Abstract
Long chain triglyceride (>C12) in the intestinal lumen potently inhibits gastric emptying and acid secretion via the vagal afferent pathway. While the mechanism of inhibition involves the formation of chylomicrons, the essential role of the apolipoprotein apo A-IV is unclear. Using apo A-IV−/− mice, we tested the hypothesis that inhibition of gastric emptying and gastric acid secretion in response to dietary lipid is dependent upon apo A-IV. As measured by nuclear scintigraphy in awake mice, gastric emptying of an ingested whole-egg meal was significantly faster in apo A-IV−/− knockout versus A-IV+/+ controls (34 ± 1 versus 54 ± 3 min, P < 0.0001). In anaesthetized A-IV+/+ mice, meal-stimulated gastric acid secretion was 59% inhibited by intestinal lipid infusion; this was abolished in apo A-IV−/− mice. Oral gavage of lipid in awake mice activated neurones throughout the nucleus of the solitary tract (NTS) in A-IV+/+ mice, measured by immunohistochemical localization of Fos protein expression. However, in the mid region of the NTS (bregma −7.32 to −7.76 mm), Fos expression in response to intestinal lipid was significantly decreased by 50% in apo A-IV−/− mice compared to A-IV+/+ controls. We conclude that activation of the vagal afferent pathway and inhibition of gastric function in response to dietary lipid is partly dependent upon apo A-IV.
Obesity is a growing global epidemic that involves genetic, environmental, and behavioural factors. In the United States it is estimated that 64% of the population is overweight or obese (Shortt, 2004). A diet high in fat content is an environmental factor that is positively correlated with the incidence of obesity (Lichtenstein et al. 1998), and dietary fat plays an important role in the regulation of food intake and body mass control (Wenk, 2004). Regulation of fat intake and fate is controlled by a number of mechanisms, including those that are sensitive to the amount of stored fat (Elmquist et al. 1999) and those that are sensitive to the macronutrient content of the food being consumed (Moran & Kinzig, 2004). The chemosensory transduction system in the gastrointestinal tract is the first point at which information regarding the macronutrient content of food can be monitored. The vagal afferent pathway is sensitive to luminal triglyceride, and aids in the control of meal size and the sensation of satiety (Berthoud & Neuhuber, 2000). The densest innervation of vagal afferents is at the duodenum (Berthoud et al. 1995); during the intestinal phase of digestion, signals from the duodenum act to tightly regulate feedback inhibition of gastric motility and secretion, stimulation of pancreatic secretion, gall bladder contraction, and relaxation of the sphincter of Oddi (Raybould, 2002). These feedback and feedforward responses allows for the matching of the digestive and absorptive capacity of the intestine with the entry of food from the stomach and secretions from the gall bladder and pancreas.
Chylomicron formation is required for the absorption of long chain fatty acids of carbon chain length C12 or greater, which are critical for lipid-induced inhibition of gastric function (Hunt & Knox, 1968) and the release of cholecystokinin (CCK) from endocrine cells (Isaacs et al. 1987; Matzinger et al. 2000). Apolipoprotein A-IV (apo A-IV) is a protein secreted by the enterocyte of the small intestine in humans (Elshourbagy et al. 1987) and by both the small intestine and the liver in rodents; however, the primary site of secretion in rodents is the intestine (Wu & Windmueller, 1979). Apo A-IV appears to be the only lipoprotein that is directly influenced by dietary lipid. Active lipid absorption stimulates apo A-IV expression, synthesis and release (Hayashi et al. 1990; Apfelbaum et al. 1987; Rodriguez et al. 1997) and stimulation of apo A-IV production by lipid feeding is associated with the formation of chylomicrons (Hayashi et al. 1990). Chylomicron formation and apo A-IV synthesis can be rapid (Tso et al. 2001), which lends itself to the idea of its involvement in signalling of intestinal lipid content to other organs. Apo A-IV seems to have important roles in lipid and lipoprotein metabolism, including the inhibition of lipid oxidation (Qin et al. 1998), the increase of circulating low-density lipoprotein cholesterol (Weinstock et al. 1997), and prevention against atherosclerotic lesions (Duverger et al. 1996; Cohen et al. 1997; Baroukh et al. 2001). However, in addition to effects on lipid fate and metabolism, apo A-IV has been shown to inhibit food intake (Fujimoto et al. 1992, 1993; Tso et al. 2001), inhibit gastric motility (Glatzle et al. 2002, 2003, 2004) and gastric emptying (Okumura et al. 1996) and gastric acid secretion (Okumura et al. 1994, 1995). Apo A-IV may be physiologically involved in the regulation of food intake; an increase in short-term food intake was observed in male apo A-IV knockout mice following an overnight fast. However, overall growth and ad libitum food intake were not significantly different between apo A-IV knockout mice and their wildtype controls (Weinstock et al. 1997). Apo A-IV acts to inhibit gastric motility via CCK-responsive vagal afferent fibre discharge and a CCK1 receptor pathway (Glatzle et al. 2004), and this is consistent with a role for apo A-IV in lipid-induced inhibition of gastric emptying.
The present study tested the hypothesis that apo A-IV is involved in lipid sensing in the intestine. The specific aims were to demonstrate that: (1) inhibition of gastric emptying and gastric acid secretion in response to dietary lipid is dependent upon apo A-IV and (2) lipid-induced activation of the vagal afferent pathway is dependent upon apo A-IV. To establish a role for apo A-IV in the gastrointestinal response to lipid, we used a mouse strain with a deletional mutation of the apo A-IV gene, and their wildtype counterparts.
Methods
Animals
Experiments were performed using male C57BL/6J mice (JAX West, University of California, Davis), male apo A-IV knockout mice and the control strain (Weinstock et al. 1997). These mice were generated by Dr Jan Breslow (Rockefeller University, New York) using homologous recombination in embryonic stem cells. They were found to have normal lipid absorption, weight gain and food consumption up to 5 months of age. C57BL/6J mice were used in addition to the control strain for the apo A-IV knockout mice; the control strain (apo A-IV+/+, referred to as wildtypes in the present study) and the apo A-IV mutant strain were found to be 98.15–99.07% genetically homologous with the C57BL/6J background. Mice were of initial weight 18–20 g (6 to 10 weeks of age), and were maintained on regular laboratory chow (Purina Laboratory Chow). Mice were fasted overnight but allowed water ad libitum prior to all experimental procedures. The institutional guidelines for care and use of laboratory animals were followed throughout the study.
