Abstract
The vertebrate retina receives centrifugal input from the brain. In zebrafish, the major centrifugal input originates in the terminal nerve (TN). TN cell bodies are located in the olfactory bulb and ventral telencephalon. The TN projects axons to the retina where they branch in the inner plexiform layer (IPL) and synapse onto several inner retinal cell types, including dopaminergic interplexiform cells (DA-IPCs). This olfactoretinal centrifugal input plays a role in modulating retinal ganglion cell (RGC) activity, probably via dopamine-mediated Ca2+ signalling pathways. Normally, dopamine inhibits RGC firing by decreasing the inward Ca2+ current. Olfactory stimulation with amino acids decreases dopamine release in the retina, thereby reducing dopaminergic inhibition of RGCs. This model of olfacto-visual integration was directly tested by recording single-unit RGC activity in response to olfactory stimulation in the presence or absence of dopamine receptor blockers. Stimulation of the olfactory neurones increased RGC activity. However, this effect diminished when the dopamine D1 receptors were pharmacologically blocked. In isolated RGCs, the application of dopamine or a dopamine D1 receptor agonist decreased voltage-activated Ca2+ current and lowered Ca2+ influx. Together, the data suggest that olfactory input has a modulatory effect on RGC firing, and that this effect is mediated by dopamine D1 receptor-coupled Ca2+ signalling pathways.
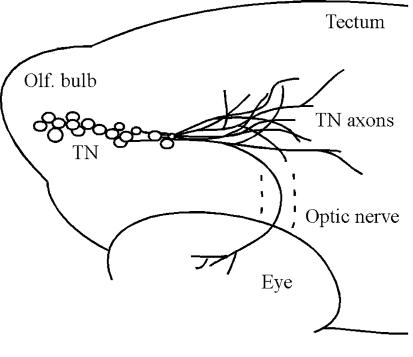
The vertebrate retina receives centrifugal input from the brain (for reviews, see Uchiyama, 1989; Behrens & Wagner, 2004). The input may originate in different brain areas, for example, the posterior hypothalamus in monkeys (Gastinger et al. 1999), the ventral hypothalamus in dogs (Terubayashi et al. 1983), and the diencephalon in rats (Itaya, 1980). In fish, the centrifugal input originates in the terminal nerve (TN). TN cell bodies are located in the olfactory bulb and ventral telencephalon (Arey, 1916; Münz et al. 1982). Most of the TN axons project to the forebrain and midbrain, but some enter the optic nerve and extend to the neural retina (Fig. 1; see also Stell et al. 1984; Maaswinkel & Li, 2003). In the retina, TN axons branch in the IPL and synapse onto several retinal cell types, including dopaminergic interplexiform cells (DA-IPCs) (Zucker & Dowling, 1987).
Figure 1. Diagram of the terminal nerve (TN) pathway in zebrafish.
TN cell bodies (indicated by open circles) are located in the olfactory bulb and ventral telencephalon. Most TN fibres project to the forebrain and midbrain, but some enter the optic nerve and extend to the retina. Dashed lines outline the optic nerve.
The effect of olfactoretinal centrifugal input in the retina has been previously studied by using in vitro preparations, such as isolated retinas (e.g. Stell et al. 1984; Umino & Dowling, 1991; Gastinger et al. 2004). In goldfish, the application of molluscan cardioexcitatory tetrapeptide (a neuropeptide that has some resemblance to RFamide-like peptide released by the teleostean TN; see Kyle et al. 1995; Fischer & Stell, 1997) increased the firing rate of retinal ganglion cells (RGCs) (Stell et al. 1984). In perch, the application of gonadotropin-releasing hormone (GnRH, another neuropeptide contained in the TN; see Münz et al. 1982; Stell et al. 1984) depolarized horizontal cell membrane potentials and increased their responses to spots of light (Umino & Dowling, 1991). The effect of GnRH on horizontal cells can be mimicked by dopamine, suggesting that GnRH may stimulate the release of dopamine from DA-IPCs. In many cases, the application of FMRFamide alone did not produce any effect. However, when applied with GnRH, FMRFamide suppressed the effect of GnRH (Umino & Dowling, 1991). GnRH receptors were found to be expressed in both DA-IPCs and RGCs (Grens et al. 2005).
Zebrafish (Danio rerio) have recently emerged as a model vertebrate for genetic and physiological studies of visual modulation (for reviews, see Baier, 2000; Li, 2001). By screening the F1 generation of chemically mutagenized zebrafish, Li & Dowling (2000a) isolated a dominant mutant (nbb) characterized by disruption of TN networks and degeneration of DA-IPCs. During prolonged dark adaptation (> 1 h), the behavioural visual sensitivity of nbb mutants decreased maximally by 2 log units. Depletion of DA-IPCs in wild-type fish also resulted in decreased visual sensitivity, which is due to a disruption of inner retinal rod signal transmission (Li & Dowling, 2000b). In a recent study which was aimed at investigating the role of TN input in the retina, Maaswinkel & Li (2003) examined the effect of olfactory stimulation on behavioural visual sensitivity in zebrafish. They found that stimulation of olfactory neurones with amino acids increased visual sensitivity. When the DA-IPCs were depleted, however, olfactory stimulation produced no effect on visual sensitivity. This suggests that the effect of olfactory input in the retina is mediated by dopaminergic signalling pathways.
The mechanisms that underlie dopaminergic modulation of olfacto-visual integration remain unknown. Some studies suggest that stimulation of olfactory neurones increases TN activity (Demski & Northcutt, 1983; Springer, 1983; Wirsig-Wiechmann et al. 2002). Because TN fibres synapse onto DA-IPCs and dopamine is known to have a role in the regulation of visual sensitivity, we propose that stimulation of the TN may alter DA-IPC activities. This alteration may change the dynamics of dopamine release, thereby modifying retinal sensitivity. In the present study, we tested this hypothesis in zebrafish by recording single-unit RGC activity in response to olfactory stimulation or dopamine treatment. Using an in vivo microdialysis assay, we determined dopamine concentrations in the retina. We also measured voltage-activated Ca2+ currents and Ca2+ influx in isolated RGCs. The data suggest that olfactory stimulation has a modulatory role in RGC activity via dopamine D1 receptor-coupled Ca2+ signalling pathways.
