Abstract
Glycinergic synapses are implicated in the coordination of reflex responses, sensory signal processing and pain sensation. Their activity is pre- and postsynaptically regulated, although mechanisms are poorly understood. Using patch-clamp recording and Ca2+ imaging in hypoglossal motoneurones from rat and mouse brainstem slices, we address here the role of cytoplasmic Ca2+ (Cai) in glycinergic synapse modulation. Ca2+ influx through voltage-gated or NMDA receptor channels caused powerful transient inhibition of glycinergic IPSCs. This effect was accompanied by an increase in both the failure rate and paired-pulse ratio, as well as a decrease in the frequency of mIPSCs, suggesting a presynaptic mechanism of depression. Inhibition was reduced by the cannabinoid receptor antagonist SR141716A and occluded by the agonist WIN55,212-2, indicating involvement of endocannabinoid retrograde signalling. Conversely, in the presence of SR141716A, glycinergic IPSCs were potentiated postsynaptically by glutamate or NMDA, displaying a Ca2+-dependent increase in amplitude and decay prolongation. Both presynaptic inhibition and postsynaptic potentiation were completely prevented by strong Cai buffering (20 mm BAPTA). Our findings demonstrate two independent mechanisms by which Ca2+ modulates glycinergic synaptic transmission: (i) presynaptic inhibition of glycine release and (ii) postsynaptic potentiation of GlyR-mediated responses. This dual Ca2+-induced regulation might be important for feedback control eof neurotransmission in a variety of glycinergic networks in mammalian nervous systems.
Glycinergic synapses in mature central nervous systems of vertebrates are implicated in the coordination of reflex responses, in the processing of sensory signals and in pain sensation. These synapses provide inhibitory neurotransmission by postsynaptic activation of Cl−-selective glycine receptor (GlyR) channels (Hamill et al. 1983; Bormann et al. 1987). The functioning of GlyRs is regulated by several pathways, including cytoplasmic Ca2+ (Cai) (Ragozzino & Eusebi, 1993; Xu et al. 1999; Fucile et al. 2000), phosphorylation (Gu & Huang, 1998) and G-proteins (Yevenes et al. 2003). Particularly intriguing is the phenomenon of Ca2+-induced fast potentiation of GlyRs (Fucile et al. 2000). This rapid transient increase in glycine-induced currents, resulting from Ca2+ elevation, is independent of both phosphorylation and G-proteins and, presumably, involves an unknown Ca2+-binding protein. The phenomenon of Ca2+-induced potentiation has been well documented for extrasynaptic GlyRs (Fucile et al. 2000; Zhu et al. 2002; Diana & Bregestovski, 2005). Whether synaptically activated GlyRs are regulated by Cai during synaptic transmission is not known.
To answer this question, we analysed the effects of Cai on glycinergic inhibitory postsynaptic currents (IPSCs) in hypoglossal motoneurones (HMs) from rat and mouse brainstem acute slices. HMs are characterized by rhythmic activity and they are involved in a variety of motor functions, including breathing, chewing, sucking, swallowing and phonation (Peever & Duffin, 2001). HMs receive synaptic information from various parts of the brain due to dendritic arborization, and exhibit two remarkable features. The first is a low endogenous Ca2+-buffering capacity, which determines both the selective vulnerability of HMs to Ca2+-related excitotoxicity and the rapid dynamics of Cai (Lips & Keller, 1998; Palecek et al. 1999). The second is powerful glycinergic synaptic inputs, which present the major inhibitory drive in this brainstem nucleus (Umemiya & Berger, 1995; Singer et al. 1998; Donato & Nistri, 2000; Singer & Berger, 2000). HMs thus provide a convenient model for examination of the action of intracellular Ca2+ on glycinergic synapses.
We report here that Cai elevation in HMs modulates glycinergic synaptic transmission by two independent mechanisms of opposite sign: (i) a decrease in glycinergic IPSCs due to the reduction in presynaptic glycine release induced, predominantly, by retrograde action of endogenous cannabinoids; (ii) potentiation of postsynaptic GlyRs. Under normal physiological conditions in HMs, the postsynaptic effect is masked by powerful presynaptic inhibition.
Methods
Slice preparation
Experiments were carried out on brainstem slices from postnatal (P5–P9) Sprague-Dawley or Wistar rats and C57BL/6J or Swiss mice. Animals were anaesthetized with ether and killed by decapitation in agreement with the European Directive 86/609/EEC requirements. The brainstem was rapidly removed and placed in an oxygenated ice-cold saline buffer. Transverse 250-μm-thick brainstem slices were cut using a DSK (Dosaka, Japan) or HM 650V (Microm, Germany) vibrating microslicer in ice-cold oxygenated solution containing (mm): NaCl, 125; KCl, 3.5; CaCl2, 2; MgCl2, 1.3; NaH2PO4, 1.25; NaHCO3, 26; and glucose, 10; equilibrated at pH 7.3 with 95% O2 and 5% CO2. Prior to recording, slices were incubated at room temperature (22–25°C) for at least one hour to allow recovery. Experiments were performed in the same solution at room temperature.
Electrophysiological recordings
For patch-clamp recordings brainstem acute slices were visualized through a ×40 water-immersion objective using an upright microscope (Axioskop, Zeiss, Germany). HMs were identified by their location in the hypoglossal nucleus (n.XII), their large somata (25–40 μm) and their dendritic arborization.
During measurements, slices were superfused with oxygenated saline (1.0–1.5 ml min−1). Whole-cell borosilicate glass pipettes (Hilgenberg or Clark Capillaries) with a tip resistance of 2–5 MΩ were filled with a KCl-based intracellular solution containing (mm) KCl, 135; MgCl2, 1; Hepes, 10; Mg-ATP, 4; Na-GTP, 0.3; equilibrated at pH 7.3 with KOH. Different BAPTA concentrations (0.1–20 mm) were used as indicated in the text. To obtain about 50 nm free Cai in the internal solution, corresponding concentrations of CaCl2 (0.03–6 mm) were added (calculating using developed by Dr F. Mendez (Gottingen) the ‘Patcher's Power Tools’ subroutine of the ‘Igor’ program). In some experiments an alternative CsCl-based intracellular solution was used, containing (mm) CsCl, 140; MgCl2, 2; Hepes, 10; MgATP, 2; Na2GTP, 0.4; CaCl2, 0.11; and EGTA, 0.5 (pH 7.3).
Membrane currents were recorded at 3–10 kHz with an Axopatch 200A (Axon Instruments, USA) or EPC-9 (HEKA Elektronik, Germany) amplifiers. Stored data were analysed using pClamp software (Axon Instruments) or the PulseFit program (HEKA Elektronik). The series resistances, ranging between 5 and 15 MΩ as estimated from slow transient cancellation, were compensated by 30–80% depending on the cell. The holding potential (Vh) was −70 mV. IPSCs were elicited by stimulating (2–100 V, 200–300 μs) at 0.5–1 Hz with glass bipolar electrodes. The stimulus intensity was adjusted to obtain about 20–50% failures. Stimulating electrodes were obtained by pulling theta-glass tubes to a final tip diameter of 1–2 μm and filling with external solution. Pairs of stimuli (at 50 ms intervals) were applied.