Measurement of gastric emptying by nuclear scintigraphy
This method was described in detail in a previous publication (Whited et al. 2004). Briefly, during the experimental session, uncooked egg product was radioactively labelled with 15 MBq (0.4 millicuries) of Tc99m-Mebrofenin (Amersham Health, Sacramento, CA, USA) per 25 ml egg product, and cooked in a microwave oven. The sample was weighed and the radioactivity of the sample measured using a gamma camera. Mice were allowed to freely feed on cooked egg product for 5 min. The initial radioactivity of the fed mouse was measured in order to permit calculation of amount of the diet consumed by the mouse. Mice were immediately placed in the restraints, and a series of images of the mice were obtained by collecting a dynamic series of images continuously for 60 min, then again at 120–125 min. The mice were imaged using a Technicare Omega 500 Gamma camera equipped with a high-resolution parallel hole collimator and Nuclear Mac 5.2.1 software was used.
Image analysis was facilitated using custom software developed using MATLAB 6.5. To aid in viewing and region of interest (ROI) selection, images were expanded by linear interpolation to 1024 × 1024 pixels. This image was then magnified to twice screen resolution, which allowed images of reasonable size from a single mouse to be viewed in cine format prior to ROI selection. Viewing the images from a single mouse in cine format aided the user in identifying anatomy by observing the temporal change in position of the meal during the acquisition sequence, and determining which images were free of subject motion. Beginning with the first image determined to be motion free, a circular region of interest was then manually positioned over each subsequent image that the user determined was free of motion, in a location that the user decided included only stomach. The sum of the pixel values in each region along with the time after feeding that the image was acquired were recorded in a table. These count rates were then corrected for the physical decay of Tc99m and gastric half emptying time (t½ min) was calculated.
Each mouse was used several times in different imaging sessions. At the end of all the sessions, mice were killed by an overdose of sodium pentobarbital (100 mg kg−1i.p.), followed by bilateral thoracotomy.
Measurement of gastric acid secretion
Mice (apo A-IV+/+ and apo A-IV −/−, n = 5 in each group) were anaesthetized initially with tribromoethanol (Avertin, 250 mg kg−1, 12.5 mg ml−1i.p., Sigma, St. Louis, MO, USA) for induction and maintained with thiobutabarbital (Inactin, mg ml−1, 50 mg kg−1s.c., Sigma). A tracheal cannula was placed, and after a midline abdominal incision the pylorus was isolated and tied off. A double-lumen catheter to simultaneously perfuse and collect gastric perfusate was placed in the stomach via the oesophagus. A cannula was placed into the duodenum for perfusion of lipid and the abdominal incision was closed. Saline was infused (4 ml h−1) into the gastric cannula, and the collected gastric acid perfusate was back-titrated to a pH of 7 with 0.001 m NaOH using a Radiometer Copenhagen ABU 901 Autoburette attached to a Radiometer Copenhagen PHM 290 pH meter. The preparation was allowed to stabilize for 30 min, after which basal gastric acid secretion was recorded for 15 min. From t15 to t150 (min), 8% peptone (Becton Dickinson, Franklin Lakes, NJ, USA) was infused continuously into the stomach to stimulate gastric acid secretion. From t90 to t150, 6% Intralipid (2 ml h−1) was infused into the duodenum. Following completion of the experiment, mice were euthanised by cervical dislocation.
Measurement of Fos protein expression in the NTS
This method has been described in detail previously (Sagar et al. 1988). Briefly, following treatment, mice were anaesthetized with sodium pentobarbital (100 mg kg−1i.p., 50 mg ml−1 Western Medical Supply, Arcadia, CA, USA) and transcardially perfused with 20 ml of heparinized 0.9% saline (0.1 ml heparin (100 ml saline)−1) followed by 25 ml 4% paraformaldehyde (Sigma). The brainstem was removed and postfixed in 4% paraformaldehyde for 1 h. Sections were cut at 100 μm using a vibratome. Sections were incubated for 1 h in goat serum-phosphate-buffered saline (PBS) (Chemicon, Temecula, CA, USA), incubated in primary antibody (1 : 2000 rabbit antifos; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 3 h, followed by incubation with the secondary antibody (1: 200 biotinylated goat antirabbit; Vector Laboratories, Burlingame, CA, USA) for 2 h. Tissue was incubated for 3 h in ABC solution (Standard Elite Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA). DAB solution (Sigma) was added for a 5 min incubation, and then 50 μl H2O2-PBS (0.1 ml 30% H2O2 : 10 ml PBS) was added to catalyse the DAB reaction; the reaction was stopped with a PBS wash. Tissue was thoroughly washed between each incubation period.
Images were taken on a Provis microscope and analysed using Paint Shop Pro, Edition 7. A stereotaxic mouse brain atlas was used to determine the location of the nucleus of the solitary tract (NTS) in each section of tissue (Paxinos & Franklin, 2001). A region of interest was drawn around the NTS) and the area postrema (AP) and all activated neurones in the NTS region of interest were counted. Neurones were determined to be immunopositive (above threshold) by their colour and size. Representative sections were chosen to represent regions of the NTS pre- (bregma −8.00 to −7.92 mm), at (−7.76 to −7.32 mm) and post-AP (−7.08 to −6.48 mm). Three sections were chosen for each region for a total of nine sections per mouse. The numbers of labelled neurones per section were summed for each region for each mouse; this value was used in subsequent statistical analyses.
Measurement of plasma triglyceride
C57BL/6J mice/apo A-IV+/+ (n = 13) and apo A-IV−/− (n = 12) mice were used for these experiments. C57BL/6J mice were used in addition to the control strain for apo A-IV−/− mice, because the apo A-IV+/+ mice were found to be 98.15–99.07% genetically homologous with the C57BL/6J background. Fasted mice or fasted mice gavaged with 0.2 ml 20% Intralipid (15 min pretreatment, 0.04 g lipid) were deeply anaesthetized with sodium pentobarbital (100 mg kg−1i.p., 50 mg ml−1 Western Medical Supply, Arcadia, CA, USA). The abdominal cavity was opened and the inferior vena cava was isolated. Using a heparinized syringe, blood was collected from the inferior vena cava, centrifuged, and the plasma was separated and stored at −80°C. Plasma triglyceride (TAG) levels were assessed using a TAG kit (Sigma).
