Abstract
This rat renal blood flow (RBF) study quantified the impact of nitric oxide synthase (NOS) inhibition on the myogenic response and the balance of autoregulatory mechanisms in the time domain following a 20 mmHg-step increase or decrease in renal arterial pressure (RAP). When RAP was increased, the myogenic component of renal vascular resistance (RVR) rapidly rose within the initial 7–10 s, exhibiting an ∼5 s time constant and providing ∼36% of perfect autoregulation. A secondary rise between 10 and 40 s brought RVR to 95% total autoregulatory efficiency, reflecting tubuloglomerular feedback (TGF) and possibly one or two additional mechanisms. The kinetics were similar after the RAP decrease. Inhibition of NOS (by l-NAME) increased RAP, enhanced the strength (79% autoregulation) and doubled the speed of the myogenic response, and promoted the emergence of RVR oscillations (∼0.2 Hz); the strength (52%) was lower at control RAP. An equi-pressor dose of angiotensin II had no effect on myogenic or total autoregulation. Inhibition of TGF (by furosemide) abolished the l-NAME effect on the myogenic response. RVR responses during furosemide treatment, assuming complete inhibition of TGF, suggest a third mechanism that contributes 10–20% and is independent of TGF, slower than the myogenic response, and abolished by NOS inhibition. The hindlimb circulation displayed a solitary myogenic response similar to the kidney (35% autoregulation) that was not enhanced by l-NAME. We conclude that NO normally restrains the strength and speed of the myogenic response in RBF but not hindlimb autoregulation, an action dependent on TGF, thereby allowing more and slow RAP fluctuations to reach glomerular capillaries.
Autoregulation of blood flow is found in virtually every tissue. The pressure-induced myogenic response of vascular smooth muscle is an integral part of this regulation (Johnson, 1986). The degree of autoregulation varies between tissues and is particularly strong in the kidney (Johnson, 1986). Autoregulation of renal blood flow (RBF) is mediated by a tubuloglomerular feedback (TGF) system in addition to the myogenic response (Navar et al. 1996) and possibly a third regulatory component (Just & Arendshorst, 2003). However, little is known about the relative contribution of these mechanisms to overall regulation and changes in their balance in various situations. Micropuncture studies of single nephron glomerular filtration rate (GFR) in the superficial cortex have estimated that the myogenic response and TGF contribute equally under basal conditions (Moore et al. 1979). More recent transfer function analyses of spontaneous fluctuations of RBF and renal arterial pressure (RAP) indicate that both mechanisms are active in vivo at the whole kidney level, albeit precise quantification of the relative contributions is a limitation of this technique (Ajikobi et al. 1996; Just et al. 1998). A more reliable quantitative assessment is based on analysis of transients of RBF to a rapid step change in RAP. Such a dynamic analysis reveals approximately equal participation of the myogenic response (55%) and TGF (35–45%) in both dog and rat; a third system appears to contribute about 10% during euvolaemia (Just et al. 2001; Just & Arendshorst, 2003; Wronski et al. 2003).
Little is known about whether this balance between the mechanisms is modulated, and, if so, what the major modulating factors are. A shift in the predominance of autoregulatory mechanisms is likely to have important functional consequences because of their different response times. Whereas the myogenic response to a step change in RAP is usually complete within 10 s (Clausen et al. 1992; Young & Marsh, 1981), TGF is much slower, with an initial delay of ∼10 s and completion in 20–30 s (Daniels & Arendshorst, 1990). One or two additional regulatory components that are of similar or even slower speed than TGF may also be involved (Just & Arendshorst, 2003). Accordingly, a more pronounced contribution of the fast myogenic response would accelerate overall regulation and prevent more and faster fluctuations of RAP from reaching glomerular and peritubular capillaries. In this manner, the myogenic response is poised to buffer changes in RAP on glomerular filtration, pressure natriuresis, and glomerular damage.