To isolate strychnine-sensitive glycinergic synaptic currents all experiments were performed in the presence of blockers of glutamate (CNQX, 10 μm; AP-5, 40 μm) and GABA (bicuculline, 20 μm) receptors unless otherwise mentioned. Control experiments (n = 5) showed that, at the concentrations used, bicuculline did not affect the amplitude of glycinergic IPSCs (see supplementary material). To record glycinergic miniature IPSCs (mIPSCs) TTX (1 μm) was added to the external solution in addition to the antagonists of glutamate and GABA receptors. Cannabinoid (CB) receptor agonist WIN55,212-2 (2,3-dihydro-5-methyl-3-(4-morpholinymethyl)pyrrolo[1,2,3de]-1,4-benzoxazin-6-yl]-1naphthalenylmethanone mesylate) and antagonist SR141716A (N-piperidini-5(4chlorophenyl)-4-methyl-3pyrazole-carboxamide) were bath-applied at concentrations of 3–5 μm. These concentrations are far above affinity constant (Ki) for CB1 receptors (Howlett et al. 2002) and similar concentrations have been used in a number of studies on brain slices (Hajos et al. 2001; (Jennings et al. 2001; Diana et al. 2002; Marsicano et al. 2002; Yoshida et al. 2002). Adding WIN55,212-2 or SR141716A did not change leakage currents or input resistance of recorded HMs.
To increase the cytosolic Ca2+, the recorded neurones were repetitively depolarized to 0 mV (1 s pulse duration). Each single depolarization was preceded by a 100 ms step to −100 mV to allow complete activation of voltage-dependent Ca2+ channels, and was followed by electrical stimulation to produce an IPSC after a delay of 100–300 ms. This protocol was repeated 20–40 times (30 times in most of experiments) and properties of averaged glycinergic IPSCs were compared in control and following depolarizing series.
For external application, agonists were applied via a puff pipette close to the cell soma. For glycine application, pipettes contained either low (30 μm) or high (0.5–5 mm) concentrations of the agonist. Glutamate and NMDA were applied at a concentration of 200 μm. Glycine and NMDA were dissolved in the standard external solution (Ca2+ 2 mm; Mg2+ 1.3 mm). In experiments with application of glutamate, to maximize the influx of Ca2+ the puff pipette contained Mg2+-free extracellular solution with elevated Ca2+ (10 mm). In some experiments, at NMDA application cells were concomitantly depolarized (−20 mV) to give relief from Mg2+ block (Nowak et al. 1984). Effects of NMDA and glutamate were studied with AP-5 omitted from the external solution.
Antagonists and agonists were bath-applied via a gravity-driven perfusion system (unless otherwise specified). AP-5, bicuculline and WIN55,212-2 were from Tocris (UK), SR141716A was the gift from Sanofi–Aventis and TTX was from Latoxan (France). All the other chemicals were from Sigma (USA).
The results presented here have been obtained on 70 HMs from rat and 79 HMs from mouse brain slices. As data obtained on the two preparations were similar they have been pooled (unless otherwise specified).
Statistical analysis was done in Origin (OriginLab Corporation, USA) and SigmaStat (Systat Software Inc., USA) programs using Student's paired t test or Wilcoxon's signed-rank test for non-parametric data. The Kolmogorov–Smirnov statistical test was used to assess differences in distribution of glycinergic mIPSC intervals. Results are given as means ± s.e.m.
Calcium imaging
Monitoring of Cai was conducted using a customized digital imaging microscope allowing simultaneous monitoring of whole-cell currents and fluorescence signals. Fluo-5F (50–100 μm) or, in some experiments, Fura-2 (50–200 μm) (both from Molecular Probes, Netherlands) were included in the internal solution and allowed to diffuse into the neurone for at least 15 min before the beginning of the records. The single (480-nm) or dual (350/380-nm) wavelength excitation was achieved using a 1 nm bandwidth polychromatic light selector equipped with a 100 W xenon lamp (Polychrome II, TILL Electronics, Germany). Fluorescence was visualized using an upright microscope (Axioskop, Zeiss, Germany) and a 12-bit charge-coupled device (CCD) camera equipped with an image intensifier (PentaMax, Princeton Instruments, USA). Fluorescence signals were acquired with variable sampling rate (from 0.2 to 10 Hz), using MetaFluor software (Universal Imaging Co, USA). Because of high Cai transients in HMs, fluorescence signals recorded with Fura-2 showed a tendency to saturation. To avoid this problem, a lower sensitivity Ca2+ dye, Fluo-5F, was used in the experiments described here. To ensure that Ca2+ signals were recorded within the dynamic range of Fluo-5F, the membrane permeability of HMs was artificially increased by a series of electrical discharges at the end of some experiments. Under these conditions the estimated maximal change in fluorescence (ΔF) was 2–5-fold higher than that induced by depolarization-induced Cai transients.
Results
Ca2+-dependent modulation of glycinergic synaptic currents in brainstem motoneurones
Properties of glycinergic IPSCs were studied in visually identified HMs. IPSCs were evoked with a theta-tube glass pipette placed either close (100–150 μm) to the recorded neurone or on the surface of small neurones (<10 μm) in the reticular formation, ventral to and immediately outside the hypoglossal nucleus, allowing extracellular monosynaptic stimulation (Umemiya & Berger, 1995). In the presence of antagonists of GABA and glutamate ionotropic receptors, stable IPSCs (range in different cells 50–800 pA, n = 149) were recorded at −70 mV, and their activity was completely abolished by strychnine (3 μm; Fig. 1A). IPSC deactivation kinetics was best fitted by a single exponential. The decay time constant varied in different experimental conditions from 6 to 20 ms, consistently with previously described kinetic properties (Takahashi et al. 1992; Singer et al. 1998; Singer & Berger, 1999; Donato & Nistri, 2000).
Figure 1. Glycinergic synaptic currents and depolarization-induced Cai dynamics in the hypoglossal motoneurones (HMs).
A, top trace, glycinergic IPSCs recorded in a voltage-clamped HM from an acute brainstem slice in the presence of bicuculline (20 μm), AP-5 (40 μm) and CNQX (10 μm). The trace was selected to also show spontaneous IPSCs. These currents were abolished by 3 μm strychnine (bottom trace). B, Cai fluorescence transients induced by repetitive depolarizations in HM dialysed with 0.1 mm BAPTA and 100 μm Fluo-5F. C, dependence of the amplitude of Cai fluorescence on the number of cell depolarizations; average data (± s.e.m.) from seven cells are represented.
To study Ca2+-dependent modulation of glycinergic currents with whole-cell recordings, we used relatively low concentrations of Ca2+ buffers in pipette solutions (0.1 mm BAPTA or 0.5 mm EGTA). An increase in Cai was induced by a series of somatic depolarizations of the recorded HMs (see details in Methods).
When the recording pipette contained 0.1 mm BAPTA and 0.1 mm Fluo-5F, depolarization caused a rapid Cai rise, whose amplitude was proportional to the number of depolarizing pulses (Fig. 1B). In these conditions, after 10–30 depolarizing pulses, the quasi-maximum values for Cai-induced relative fluorescence amplitudes (Fig. 1C) were reached. For this reason a depolarization protocol employing 30 pulses (otherwise mentioned) was chosen in the subsequent investigations.
