppression, of glutamate release, it lasts for minutes, rather than hundreds of milliseconds, and it is induced by a presynaptic, rather than a postsynaptic, mechanism. Although occurring with apparently different kinetics, the two phenomena appear to overlap in time, presenting the possibility that they could interact with each other. Whereas high frequency stimulation-induced facilitation appears to be a circuit phenomenon affecting afferent excitatory circuits, activity-dependent endocannabinoid modulation may be specific to individual synapses, allowing for a synapse-to-synapse regulation of synaptic transmission. How these activity-dependent, opposing actions on synaptic glutamate release might interact to regulate magnocellular neuronal excitability is a subject for future study.
Presynaptic CB1 receptor modulation of transmitter release is mediated in most brain areas primarily by the suppression of voltage-gated calcium currents (Wilson et al. 2001; Brown et al. 2004). Our findings in the SON, however, like those of Hirasawa et al. (2004), indicate that the CB1 receptor modulation of glutamate release in the SON is not mediated by actions on voltage-gated calcium currents because, unlike in the hippocampus, cannabinoids suppressed action potential-independent (i.e. quantal) release of glutamate onto magnocellular neurones. Quantal release of glutamate in the SON is not influenced by extracellular calcium levels and is, thus, not dependent on calcium influx through voltage-gated calcium channels (Inenaga et al. 1998; Stern et al. 2000; Bourque & Richard, 2001). Therefore, the cannabinoid modulation of glutamate release in the SON must be mediated by an alternative mechanism, such as a reduction of store-dependent intraterminal calcium levels and/or a direct effect on the synaptic release machinery. CB1 receptors are negatively coupled to the cAMP signalling cascade via Gαi (Howlett et al. 2004), which is capable of modulating glutamate release at the calyx of Held independent of calcium influx (Kaneko & Takahashi, 2004) and therefore provides a potential mechanism of cannabinoid suppression of glutamate release in the SON. CB1 receptor activation has been shown to promote neuroprotection by reducing cAMP-dependent, ryanodine receptor-mediated calcium release from intracellular stores (Zhuang et al. 2005). This represents another potential mechanism of cannabinoid modulation of glutamate release onto magnocellular neurones; however, reducing cAMP does not appear to mediate the cannabinoid suppression of glutamate release in the nucleus accumbens (Robbe et al. 2001). Additional studies will be necessary to determine the cellular mechanism of cannabinoid modulation of glutamate release in the SON.
We found that blocking cannabinoid reuptake with transporter blockers caused an elevation of extracellular endocannabinoid levels that was capable of suppressing spiking activity elicited by a combination of direct postsynaptic depolarization and enhanced synaptic glutamate release. This suggests that there is a basal synthesis and release of endocannabinoids in the SON. However, the CB1 receptor antagonist AM251 applied alone had no effect on mEPSCs, indicating that there was little tonic activation of CB1 receptors at presynaptic glutamate terminals by basal levels of endocannabinoids. Therefore, it appears that endocannabinoid levels in the SON are tightly regulated by reuptake mechanisms, which is consistent with our previous findings showing that there is little or no spillover of endocannabinoids from one neurone to another (Di et al. 2003, 2005).
The suppression of synaptically mediated spiking activity by blocking endocannabinoid reuptake raises the interesting possibility that the retrograde release of endocannabinoids might be capable of shaping the patterns of spiking activity in magnocellular neurones, including bursting patterns, under certain conditions. The magnocellular neuroendocrine system has the capacity for dramatic structural and functional plasticity under different physiological and hormonal conditions, including proliferation of glutamatergic, GABAergic and noradrenergic synapses and diminished astrocytic coverage with lactation and chronic dehydration (Miyata et al. 1994; Hatton, 1997; Theodosis & Poulain, 2001; Theodosis, 2002; Mueller et al. 2005). Glutamate inputs are increased and glutamate clearance from the extracellular milieu is reduced under these conditions, allowing for increased presynaptic effects of endogenous glutamate at metabotropic glutamate receptors (Oliet et al. 2001; Boudaba et al. 2003). Also, chronic dehydration leads to enhanced presynaptic noradrenergic modulation of glutamate release (Di et al. 2004), indicating that presynaptic modulatory mechanisms are altered in these conditions. The increased propensity of the magnocellular neurones for bursting in these circumstances suggests that the plastic changes may provide a structural substrate that facilitates burst generation. A key player contributing to burst generation in oxytocin neurones is oxytocin itself (Lambert et al. 1993; Israel et al. 2003), and a recent study by Pittman and colleagues showed that the inhibitory actions of activity-dependent dendritic release of oxytocin on afferent glutamatergic inputs to oxytocin neurones are mediated by the retrograde release of endocannabinoids (Hirasawa et al. 2004). Thus, it will be interesting in the future to determine whether the structural plasticity of the oxytocinergic system alters endocannabinoid signalling in the SON and PVN, and whether retrograde endo-cannabinoid release plays a key role in determining the bursting patterns characteristic of oxytocin and vasopressin neurones under conditions of enhanced hormone release.