An attractive paracrine candidate for such a modulating role is nitric oxide (NO). NO exerts a strong tonic dilator effect that is more pronounced in the kidney than in other vascular beds (Sonntag et al. 1992; Sigmon et al. 1993). On the other hand, acute inhibition of NO production has little, if any, effect on steady-state RBF autoregulation (Beierwaltes et al. 1992; Majid & Navar, 1992; Baumann et al. 1992). Nevertheless, subtle NO-dependent effects are evident as a reduction in the lower pressure limit of autoregulation during NOS-inhibition (Turkstra et al. 2000; Kramp et al. 2001). Isolated renal vessel studies using blood-free perfusates indicate that NO can attenuate the myogenic response (Imig et al. 1993; Bouriquet & Casellas, 1995; Juncos et al. 1995), although others have failed to detect such an effect of NO. The NO effects, however, seem to be limited to certain vascular segments (Imig & Roman, 1992; Hoffend et al. 1993) and others have not detected a modulatory effect of NO (Hayashi et al. 1995; Yip & Marsh, 1996). Perfusion with haemoglobin-containing solutions usually attenuates the impact of NO in isolated preparations. At the whole kidney level in vivo, transfer function analyses of regulatory mechanisms suggest a more active myogenic response in the absence of NO (Wang et al. 1999; Wang & Cupples, 2001), although such assessments are not well suited to reliably estimate the overall autoregulatory efficiency and the contributions of individual mechanisms (Bidani et al. 2003). Mathematical modelling of the dynamics of RBF responses to a step increase in RAP after complete ischaemia also suggests NO counteracts myogenic contrictor responsiveness (Wronski et al. 2003).
Although an attenuating effect of NO on the myogenic response is reported for several extrarenal vessels including skin (Griffith & Edwards, 1990), myocardium (Rubanyi et al. 1986; Ueeda et al. 1992; Pohl et al. 1994), mesentery (Pohl et al. 1991), spleen (Brookes & Kaufman, 2003) and skeletal muscle (Johnsson et al. 1991; de Wit et al. 1998; Nurkiewicz & Boegehold, 1999) other studies failed to confirm this particularly in the skeletal muscle circulation (Falcone et al. 1991; Ekelund et al. 1992; Sun et al. 1994).
An aim of the present in vivo study was to quantify the impact of NOS inhibition on the relative contributions of myogenic response and TGF to RBF autoregulation in the time domain. To this end, we employed small changes in RAP within the autoregulatory range and our data analyses were independent of modelling assumptions. Another goal was to determine whether the modulatory influence of NO on the myogenic response was dependent on TGF activity. A third objective was to test whether NO inhibition enhanced the myogenic response in the hindlimb circulation as it did in the kidney.
Methods
Experiments were conducted on 23 male Sprague-Dawley rats (age 9–15 weeks, body weight 320–470 g, left kidney weight 1.6–2.4 g) from our local breeding colony (18 rats) or purchased from Charles River Laboratories Inc. (Raleigh, NC, USA; 5 rats) and were approved by the Institutional Animal Care and Use Committee of the University of North Carolina. The animals were fed a standard lab chow with free access to tap water, and were kept on a 12 h : 12 h light–dark cycle. Surgery and experimental procedures were similar to those reported earlier (Just & Arendshorst, 2003).
Surgical procedures
After induction of anaesthesia by pentobarbital (50–60 mg (kg body wt)−1i.p., Nembutal, Abbott, Chicago, IL, USA), a rat was placed on a temperature-controlled table kept at 37°C. The depth of anaesthesia was monitored by the response to ear and toe pinching. Additional doses of pentobarbital (3–8 mg kg−1i.v.) were given as needed (∼every 30–60 min). The left femoral artery was catheterized (PE-50) to measure arterial pressure, and three catheters (PE-10) were placed into the left femoral vein for infusion of volume replacement and injections of pentobarbital, and drugs. An isoncotic bovine serum albumin solution (4.75 g dl−1) was infused initially at 100 μl min−1 to replace surgical losses (1.25 ml (100 g body wt)−1), followed by a maintenance rate of 5 μl min−1 (100 g body wt)−1. The trachea was cannulated (PE-240) to facilitate respiration. Via a midline abdominal incision, the abdominal aorta, left renal artery, and origins of right renal and superior mesenteric artery were exposed. An inflatable vascular occluder (1.5 mm; IVM, Healdsburg, CA, USA) was implanted around the aorta above the left renal artery. In six rats an additional occluder was implanted above the coeliac artery. A non-cannulating flowprobe (1 RB; Transonic, Ithaca, NY, USA) was placed around the left renal artery and filled with ultrasonic coupling gel (HR Lubricating Jelly; Carter-Wallace, New York, NY, USA). In five animals, an additional flowprobe of the same type was placed on the left iliac artery. A 23-gauge needle with a catheter (PE-50) allowed bladder urine to drain by gravity. Sixty minutes were allowed for stabilization after surgery.