Methods
Animals and maintenance
Zebrafish (Danio rerio) were maintained as described (Westerfield, 1995). The fish (6–12 months of age) were kept in a 14 h–10 h light–dark cycle (room fluorescent light, 07:00 h to 21:00 h). The experiments were performed between 10:00 h and 18:00 h. All experimental procedures adhered to the NIH guidelines for animals in research.
Single-unit RGC recordings
The fish was anaesthetized with 0.04% 3-amino benzoic acid and immobilized by intraperitoneal injections of 3–5 μl of 0.5 mg ml−1 gallamine triethiodide dissolved in phosphate-buffered saline (PBS) (Maaswinkel et al. 2005). Then, the fish was placed on a wet sponge with most of its body covered by a wet paper towel. A slow stream of system water (distilled water with ocean salt added, 3 g gal−1, pH 7.0; Westerfield, 1995) was directed into the mouth to keep the fish oxygenized. The eye was slightly pulled out of its socket and held in place by glass rods, thus exposing the optic nerve. Single-unit RGC responses (determined by the spike waveform) were recorded from the optic nerve by using a Tungsten microelectrode (resistance, 5–10 MΩ). Electrical signals were filtered with a band pass filter between 30 and 3000 Hz.
The fish was dark adapted for 30 min before the first RGC recording was made. The light stimuli (full-field dim white light, generated by a halogen bulb) were directed to the fish eye via a mirror system (Ren & Li, 2004a,b). The intensity of the unattenuated light beam (logI = 0) measured in front of the fish eye was 670 μW cm−2 (Optical Power Meter, UDT Instruments, MD, USA). Full-field stimuli may not be suitable for studying receptive field properties of individual cells, but they are effective in ascertaining the threshold of On or Off responses (see also Li & Dowling 2000a,b). To determine the threshold, the light intensity was first set at below threshold level (e.g. logI = −6.0) and then increased by 0.5 log-unit steps until the first light-evoked RGC responses were recorded (criteria, 20% above or below the rate of spontaneous firing). This light intensity was noted as the threshold. For each recording, 10 stimuli (600 ms flashes) were delivered at 3 s intervals.
Olfactory stimulation and intraocular injections
Amino acids are primary odourants for zebrafish (Michel & Lubomudrov, 1995; Friedrich & Korsching, 1997, 1998). We chose methionine as a stimulus because it is more effective in increasing visual sensitivity than other amino acids tested, such as alanine, arginine, or aspartate (Maaswinkel & Li, 2003). Methionine (Sigma, MO, USA) was dissolved and diluted to 1 mm in regular system water. The fish was anaesthetized and immobilized as described above. Methionine solution (8–10 μl) was dripped into the nostril that was contralateral to the recorded eye (the eye receives mainly contralateral olfactoretinal centrifugal input; authors' unpublished observations). The light threshold required to evoke RGC responses was measured before the application of methionine, and was measured again within 10 s following the application of methionine. Thereafter, the measurement was repeated at 1 min intervals for 10 min. During this time, no methionine solutions were dripped into the nostril of the fish. For all the experiments, only naive fish were used.
Dopamine or dopamine receptor agonists or antagonists (Sigma) were dissolved and diluted to 500 μm in PBS. Drug solutions (up to 0.2 μl) were injected into the vitreous by using a microinjector. The final drug concentration in the eye was estimated to be 25–50 μm, considering that the total volume of the vitreous is approximately 2 μl.
Whole-cell recordings
RGCs were labelled retrogradely with DiI (Molecular Probes, OR, USA). DiI was sonicated in ethanol (1: 40) and dissolved in PBS (1: 100) and was injected into the optic nerve using a microinjector. Two days after the injection, the fish were killed by decapitation. Isolated retinas were transferred into enzyme solutions (see below) and were dissociated by gentle trituration with a wide-tipped fire-polished Pasteur pipette. The resulting cell suspension was plated onto culture dishes containing bath solutions (see below).
Solutions used in this study were modified from Liu & Lasater (1994) and Lee et al. (2003). Enzyme cocktail: papain 20 U ml−1, l-cysteine 1 mg ml−1, EDTA 0.1 mm, NaCl 140 mm, KCl 5 mm, NaH2PO4 0.3 mm, Na2HPO4 0.3 mm, glucose 10 mm, pH 7.4. Bath solution: NaCl 100 mm, KCl 5 mm, caesium acetate 10 mm, TEA-Cl 30 mm, Hepes 5 mm, TTX 0.001 mm, CaCl2 10 mm, MgCl2 1 mm, glucose 10 mm, pH 7.4. Pipette solution: CsCl 40 mm, caesium acetate 80 mm, TEA-Cl 30 mm, CaCl2 0.5 mm, MgCl2 1 mm, EGTA 5 mm, Hepes 10 mm, Mg-ATP 2 mm, Na-GTP 0.5 mm, pH 7.4.
Voltage-activated Ca2+ currents were recorded with an Axopatch amplifier (Axon Instruments, CA, USA). Electrical signals were filtered with a low-pass filter at 2 kHz and acquired at 5 kHz. The resistance of the recording micropipette was between 6 and 10 MΩ when back-filled with the pipette solution and measured in the bath solution. Drug solutions (dopamine, dopamine receptor agonists or antagonists; final concentrations in the media, 10 μm) were applied to bath solutions through local perfusion by using a gravity-driven superfusion system (Warner Instruments, CT, USA). Data were analysed off-line by Clampfit (Axon Instruments).
Ca2+ imaging
RGCs were labelled retrogradely with DiI and were dissociated as described above. Cytoplasmic free-Ca2+ was labelled with the acetoxymethyl ester form of Fluo-3 (Fluo-3 AM; 5 μm) and Pluronic F-127 (0.03%) (Molecular Probes) in culture medium (Gibco, NY, USA). Time lapse images were acquired by using MetaMorph (Universal Imaging Co., PA, USA) with an inverted Zeiss Axiovert S100TV microscope and a ×40 plan-NeoFluar oil immersion objective lens. Images were taken at 10 s intervals controlled by a Lambda 10-2 shutter (Sutter Instrument Co., CA, USA).