CCK immunohistochemistry
Fed mice (apo A-IV+/+ and apo A-IV−/−, n = 3 in each group) were anaesthetized with sodium pentobarbital (100 mg kg−1i.p., 50 mg ml−1 Western Medical Supply, Arcadia, CA, USA) and transcardially perfused with 20 ml heparinized 0.9% saline (0.1 ml heparin (100 ml saline)−1) followed by 25 ml 4% paraformaldehyde (Sigma). The duodenum was removed and postfixed in 4% paraformaldehyde for 1 h. The tissue was transferred to a 25% sucrose solution with 1% sodium azide (Sigma) for 2 h at room temperature, and then refrigerated overnight. Sections were cut at 12 μm (longitudinal gut) or 20 μm (transverse gut) using a cryostat. Sections were incubated for 1 h in goat serum-PBS (Chemicon, Temecula, CA, USA), incubated in primary antibody (1: 5000 rabbit X CCK; AB1972, Chemicon, Temecula, CA, USA) for 3 h, followed by incubation with the secondary antibody (1: 200 Alexa 488 goat anti-rabbit; Molecular Probes, Eugene, OR, USA) for 2 h. Tissue was thoroughly washed between each incubation period. Images were taken on a Provis microscope, and a representative image was selected from each mouse strain (apo A-IV+/+ and apo A-IV−/−) for both transverse and longitudinal sections of the duodenum, in order to compare endocrine cell morphology.
Experimental protocols
Effect of intestinal lipid on gastric emptying
Apo A-IV+/+ (n = 9) and apo A-IV−/− (n = 13) mice were used for these experiments. Mice were acclimatized to restraint and to the experimental diets during training sessions 2–3 times a week for 2 weeks prior to the first experimental session. Each mouse participated in four imaging sessions; egg white, whole egg, or egg yolk, and whole-egg diet plus CCK (20 min prior to the whole-egg meal, 22 pmol i.p., Sigma; Mantella et al. 2003). The mice were offered approximately 1 g labelled egg product and allowed to freely feed for 5 min prior to the imaging session.
Effect of intestinal lipid on Fos expression in the NTS
Apo A-IV+/+ (n = 20) mice and apo A-IV−/− (n = 20) mice were used for these experiments. Fasted mice were gavaged with 0.2 ml 20% Intralipid (0.04 g lipid, Fresenius Kabi, Germany) or 0.9% saline, or treated with CCK (22 pmol i.p., Sigma). A group of mice was pretreated with the CCK1 receptor antagonist, devazepide (15 min; 100 μg kg−1i.p.; Whited et al. 2004) followed by either 0.2 ml saline or 20% Intralipid. The dose of 0.2 ml Intralipid was used because it contains approximately the same amount of lipid as is in the average amount of whole egg consumed by the mice (0.04 g TAG in 0.2 ml Intralipid/0.035 g TAG in 0.31 mg whole egg). After 120 min, mice were deeply anaesthetized with sodium pentobarbital (100 mg kg−1i.p., 50 mg ml−1 Western Medical Supply, Arcadia, CA, USA), transcardially perfused with fixative, and tissue removed.
Statistical analysis
Scintigraphy
Decay-corrected counts versus time were analysed using non-linear regression, and fitted to a one-phase exponential decay curve, and the half-emptying time (t½) calculated. Significant differences in t½ emptying between treatment groups were calculated using an unpaired t test and a one-way ANOVA followed by Bonferroni's multiple comparison test. P < 0.05 was taken as significantly different. All reported results are the t½ emptying time ± s.e.m.
Fos protein expression in the NTS
Significant differences in Fos protein expression between treatment groups were calculated using a one-way ANOVA followed by Bonferroni's multiple comparison test. P < 0.05 was taken as significantly different. All reported results are the number of Fos-positive neurones ± s.e.m.
Gastric acid secretion and plasma triglyceride levels
Significant differences between treatment groups were calculated using an unpaired t test. P < 0.05 was taken as significantly different. All reported results are the number of positive neurones, meq gastric acid secretion min−1 ml−1 or mg plasma triglyceride ml−1± s.e.m.
Results
Lipid-induced inhibition of gastric emptying in apo A-IV+/+ and apo-IV−/− mice
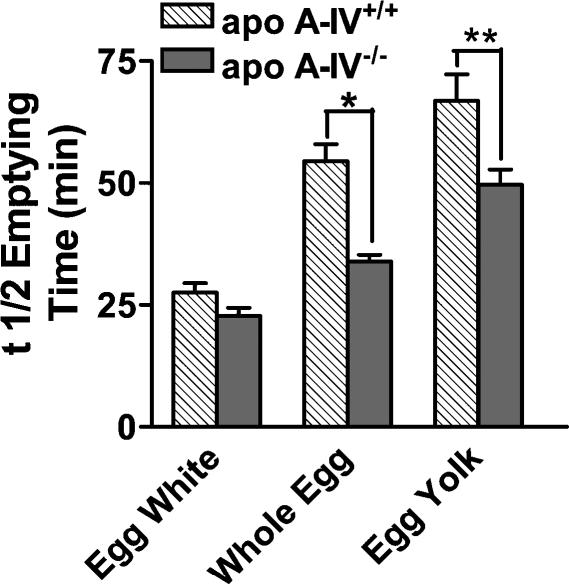
Increasing amounts of lipid in the ingested diet produced a significant slowing of gastric emptying in apo A-IV+/+ as previously demonstrated in C57BL/6J mice (Whited et al. 2004). However, in apo —IV−/− mice, there was a significant attenuation in the inhibition of gastric emptying induced by lipid (Fig. 1). The difference in mean t½ emptying time between the three diets for both apo A-IV+/+ and apo A-IV−/− mice was significantly different for whole egg (P < 0.0001) and egg yolk (P < 0.01), but not for egg white.
Figure 1. Lipid-induced inhibition of gastric emptying is significantly attenuated in apo A-IV knockout mice.
Gastric emptying, expressed as half emptying time, of test meals differing in fat content was measured in freely fed apo A-IV wildtype and knockout mice using nuclear scintigraphy. In wildtype mice, the rate of gastric emptying was significantly inhibited by an increase in the fat content of the ingested test meals. In apo A-IV−/− mice, the inhibitory effect of lipid on gastric emptying was significantly attenuated (*P < 0.0001 and **P < 0.01, wildtype versus knockout, n = 9 apo A-IV+/+ and n = 13 apo A-IV−/−). Error bars are s.e.m.
There was no significant difference between apo A-IV+/+ or apo A-IV−/− mice in the amount of test diet eaten (egg white, whole egg and egg yolk, Table 1).