Pressure in the left renal artery (RAP) was measured via the femoral artery catheter and a pressure transducer (Statham P23B). Renal blood flow (RBF) and blood flow in the iliac artery (IBF) were measured with flowprobes connected to ultrasound transit-time flowmeters (TS-420 for RBF, T-206 for IBF; Transonic, low-pass filter set to 30–40 Hz). Zero offset was determined at the end of each experiment after cardiac arrest. The pressure in the vascular occluder was monitored by a transducer (MSP 3101P2-ND; Measurement Specialties Inc., Fairfield, NJ, USA) to allow for automatic detection of the time points of increase or decrease in RAP. All data were recorded on a computer (Pentium III, DataTranslation A/D converter, Labtech Notebook-Pro) at 100 Hz (RAP and RBF) or 10 Hz (occluder pressure). Urinary flow was measured using a graduated cylinder.
The autoregulatory response of RBF to a rapid change of RAP within the autoregulatory pressure range was measured after RAP was reduced by 20 mmHg with controlled inflation of the aortic occluder for a period of 60 s. A 20 mmHg step increase in RAP then was produced by rapid deflation of the occluder. RAP reductions were repeated every 5 min, thus allowing 4 min for recovery. At least three RAP steps were made in each period. At the conclusion of the experiments the animals were killed by an overdose of pentobarbital (> 150 mg kg−1i.v.).
Experimental protocols
Group A. NOS-inhibition (n = 8)
To investigate the effect of NO on the myogenic response and the dynamics of RBF autoregulation, the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) was injected (25 mg kg−1 in 1 ml kg−1 saline i.v.) after a control period. After l-NAME, there was a 15 min equilibration period. Since NOS-inhibition elevates baseline RAP, we wanted to separate direct effects of l-NAME from those of elevated RAP and to test for pressure dependency of NO inhibitory effects. Thus, baseline RAP was reduced in a third experimental period to match the control level before addition of l-NAME by either inflating an additional proximal occluder (4 rats) or by partially pre-inflating a single occluder (4 rats). After an equilibration period of 5–10 min, 20 mmHg RAP steps were superimposed on the restored level of RAP.
Group B. Angiotensin II (n = 6)
Since NOS inhibition produces vasoconstriction, similar experiments were conducted in which similar degrees of hypertension and renal vasoconstriction were induced by i.v. infusion of Ang II (100 ± 24 ng kg−1min−1). Previous studies indicate that a pressor dose of Ang II has only minor effects on the dynamics of RBF autoregulation (Just et al. 2002). A period of 15 min was allowed for equilibration after final dose adjustment. Subsequently, the baseline level of RAP was reduced to match the control level before addition of Ang II by inflation of an aortic occluder. After the target RAP was reached, 5–10 min were allowed for stabilization.
Group C. NOS inhibition during TGF inhibition (n = 4)
To test whether the effects of NOS inhibition on RBF autoregulatory dynamics are intrinsic to the myogenic response or depend on an intact TGF, l-NAME was given during inhibition of TGF by furosemide. After a control period, the albumin solution was diluted 1 : 10 with isotonic saline for volume replacement, the infusion rate was increased to 50 μl−1 kg−1 min−1 and furosemide was injected (10 mg kg−1 in 1 ml kg−1 saline i.v.; American Regent Laboratory Inc., Shirley, NY, USA). Urine output was measured every 5 min and the infusion rate was adjusted to match urine flow. To avoid augmentation of TGF by volume contraction (Schnermann & Briggs, 1990), the cumulative infused volume was intentionally kept 1–2 ml above that of the excreted urine. After a 15 min equilibration period, at least three step responses were recorded. Subsequently, l-NAME was injected (25 mg kg−1i.v.) and, after 15 min equilibration, additional step responses were recorded.