Microdialysis and high performance liquid chromatography (HPLC)
Methods for microdialysis were similar to those described in Puppala et al. (2004). The corpus vitreum was dialysed with sterile Ringer solution (147 mm Na+, 2.3 mm Ca2+, 4 mm K+, and 155.6 mm Cl−; pH 7.0) at a flow rate of 2 μl min−1. The fish was anaesthetized and immobilized as described above. For each fish, two dialysis samples were collected, each for a duration of 30 min: one before olfactory stimulation and one during olfactory stimulation. During the stimulus session, the experimental fish received three methionine stimulations (at 0, 10 and 20 min; dripped into the nostril contralateral to the recorded eye) alternated with three sham stimulations using system water (at 5, 15 and 25 min). This was done to prevent habituation. The control fish received only sham stimulation with system water at all six time points. Dialysate fractions were collected in tubes containing 15 μl of 100 mm citric acid that were kept in the dark at 4°C.
Dopamine content in the dialysates was determined by HPLC using a microdialysis MD-150 analytical column (ESA, MA, USA). The mobile phase consisted of 75 mm sodium dihydrogen phosphate monohydrate, 1.7 mm 1-octanesulphonic acid sodium salt, 0.01% triethylamine, 25 µm EDTA, and 10% acetonitrile, pH 3.85. Samples were first oxidized at +250 mV and then reduced at −250 mV. The flow rate was 0.7 ml min−1. Dopamine was diluted in 0.1 m perchloric acid to generate a standard curve. The peak area was calculated by using Millennium software (Waters, MA, USA) and converted into the total amount (Aragona et al. 2003; Curtis et al. 2003).
Data analysis
We used the paired t test to compare the changes (means ± s.e.m.) of the parameters of interest (e.g. RGC spike frequency and threshold, Ca2+ current, vitreal dopamine content) before and after a given treatment (olfactory stimulation, drug application) and the unpaired t test to compare the changes between the groups that received different treatments (e.g. vitreal dopamine content in fish that received either methionine or sham stimulation). For all the statistical analyses, we applied two-tailed tests and the significance level was α = 0.05. We also reported statistical ‘tendencies’ in cases of 0.05 < P < 0.1.
Results
Olfactory stimulation modulates RGC activity
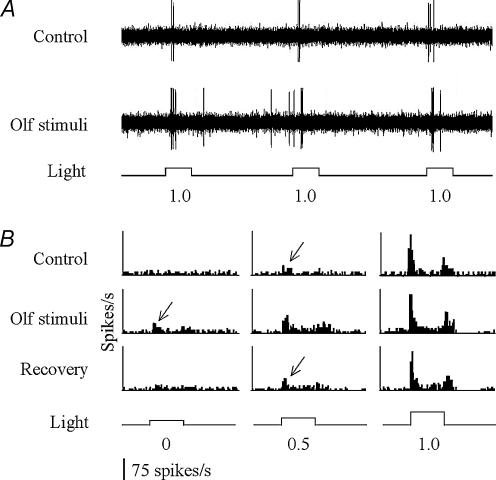
To examine the effect of olfactory input in the retina, we recorded single-unit RGC activity in response to olfactory stimulation with methionine. In most cases, the RGC firing rate increased after the application of methionine (Fig. 2A). On average, the number of RGC spikes (either On-Off- or On–Off responses) evoked by the same light intensity that was used before olfactory stimulation increased by 54.0 ± 12.6% (bin size, 20 ms) (P < 0.001; n = 24) (Table 1). In addition, the light threshold that was required to evoke RGC responses decreased, on average, by 0.65 ± 0.06 log units (P < 0.001; n = 24) (Fig. 2B; Table 1). The increase of the rate of spike firing was seen in 18 out of 24 cells recorded. In the remaining cells, the spike rate remained either unchanged (n = 3) or slightly decreased (n = 3). The decrease of light threshold was seen in 17 out of 24 cells after olfactory stimulation. In the remaining cells, the threshold remained either unchanged (n = 6) or increased (n = 1) (Table 1).
Figure 2. Single-unit retinal ganglion cell (RGC) responses recorded before and after olfactory stimulation.
A, representative RGC responses recorded from a single RGC fibre in the optic nerve. The stimulus light intensity was approximately 1 log unit above the RGC threshold level as determined before olfactory stimulation (designated 1.0). After olfactory stimulation, the number of spikes (both spontaneous and light-evoked) increased in response to the same light stimulation. B, histograms of light-evoked RGC responses before (top) and during (middle) olfactory stimulation, and after 5 min of recovery (bottom). Threshold RGC responses are indicated by arrows. Note that the threshold intensity that was required to evoke RGC responses decreased after olfactory stimulation. After 5 min of recovery, the threshold returned to the control level. The intensity of the light stimuli (at the bottom of the histograms) increased by 0.5 log-unit steps (from left to right), starting below or near threshold level (designated 0).
Table 1.
Effect of olfactory stimulation and intraocular injection of dopamine, or receptor agonist or antagonist, on RGC activity
| Increase (no. of cells) | Decrease (no. of cells) | No change (no. of cells) | Average changes (means ± s.e.m.) | P value | ||
|---|---|---|---|---|---|---|
| Methionine | Sensitivity | 17 (72%) | 1 | 6 | 0.65 ± 0.06 log units | < 0.001 |
| No. of spikes | 18 (75%) | 3 | 3 | 54.0 ± 12.6% | < 0.001 | |
| Met + SCH | Sensitivity | 3 | 1 | 8 (67%) | 0.13 ± 0.10 log units | n.s. |
| No. of spikes | 3 | 2 | 7 (58%) | 5.7 ± 4.7% | n.s. | |
| Met + Sul | Sensitivity | 5 (83%) | 1 | 0 | 0.42 ± 0.20 log units | < 0.05 |
| No. of spikes | 5 (83%) | 1 | 0 | 39.6 ± 14.8% | 0.08 | |
| Dopamine | Sensitivity | 1 | 8 (80%) | 1 | −0.75 ± 0.14 log units | < 0.05 |
| No. of spikes | 1 | 8 (80%) | 1 | −51.0 ± 10.2% | < 0.05 | |
| SKF | Sensitivity | 0 | 5 (71%) | 2 | −0.53 ± 0.13 log units | < 0.05 |
| No. of spikes | 1 | 6 (86%) | 0 | −24.1 ± 10.1% | < 0.05 | |
| SCH | Sensitivity | 8 (89%) | 0 | 1 | 0.44 ± 0.06 log units | < 0.05 |
| No. of spikes | 7 (78%) | 1 | 1 | 32.6 ± 10.9% | < 0.05 | |
| Quinpirole | Sensitivity | 1 | 1 | 2 (50%) | 0.1 ± 0.20 log units | n.s. |
| No. of spikes | 1 | 1 | 2 (50%) | 6.7 ± 11.6% | n.s. | |
| Sulpiride | Sensitivity | 2 | 0 | 5 (71%) | 0.14 ± 0.09 log units | n.s. |
| No. of spikes | 2 | 1 | 4 (57%) | 4.9 ± 7.1% | n.s. |
Met, methionine; SCH, SCH 23390; SKF, SKF 38393; Sul, sulpiride. P values were obtained from the paired t test to compare the mean sensitivity or spike activity changes before and after a given treatment. n.s., not significant.