Table 1.
Amount of test diet eaten and corresponding lipid content in gastric emptying studies
| Egg white | Whole egg | Egg yolk | |
|---|---|---|---|
| Apo A-IV+/+ | 0.22 ± 0.05 g | 0.27 ± 0.06 g | 0.35 ± 0.05 g |
| n = 9 mice | 0.00 g lipid | 0.03 g lipid | 0.11 g lipid |
| Apo A-IV−/− | 0.30 ± 0.04 g | 0.35 ± 0.04 g | 0.42 ± 0.05 g |
| n = 13 mice | 0.00 g lipid | 0.04 g lipid | 0.13 g lipid |
There was no significant difference in the amount of test diet eaten (egg white, whole egg and egg yolk) between apo A-IV wildtype and knockout mice. In addition, there was no significant different in the amount of test diet eaten by Apo A-IV wildtype and knockout mice for all three test diets.
Effect of exogenous CCK on gastric emptying
In order to determine whether the attenuated inhibitory response to lipid in apo A-IV−/− mice was not dependent on a decrease in sensitivity to CCK, t½ was measured in apo A-IV−/− mice in response to exogenous CCK. CCK (22 pmol i.p., 20 min prior to the whole-egg meal) significantly inhibited gastric emptying of whole egg in apo A-IV−/− mice (vehicle versus CCK: t½ 34 ± 1 min versus 57 ± 3 min, n = 13, P < 0.0001). Gastric emptying rate was not altered by exogenous administration of CCK in apo A-IV+/+ (t½= 54 ± 3 min versus 63 ± 3, respectively, n = 9, not significant (n.s.)). There was no significant effect of CCK treatment on the amount of whole egg consumed between apo A-IV+/+ mice (0.38 ± 0.04 g, n = 9) and apo A-IV−/− mice (0.47 ± 0.03 g, n = 13, n.s.).
Lipid-induced inhibition of gastric acid secretion
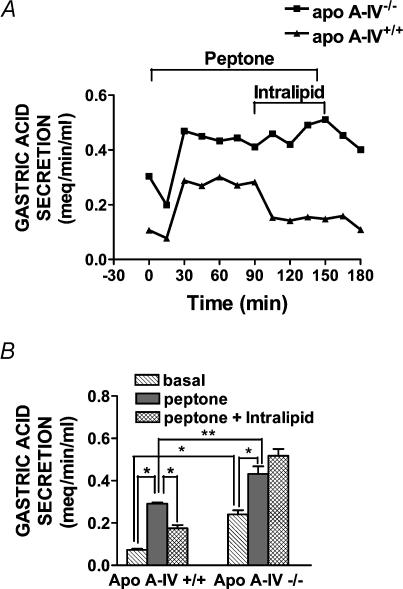
In apo A-IV+/+ mice, intragastric perfusion with peptone significantly stimulated gastric acid secretion (n = 5, P < 0.0001, Fig. 2). Intestinal perfusion with lipid inhibited peptone-stimulated gastric acid secretion in apo A-IV+/+ mice by 59% (n = 5, P < 0.0001, Fig. 2). In apo A-IV−/− mice, basal and peptone-stimulated gastric acid secretion were significantly higher compared to apo A-IV+/+ mice (basal; P < 0.0001, peptone stimulated; P < 0.01, Fig. 2). However, in apo A-IV−/− mice, in contrast to A-IV+/+ controls, intestinal lipid had no significant effect on peptone-stimulated gastric acid secretion (n = 5, n.s., Fig. 2).
Figure 2. Lipid-induced inhibition of gastric acid secretion is abolished in apo A-IV knockout mice.
A, time course changes in gastric acid secretion in individual apo A-IV wildtype and knockout mice. Gastric acid secretion in anaesthetized mice was continuously measured by back titration in response to intragastric perfusion with peptone (t0-t150) and duodenal perfusion with Intralipid (t90-t150). In both the wildtype mouse and knockout mouse, intragastric peptone stimulated gastric acid secretion, but duodenal lipid infusion inhibited meal-stimulated gastric acid secretion only in the wildtype control. B, intestinal lipid inhibition of gastric acid secretion in apo A-IV knockout (n = 5) and wildtype controls (n = 5) during basal, intragastric peptone and intragastric peptone, together with intestinal lipid perfusion. In apo A-IV−/− mice, basal and peptone-stimulated gastric acid secretion were significantly higher compared to apo A-IV+/+ mice (basal; *P < 0.0001, peptone stimulated; **P < 0.01). In both wildtype and apo A-IV knockout mice, intragastric peptone significantly stimulated gastric acid secretion, but duodenal lipid infusion inhibited meal-stimulated gastric acid secretion only in the wildtype control (*P < 0.0001). Error bars are s.e.m.
Lipid-induced activation of neurones in the NTS
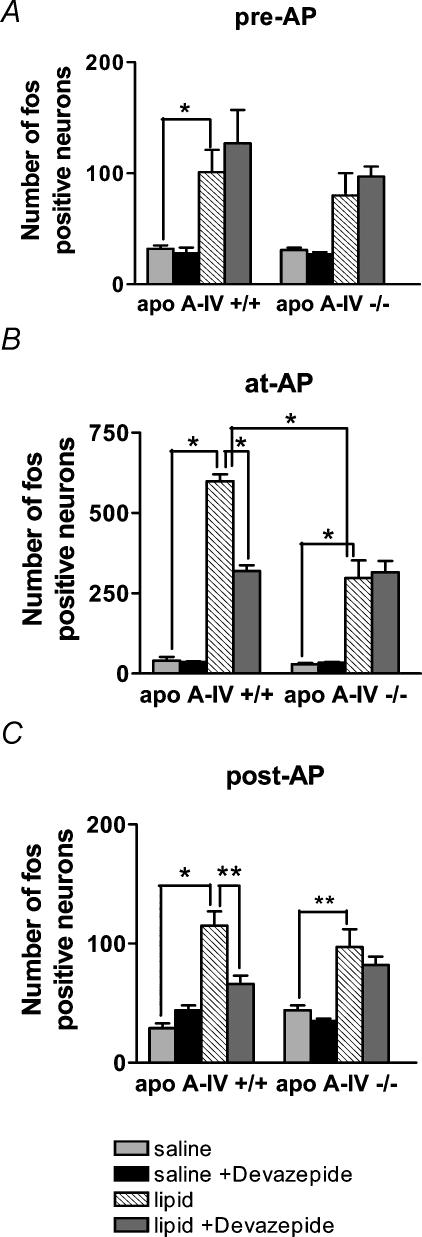
The number of Fos-positive NTS neurones was analysed with respect to region within the NTS (pre-, at- and post- AP) (Fig. 3). In apo A-IV+/+ and apo A-IV−/− mice gavaged with saline, expression of Fos protein in neurones in the NTS was not significantly different between the two groups in all regions of the NTS (n.s., n = 4 mice in each group, Figs 3 and 4). In apo-IV+/+ mice, gavage with Intralipid significantly increased the number of Fos-positive neurones in all regions of the NTS compared to saline gavage (pre-AP, P < 0.05; at-AP, P < 0.001; post-AP, P < 0.001, n = 4 mice in each group, Figs 3 and 4). However, in apo A-IV−/− mice, gavage with Intralipid significantly increased Fos expression in the region of the NTS at- and post-AP, but not in pre-AP (Intralipid versus saline; at-AP, P < 0.001; pre-AP, NS; post-AP, P < 0.01, n = 4 mice in each group, Figs 3 and 4). The increase in the number of Fos-positive neurones in the NTS at-AP in response to Intralipid gavage was significantly different between apo A-IV+/+ and apo A-IV−/− mice (P < 0.001, n = 4 mice in each group, Figs 3 and 4B). There was no significant difference in the regions of the NTS pre- and post-AP between apo A-IV+/+ and apo A-IV−/− mice in response to Intralipid gavage (n.s., n = 4 mice in each group, Fig. 4A and C).