Group D. Effect of NOS inhibition in the hindlimb circulation and TGF inhibition during NOS inhibition (n = 5)
To determine whether the observed effects of NOS inhibition are a generalized phenomenon or unique to the kidney, l-NAME (25 mg kg−1i.v.) was given to a group of rats in which IBF was measured simultaneously with RBF. After the control and l-NAME periods, furosemide was injected (10 mg kg−1i.v.) in a third period, the infusion rate adjusted as described above, and step responses recorded after an equilibration period of 15 min.
Data analysis
Data analyses were done off-line by dedicated computer programs. The 100 Hz data of RAP and RBF were smoothed by a sliding average over 100 values each. Zero-offsets of RBF and IBF were subtracted from the recorded values. RVR was calculated as RPP/RBF, where renal perfusion pressure (RPP) = RAP − 4 mmHg; 4 mmHg was assumed as renal venous pressure. These data sets of RAP, RBF and RVR were then resampled to a sampling rate of 10 Hz. Short segments were then extracted into single files for each RAP reduction containing the last 10 s before RAP reduction, followed by the segment between the last 10 s before release through 120 s after release. For analysis of the responses to a RAP decrease, segments were extracted containing the data from 10 s before RAP reduction until the time of RAP release. The exact time points for reduction and release of RAP were derived from changes in the occluder pressure, i.e. at an accuracy of ±10 ms. Baseline values given in Table 1 are the mean values during the last 10 s before each RAP reduction of a particular experimental period, except for urine flow rates, which are averaged over an observation period.
Table 1.
Baseline values of haemodynamic parameters and urine excretion during the experimental periods
| Experimental period | n | MAP (mmHg) | RBF (ml min−1 gKW−1) | HR (beats min−1) | UV (μl min−1) |
|---|---|---|---|---|---|
| Control | 8 | 108 ± 2 | 6.4 ± 0.3 | 391 ± 7 | 18 ± 3 |
| l-NAME | 8 | 136 ± 4* | 2.8 ± 0.3*** | 382 ± 11 | 19 ± 3 |
| l-NAME + RAP-servo | 8 | 111 ± 2# | 2.8 ± 0.3*** | 383 ± 16 | 17 ± 6 |
| Control | 6 | 109 ± 4 | 6.1 ± 0.6 | 362 ± 19 | 29 ± 7 |
| Ang II | 6 | 133 ± 3*** | 3.5 ± 0.4*** | 352 ± 24 | 41 ± 15 |
| Ang II + RAP-servo | 6 | 112 ± 3### | 3.1 ± 0.3*** | 355 ± 33 | 34 ± 6 |
| Control | 4 | 123 ± 4 | 6.3 ± 0.5 | 313 ± 15 | 24 ± 4 |
| Furosemide | 4 | 122 ± 4 | 6.2 ± 0.3 | 304 ± 12 | 344 ± 16*** |
| Furosemide +l-NAME | 4 | 139 ± 4**, ## | 4.4 ± 0.4***, ### | 312 ± 42 | 523 ± 47***, ## |
| Control | 5 | 118 ± 3 | 5.1 ± 0.4 | 317 ± 18 | 12 ± 2 |
| l-NAME | 5 | 147 ± 8** | 2.3 ± 0.3*** | 264 ± 16** | 15 ± 6 |
| l-NAME + Furosemide | 5 | 131 ± 6 | 3.7 ± 0.6**, ## | 275 ± 20** | 471 ± 87***, ### |
P < 0.05,
P < 0.01,
P < 0.001 versus respective control;
P < 0.05,
P < 0.01,
P < 0.001 versus preceding group. gKW−1, per gram kidney weight.
The first and second derivatives of RVR were calculated by the Savitzky-Golay algorithm (Savitzky & Golay, 1964) with a window size of 11 points and coefficients for 3rd order fitting. The speed of the myogenic response was derived from the average 1st derivative between 2 and 3 s after the RAP step. To account for changes in baseline RVR as well as differences from resistance of IBF (IVR), changes in RVR and IVR in ((mmHg (ml min−1)−1) s−1) were normalized by dividing through the respective baseline resistance in (mmHg (ml min−1)−1); accordingly, results are expressed as fractional resistance change in normalized units per second (U s−1).