Acknowledgments
We thank Katalin Halmos for her expert technical assistance with the study, and Quentin Pittman for providing critical feedback on the manuscript. This work was supported by NIH grants MH066958 (J.G.T.), NS23002 (N.G.B.), and P20RR016816 (N.G.B. and J.G.T.).
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Ames F. A clinical and metabolic study of acute intoxication with Cannabis sativa and its role in the model psychoses. J Ment Sci. 1958;104:972–999. doi: 10.1192/bjp.104.437.972. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Stern JE. Electrophysiological and morphological characteristics of neurons in perinuclear zone of supraoptic nucleus. J Neurophysiol. 1997;78:2427–2437. doi: 10.1152/jn.1997.78.5.2427. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol. 2003;551:815–823. doi: 10.1113/jphysiol.2003.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Osmoregulation of vasopressin neurons: a synergy of intrinsic and synaptic processes. Prog Brain Res. 1998;119:59–76. doi: 10.1016/s0079-6123(08)61562-9. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Richard D. Axonal projections from the organum vasculosum lamina terminalis to the supraoptic nucleus: functional analysis and presynaptic modulation. Clin Exp Pharmacol Physiol. 2001;28:570–574. doi: 10.1046/j.1440-1681.2001.03488.x. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Brown TT, Dobs AS. Endocrine effects of marijuana. J Clin Pharmacol. 2002;42:90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42:993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and GABA inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl. 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Honda E, Hirakawa T, Nakamura S, Yamashita H. Glutamatergic synaptic inputs to mouse supraoptic neurons in calcium-free medium in vitro. J Neuroendocrinol. 1998;10:1–7. doi: 10.1046/j.1365-2826.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- Israel JM, Le Masson G, Theodosis DT, Poulain DA. Glutamatergic input governs periodicity and synchronization of bursting activity in oxytocin neurons in hypothalamic organotypic cultures. Eur J Neurosci. 2003;17:2619–2629. doi: 10.1046/j.1460-9568.2003.02705.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neuroscience. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Chen X, Pittman QJ. Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons. J Neurophysiol. 2000;83:2542–2553. doi: 10.1152/jn.2000.83.5.2542. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Moos FC, Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993;57:1027–1038. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001a;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res. 2001b;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Nakashima T, Kiyohara T. Structural dynamics of neural plasticity in the supraoptic nucleus of the rat hypothalamus during dehydration and rehydration. Brain Res Bull. 1994;34:169–175. doi: 10.1016/0361-9230(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Di S, Paden CM, Herman JP. Activity-dependent modulation of neurotransmitter innervation to vasopressin neurons of the supraoptic nucleus. Endocrinology. 2005;146:348–354. doi: 10.1210/en.2004-0539. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Munoz RM, Adrian BA, Villanua MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5:432–446. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Meth Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostagland Leukotr Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Puder M, Weidenfeld J, Chowers I, Nir I, Conforti N, Siegel RA. Corticotrophin and corticosterone secretion following Δ1-tetrahydrocannabinol, in intact and in hypothalamic deafferentated male rats. Exp Brain Res. 1982;46:85–88. doi: 10.1007/BF00238101. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC, Harakal JJ, Erikson DJ. Comparative diuretic activity of Δ9-tetrahydrocannabinol, cannabidiol, cannabinol and hydrochlorothiazide in the rat. Arch Int Pharmacodyn Ther. 1977;225:77–87. [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol. 2000;526:109–114. doi: 10.1111/j.1469-7793.2000.t01-1-00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT. Oxytocin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol. 2002;23:101–135. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Maternity leads to morphological synaptic plasticity in the oxytocin system. Prog Brain Res. 2001;133:49–58. doi: 10.1016/s0079-6123(01)33004-2. [DOI] [PubMed] [Google Scholar]
- Tyrey L, Murphy LL. Effects of delta-9-tetrahydrocannabinol on reproductive neuroendocrine function in the female: animal studies. NIDA Res Monogr. 1984;55:42–51. [PubMed] [Google Scholar]
- Tyrey L, Murphy LL. Inhibition of suckling-induced milk ejections in the lactating rat by delta 9-tetrahydrocannabinol. Endocrinology. 1988;123:469–472. doi: 10.1210/endo-123-1-469. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger T, Jamali KA, Juaneda C, Leonardelli J, Tramu G. Arachidonyl ethanolamide (anandamide) activates the parvocellular part of hypothalamic paraventricular nucleus. Biochem Biophys Res Commun. 1997;237:724–728. doi: 10.1006/bbrc.1997.7222. [DOI] [PubMed] [Google Scholar]
- Wenger T, Ledent C, Tramu G. The endogenous cannabinoid, anandamide, activates the hypothalamo-pituitary-adrenal axis in CB1 cannabinoid receptor knockout mice. Neuroendocrinology. 2003;78:294–300. doi: 10.1159/000074882. [DOI] [PubMed] [Google Scholar]
- Wenger T, Moldrich G. The role of endocannabinoids in the hypothalamic regulation of visceral function. Prostaglandins Leukot Essent Fatty Acids. 2002;66:301–307. doi: 10.1054/plef.2001.0353. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang SY, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA. Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology. 2005;48:1086–1096. doi: 10.1016/j.neuropharm.2005.01.005. [DOI] [PubMed] [Google Scholar]