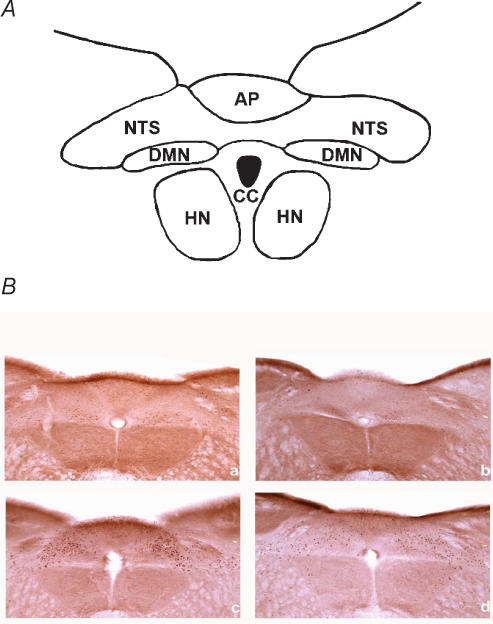
Figure 3. Lipid-induced Fos protein expression in the nucleus of the solitary tract (NTS) is attenuated in apo A-IV knockout mice.
A, cytoarchitecture map for photomicrographs of mice brainstem. NTS = nucleus of the solitary tract; CC = central canal; AP = area postrema; DMN = dorsal motor nucleus of vagus; HN = hypoglossal nucleus. B, photomicrographs of mouse brainstem showing activation of Fos expression in neurones in the nucleus of the solitary tract at the area postrema in apo A-IV wildtype control and knockout mice following saline or Intralipid gavage. a, apo A-IV+/+ saline; b, apo A-IV−/− saline; c, apo A-IV+/+ Intralipid d, apo A-IV−/− Intralipid.
Figure 4. Lipid-induced Fos protein expression in the nucleus of the solitary tract (NTS) is attenuated in apo A-IV knockout mice.
Activation of Fos expression at different levels of the NTS in apo A-IV knockout and wildtype control mice with saline (n = 4), saline and devazepide i.p. (n = 4), Intralipid gavage (n = 4) or Intralipid gavage and devazepide i.p. (n = 4). A, pre-area postrema (*P < 0.05); B, at-area postrema (*P < 0.001, note a change in axis scale for this graph); C, post-area postrema (*P < 0.001, **P < 0.01). Error bars are s.e.m.
In apo A-IV+/+ mice, administration of the CCK1 receptor antagonist, devazepide (15 min pretreatment; 100 μg kg−1i.p.) with Intralipid gavage (0.04 g lipid) significantly decreased the number of Fos-positive neurones in the NTS (at-AP, post-AP) in comparison to Intralipid alone (pre-AP, n.s.; at-AP, P < 0.001; post-AP, P < 0.01, n = 4 mice in each group, Fig. 4A–C). However, administration of the CCK1 receptor antagonist devazepide had no significant effect on Fos expression in response to intestinal lipid in apo A-IV−/− mice at any region of the NTS (n.s., n = 4 mice in each group, Fig. 4A-C). Thus, after devazepide treatment, there was no significant difference in Fos expression in response to Intralipid gavage between apo A-IV+/+ and apo A-IV−/− mice.
Administration of CCK (15 min pretreatment; 22 pmol i.p.) significantly increased Fos expression in the NTS of both apo A-IV+/+ and apo A-IV−/− mice; the increase was not significantly different between the two groups at any region of the NTS (pre-AP: 73 ± 6 versus 61 ± 7 neurones, n.s.; at-AP: 275 ± 26 versus 239 ± 16 neurones, n.s.; post-AP: 75 ± 13 versus 76 ± 9 neurones, n.s., n = 4 mice in each group).
Plasma TAG assay
In apo A-IV+/+/C57BL/6J and apo A-IV−/− mice, Intralipid gavage significantly increased plasma TAG in comparison with fasting TAG levels (P < 0.008 and P < 0.03, respectively, n = 4–9; Table 2). However, there was no significant difference between fasting or lipid-fed TAG levels in apo A-IV+/+ and apo A-IV−/− mice.
Table 2.
Plasma levels of triglyceride
| Fasted | Intralipid | |
|---|---|---|
| Apo A-IV+/+ | 67 ± 3 (n = 4)* | 91 ± 5 (n = 9)* |
| Apo A-IV−/− | 51 ± 5 (n = 5)** | 77 ± 7 (n = 7)** |
There was no significant difference in lipid absorption between Apo A-IV wildtype and knockout mice. Intralipid gavage significantly increased fasting plasma triglyceride levels (mg TAG (ml plasma)−1) in comparison with fasting TAG levels in both wildtype (*P < 0.008) and knockout (** P < 0.03) mice. However, there was no significant difference between fasting or lipid-fed TAG levels in wildtype and knockout mice.