The contribution of the myogenic response was estimated from the RVR change reached within 7–10 s after the RAP reduction and 7–9 s after RAP release. These time windows were chosen based on previous results (Just & Arendshorst, 2003) as well as on the present data, in which the transition between the initial (myogenic response) and the secondary (TGF) and possible other autoregulatory mechanisms was determined from the time of lowest speed of RVR adaptation between 3 and 10 s after the RAP step, as derived from the zero-crossing of the 2nd derivative of RVR. The exact time of zero-crossing was determined from the individual time course of the 2nd derivative of RVR during control conditions of each animal by calculating a linear regression over the values between 3 and 10 s and subsequent arithmetic determination of the zero-crossing of the regression line.
Autoregulatory efficiency was expressed as a percentage of perfect autoregulation, with 100% denoting a RVR adjustment matched to keep RBF exactly constant in the face of a change in RAP; 0% indicates unchanged RVR and the absence of autoregulation. The efficiency of total RBF autoregulation was calculated as (RVRend− RVRpre)/[(RPPend/RBFpre) – RVRpre]× 100, where RPPpre, RBFpre and RVRpre are averages for the last 10 s before release of the aortic occluder, and RVRend and RPPend are the average values during 90–120 s after the step increase in RAP. As a measure of the autoregulation provided by the myogenic response, the same formula was applied but instead of RVRend, the RVR averaged at 7–10 s was used. Autoregulatory efficiency for the response to decreased RAP was calculated by taking for RPPpre, RBFpre and RVRpre the averages for the last 10 s before RAP reduction, and for RVRend and RPPend the average values during 50–60 s after RAP reduction.
Within the initial 1 s after the increase or decrease in RAP, RVR transiently changed in opposite direction to the following autoregulatory response, which most likely reflects passive distention or collapse of the resistance vessels. To determine the time point of maximum or minimum RVR during this period as a measure of the delay time of the active response, the first derivative was calculated from the time course of changes in RVR. A linear regression was then calculated over all derivative values versus time between t = 0.2 s and t = 0.8 s. Zero-crossing of this regression line indicated the time of maximum or minimum RVR.
Statistical analysis
The effects of experimental interventions were determined by comparison of the results from the respective experimental periods within the same animal. Statistical significance was tested by ANOVA for repeated measures in conjunction with the Holm-Sidak test (SigmaStat 3.10, SPSS Inc., Chicago, IL, USA). In the case of non-normal distribution, data were log-transformed before testing. If the speed of the myogenic response was not normally distributed by log-transformation (‘l-NAME’ and ‘All l-NAME’ groups), the Tukey test for multiple comparisons was used as a post hoc test. A P value < 0.05 was considered statistically significant. Data are represented as mean ± s.e.m.
Results
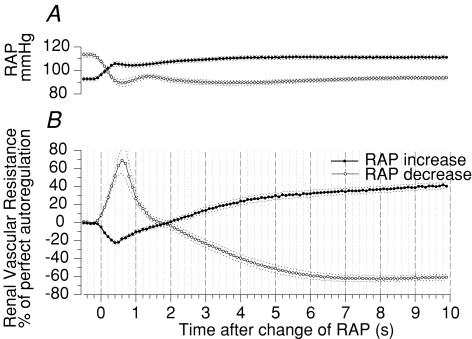