Depolarization-induced Cai rise in HMs caused a significant decrease in glycinergic IPSC amplitude. IPSC depression was followed by a recovery of the amplitude within 3–5 min (Fig. 2A and B). As illustrated in Fig. 2C and D, the inhibition become more prominent with elevation of a number of depolarisations, and reached the quasi-maximum values after 20–30 pulses. These results were observed in rat and mouse HMs. In the rat HMs, the depolarization-induced inhibition was recorded with different Ca2+ buffers in the pipette solution. With BAPTA 0.1 mm, the mean depression was 49 ± 3% (n = 10, P < 0.001, t test; values are given throughout as mean ± s.e.m.); with BAPTA 0.5 mm or 1 mm and with EGTA 0.5 mm depression was, respectively, 44%± 4% (n = 5, P = 0.002, t test; Fig. 2B), 38 ± 3% (n = 10, P < 0.001, t test) and 41 ± 3% (n = 12, P < 0.001, t test). A similar degree of inhibition was observed in the mouse HMs; at recordings with BAPTA 0.1 mm in the pipette the mean depression was 51 ± 2% (n = 30, P < 0.001, t test). These results demonstrate that even at relatively strong Cai buffering (BAPTA 1 mm), a series of depolarizations caused a substantial inhibition of glycinergic IPSCs.
Figure 2. Ca2+-dependent inhibition of glycinergic synaptic currents in HMs.
A, effect of depolarization on glycinergic IPSCs recorded in HM with 0.5 mm BAPTA. Average traces, representing 30 individual evoked IPSCs, before (Control), in the first minute after (Depo) and 5 min after (Recovery) the series of depolarizations. B, average inhibition of glycinergic IPSCs induced by repeated depolarization (mean (± s.e.m.) from five experiments). *significant difference (P = 0.002). C, average traces of glycinergic IPSCs before (Control) and after (Depo) of 5 (left traces) or 30 (right traces) repetitive depolarizations of HM. Note stronger inhibition with elevation of depolarizing pulses. D, dependence of degree of glycinergic IPSCs' inhibition on the number of cell depolarizations. Data (± s.e.m.) from seven neurones.
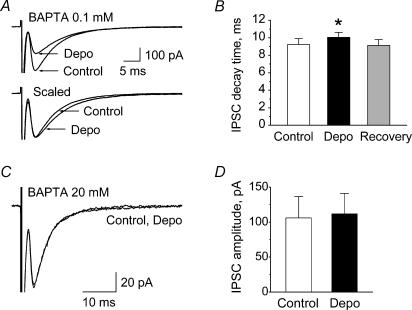
In addition to amplitude decrease, a weak but consistent reversible prolongation of the IPSC decay time constant was observed following depolarization-induced elevation of Cai (Fig. 3A and B). As summarized in Fig. 3B, at 0.1 mm BAPTA in the recording pipette, the mean decay time of glycinergic IPSCs before depolarization was 9.2 ± 0.7 ms; after depolarization it was 10% longer (10.1 ± 0.8 ms, n = 10, P = 0.01, Wilcoxon test) and 5 min later the decay kinetics had recovered to the control value (9.1 ± 0.8 ms).
Figure 3. Ca2+-dependent prolongation of glycinergic synaptic currents in HMs.
A, depolarization-induced prolongation of glycinergic IPSCs. Top traces, average traces of IPSCs before (Control) and after (Depo) the series of depolarizations. Bottom traces, same traces as the top, scaled to control amplitude, showing the increase in the decay time of IPSCs after depolarization. B, summary of depolarization-induced decay time prolongation of glycinergic IPSCs (mean (± s.e.m.) from 10 experiments). *Significant difference (P = 0.01). C and D, strong Ca2+ buffering suppressed depolarization-induced modulation of glycinergic IPSCs. C, superimposed traces representing IPSCs recorded in BAPTA 20 mm solution before (Control) and after (Depo) series of depolarizations. D, average of IPSC amplitudes in BAPTA 20 mm internal solution before (Control) and after (Depo) depolarization. Data (± s.e.m.) from seven neurones.
With very strong Cai buffering (BAPTA 20 mm internal solution), depolarization failed to modulate the amplitude and kinetics of glycinergic IPSCs (n = 7, Fig. 3C and D). These results indicate that: (i) postsynaptic depolarization of HMs causes depression of IPSC amplitude and weak prolongation of its decay time; and (ii) both phenomena are Ca2+ dependent.
Ca2+-induced potentiation of glycine-induced currents
Ca2+-dependent depression of glycinergic IPSCs could be presynaptic in origin, due to inhibition of neurotransmitter release. Alternatively, it could be induced postsynaptically if the rise in Cai caused downregulation of GlyRs expressed in HMs, i.e. these receptors exhibit properties different from those described in spinal neurones and in HEK 293 cells expressing GlyRs (Fucile et al. 2000).
To address this point, we analysed the whole-cell currents evoked by pressure application of exogenous glycine from a puff pipette positioned close to the recorded HMs. Depending on the agonist concentrations and duration of application pulses, at a holding potential (Vh) of −70 mV, glycine induced inward currents (IGly) in the range 50–1000 pA.
When the Cai was increased by repeated depolarization, systematic modulation of IGly was not observed (not shown). A previous study (Fucile et al. 2000) demonstrated that, in spinal cord neurones, conditioning pulses of glutamate induce the potentiation of responses to exogenously applied glycine. As HMs express both NMDA and Ca2+-permeable AMPA receptors (O'Brien et al. 1997; Essin et al. 2002), we used glutamate receptor ionic channels as a tool to elevate Cai during whole-cell recordings.
When a second puff pipette, containing glutamate (200 μm), was positioned close to the motoneurone's soma, a conditioning application of glutamate caused a large and reversible increase in the peak amplitude of IGly (Fig. 4A and B). No changes in the decay time of glycine-evoked currents were observed. This is in agreement with the fact that at puff application the decay time of IGly is determined by the relatively slow rate of glycine washout from the vicinity of recorded HM. On average, when recording with 0.1 mm BAPTA in the intracellular solution, after glutamate application IGly increased to 154 ± 8% (n = 6, P = 0.001, t test) and recovered to the control value (110 ± 3%) within 2–3 min (Fig. 4A–C). The extent of potentiation increased with prolongation of the conditioning glutamate pulse (Fig. 4B). Similar augmentation of IGly was observed with a conditioning application of NMDA (200 μm) dissolved in standard extracellular solution. After NMDA pulses (5–10 s), the IGly amplitude reversibly increased to 127 ± 3% (n = 3, P = 0.013, t test; Fig. 4D).
Figure 4. Ca2+-dependent potentiation of glycine-evoked currents in HMs.