The responsiveness of RGCs declined steadily after olfactory stimulation. Within 5 min, both the firing rate and the threshold returned to levels that were recorded before the application of methionine (Fig. 2B).
The effect of olfactory input on RGCs is mediated by dopamine
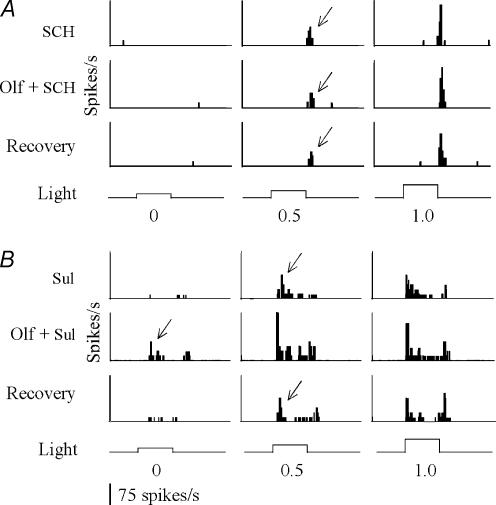
In the retina, TN fibres synapse onto DA-IPCs. To test whether the effect of olfactory stimulation on RGCs is mediated by dopamine, we recorded single-unit RGC activity in response to olfactory stimulation in zebrafish that were treated with either dopamine D1 or D2 receptor antagonists. Intraocular injections of SCH 23390 (a dopamine D1 receptor antagonist) blocked the effect of olfactory stimulation on RGCs. For example, in 8 out of 12 cells tested, the threshold intensity that was required to evoke RGC responses remained unchanged after the application of methionine. The number of spikes also remained unchanged in 7 out of 12 cells tested (Fig. 3A; Table 1). By contrast, intraocular injections of sulpiride (a D2 receptor antagonist) did not inhibit methionine-induced increases in RGC sensitivity. In response to olfactory stimulation, the threshold intensity required to evoke RGC responses decreased by 0.42 ± 0.20 log units (P < 0.05; n = 6) and the number of spikes increased by 39.6 ± 14.8% (P = 0.08; n = 6) (Fig. 3B; Table 1).
Figure 3. Histograms of light-evoked RGC responses to olfactory stimulation in zebrafish that were treated with SCH 23390 (a dopamine D1 receptor antagonist, A) or sulpiride (a dopamine D2 receptor antagonist, B).
When the D1 receptors were blocked, the light intensity that was required to evoke RGC responses remained unchanged after olfactory stimulation (A). By contrast, the application of D2 blockers did not inhibit olfactory stimulation-induced changes in RGC activity (B). Threshold RGC responses are indicated by arrows. The intensity of the light stimuli (at the bottom of the histograms) increased by 0.5 log-unit steps (from left to right), starting below or near threshold level (designated 0). SCH, SCH 23390; Sul, sulpiride.
Dopamine inhibits RGC activity
To investigate whether or not dopamine has a direct role in RGC activity, we recorded single-unit RGC responses in zebrafish that received intraocular injections of dopamine. On average, the number of spikes decreased by 51.0 ± 10.2% after the application of dopamine (P < 0.05; n = 10). In addition, the threshold intensity that was required to evoke RGC responses increased, on average, by 0.75 ± 0.14 log units (P < 0.001; n = 10) (Fig. 4A; Table 1). Intraocular injections of SKF 38393 (a dopamine D1 receptor agonist; n = 7) produced a similar result, i.e. a decrease in spike numbers (24.1 ± 10.1%, P < 0.05, n = 7) and an increase in light threshold (0.53 ± 0.13 log units, P < 0.05, n = 7) (Fig. 4B; Table 1). When the D1 receptors were selectively blocked with SCH 23390, RGC activity increased. On average, the number of spikes increased by 32.6 ± 10.9% (P < 0.05, n = 9), and the threshold intensity decreased by 0.44 ± 0.06 log units (P < 0.05, n = 9) (Fig. 4C, Table 1). Intraocular injections of quinpirole (a D2 receptor agonist; n = 4) or sulpiride (a D2 receptor antagonist; n = 7) did not change RGC activities obviously (Table 1).
Figure 4. Histograms of light-evoked RGC responses before and after intraocular injections of dopamine (A), SKF 38393 (a dopamine D1 receptor agonist, B) or SCH 23390 (a dopamine D1 receptor antagonist, C).
Intraocular injections of dopamine or SKF 38393 (SKF) increased the threshold intensity that was required to evoke RGC responses (arrows). By contrast, injections of SCH 23390 (SCH) decreased the threshold intensity (arrows). In all cases, after 5–10 min of recovery, the thresholds returned to control levels. The intensity of the light stimuli (at the bottom of the histograms) increased by 0.5 log-unit steps (from left to right), starting below or near threshold level (designated 0).
Olfactory stimulation decreases vitreal dopamine concentrations
Because methionine (added to the nostril) and dopamine (injected into the eye) produced opposite effects on RGC firing, and because the effect of olfactory stimulation on RGCs was mediated by dopamine, we reasoned that stimulation of olfactory neurones decreases the release of dopamine by DA-IPCs. To test this hypothesis, we measured dopamine content in the vitreous by using microdialysis and HPLC. Before olfactory stimulation, the dopamine content of the dialysate samples was on average 2.97 pg (n = 15). After the application of methionine (to the nostril), dopamine content decreased on average by 0.43 ± 0.19 pg (P = 0.07; n = 7). The decrease of dopamine content was seen in 6 out of 7 fish. In one fish, dopamine content slightly increased. The application of system water to the nostril (control stimuli, n = 8) did not alter dopamine concentrations in a clear pattern: dopamine content increased in five samples and decreased in three samples. Overall, there was a slight (not significant) increase of dopamine content (0.32 ± 0.27 pg). The unpaired t test revealed that the effect of olfactory stimulation on vitreal dopamine content in the experimental group was significantly different (P < 0.05) from the effect of the sham stimulation in the control group.