CCK immunohistochemistry
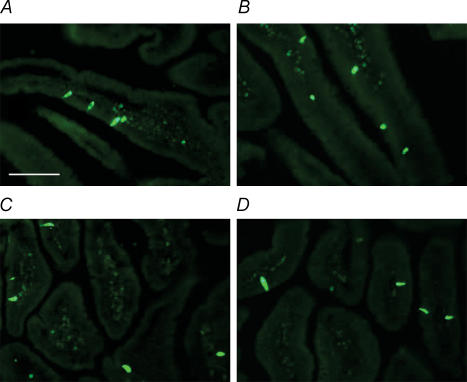
Representative images chosen for transverse and longitudinal gut sections were compared to determine if there were any gross morphological differences in endocrine cell expression between apo A-IV+/+ (n = 3) and apo A-IV−/− mice (n = 3). CCK-expressing endocrine cell distribution and overall morphology appears to be the same between the two strains of mice (Fig. 5).
Figure 5. No gross difference in expression of duodenal CCK endocrine cells in apo A-IV wildtype and knockout mice.
Photomicrographs of mice duodenum showing distribution of CCK-expressing endocrine cells in a chow-fed apo A-IV wildtype control and knockout mouse. A, apo A-IV+/+ transverse section; B, apo A-IV−/− transverse section; C, apo A-IV+/+ longitudinal section; D, apo A-IV−/− longitudinal section. Scale bar = 100 μs.
Discussion
The present results support the hypothesis that lipid-induced stimulation of intestinal feedback is mediated, at least in part, by apolipoprotein A-IV. We have demonstrated that inhibition of gastric emptying and gastric acid secretion induced by lipid in the small intestine was markedly attenuated in the apo A-IV−/− mice compared to the wildtype controls. In addition, the data show that activation of the vagal afferent pathway, as determined by activation of neurones within the NTS, in response to intestinal lipid was significantly reduced in apo A-IV−/− mice. It is well established that intestinal lipid activates vagal afferents via a CCK1 receptor-dependent mechanism, resulting in activation of NTS neurones and reflex changes in gastric motor function and gastric acid secretion (Lloyd et al. 1992; Hölzer et al. 1994; Zittel et al. 1994; Glatzle et al. 2002, 2003). We have previously shown that apo A-IV acts to inhibit gastric motor function via activation of CCK-responsive vagal afferent fibres, and by a mechanism dependent on CCK1 receptors (Glatzle et al. 2004). Taken together, these data suggest that active lipid absorption results in apo A-IV release from enterocytes, which in turn acts to stimulate release of CCK from enteroendocrine cells in the intestinal epithelium, followed by activation of vagal afferents via the CCK1 receptor.
The first aim of this study was to determine whether inhibition of gastric motor and secretory function in response to dietary lipid is dependent upon apo A-IV. We were able to quantify the gastric emptying rate of three test meals (egg white, whole egg, and egg yolk) under basal conditions, and have previously demonstrated that the rate of gastric emptying is proportional to the fat consumed (Whited et al. 2004). In apo A-IV−/− mice, the inhibitory effect of lipid on gastric emptying was significantly attenuated. This observation is in agreement with our previous data showing that administration of exogenous apo A-IV can inhibit proximal gastric motor function in rats (Glatzle et al. 2004). A decrease in intraluminal pressure in the proximal stomach will decrease gastric emptying by decreasing the delivery of chyme to the antral pump (Moragas et al. 1993). It is unlikely that the attenuated response to lipid is explained by a reduced sensitivity to CCK; administration of exogenous CCK was able to inhibit gastric emptying in the knockout mice. Administration of exogenous CCK in wildtype mice had no significant effect on the emptying rate of whole egg; this is presumably because in these animals, whole egg released endogenous CCK, which activated vagal afferents resulting in inhibition of gastric emptying. The effectiveness of CCK in the apo A-IV−/− mice suggests that the difference in t½ gastric emptying time between apo A-IV−/− and apo A-IV+/+ mice in response to lipid challenge is due to the lack of apo A-IV gene expression in the knockout mice, rather than altered sensitivity to CCK. Further evidence to support this conclusion is provided by the results from the studies determining activation of the vagal afferent pathway using expression of Fos in the NTS (see later).
Inhibition of gastric acid secretion in response to dietary lipid was completely absent in apo A-IV−/− mice. It has been previously shown that intracisternal injection of apo A-IV inhibits gastric acid secretion through α2-adrenergic receptors (Okumura et al. 1994, 1995). Through the use of transgenic mice expressing human apo A-IV (hapo A-IV), Vergnes et al. (1999) showed that the overexpression of apo A-IV in these mice decreases gastric acid secretion without alteration of the gastric mucosa. Consistent with these published observations, in the present study, we observed that basal gastric acid secretion was significantly higher in apo A-IV−/− mice in comparison with apo A-IV+/+ animals. Gastric acid secretion stimulated in response to intragastric peptone was also higher; however, the increase in response to peptone was not different between wildtype and knockout mice. The reason for the increase in basal gastric acid secretion is not clear, but might involve central apo A-IV rather than the intestinal apo A-IV, since central administration of apo A-IV inhibits gastric acid secretion. It is interesting to note that lipid-induced inhibition of gastric acid was completely absent in the apo A-IV−/−; in contrast, gastric emptying of whole egg or egg yolk was still slower than for egg white in these mice, suggesting residual lipid-induced inhibition of gastric emptying. It is possible that the gastric secretory response and motor response are mediated by different pathways. This is unlikely because it has been shown that lipid-induced inhibition of gastric acid secretion is also mediated by a vago-vagal reflex CCK1 receptor-dependent pathway, similarly to inhibition of gastric motor function (Lloyd et al. 1992). The reason for this discrepancy is unclear; however, two different lipids were used in these two studies; in the gastric emptying studies, we used egg products containing different amounts of lipid but also other nutrients, including protein. In the gastric acid secretion studies, we were able to use a lipid emulsion. This difference may in part account for the different degree of attenuation seen in the knockout mice.