A, glycine-evoked currents (IGly) recorded in HM with 0.1 mm BAPTA in intracellular solution are potentiated by a conditioning pulse of glutamate. Traces of whole-cell currents induced by pressure application of glycine (2 mm, 20 ms, arrows) recorded before, immediately after and 2 min after a glutamate pulse (200 μm, 24 s). B, time course of successive IGly peaks in an experiment when two conditional pulses of glutamate, respectively, of 12 and 24 s (indicated by bars), were applied to HM. Note reversible potentiation of IGly amplitude by glutamate. C, glutamate-induced potentiation of IGly from six neurones obtained in BAPTA 0.1 mm. Columns represent the amplitudes of Igly normalized with respect to control. *Significant difference (P = 0.008). D, average potentiation of IGly by NMDA (200 μm, 5 s) obtained in BAPTA 0.1 mm (three cells) and in BAPTA 20 mm (five cells) internal solutions. Current amplitudes are normalized to control amplitude before NMDA application. Note that in BAPTA 20 mm solution there is no potentiation of IGly. Data are mean ± s.e.m.*significant difference (P = 0.013).
When the same protocol was performed in cells recorded in the presence of high-Cai buffer, no effect on the glycine-induced currents was observed. In five cells recorded with BAPTA 20 mm intracellular solution, amplitudes of IGly after NMDA application were similar to control (102 ± 1%, n = 5; Fig. 4D).
These results indicate that glutamate- or NMDA-dependent elevation of glycine-evoked currents is a Ca2+-dependent phenomenon. They also demonstrate that glycine receptors expressed in HMs are potentiated by Cai, similarly to GlyRs previously described (Fucile et al. 2000), raising the question of the mechanism of Ca2+-dependent depression of glycinergic IPSCs shown in Fig. 2.
Presynaptic nature of Ca2+-dependent depression at glycinergic synapses
Depression of glycinergic IPSCs in HMs resembles the phenomenon called depolarization-induced suppression of inhibition (DSI), described originally for GABAergic synapses in cerebellar Purkinje cells (Llano et al. 1991). DSI is initiated postsynaptically due to a rise in Cai and expressed presynaptically as a decrease in neurotransmitter release. This phenomenon is accompanied by an increase in the failure rate (Vincent et al. 1992; Diana & Marty, 2003) and also an elevation in the paired-pulse ratio (PPR) (Yoshida et al. 2002; Diana & Marty, 2003).
To clarify whether the Ca2+-dependent depression of glycinergic IPSCs was of presynaptic origin, we analysed changes in these parameters before and after Cai elevation in HMs. To measure the PPR, two consecutive pulses separated by 50 ms were delivered to the stimulating pipette. In the majority of neurones, we observed an increase in IPSC amplitude evoked by the second stimulus (I2) with respect to the first one (I1) (Fig. 5A and B). In whole-cell recordings with 0.5 mm EGTA in the intracellular solution, the average value of the PPR, expressed as the ratio I2/I1, was 1.13 ± 0.05 (n = 10, range 0.91–1.34; Fig. 5C). When a series of depolarizing steps was delivered to the recorded neurone, the PPR significantly increased. In the first minute after depolarization-induced Ca2+ influx, PPR increased to 1.36 ± 0.08 (n = 10, P = 0.002, Wilcoxon test) and recovered to the control value within 5 min (1.11 ± 0.05; Fig. 5C). The failure rate of glycinergic IPSCs also increased after the series of depolarizing pulses. On average, the failure rate increased from 34 ± 4% to 62 ± 6% (n = 10, P = 0.002, Wilcoxon test; Fig. 5D).
Figure 5. Depolarization-induced inhibition alters paired-pulse facilitation of glycinergic IPSCs.
A, superimposed traces showing glycinergic IPSCs evoked by a paired stimulation (50 ms interval) before (Control) and after (Depo) the series of depolarizations. B, same traces as in A, scaled to control amplitude, showing the increase in the paired-pulse facilitation after depolarization. C, paired pulse ratio (I2/I1) observed in 10 neurones before (Control), in the first minute after (Depo) and 5 min after (Recovery) the series of depolarizations. The mean values of PPR (± s.e.m.) are represented by open circles. D, failure rate of glycinergic IPSCs estimated in the same conditions as in C for 10 cells. The mean values of failure rate are represented by open circles.
As another way of clarifying the origin of depolarization-induced depression, we analysed properties of spontaneous IPSCs (sIPSCs) as well as miniature IPSCs (mIPSCs) recorded in the presence of TTX (1 μm). In control conditions, the average values of amplitude and frequency of glycinergic mIPSCs in HMs were: 50 ± 5 pA and 0.83 ± 0.36 s−1 (n = 7), correspondingly. Series of depolarizations caused a reduction in frequency of glycinergic mIPSCs (Fig. 6A), as further illustrated by the cumulative interevent interval distribution (n = 7, P < 0.001, Kolmogorov–Smirnov test; Fig. 6B). On average, depolarization resulted in a decrease in the frequency of mIPSCs by 53 ± 2% (n = 7, P = 0.016, Wilcoxon test) without significant changes in their amplitude (Fig. 6C and D). Recorded in the absence of TTX, glycinergic sIPSCs exhibited depolarization-induced decreases in both the amplitude and frequency, by 35 ± 10% (n = 7, P = 0.007, t test; Fig. 6E) and 39 ± 4% (n = 7, p = 0.027, Wilcoxon test; Fig. 6F), correspondingly.
Figure 6. Effect of depolarization on glycinergic mIPSCs and sIPSCs.
A, glycinergic mIPSCs recorded in the presence of TTX (1 μm) before and after the series of 20 depolarizations. Note the clear reduction in frequency of mIPSCs following depolarization. Inset shows average traces of mIPSCs recorded during 20 s intervals before and immediately after the series of depolarizations. B, cumulative interevent interval histograms of glycinergic mIPSCs, showing the increase in the time intervals between mIPSCs after a series of 20 depolarizations. Data from seven motoneurones. C and D, average amplitude and frequency values of glycinergic mIPSCs from seven cells before (Control), in the first minute after (Depo) and 3–5 min after (Recovery) the series of depolarizations. Note the significant decrease only in the frequency of mIPSCs. *Significant difference (P = 0.016). E and F, average amplitude and frequency values of glycinergic sIPSCs before (Control), in the first minute after (Depo) and 3–5 min after (Recovery) the series of depolarizations. Note the significant decrease in both the amplitude and frequency of sIPSCs. Data (± s.e.m.) from seven motoneurones. *Significant difference (P = 0.007, E; P = 0.027, F).
These results strongly suggest that Ca2+-dependent depression of glycinergic IPSCs is of presynaptic origin and resembles DSI. For this reason we have called this phenomenon ‘glycinergic DSI’.
Involvement of endocannabinoids in Ca2+-dependent depression of glycinergic IPSCs
Several recent studies have demonstrated that the retrograde signalling for DSI is mediated by endo-cannabinoids, whose synthesis is stimulated by the depolarization-induced rise in Cai (Maejima et al. 2001; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001). To clarify whether such a mechanism could also be responsible for Ca2+-dependent glycinergic DSI, we analysed the effects of the cannabinoid (CB) receptor agonist WIN55,212-2 and the CB receptor antagonist SR141716A on IPSCs.