Dopamine decreases Ca2+ current in RGCs
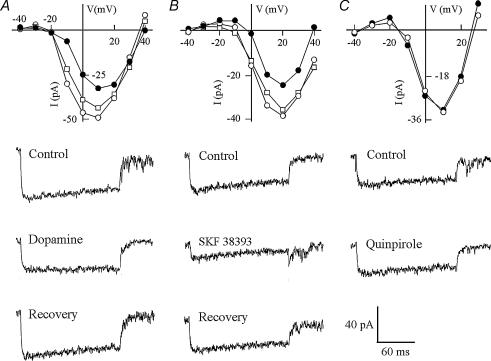
The mechanisms that regulate RGC spike activity during olfactory stimulation involve dopamine D1 receptor-coupled Ca2+ signalling pathways. Ca2+ currents have been shown to play important roles in generating RGC spikes (Ishida, 1995). In zebrafish RGCs, two types of voltage-activated Ca2+ currents were recorded: one was activated by high membrane voltages (maximum activation, between 0 and +20 mV) and one was activated by low membrane voltages (maximum activation, between −40 and −10 mV). Dopamine decreased both high and low voltage-activated Ca2+ currents. On average, the Ca2+ current decreased by 56.8 ± 11.0% (P < 0.05; n = 22) (Fig. 5A; Table 2). A decrease in Ca2+ current (by 53.8 ± 9.7%) was also seen when the D1 receptors were selectively activated by SKF 38393 (P = 0.06; n = 13) (Fig. 5B; Table 2). By contrast, the D2 receptor agonist quinpirole (n = 7) produced no effect on Ca2+ current (Fig. 5C; Table 2).
Figure 5. Treatments (•) with dopamine (A), SKF 38393 (a dopamine D1 receptor agonist, B) or quinpirole (a dopamine D2 receptor agonist, C) on voltage-activated Ca2+ current in isolated RGCs.
Dopamine or SKF 38393 decreased the Ca2+ current, whereas quinpirole produced no effect. After 3 min of washout (□), the current returned to levels as measured in normal bath solutions (○). Traces under each current–voltage curve show the Ca2+ current recorded in normal bath solutions (top), in drug solutions (middle) and after 3 min of washout (bottom). Testing potential, +10 mV; holding potential, −90 mV.
Table 2.
Effect of dopamine or dopamine receptor agonists on voltage-activated Ca2+ current and Ca2+ influx
| Increase (no. of cells) | Decrease (no. of cells) | No change (no. of cells) | Average changes (means ± s.e.m.) | P value | ||
|---|---|---|---|---|---|---|
| Dopamine | Current | 2 | 16 (73%) | 4 | −56.8 ± 11.0% | < 0.05 |
| Influx | 4 | 19 (63%) | 7 | −32.7 ± 6.4% | < 0.001 | |
| SKF | Current | 3 | 8 (62%) | 2 | −53.8 ± 9.7% | 0.06 |
| Influx | 0 | 16 (73%) | 6 | −34.0 ± 8.3% | < 0.001 | |
| Quinpirole | Current | 0 | 1 | 6 (86%) | −4.2 ± 5.7% | n.s. |
| Influx | 1 | 2 | 8 (73%) | −1.9 ± 3.46% | n.s. |
SKF, SKF 38393. P values were obtained from the paired t test to compare the means before and after dopamine or dopamine receptor agonist treatment.
Ca2+ may enter RGCs via ligand-gated ion channels (Ishida et al. 1991). Dopamine decreased the steady-state Ca2+ influx by 32.7 ± 6.4% (P < 0.001; n = 30) (Fig. 6; Table 2). A decrease in Ca2+ influx (by 34.0 ± 8.3%) was also seen when the D1 receptors were activated by SKF 38393 (P < 0.001; n = 22) (Fig. 6; Table 2). No obvious changes in cytoplasmic Ca2+ concentrations were seen when the D2 receptor agonist quinpirole was applied (n = 11) (Table 2).
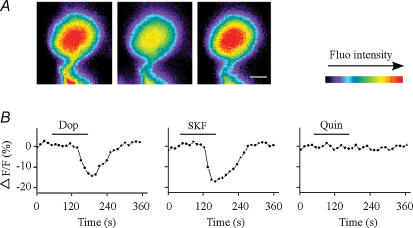
Figure 6. Effect of dopamine or dopamine receptor agonists on Ca2+ influx in isolated RGCs.
A, time lapse images showing cytoplasmic free-Ca2+ concentrations measured before (left), during (middle) and after washout (right) of dopamine. Ca2+ concentrations decreased in response to dopamine treatment, and returned to control levels after washout. Calibration bar, 5 μm. B, cytoplasmic free-Ca2+ concentrations (relative fluorescence intensities) in response to dopamine (Dop), SKF 38393 (SKF; a D1 receptor agonist) or quinpirole (Quin; a D2 receptor agonist) in isolated RGCs. Activation of the D1 but not D2 receptors decreased Ca2+ influx. Horizontal lines indicate the time of drug treatment.
Discussion
In teleosts, the TN signals transmitted to the neural retina play a role in modulating retinal cell functions. They may affect retinal signalling transmission directly by targeting the cells that are part of the main signalling pathway (e.g. RGCs, Stell et al. 1984; Malz et al. 1999; Grens et al. 2005) or indirectly by targeting cells that modulate the function of other neurones (e.g. DA-IPCs, Zucker & Dowling, 1987; Umino & Dowling, 1991). We found that the TN modulates RGC activity through DA-IPCs. Normally, dopamine inhibits RGC activity (Liu & Lasater, 1994; also this study). However, this dopaminergic inhibition can be reduced by olfactory stimulation, probably through the olfactoretinal centrifugal pathway. Based on our study, we have constructed a straightforward model that demonstrates how this pathway functions. Olfactory stimulation alters the activity of the TN pathway, which in turn, decreases the release of dopamine by DA-IPCs, thereby reducing dopaminergic inhibition of RGCs. As the dopamine concentration decreases, Ca2+ influx increases, and thus, RGC activity increases. This effect is probably mediated by dopamine D1 receptors that are located on RGCs.