We characterized the myogenic response and the efficiency of blood flow autoregulation in the renal and hindlimb vasculature based on the dynamics of the time-dependent changes in vascular resistance after a rapid 20 mmHg step change of RAP. Absolute values of MAP, RBF, heart rate (HR) and urine flow for the individual groups are summarized in Table 1. The normal autoregulatory response pattern of RVR as a function of time is shown in the control curves of Figs 1, 2, 3. When RAP was increased, RVR transiently fell, and then rapidly rose within the first 7–10 s to provide 32–41% of perfect autoregulation (Figs 1A and C, 2A and 4; Table 2). After a short delay, a secondary rise brought RVR to the final level of actual autoregulatory strength of 95% for all control animals (n = 23) (Table 2). As we have noted earlier (Just & Arendshorst, 2003), these temporal characteristics are consistent with the notion that the myogenic response mediates the initial rise in RVR, with slower responses due to TGF and possibly a third mechanism. The transition between the initial and secondary responses, determined from the turning point of RVR changes, was at 8.6 ± 0.4 s. Further analysis of these data indicates that the maximum speed of the myogenic response was 2.8 U s−1 (Table 2). In response to step reduction of RAP, RVR fell in a quantitatively similar fashion, except that the myogenic response provided an autoregulatory efficiency of 62% (P < 0.001 versus RAP increase) (Figs 1B and D, and 3D; Table 3). The turning point between the myogenic response and TGF was 9.6 ± 1.1 s. A more detailed comparison between the dynamics of the responses to increase and decrease of RAP is given in Fig. 5, showing the time course of the initial 10 s from the control periods for all 23 rats. The maximum or minimum RVR during the initial transient change of RVR indicating the delay time before beginning of the active vasoconstriction or –dilation, occurred slightly earlier in response to the increase (∼0.4 s) than to the decrease in RAP (∼0.6 s, Fig. 5). Determination of the maximum/minimum RVR from the first derivative for each animal revealed that the delay times were statistically different (0.39 ± 0.01 versus 0.53 ± 0.05 s, P < 0.001). Choosing different time windows between 0 and 1.1 s for the regression analysis of the time point (see Methods) gave similar results. The time course of the subsequent active responses was slightly faster for the dilator response to an decrease of RAP than for the constriction to an increase of RAP; bi-exponential fitting of the averaged time courses detected only mono-exponential dynamics with shorter time constants for the dilator (2.6 s) than the constrictor response (5.1 s).
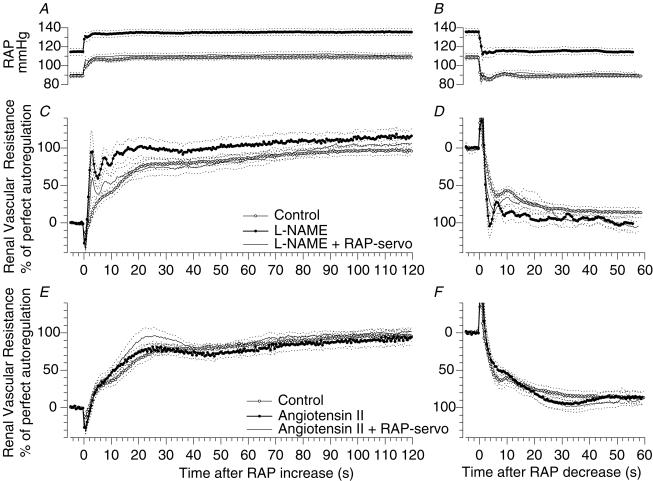
Figure 1. Step responses of renal blood flow autoregulation. Influence of NO and angiotensin II.
Time course of renal artery pressure (RAP) and of the autoregulatory response of renal vascular resistance during a step increase (A, C and E) and a step decrease (B, D and F) of RAP by 20 mmHg. A and B, time course of RAP during control (○), during NOS inhibition by l-NAME (•), and during l-NAME with mechanical restoration of RAP to the control level (line without circles), n = 8. C and D, responses of vascular resistance to the pressure steps shown in A and B during control (○), l-NAME (•), and l-NAME with restoration of RAP (line without circles), n = 8. E and F, responses during control (○), during infusion of Ang II to match the pressor and renal vasoconstrictor effects of l-NAME (•), and during Ang II with restoration of RAP (line without circles), n = 6. Mean (continuous lines) ± s.e.m. (dashed lines).
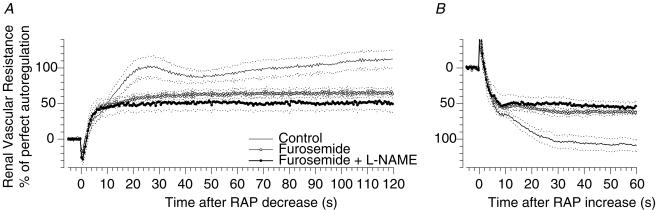
Figure 2. Step responses of renal blood flow autoregulation. Influence of NO in the absence of TGF.