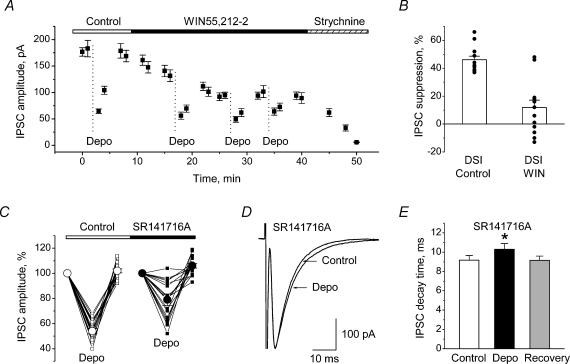
The CB receptor agonist WIN55,212-2 (3 μm) caused decrease in IPSCs and partially or completely occluded glycinergic DSI (Fig. 7A). In 13 recorded cells, in low-Ca2+-buffering conditions (0.5 mm EGTA or 0.1 mm BAPTA), IPSC suppression by WIN55,212-2 was 35 ± 3% (P < 0.001, t test; not shown). In these cells, control DSI achieved 46 ± 3% (Fig. 7B) while, in the presence of WIN55,212-2, the DSI value was, on average, only 12 ± 5% (n = 13, P < 0.001, t test; Fig. 7B). In two cells WIN55,212-2 was not effective while in the other 11 cells the DSI values varied from −13 to +19% (Fig. 7B). Administration of WIN55,212-2 caused augmentation of the mean PPR value from 1.20 ± 0.10 to 1.45 ± 0.13 (n = 13, P < 0.001, Wilcoxon test; not shown), suggesting the presynaptic origin of glycinergic IPSC suppression by this CB receptor agonist.
Figure 7. Effect of cannabinoid receptor agonist and antagonist on depolarization-induced inhibition of glycinergic IPSCs.
A and B, effect of the cannabinoid receptor agonist WIN55,212-2 on glycinergic IPSCs. A, time course of IPSC amplitudes in control conditions, during application of WIN55,212-2 (3 μm) and after addition of strychnine (1 μm) to the external solution. Four depolarization series (Depo) were applied during the course of experiment. Note that WIN55,212-2 caused a decrease in IPSC amplitude and partially occluded DSI; strychnine completely antagonized IPSCs, confirming the glycinergic nature of the currents. B, average values of DSI in control and in the presence of 3 μm WIN55,212-2. Data from 13 neurones. C, effect of cannabinoid receptor antagonist SR141716A on glycinergic DSI; the data from 16 neurones for depolarization-induced inhibition of IPSCs in control (□) and in the presence of 3–5 μm SR141716A (▪). The average values are shown by ○ (Control) and • (SR141716A). Note that the effect of SR141716A varied markedly in the different cells. D, superimposed traces of glycinergic IPSCs recorded in the presence of SR141716A (3–5 μm) before (Control) and after (Depo) depolarization; each trace is the average of 30 single IPSCs. Note decay time prolongation of glycinergic IPSCs after depolarization. E, averaged data from 16 neurones for decay time of IPSCs before (Control), in the first min after (Depo) and 5 min after (Recovery) depolarization. Data are mean ± s.e.m.*Significant difference (P < 0.001).
In 16 HMs recorded in low Ca2+-buffering conditions (0.5 mm EGTA or 0.1 mm BAPTA), we compared glycinergic DSI before and after SR141716A. In control, the DSI value varied from 32% to 60% (mean 46 ± 2%; Fig. 7C). SR141716A (3–5 μm) antagonized glycinergic DSI, however, with varying efficacy. In six cells the DSI value was nearly completely eliminated (to 0–10%) without changes in the control amplitude of IPSCs; in four cells DSI decreased to 18–30%, and in the remaining six neurones it stayed in the range 35–48% (Fig. 7C). On average, in the presence of SR141716A the DSI value was 21 ± 5% (n = 16, P < 0.001, t test; Fig. 7C). In addition, SR141716A prevented the effect of WIN55,212-2 on the IPSC amplitude (n = 10; not shown).
These results indicate that, in the hypoglossal nucleus, the release of glycine is modulated presynaptically through the Ca2+-induced cannabinoid machinery (Wilson & Nicoll, 2002; Freund et al. 2003; Diana & Marty, 2004), while the extent of this modulation varied for different HMs.
Similarly to the control conditions (Fig. 3A and B), in the presence of SR141716A (3–5 μm), glycinergic IPSCs exhibited depolarization-induced prolongation of decay kinetics (Fig. 7D and E). The mean decay time of IPSCs before depolarization was 9.2 ± 0.5 ms; after depolarization it was 11% longer (10.3 ± 0.6 ms; n = 16, P < 0.001, Wilcoxon test) and 5 min later it had recovered to the control value (9.1 ± 0.4 ms; Fig. 7E).
This weak but significant reversible increase of glycinergic IPSC decay time might reflect prolongation of postsynaptic glycine receptor channel openings during Cai elevation (Fucile et al. 2000). To check this hypothesis, we analysed glycinergic IPSCs when presynaptic modulation was suppressed by the CB receptor antagonist SR141716A and the Cai rise was transiently induced by activation of NMDA receptor channels.
Potentiation by NMDA of glycinergic IPSCs in the presence of a CB receptor antagonist
In the absence of a CB receptor antagonist, when the intracellular solution contained 0.1 mm BAPTA, application of NMDA (5–10 s pulses, Vh=−70 mV) resulted in a strong suppression of glycinergic IPSCs, similar to that caused by depolarization (Fig. 8A and B). On average, in six neurones analysed, the amplitude of glycinergic IPSCs after the NMDA pulse decreased to 47 ± 8% (P = 0.001, t test; Fig. 8B).
Figure 8. NMDA potentiates and prolongs glycinergic IPSCs in the presence of SR141716A.
A, B and C, NMDA-induced suppression of glycinergic IPSCs in physiological conditions. A, superimposed traces before and after NMDA application (left) and normalized to the amplitude of control (right). Recording with BAPTA 0.1 mm intracellular solution; NMDA was applied focally from a puff pipette (200 μm, 5–10 s, Vh=−70 mV). Each trace is the average of 10–20 single IPSCs. B, mean IPSC amplitude and C, decay time constant before (Control), in the first minute after (NMDA) and 5 min after NMDA application (Wash). Data (± s.e.m.) from six neurones. *Significant difference (P = 0.001, B; P = 0.031, C). Note pronounced increase in mean decay time after NMDA application. D, E and F, NMDA-induced potentiation of glycinergic IPSCs in the presence of the cannabinoid receptor antagonist SR141716A (3 μm). D, superimposed traces before and after NMDA application. Each trace is the average of 5–15 single IPSCs. E and F, similar presentation as in B and C. Mean data from six cells. Recording with 0.1 mm BAPTA. Note that NMDA caused an increase in IPSC amplitude and a prolongation of decay time, correspondingly by 23% and 80%. In this series of experiments, during the pulse of NMDA application the neurones were depolarized to −20 mV, which abolished Mg2+ block and, thus, elevated Ca2+ influx through NMDA receptor channels. *Significant difference (P = 0.012, E; P = 0.006, F). G, H and J, strong Ca2+ buffering in postsynaptic neurone abolishes NMDA-induced modulation of glycinergic IPSCs. Superimposed traces G, mean amplitudes H and decay times J of IPSCs in control and after NMDA application. For H and J, data are from six neurones. Recording with BAPTA 20 mm intracellular solution and with SR141716A (3 μm) in the extracellular solution.