The TN input may also regulate RGC activity via other pathways. TN fibres have been found to synapse not only onto DA-IPCs, but also onto GABAergic cells (Stell et al. 1987; Ball et al. 1989; Malz et al. 1999). Fischer & Stell (1997) showed that the release of RFamide-like peptide by the TN may be regulated by the neurotransmitter GABA. In dark-adapted retinas, for example, the application of GABA receptor antagonists decreased the level of RFamide-like peptide. In light-adapted retinas, the receptor agonists increased the content of the peptide. Fischer & Stell (1997) suggest this may be due to a modulatory effect of signals generated from retinal bipolar cells. Additionally, Grens et al. (2005) have demonstrated that GnRH receptors are located on RGCs. This introduces the possibility that the TN directly modulates RGC activity, therefore bypassing the dopaminergic or GABAergic systems. However, because dopamine D1 receptor antagonists blocked the effect of olfactory stimulation on RGC spike firing, the GnRH receptors are not likely to play a major role in olfactory modulation of RGC activity.
The exact mechanisms by which olfactory stimulation regulates RGC activity remain to be studied further. It is possible that different neuronal pathways are involved (Fig. 7). However, our current research demonstrates that the dopaminergic system plays an important role. The data confirm our previous findings (Maaswinkel & Li, 2003), which suggest that olfactory modulation of visual sensitivity requires an intact dopaminergic system. Because dopamine may act at a distance by volume transmission (Yazulla & Zucker, 1988; Negishi et al. 1990), the dopamine receptors located on the RGCs are most likely directly affected by changes in dopamine concentration. Dopamine may also modulate RGC activity via voltage-gated Na+ channels (Vaquero et al. 2001; Hayashida & Ishida, 2004) or by modulating the function of other cells that synapse onto RGCs (Witkovsky & Dearry, 1991). Further studies are required to address these open questions, for example, by local application of pharmacological reagents in slice preparations in order to isolate selected circuits.
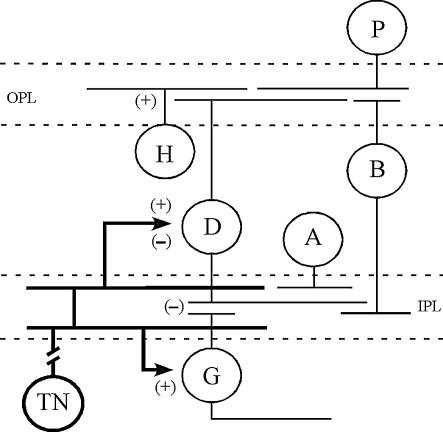
Figure 7. Diagram of possible synaptic connections between TN fibres (bold lines) and retinal cells.
TN fibres branch in the IPL and synapse with DA-IPCs and perhaps other retinal cell types such as RGCs. GnRH and RFamide-like peptide may have different effects on DA-IPCs, i.e. GnRH stimulates (+) dopamine release and RFamide-like peptide decreases (−) dopamine release. Dopamine increases (+) horizontal cell responses but inhibits (−) RGC activities. Dashed lines outline the inner and outer plexiform layers. A, amacrine cell; B, bipolar cell; D, DA-IPC; G, RGC; H, horizontal cell; P, photoreceptor cell; IPL, inner plexiform layer; OPL, outer plexiform layer.
There are some other factors that may complicate our model. For example, Umino & Dowling (1991) found that GnRH and FMRFamide might have an opposite effect on dopamine release by DA-IPCs. In dark-adapted perch retinas, GnRH promoted the release of dopamine. However, this effect was suppressed by FMRFamide. Stell et al. (1984) and Walker & Stell (1986) reported that in goldfish retinas GnRH was in most cases excitatory, whereas FMRFamide could be either excitatory or inhibitory. Thus, it is not clear what TN signals are received by DA-IPCs and how the process of dopamine release is regulated. Furthermore, Fischer & Stell (1997) and Kyle et al. (1995) reported that the RFamide-like peptide released by the teleostean TN has a slightly different structure as compared to FMRFamide used in most of the previous experimental studies (Walker & Stell, 1986; Umino & Dowling, 1991). It is not clear whether the molluscan cardioexcitatory tetrapeptide FMRFamide can be expected to reproduce exactly the action of the endogenous RFamide-like peptide.
In an earlier study, Li & Dowling (2000b) reported that dopamine played a role in modulating rod signalling transmission in the inner retina. In zebrafish that were treated with 6-OHDA (which selectively destroys DA-IPCs), the threshold intensity that was required to evoke RGC spikes was elevated by nearly 2 log units. At first glance, this seems to contradict findings presented in the current study, which suggest that decreases in dopamine concentrations result in increases in RGC activity. There are fundamental differences between these two studies. First, the chronic depletion of dopamine may not have the same effect on retinal cell functions as a transient decrease of dopamine concentration (i.e. by olfactory stimulation). In goldfish, the depletion of dopamine by 6-OHDA did not affect the K+ currents in retinal bipolar cells, for example, the electroretinogram (ERG) b-wave, which partially reflects the K+ currents of bipolar cells, was normal after 6-OHDA treatment (Lin & Yazulla, 1994). However, a transient change in vitreal dopamine concentrations resulted in either a decrease or an increase in K+ current (Fan & Yazulla, 1999; Yu & Li, 2005). Second, the former study (Li & Dowling, 2000b) examined the effect of dopamine depletion on inner retinal sensitivity. The decrease of inner retinal sensitivity after dopamine depletion may be due to actions that take place in any cell type. By contrast, the current study directly tests the effect of dopamine on RGC activity by using single-unit RGC recording methods.
The significance of the olfactoretinal projection of the TN in fish remains to be elucidated. At the moment, we can only forward some speculations. The olfactoretinal projection might play a role in visually guided behaviours, such as feeding or mating. Food extracts or amino acids applied to the nostrils increase ERG b-wave amplitude in fish (Weiss & Meyer, 1988; Maaswinkel & Li, 2003). Amino acids presented as olfactory stimuli also increase RGC sensitivity (this study) and behavioural visual sensitivity (Maaswinkel & Li, 2003). Amino acids can elicit feeding-related behaviours in fish (Valentinèiè & Caprio, 1997; Lindsay & Vogt, 2004). Further study is needed to determine whether olfactory stimulation can improve visually based foraging or prey catching. In addition, the TN has been suggested to play a role in mating behaviour (Demski & Northcutt, 1983; Wirsig-Wiechmann et al. 2002; however, see Fujita et al. 1991). Walker & Stell (1986) found in goldfish that retinal responsiveness to GnRH and FMRFamide decreases in autumn, when the fish are minimally sexual active. This suggests that the TN has a role in reproduction. Interestingly, retinal GnRH content also depends on diurnal and seasonal factors (Ball et al. 1989).