Time course of the autoregulatory response of renal vascular resistance to a step increase (A) and a step decrease (B) of renal artery pressure (RAP) during control (line without circles), during TGF inhibition by furosemide (○), and during furosemide combined with NOS inhibition by l-NAME (•). Mean (continuous lines) ± s.e.m. (dashed lines), n = 4.
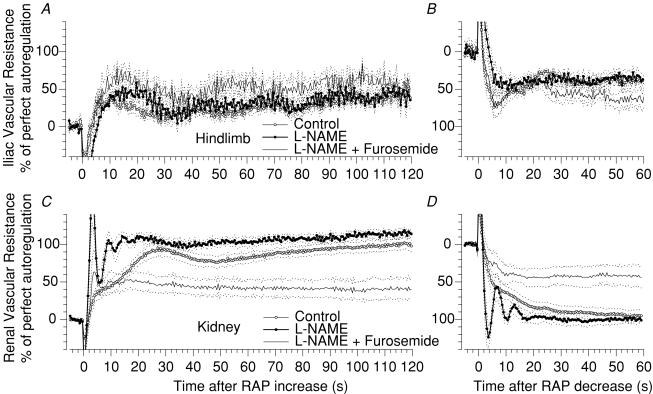
Figure 3. Step responses of hindlimb and renal blood flow autoregulation. Influence of NO and TGF.
Time course of the autoregulatory response of vascular resistance in response to a step increase (A and C) and a step decrease (B and D) of arterial pressure in the hindlimb (A and B) and in the kidney (C and D). Responses during control (○), during NOS inhibition by l-NAME (•) and during additional TGF inhibition by furosemide (lines without circles). Mean (continuous lines) ± s.e.m. (dashed lines), n = 5.
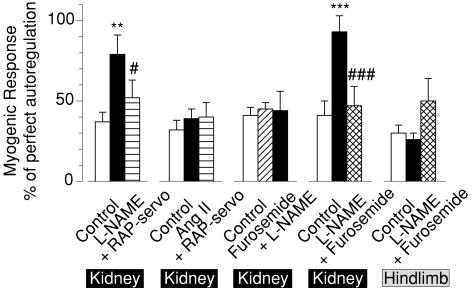
Figure 4. Autoregulatory strength of the myogenic response in renal and hindlimb blood flow.
Strength of the myogenic response as derived from the autoregulatory efficiency reached at 7–10 s following a step increase of renal artery pressure (RAP). Open bars denote results in control conditions for each experimental group. Other bars show data during NOS inhibition by l-NAME (filled bar, l-NAME) alone and with mechanical normalization of RAP (striped bar, + RAP-servo), during infusion of Ang II alone (filled bar, Ang II) and with normalization of RAP (striped bar, + RAP-servo), during TGF inhibition by furosemide alone (hatched bar, Furosemide) and with l-NAME (filled bar, l-NAME), during l-NAME alone (filled bar, l-NAME) and with furosemide (cross-hatched bar, + Furosemide) in the kidney, and the latter also in the hindlimb. Mean ± s.e.m.
Table 2.