Moreover, pulses of NMDA caused a pronounced prolongation of glycinergic IPSC decay time constants (by 29 ± 4%; n = 6, p = 0.031, Wilcoxon test; Fig. 8C), suggesting that, in addition to inhibition of presynaptic glycine release, postsynaptic GlyRs are profoundly modulated by this route of Cai elevation. This assumption was confirmed in the experiments when the presynaptic modulatory action of Ca2+ was diminished by a CB receptor antagonist.
In the presence of SR141716A (3 μm), following NMDA pulses, synaptic currents exhibited both significant increases in peak amplitudes and prolongation of decay time constants (Fig. 8D–F). Both parameters reverted almost completely to control values within 1–2 min. On average, in the first minute following NMDA application, the amplitude of IPSCs increased to 123 ± 6% of control (n = 6, p = 0.012, t test; Fig. 8E). In these experiments, to give relief from Mg2+ block, cells were concomitantly depolarized to −20 mV during NMDA applications. This facilitation of Ca2+ influx resulted in a particularly powerful prolongation of the IPSC decay time constant (by 80 ± 17%; n = 6, P = 0.006, Wilcoxon test; Fig. 8F). After the NMDA pulse, the IPSC amplitude and decay kinetics recovered to, respectively, 103 ± 5% and 119 ± 13% of control (n = 6; Fig. 8E and F).
When the same experiments were repeated with the BAPTA 20 mm internal solution, the NMDA-induced potentiation was absent: both IPSC amplitude (103 ± 6%, n = 6) and decay time constant (105 ± 4%, n = 6) measured following NMDA application were equal to control values (Fig. 8G, H and J).
Our observations demonstrate that GlyRs at glycinergic synapses can be strongly modulated by Ca2+ influx through glutamate receptor channels. This postsynaptic modulation could cause an increase in glycinergic transmission. However, in the HMs this phenomenon is masked by a powerful retrograde inhibition mediated partially by the endocannabinoid system.
Discussion
We have shown two novel roles for intracellular Ca2+ in modulation of glycinergic synaptic transmission in HMs: (i) presynaptic depression of neurotransmitter release; and (ii) postsynaptic potentiation of GlyR channels (Fig. 9). An inhibition of neurotransmitter release was induced by postsynaptic Ca2+ influx through voltage-gated Ca2+ channels or glutamate receptor channels, and it was observed even at relatively strong Ca2+ buffering (1 mm BAPTA in the recording pipette). This phenomenon is of presynaptic nature and the mechanism resembles endocannabinoid-stimulated inhibition of neurotransmitter release from presynaptic terminals (Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001; Diana & Marty, 2003). Potentiation of postsynaptic GlyRs was observed preferably at activation of glutamate receptors, and it was only detectable when endocannabinoid-mediated depression was suppressed. This effect results in an increase in amplitude and prolongation of decay time of glycinergic IPSCs, and resembles the mechanism of a rapid Ca2+-induced potentiation (Fucile et al. 2000). In hypoglossal brainstem motoneurones, the first phenomenon is highly predominant.
Figure 9. Scheme summarizing the mechanisms of glycinergic synaptic transmission modulation by intracellular Ca2+ in HMs.
Presynaptic depression. Activation of postsynaptic voltage-gated Ca2+ channels (1) or NMDA receptor (NMDAR) channels (2), increase in Cai (3) and synthesis of endocannabinoids (EC) (4). Diffusion of endocannabinoids to presynaptic terminals leads to activation of CB receptors (CBR) (5), which causes the reduction in Cai transients in the presynaptic terminals and, consequently, a decrease (−) in release probability of glycine-containing vesicles (6); this results in a decrease in glycinergic IPSC amplitude (7). WIN55,212-2 (WIN), a CB receptor agonist, causes similar inhibition of IPSCs. In addition the increase in Cai modulates GlyRs (8), causing slight IPSC prolongation. Postsynaptic potentiation. In the presence of a CB receptor antagonist (SR141716A), the presynaptic mechanism of IPSC depression (steps 5–7) is strongly reduced. On the other hand, the increase in Cai generated by depolarization (1) and, much more effectively, by activation of NMDA receptor channels (2), potentiates postsynaptic GlyRs (8), increasing (+) the open-channel probability (Fucile et al. 2000). This results in the increase in amplitude and prolongation of decay time of glycinergic IPSCs (9). In brainstem HMs, under normal physiological conditions, IPSC presynaptic depression is highly predominant. Potentiation of postsynaptic GlyRs is only detectable when endocannabinoid-mediated depression is suppressed.
Depolarization-induced inhibition
Several arguments indicate that the glycinergic DSI has a presynaptic origin and is largely mediated by the endo-cannabinoid system. First, we find that depolarization: (i) increases PPR and (ii) increases the failure rate of stimulation-induced IPSCs. Second, depolarization caused a decrease in the frequency of mIPSCs without changes in their amplitude. Third, SR141716A, a selective antagonist of the brain CB receptors, decreased glycinergic DSI without changes in IPSC amplitude. Finally, the CB receptor agonist WIN55,212-2 decreased the amplitude of IPSCs and occluded glycinergic DSI. The mechanism of Ca2+-induced feedback regulation of inhibitory and excitatory synaptic transmission via retrograde endo-cannabinoid signalling is widely used in the mammalian nervous system (Jennings et al. 2001; Wilson & Nicoll, 2001; Yoshida et al. 2002; Azad et al. 2003).
Endocannabinoids can modulate the function of ionic channels independently on CB receptors. It has been shown that the acid-sensitive potassium (K+) channel TASK-1 is directly blocked by submicromolar concentrations of anandamide (Maingret et al. 2001). Moreover, anandamide, as well as WIN55,212-2, can also inhibit the activity of glycine receptors in heterologous systems (Lozovaya et al. 2005). While for HMs, a direct modulation of ionic channels by endocannabinoids is not excluded, several arguments suggest that this mechanism plays a minor role in glycinergic DSI. First, presynaptic inhibition of K+ channels should lead to membrane depolarization and, consequently, stimulation of neurotransmitter release. In our experiments we observed the opposite effect. Second, postsynaptic inhibition by a CB receptor agonist of K+ TASK-1 channels should result in changes of both the input resistance and level of background current. However, WIN55,212-2 caused inhibition of glycinergic IPSCs without affecting the membrane properties of HMs. Third, SR141716A does not prevent the effects of anandamide and WIN55,212-2 on TASK-1 channels (Maingret et al. 2001), while in HMs this CB receptor antagonist effectively suppressed the action of WIN55,212-2. Finally, postsynaptic inhibition of ionic channels should be observed without changes in failure rate and PPR. In our experiments, these parameters were clearly modified by WIN55,212-2.