Lastly, we found that the effects of dopamine on RGCs varied. In most cells, dopamine decreased RGC spike firing, but in other cells, dopamine increased RGC spike activity or produced no effect. The different effects of dopamine on RGCs may be attributed to the physiological diversity of RGCs. In zebrafish, 11 different morphological types of RGCs have been identified (Mangrum et al. 2002), and there may well be other types that have not yet been recognized. Among them, some possess stratified dendritic arborizations in the On or Off sublaminae of the IPL, whereas others possess dendritic trees that ramify diffusely throughout the entire thickness of the IPL. Different types of RGCs may respond differently to dopamine. For example, in rabbit retinas that were treated with dopamine receptor blockers, the spontaneous activity of Off cells decreased but the activity of On cells increased (Jensen & Daw, 1984). We could not correlate the response patterns to cell types by recording responses from the optic nerve or dissociated cells.
In summary, this study provides insight on the mechanisms that underlie olfacto–visual sensory integration. In zebrafish, olfactory TN signals are transmitted to the retina where they modulate retinal cell functions. The effect of the TN on RGCs is mediated, either directly or indirectly, and at least in part, by dopamine D1 receptor-coupled Ca2+ signalling pathways.
Acknowledgments
The authors thank Drs J. T. Curtis and Z. X. Wang for their assistance in HPLC experiments, and Dr D. Q. Zhang for advice regarding intracellular recordings. The authors thank A. C. Carr for proof reading the manuscript. This work was supported in part by NIH grants R01 EY13147 and EY13680.
References
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey LB. The function of the afferent fibers of the optic nerve of fishes. J Comp Neurol. 1916;26:213–245. [Google Scholar]
- Baier H. Zebrafish on the move: towards a behavior-genetic analysis of vertebrate vision. Curr Opin Neurobiol. 2000;10:451–455. doi: 10.1016/s0959-4388(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Ball AK, Stell WK, Tutton DA. Efferent projections to the goldfish retina. In: Weiler R, Osborne NN, editors. Neurobiology of the Inner Retina. Berlin: Springer; 1989. pp. 103–116. [Google Scholar]
- Behrens U, Wagner HJ. Terminal nerve and vision. Microsc Res Tech. 2004;65:25–32. doi: 10.1002/jemt.20108. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Stowe JR, Wang Z. Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience. 2003;118:1165–1173. doi: 10.1016/s0306-4522(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Demski LS, Northcutt RG. The terminal nerve: a new chemosensory system in vertebrates? Science. 1983;220:435–437. doi: 10.1126/science.6836287. [DOI] [PubMed] [Google Scholar]
- Fan SF, Yazulla S. Modulation of voltage-dependent K+ currents (IKV) in retinal bipolar cells by ascorbate is mediated by dopamine D1 receptors. Vis Neurosci. 1999;16:923–931. doi: 10.1017/s095252389916512x. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Stell WK. Light-modulated release of RFamide-like neuropeptide from nervus terminalis axon terminals in the retina of goldfish. Neuroscience. 1997;77:585–597. doi: 10.1016/s0306-4522(96)00454-x. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci. 1998;18:9977–9988. doi: 10.1523/JNEUROSCI.18-23-09977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita I, Sorensen PW, Stacey NE, Hara TJ. The olfactory system, not the terminal nerve, functions as the primary chemosensory pathway mediating responses to sex pheromones in male goldfish. Brain Behav Evol. 1991;38:313–321. doi: 10.1159/000114397. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, O'Brien JJ, Larsen NB, Marshak DW. Histamine immunoreactive axons in the macaque retina. Invest Ophthalmol Vis Sci. 1999;40:487–495. [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Yusupov RG, Glickman RD, Marshak DW. The effects of histamine on rat and monkey retinal ganglion cells. Vis Neurosci. 2004;21:935–943. doi: 10.1017/S0952523804216133. [DOI] [PubMed] [Google Scholar]
- Grens KE, Greenwood AK, Fernald RD. Two visual processing pathways are targeted by gonadotropin-releasing hormone in the retina. Brain Behav Evol. 2005;66:1–9. doi: 10.1159/000085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Ishida AT. Dopamine receptor activation can reduce voltage-gated Na+ current by modulating both entry into and recovery from inactivation. J Neurophysiol. 2004;92:3134–3141. doi: 10.1152/jn.00526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT. Ion channel components of retinal ganglion cells. Prog Retinal Eye Res. 1995;15:261–280. [Google Scholar]
- Ishida AT, Bindokas VP, Nuccitelli R. Calcium ion levels in resting and depolarized goldfish retinal ganglion cell somata and growth cones. J Neurophysiol. 1991;65:968–979. doi: 10.1152/jn.1991.65.4.968. [DOI] [PubMed] [Google Scholar]
- Itaya SK. Retinal efferents from the pretectal area in the rat. Brain Res. 1980;201:436–441. doi: 10.1016/0006-8993(80)91049-5. [DOI] [PubMed] [Google Scholar]
- Jensen RJ, Daw NW. Effects of dopamine antagonists on receptive fields of brisk cells and directionally selective cells in the rabbit retina. J Neurosci. 1984;4:2972–2985. doi: 10.1523/JNEUROSCI.04-12-02972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle AL, Luo B, Magnus TH, Stell WK. Substance P-, F8Famide-, and A18Famide-like immunoreactivity in the nervus terminalis and retina of goldfish, Carassius auratus. Cell Tiss Res. 1995;280:605–615. doi: 10.1007/BF00318363. [DOI] [PubMed] [Google Scholar]
- Lee SC, Hayashida Y, Ishida AT. Availability of low-threshold Ca2+ current in retinal ganglion cells. J Neurophysiol. 2003;90:3888–3901. doi: 10.1152/jn.00477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. Zebrafish mutants: Behavioral genetic studies of visual system defects. Dev Dyn. 2001;221:365–372. doi: 10.1002/dvdy.1159. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J Neurosci. 2000a;20:1883–1892. doi: 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE. Effects of dopamine depletion on visual sensitivity of zebrafish. J Neurosci. 2000b;20:1893–1903. doi: 10.1523/JNEUROSCI.20-05-01893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZS, Yazulla S. Depletion of retinal dopamine does not affect the ERG b-wave increment threshold function in goldfish in vivo. Vis Neurosci. 1994;11:695–702. doi: 10.1017/s095252380000300x. [DOI] [PubMed] [Google Scholar]