The second aim of this study was to determine whether activation of the vagal afferent pathway by intestinal lipid is dependent upon apo A-IV. We measured Fos protein expression in neurones in the NTS as a measure of activation of the vagal afferent pathway. Stimulation of neurones induces transcriptional and translational activity of the c-fos oncogene, and results in the production of intracellular regulatory factors like Fos protein, whose expression is an indicator of neuronal activation. Duodenal perfusion with lipid emulsion significantly increases Fos protein expression in the NTS of the rat (Zittel et al. 1994). In the mid-region of the NTS (at the AP), the region where vagal afferents from the duodenum terminate, Fos expression in response to intestinal lipid was significantly reduced in apo A-IV−/− mice compared to apo A-IV+/+ mice. Importantly, there was no significant difference between apo A-IV−/− mice treated with lipid and wildtypes treated with lipid in the presence of the CCK1 receptor antagonist. This suggests that the attenuated response to lipid in apo A-IV−/− mice is due to an alteration in the ability of lipid to activate the CCK1 receptor pathway. This observation, taken together with the observation that CCK was equally effective in activating NTS neurones in both wildtype and knockout mice in response to i.p. CCK injection, strongly suggests that apo A-IV knockout mice possess a functional afferent neuronal pathway, and are able to respond to CCK challenge in a similar manner to their wildtype counterparts. It is interesting to note that there is a residual response to lipid in both wildtypes and knockouts in the presence of the CCK1 receptor antagonist; this suggests a CCK1 receptor-independent pathway by which lipid activates vagal afferents. This may involve activation of vagal afferents by gastric distention or post-absorptive signals from the liver.
Apo A-IV is synthesized not only in the intestine, but also in the hypothalamus, a site intimately involved in the integration of signals for regulation of food intake and energy metabolism (Liu et al. 2001) and the deficit in reflex regulation of gastric function between apo A-IV+/+ and apo A-IV−/− mice may be due, in part, to a lack of apo A-IV synthesis in the brain. However, the observation that activation of the vagal afferent pathway is attenuated in apo A-IV−/− mice suggests that the defect is in detection of lipid in the gut wall and activation of vagal afferent pathway.
The mechanism by which apo A-IV is involved in release or the action of CCK on vagal afferents is unclear. It is possible that apo A-IV stimulates CCK release from intestinal endocrine cells, but this has yet to be demonstrated. The mechanism by which endocrine cells release CCK in response to long-chain triglyceride is unclear. It has previously been demonstrated that infusion of long-chain fatty acids or long-chain triglyceride emulsions increases plasma levels of CCK (Liddle, 1994), specifically fatty acids of chain length C12 or longer (McLaughlin et al. 1998). This carbon chain length requirement suggests that chylomicron formation is involved, as well as apo A-IV, because stimulation of apo A-IV production by lipid feeding is associated with the formation of chylomicrons (Hayashi et al. 1990). A direct effect of long-chain triglyceride has been shown in the enteroendocrine cell model, STC-1 cells, although whether this occurs in native endocrine cells in not known (McLaughlin et al. 1998; Sidhu et al. 2000). It is possible that apo A-IV mediates release of CCK, but the residual response to lipid for inhibition of gastric emptying and activation of NTS neurones may be mediated by a direct effect of free fatty acid on endocrine cells and release of CCK. Alternatively, it is possible that free fatty acid directly stimulates vagal afferent nerve terminals. It has been shown that short-chain fatty acids can directly stimulate mesenteric nerve fibres, but this has not been demonstrated for long-chain triglyceride; indeed, the ability of long-chain triglyceride to stimulate vagal afferent activity is completely blocked by the CCK1 receptor antagonist, devazepide (Lal et al. 2001). The ability of apo A-IV to directly influence endocrine cell function is unknown. Measurement of plasma levels of CCK is confounding, because the action of CCK on vagal afferent nerve terminals is not an endocrine action and therefore not dependent on appearance in plasma, but occurs at the level of the lamina propria. Our observations suggest that there is no gross difference in expression of CCK endocrine cells in the apo A-IV−/− mice, but a detailed understanding of the role of apo A-IV in release of CCK remains to be determined.
It is unlikely that the differences observed in the present study between apo A-IV−/− and +/+ mice are associated with postabsorptive differences; the increase in plasma TAG in response to lipid was not significantly different between the two groups, confirming the observation that there is no difference in lipid absorption between the wildtype and knockout mice (Weinstock et al. 1997).
In summary, the present data provide evidence for a role for apo A-IV in the detection of lipid in the intestinal wall. This is the first site in the body at which intake of macronutrients can be monitored. Detection of nutrients in the wall of the gut initiates changes in function of the gastrointestinal tract and also causes decrease in food intake, processes that are important in digestion and absorption, and possibly in body weight regulation.
Acknowledgments
The wrok was funded by NIH DK41004 (HER). The authors are grateful to Jim Sharp for his expert technical assistance with immunocytochemistry.
References
- Apfelbaum TF, Davidson NO, Glickman RM. Apolipoprotein A-IV synthesis in rat intestine: regulation by dietary triglyceride. Am J Physiol. 1987;252:G662–G666. doi: 10.1152/ajpgi.1987.252.5.G662. [DOI] [PubMed] [Google Scholar]
- Baroukh N, Ostos MA, Vergnes L, Recalde D, Staels B, Fruchart J, Ochoa A, Castro G, Zakin MM. Expression of human apolipoprotein A-I/C-III/A-IV gene cluster in mice reduces atherogenesis in response to a high fat-high cholesterol diet. FEBS Lett. 2001;502:16–20. doi: 10.1016/s0014-5793(01)02621-7. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol. 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Cohen RD, Castellani LW, Qiao JH, Van Lenten BJ, Lusis AJ, Reue K. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J Clin Invest. 1997;99:1906–1916. doi: 10.1172/JCI119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverger N, Tremp G, Caillaud JM, Emmanuel F, Castro G, Fruchart JC, Steinmetz A, Denefle P. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 1996;273:966–968. doi: 10.1126/science.273.5277.966. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Elshourbagy NA, Walker DW, Paik YK, Boguski MS, Freeman M, Gordon JI, Taylor JM. Structure and expression of the human apolipoprotein A-IV gene. J Biol Chem. 1987;262:7973–7981. [PubMed] [Google Scholar]
- Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–G1006. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest. 1993;91:1830–1833. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzle J, Darcel N, Rechs AJ, Kalogeris TJ, Tso P, Raybould HE. Apolipoprotein A-IV stimulates duodenal vagal afferent activity to inhibit gastric motility via a CCK1 pathway. Am J Physiol Regul Integr Comp Physiol. 2004;287:R354–R359. doi: 10.1152/ajpregu.00705.2003. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Kalogeris TJ, Zittel TT, Guerrini S, Tso P, Raybould HE. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. Am J Physiol Gastrointest Liver Physiol. 2002;282:G86–G91. doi: 10.1152/ajpgi.2002.282.1.G86. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Wang Y, Adelson DW, Kalogeris TJ, Zittel TT, Tso P, Wei JY, Raybould HE. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol. 2003;550:657–664. doi: 10.1113/jphysiol.2003.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Fujimoto K, Cardelli JA, Nutting DF, Bergstedt S, Tso P. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am J Physiol. 1990;259:G709–G719. doi: 10.1152/ajpgi.1990.259.5.G709. [DOI] [PubMed] [Google Scholar]
- Hölzer HH, Turkelson CM, Solomon TE, Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol. 1994;267:G625–G629. doi: 10.1152/ajpgi.1994.267.4.G625. [DOI] [PubMed] [Google Scholar]
- Hunt JN, Knox MT. A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol. 1968;194:327–336. doi: 10.1113/jphysiol.1968.sp008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs PE, Ladas S, Forgacs IC, Dowling RH, Ellam SV, Adrian TE, Bloom SR. Comparison of effects of ingested medium- and long-chain triglyceride on gallbladder volume and release of cholecystokinin and other gut peptides. Dig Dis Sci. 1987;32:481–486. doi: 10.1007/BF01296030. [DOI] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Kennedy E, Barrier P, Danford D, Ernst ND, Grundy SM, Leveille GA, Van Horn L, Williams CL, Booth SL. Dietary fat consumption and health. Nutr Rev. 1998;56:S3–S19. doi: 10.1111/j.1753-4887.1998.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Liddle RA. Regulation of cholecystokinin synthesis and secretion in rat intestine. J Nutr. 1994;124:1308S–1314S. doi: 10.1093/jn/124.suppl_8.1308S. [DOI] [PubMed] [Google Scholar]
- Liu M, Doi T, Shen L, Woods SC, Seeley RJ, Zheng S, Jackman A, Tso P. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1382–R1387. doi: 10.1152/ajpregu.2001.280.5.R1382. [DOI] [PubMed] [Google Scholar]
- Lloyd KCK, Raybould HE, Walsh JA. Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am J Physiol. 1992;263:G287–G292. doi: 10.1152/ajpgi.1992.263.3.G287. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Rinaman L, Vollmer RR, Amico JA. Cholecystokinin and D-fenfluramide inhibit food intake in oxytocin-deficient rats. Am J Physiol. 2003;285:1037–1045. doi: 10.1152/ajpregu.00383.2002. [DOI] [PubMed] [Google Scholar]
- Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, D'Amato M, Rovati L, Beglinger C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut. 2000;46:688–693. doi: 10.1136/gut.46.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JT, Lomax RB, Hall L, Dockray GJ, Thompson DG, Warhurst G. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol. 1998;513:11–18. doi: 10.1111/j.1469-7793.1998.011by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moragas G, Azpiroz F, Pavia J, Malagelada J. Relations among intragastric pressure, postcibal perception, and gastric emptying. Am J Physiol. 1993;264:G1112–G1117. doi: 10.1152/ajpgi.1993.264.6.G1112. [DOI] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Intracisternal injection of apolipoprotein A-IV inhibits gastric secretion in pylorus-ligated conscious rats. Gastroenterology. 1994;107:1861–1864. doi: 10.1016/0016-5085(94)90833-8. [DOI] [PubMed] [Google Scholar]
- Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Mechanism of action of intracisternal apolipoprotein A-IV in inhibiting gastric acid secretion in rats. Gastroenterology. 1995;109:1583–1588. doi: 10.1016/0016-5085(95)90647-9. [DOI] [PubMed] [Google Scholar]
- Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Apolipoprotein A-IV acts in the brain to inhibit gastric emptying in the rat. Am J Physiol. 1996;270:G49–G53. doi: 10.1152/ajpgi.1996.270.1.G49. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Sterotaxic Coordinates. 2nd edn. San Diego: Academic Press; 2001. [Google Scholar]
- Qin X, Swertfeger DK, Zheng S, Hui DY, Tso P. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am J Physiol. 1998;274:H1836–H1840. doi: 10.1152/ajpheart.1998.274.5.H1836. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Visceral perception: sensory transduction in visceral afferents and nutrients. Gut. 2002;51(Suppl. 1):i11–i14. doi: 10.1136/gut.51.suppl_1.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MD, Kalogeris TJ, Wang XL, Wolf R, Tso P. Rapid synthesis and secretion of intestinal apolipoprotein A-IV after gastric fat loading in rats. Am J Physiol. 1997;272:R1170–R1177. doi: 10.1152/ajpregu.1997.272.4.R1170. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Shortt J. Obesity – a public health dilemma. AORN J. 2004;80:1069–1078. doi: 10.1016/s0001-2092(06)60686-8. [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Thompson DG, Warhurst G, Case RM, Benson RS. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J Physiol. 2000a;528:165–176. doi: 10.1111/j.1469-7793.2000.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso P, Liu M, Kalogeris TJ, Thomson AB. The role of apolipoprotein A-IV in the regulation of food intake. Annu Rev Nutr. 2001;21:231–254. doi: 10.1146/annurev.nutr.21.1.231. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Baroukh N, Lehy T, Moizo L, Bado A, Baralle M, Baralle FE, Zakin MM, Ochoa A. Human apolipoprotein A-IV reduces gastric acid secretion and diminishes ulcer formation in transgenic mice. FEBS Lett. 1999;460:178–181. doi: 10.1016/s0014-5793(99)01332-0. [DOI] [PubMed] [Google Scholar]
- Weinstock PH, Bisgaier CL, Hayek T, Aalto-Setala K, Sehayek E, Wu L, Sheiffele P, Merkel M, Essenburg AD, Breslow JL. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J Lipid Res. 1997;38:1782–1794. [PubMed] [Google Scholar]
- Wenk C. Implications of dietary fat for nutrition and energy balance. Physiol Behav. 2004;83:565–571. doi: 10.1016/j.physbeh.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Whited KL, Hornof WJ, Garcia T, Bohan DC, Larson RF, Raybould HE. A non-invasive method for measurement of gastric emptying in mice: effects of altering fat content and CCK A receptor blockade. Neurogastroenterol Motil. 2004;16:421–427. doi: 10.1111/j.1365-2982.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- Wu AL, Windmueller HG. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem. 1979;254:7316–7322. [PubMed] [Google Scholar]
- Zittel TT, de Giorgio R, Sternini C, Raybould HE. Fos protein expression in the nucleus of the solitary tract in response to intestinal nutrients in awake rats. Brain Res. 1994;663:266–270. doi: 10.1016/0006-8993(94)91272-6. [DOI] [PubMed] [Google Scholar]