The fact that SR141716A did not completely suppress glycinergic DSI might indicate the possibility of an additional mechanism of Ca2+-induced feedback regulation. Firstly, glycinergic neurotransmission in HMs could be regulated via a putative, as yet unidentified, cannabinoid receptor. Indeed, besides the cloned G-protein-coupled CB1 and CB2, it has been suggested that other cannabinoid-sensitive receptors (CBX) are present in the brain (Di Marzo et al. 2000; Breivogel et al. 2001; Hajos et al. 2001; Hajos & Freund, 2002). Alternatively, endocannabinoid-independent retrograde inhibition via activation of GABAB or metabotropic glutamate receptors, similar to that described for glutamatergic EPSCs (Zilberter et al. 1999) or GABAergic IPSCs (Zilberter, 2000) in rat neocortex neurones, could be involved. The GABAB modulation pathway is particularly attractive as corelease of glycine and GABA from ‘mixed’ presynaptic terminals is well documented (Jonas et al. 1998; O'Brien & Berger, 1999). Moreover, in the auditory brainstem nucleus (Lim et al. 2000) and in HMs (O'Brien et al. 2004), GABA mediates presynaptic inhibition of glycinergic synapses via GABAB receptors.
Ca2+-induced potentiation
Our observations demonstrate that in glycinergic synapses, postsynaptic GlyRs are potentiated by Cai and this modulation strongly depends on the source of Ca2+ rise. Influx of Ca2+ through NMDA receptor channels was much more effective for potentiation of GlyRs than that through voltage-gated Ca2+ channels.
The organization of synaptic inputs and specificity of the cytosolic buffering system in HMs might account for these remarkable differences in Cai action. From light- and electron-microscopic observations, the vast majority (88–98%) of axonal projections on HMs forms axodendritic synapses (Zhang et al. 2001; Zhang et al. 2003). As levels of Ca2+ are regulated in a highly local environment, this would suggest that Ca2+ signals initiated in the soma and backpropogating to dendritically localized glycinergic synapses would be rapidly attenuated. Consequently, a depolarization-induced Cai rise in the vicinity of glycinergic synapses might be much weaker than those recorded in the soma. This is particularly critical for HMs, which exhibit a low concentration of endogenous Ca2+ buffers (Ca2+ binding ratio about 40) in the cytosol (Lips & Keller, 1998), which accounts for rapid relaxation times of Cai after depolarization. Analysis of Cai transients in HMs demonstrated that peak amplitudes of voltage-induced Cai rise are more than two-fold higher in the soma than in dendrites monitored 80 μm from the soma. Moreover, the decay time constant of Cai in dendrites is faster (Ladewig et al. 2003).
On the other hand, a high efficacy of Ca2+ entry through NMDA receptor channels might suggest that glutamatergic and glycinergic synapses are in close proximity on dendrites of HMs. It has been demonstrated on young brainstem slices that NMDA and non-NMDA receptors are colocalized on glutamatergic synapses in HMs; furthermore, these neurones exhibit a markedly higher proportion of NMDA receptor-mediated mEPSCs than do non-NMDA ones (O'Brien et al. 1997). On the basis of indirect evidence, the colocalization of NMDA and glycine receptors has been proposed in neurones of spinal cord (Fern et al. 1996) and brainstem hypoglossal nucleus (Berger et al. 1998). Thus, activation of dendritically situated NMDA receptors would produce a high local rise in Cai, and consequent potentiation of neighbouring GlyRs in glycinergic synapses.
The action of NMDA might be presynaptic in origin, as in some brain areas NMDA receptors are found on both excitatory and inhibitory terminals (Casado et al. 2000; Duguid & Smart, 2004). However, several lines of evidence indicate that the potentiation of IPSCs observed in our study is of postsynaptic origin and results from the increase in GlyR activity. First, NMDA similarly potentiated glycinergic IPSCs and ionic currents induced by exogenous application of glycine. Second, the increase in IPSC amplitude is associated with prolongation of decay time, as is expected for an increase in the apparent affinity of GlyR for the agonist (Fucile et al. 1999, 2000; Li & Pearce, 2000). Finally, the effect of NMDA was absent in the strong buffering of postsynaptic Ca2+.
The most relevant feature of Ca2+-induced postsynaptic potentiation is the reversible increase in duration of glycinergic IPSC decay time. We observed this, with different extents of prolongation, after a depolarization-induced Cai rise and after application of NMDA. An early study on HMs demonstrated that GlyR deactivation is the main determinant of glycinergic IPSC decay (Singer & Berger, 1999). As deactivation kinetics correlate with the apparent affinity of GlyR (Fucile et al. 2000; Li & Pearce, 2000), IPSC prolongation is consistent with a Ca2+-induced decrease in the EC50 for glycine.
Prolongation of the IPSC decay during glycinergic DSI could be of presynaptic origin. This might result from the lower probability of liberation during glycinergic DSI and, consequently, more scattered latencies of individual vesicular release events in comparison with the control. Some presynaptic effects on IPSC decay time constant are not entirely excluded, while several observations do not support this suggestion. First, the CB receptor antagonist SR141716A, which decreased DSI, should also suppress changes in IPSC's decay time. Our results indicate that modulation of glycinergic currents' kinetics was similar in the two conditions (Fig. 3A and 7D). Second, following NMDA application, similar prolongation of IPSCs was observed in control conditions when the amplitude of IPSCs was strongly depressed (Fig. 8A–C), as well as in the presence of SR141716A when the amplitude of IPSCs was augmented (Fig. 8D–F). For a presynaptic mechanism, the kinetics should follow the directions of amplitude variations. Finally, NMDA and depolarization caused similar suppression of amplitudes while NMDA-induced Cai rise resulted in much more pronounced prolongation of IPSC decay time. In the case of changes in the latencies of individual vesicular release, the effect should be similar in the two conditions.
The increase in glycinergic IPSC peak amplitude, although limited, is somewhat surprising because glycine-activated currents are not much affected by Ca2+-dependent potentiation at saturated concentrations of glycine (Fucile et al. 2000). Augmentation of IPSCs could indicate that GlyRs at glycinergic synapses of HMs are not saturated by transmitter release; this has been shown at glycinergic synapses in the zebrafish hindbrain (Rigo et al. 2003).
The double modulation of glycinergic synaptic transmission described here may be used for selective modification of neuronal activity under different physiological conditions. In particular, at early stages of postnatal development in some brain regions, such as hippocampus and neocortex, glycinergic (Ito & Cherubini, 1991) and GABAergic (Dammerman et al. 2000) responses are excitatory. Moreover, GlyRs may be activated in a non-synaptic fashion (Flint et al. 1998), suggesting that, before the establishment of mature chloride equilibrium, Ca2+-dependent potentiation of glycinergic currents potentially represents a positive feedback in the regulation of excitation, and may have a role in synaptic stabilization (Kirsch & Betz, 1998). This mechanism, however, would not be applicable for HMs as, immediately after birth, these motoneurones exhibit inhibition by synaptic glycinergic and GABAergic inputs (Marchetti et al. 2002).
Ca2+-induced potentiation might be critical at close colocalization of GlyRs with voltage-gated or receptor-operated Ca2+-permeable ion channels. This might be the case for the giant synapse of the calyx of Held, which contains presynaptic GlyRs; glycine spillover has been observed to activate these presynaptic receptors, regulating glutamate release (Turecek & Trussell, 2001). We suggest that, in a similar manner, the process of Ca2+-dependent potentiation of GlyRs might be relevant in the physiological regulation of synaptic activity.
Acknowledgments
The authors wish to thank Drs P. Ascher, M. Diana, F. Eusebi, A. Milne and Y. Zilberter for helpful discussions and critical reading of the manuscript. We thank Sanofi–Aventis for the gift of SR141716A. This work was carried out as part of a CNR-INSERM cooperation project and D. R. was supported by FIRB grant. We thank NATO, IBRO and Fondation pour la Recherche Médicale (FRM) for financial support of M. M.