- Lindsay SM, Vogt RG. Behavioral responses of newly hatched zebrafish (Danio rerio) to amino acid chemostimulants. Chem Senses. 2004;29:93–100. doi: 10.1093/chemse/bjh009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Calcium currents in turtle retinal ganglion cells. II. Dopamine modulation via a cyclic AMP-dependent mechanism. J Neurophysiol. 1994;71:743–752. doi: 10.1152/jn.1994.71.2.743. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Li L. Olfactory input increases visual sensitivity in zebrafish: a possible function for the terminal nerve and dopaminergic interplexiform cells. J Exp Biol. 2003;206:2201–2209. doi: 10.1242/jeb.00397. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Riesbeck LE, Riley ME, Carr AL, Mullin JP, Nakamoto A, Li L. Behavioral screening of nightblindness mutants in zebrafish reveals three new loci that cause dominant photoreceptor cell degeneration. Mech Ageing Dev. 2005;126:1079–1089. doi: 10.1016/j.mad.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Malz CR, Jahn H, Meyer DL. Centrifugal Phe-Met-Arg-Phe-NH2-like immunoreactive innervation of the retina in a non-teleost bony fish, Lepisosteus osseus. Neurosci Lett. 1999;264:33–36. doi: 10.1016/s0304-3940(99)00169-x. [DOI] [PubMed] [Google Scholar]
- Mangrum WI, Dowling JE, Cohen ED. A morphological classification of ganglion cells in the zebrafish retina. Vis Neurosci. 2002;19:767–779. doi: 10.1017/s0952523802196076. [DOI] [PubMed] [Google Scholar]
- Michel WC, Lubomudrov LM. Specificity and sensitivity of the olfactory organ of the zebrafish, Danio rerio. J Comp Physiol A. 1995;177:191–199. doi: 10.1007/BF00225098. [DOI] [PubMed] [Google Scholar]
- Münz H, Claas B, Stumpf WE, Jennes L. Centrifugal innervation of the retina by luteinizing hormone releasing hormone (LHRH)-immunoreactive telencephalic neurons in teleostean fishes. Cell Tiss Res. 1982;222:313–323. doi: 10.1007/BF00213215. [DOI] [PubMed] [Google Scholar]
- Negishi K, Teranishi T, Kato S. The dopamine system of the teleost fish retina. Prog Ret Res. 1990;9:1–48. [Google Scholar]
- Puppala D, Maaswinkel H, Mason B, Legan SJ, Li L. An in vivo microdialysis study of light/dark-modulation of vitreal dopamine release in zebrafish. J Neurocytol. 2004;33:193–201. doi: 10.1023/b:neur.0000030694.88653.d6. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Li L. Rod and cone signaling transmission in the retina of zebrafish: An ERG study. Intl J Neurosci. 2004a;114:259–270. doi: 10.1080/00207450490269480. [DOI] [PubMed] [Google Scholar]
- Ren JQ, Li L. A circadian clock regulates the process of ERG b- and d-wave dominance transition in dark-adapted zebrafish. Vision Res. 2004b;44:2147–2152. doi: 10.1016/j.visres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Springer AD. Centrifugal innervation of goldfish retina from ganglion cells of the nervus terminalis. J Comp Neurol. 1983;214:404–415. [Google Scholar]
- Stell WK, Walker SE, Ball AK. Functional-anatomical studies on the terminal nerve projecting to the retina of bony fish. Ann N Y Acad Sci. 1987;519:80–96. doi: 10.1111/j.1749-6632.1987.tb36288.x. [DOI] [PubMed] [Google Scholar]
- Stell WK, Walker SE, Chohan KS, Ball AK. The goldfish nervus terminalis: a luteinizing hormone-releasing hormone and molluscan cardioexcitatory peptide immunoreactive olfactoretinal pathway. Proc Natl Acad Sci U S A. 1984;81:940–944. doi: 10.1073/pnas.81.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terubayashi H, Fujisawa H, Itoi M, Ibata Y. Hypothalamo-retinal centrifugal projection in the dog. Neurosci Lett. 1983;40:1–6. doi: 10.1016/0304-3940(83)90082-4. [DOI] [PubMed] [Google Scholar]
- Uchiyama H. Centrifugal pathways to the retina: influence of the optic tectum. Vis Neurosci. 1989;3:183–206. doi: 10.1017/s0952523800009950. [DOI] [PubMed] [Google Scholar]
- Umino O, Dowling JE. Dopamine release from interplexiform cells in the retina: Effects of GnRH, FMRFamide, bicuculline, and enkephalin on horizontal cell activity. J Neurosci. 1991;11:3034–3046. doi: 10.1523/JNEUROSCI.11-10-03034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinèiè T, Caprio J. Visual and chemical release of feeding behavior in adult rainbow trout. Chem Senses. 1997;22:375–382. doi: 10.1093/chemse/22.4.375. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, Pignatelli A, Partida G, Ishida AT. A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J Neurosci. 2001;21:8624–8635. doi: 10.1523/JNEUROSCI.21-21-08624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SE, Stell WK. Gonadotropin-releasing hormone (GnRF), molluscan cardioexcitatory peptide (FMRFamide), enkephalin and related neuropeptides affect goldfish retinal ganglion cell activity. Brain Res. 1986;384:262–173. doi: 10.1016/0006-8993(86)91162-5. [DOI] [PubMed] [Google Scholar]
- Weiss O, Meyer DL. Odor stimuli modulate retinal excitability in fish. Neurosci Lett. 1988;93:209–213. doi: 10.1016/0304-3940(88)90083-3. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) Eugene: University of Oregon Press; 1995. [Google Scholar]
- Wirsig-Wiechmann CR, Wiechmann AF, Eisthen HL. What defines the nervus terminalis? Neurochemical, developmental, and anatomical criteria. Prog Brain Res. 2002;141:45–58. doi: 10.1016/S0079-6123(02)41083-7. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. Prog Retinal Res. 1991;11:247–292. [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci. 1988;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Li L. Dopamine modulates voltage-activated potassium currents in zebrafish retinal ON bipolar cells. J Neurosci Res. 2005;82:368–376. doi: 10.1002/jnr.20637. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Dowling JE. Centrifugal fibers synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330:166–168. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]