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2005.094862
http://jp.physoc.org/cgi/content/full/jphysiol.2005.094862/DC1 and contains supplemental material consisting of a table entitled: Amplitudes and kinetics of glycinergic IPSCs in hypoglossal motoneurones at different concentrations of bicuculline
This material can also be found as part of the full-text HTML version available from http://www.blackwellsynergy.com
References
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Casado M, Dieudonne S, Ascher P. Presynaptic N-methyl-d-aspartate receptors at the parallel fiber–Purkinje cell synapse. Proc Natl Acad Sci U S A. 2000;97:11593–11597. doi: 10.1073/pnas.200354297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammerman RS, Flint AC, Noctor S, Kriegstein AR. An excitatory GABAergic plexus in developing neocortical layer 1. J Neurophysiol. 2000;84:428–434. doi: 10.1152/jn.2000.84.1.428. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Diana MA, Bregestovski P. Calcium and endocannabinoids in the modulation of inhibitory synaptic transmission. Cell Calcium. 2005;37:497–505. doi: 10.1016/j.ceca.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana MA, Marty A. Characterization of depolarization-induced suppression of inhibition using paired interneuron–Purkinje cell recordings. J Neurosci. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J Neurophysiol. 2000;84:2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron–Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- Essin K, Nistri A, Magazanik L. Evaluation of GluR2 subunit involvement in AMPA receptor function of neonatal rat hypoglossal motoneurons. Eur J Neurosci. 2002;15:1899–1906. doi: 10.1046/j.1460-9568.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- Fern R, Connolly GP, Harrison PJ. Evidence for functional co-activation of N-methyl-d-aspartate receptors by glycine. Neuroreport. 1996;7:1953–1956. doi: 10.1097/00001756-199608120-00018. [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron. 1998;20:43–53. doi: 10.1016/s0896-6273(00)80433-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fucile S, De Saint Jan D, David-Watine B, Korn H, Bregestovski P. Comparison of glycine and GABA actions on the zebrafish homomeric glycine receptor. J Physiol. 1999;517(2):369–383. doi: 10.1111/j.1469-7793.1999.0369t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, De Saint Jan D, De Carvalho LP, Bregestovski P. Fast potentiation of glycine receptor channels of intracellular calcium in neurons and transfected cells. Neuron. 2000;28:571–583. doi: 10.1016/s0896-6273(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Gu Y, Huang LY. Cross-modulation of glycine-activated Cl− channels by protein kinase C and cAMP-dependent protein kinase in the rat. J Physiol. 1998;506:331–339. doi: 10.1111/j.1469-7793.1998.331bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Bormann J, Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. Nature. 1983;305:805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ito S, Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J Physiol. 2001;534:805–812. doi: 10.1111/j.1469-7793.2001.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Kloppenburg P, Lalley PM, Zipfel WR, Webb WW, Keller BU. Spatial profiles of store-dependent calcium release in motoneurones of the nucleus hypoglossus from newborn mouse. J Physiol. 2003;547:775–787. doi: 10.1113/jphysiol.2002.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pearce RA. Effects of halothane on GABA(A) receptor kinetics: evidence for slowed agonist unbinding. J Neurosci. 2000;20:899–907. doi: 10.1523/JNEUROSCI.20-03-00899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Alvarez FJ, Walmsley B. GABA mediates presynaptic inhibition at glycinergic synapses in a rat auditory brainstem nucleus. J Physiol. 2000;525:447–459. doi: 10.1111/j.1469-7793.2000.t01-1-00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips MB, Keller BU. Endogenous calcium buffering in motoneurones of the nucleus hypoglossus from mouse. J Physiol. 1998;511:105–117. doi: 10.1111/j.1469-7793.1998.105bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Yatsenko N, Beketov A, Tsintsadze T, Burkashev N. Glycine receptors in CNS neutrons as target for nonretrograde action of cnannalinoids. J Neurosci. 2005;25:7499–7506. doi: 10.1523/JNEUROSCI.0977-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res. 2001;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Pagnotta S, Donato R, Nistri A. Inhibition of spinal or hypoglossal motoneurons of the newborn rat by glycine or GABA. Eur J Neurosci. 2002;15:975–983. doi: 10.1046/j.1460-9568.2002.01927.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Isaacson JS, Berger AJ. NMDA and non-NMDA receptors are co-localized at excitatory synapses of rat hypoglossal motoneurons. Neurosci Lett. 1997;227:5–8. doi: 10.1016/s0304-3940(97)00293-0. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Sebe JY, Berger AJ. GABA(B) modulation of GABA(A) and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol. 2004;141:35–45. doi: 10.1016/j.resp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Palecek J, Lips MB, Keller BU. Calcium dynamics and buffering in motoneurones of the mouse spinal cord. J Physiol. 1999;520:485–502. doi: 10.1111/j.1469-7793.1999.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Duffin J. Respiratory control of hypoglossal motoneurons. Adv Exp Med Biol. 2001;499:101–106. doi: 10.1007/978-1-4615-1375-9_16. [DOI] [PubMed] [Google Scholar]
- Ragozzino D, Eusebi F. Inhibition of GABA and glycine responses by glutamate in rat hippocampal neurons. Brain Res. 1993;628:115–120. doi: 10.1016/0006-8993(93)90945-j. [DOI] [PubMed] [Google Scholar]
- Rigo JM, Badiu CI, Legendre P. Heterogeneity of postsynaptic receptor occupancy fluctuations among glycinergic inhibitory synapses in the zebrafish hindbrain. J Physiol. 2003;553:819–832. doi: 10.1113/jphysiol.2003.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Development of inhibitory synaptic transmission to motoneurons. Brain Res Bull. 2000;53:553–560. doi: 10.1016/s0361-9230(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Inhibition by riluzole of glycinergic postsynaptic currents in rat hypoglossal motoneurones. Br J Pharmacol. 1995;116:3227–3230. doi: 10.1111/j.1476-5381.1995.tb15128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P, Armstrong CM, Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xu TL, Li JS, Jin YH, Akaike N. Modulation of the glycine response by Ca2+-permeable AMPA receptors in rat spinal neurones. J Physiol. 1999;514:701–711. doi: 10.1111/j.1469-7793.1999.701ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. Modulation of glycine-activated ion channel function by G-protein betagamma subunits. Nat Neurosci. 2003;6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo P, Pendlebury WW. Light and electron microscopic observations of a direct projection from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Brain Res. 2001;917:67–80. doi: 10.1016/s0006-8993(01)02911-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Pendlebury WW, Luo P. Synaptic organization of monosynaptic connections from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Synapse. 2003;49:157–169. doi: 10.1002/syn.10227. [DOI] [PubMed] [Google Scholar]
- Zhu L, Krnjevic K, Jiang Z, McArdle JJ. Ethanol suppresses fast potentiation of glycine currents by glutamate. J Pharmacol Exp Ther. 2002;302:1193–1200. doi: 10.1124/jpet.102.033894. [DOI] [PubMed] [Google Scholar]
- Zilberter Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J Physiol. 2000;528:489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.