Characteristics of renal and hindlimb blood flow autoregulation in response to a step increase of RAP
| Step increase Experimental period | n | Total autoregulation (% of perfect AR) | Myogenic response autoregulation (% of perfect AR) | Myogenic response Maximum speed (units s−1) |
|---|---|---|---|---|
| Control | 8 | 93 ± 6 | 37 ± 6 | 2.5 ± 0.4 |
| l-NAME | 8 | 110 ± 10 | 79 ± 13** | 5.4 ± 0.6* |
| l-NAME + RAP-servo | 8 | 102 ± 6 | 52 ± 11# | 7.5 ± 2.5* |
| Control | 6 | 92 ± 6 | 32 ± 6 | 2.8 ± 0.4 |
| Ang II | 6 | 87 ± 8 | 39 ± 6 | 2.8 ± 0.4 |
| Ang II + RAP-servo | 6 | 95 ± 6 | 40 ± 9 | 3.7 ± 0.8 |
| Control | 4 | 106 ± 12 | 41 ± 5 | 2.6 ± 0.6 |
| Furosemide | 4 | 63 ± 6** | 45 ± 4 | 2.9 ± 0.6 |
| Furosemide + LNAME | 4 | 51 ± 15*** | 44 ± 12 | 3.8 ± 0.7* |
| Control | 5 | 95 ± 2 | 41 ± 9 | 3.6 ± 0.3 |
| l-NAME | 5 | 112 ± 5 | 93 ± 10** | 16.5 ± 7.0* |
| l-NAME + Furosemide | 5 | 40 ± 13***, ### | 47 ± 12### | 9.1 ± 3.6 |
| Hindlimb − Control | 5 | 35 ± 5[ | 30 ± 5 | 4.9 ± 0.1 |
| Hindlimb −l-NAME | 5 | 37 ± 7[ | 26 ± 4[ | 3.3 ± 0.3 |
| Hindlimb - l-NAME + Furosemide | 5 | 55 ± 7*, # | 50 ± 14 | 4.1 ± 0.8 |
| All controls | 23 | 95 ± 4 | 37 ± 3††† | 2.8 ± 0.2 |
| Control | 13 | 94 ± 4 | 38 ± 5 | 2.9 ± 0.3 |
| All l-NAME | 13 | 111 ± 6*** | 85 ± 9*** | 9.7 ± 3.0* |
| Control | 9 | 100 ± 6 | 41 ± 5 | 3.1 ± 0.3 |
| All l-NAME + Furosemide | 9 | 45 ± 8***,2++ | 46 ± 82+ | 6.7 ± 2.1* |
P < 0.05,
P < 0.01
P < 0.001 versus respective control;
P < 0.05,
P < 0.01,
P < 0.001 versus preceding group
P < 0.01 hindlimb versus kidney;
P < 0.001 versus step decrease of RAP (Table 4);
P < 0.01,
P < 0.001 ‘All l-NAME’versus‘All l-NAME + Furosemide’; ‘units s−1’=ΔRVR/baseline RVR/time = (mmHg (ml min−1)−1) (mmHg (ml min−1)−1)−1 s−1.
Table 3.
Characteristics of renal and hindlimb blood flow autoregulation in response to a step reduction of RAP
| Step decrease Experimental period | n | Total autoregulation (% of perfect AR) | Autoregulation by the myogenic response (% of perfect AR) | Myogenic response Maximum speed (units s−1) |
|---|---|---|---|---|
| Control | 8 | 86 ± 6 | 59 ± 5 | −3.6 ± 0.5 |
| l-NAME | 8 | 99 ± 8 | 84 ± 7* | −6.9 ± 2.5 |
| L-NAME + RAP-servo | 8 | 99 ± 6 | 66 ± 9 | −5.2 ± 1.5 |
| Control | 6 | 87 ± 8 | 63 ± 6 | −3.4 ± 0.5 |
| Ang II | 6 | 87 ± 7 | 53 ± 3 | −4.0 ± 1.2 |
| Ang II + RAP-servo | 6 | 90 ± 7 | 62 ± 11 | −3.7 ± 1.6 |
| Control | 4 | 108 ± 9 | 65 ± 1 | −2.8 ± 0.2 |
| Furosemide | 4 | 62 ± 3*** | 55 ± 5 | −2.1 ± 0.5 |
| Furosemide + LNAME | 4 | 55 ± 7*** | 52 ± 14 | −3.5 ± 1.9 |
| Control | 5 | 95 ± 8 | 62 ± 10 | −3.3 ± 0.7 |
| l-NAME | 5 | 100 ± 6 | 81 ± 9 | −6.8 ± 1.3 |
| l-NAME + Furosemide | 5 | 43 ± 14**, ## | 37 ± 13# | −2.6 ± 0.4# |
| Hindlimb – Control | 5 | 39 ± 3 [$ | 66 ± 12 | −2.5 ± 1.8 |
| Hindlimb –l-NAME | 5 | 36 ± 7 [ | 45 ± 11[ | −3.4 ± 1.4 |
| Hindlimb - l-NAME + Furosemide | 5 | 72 ± 12*, # | 54 ± 11 | −2.7 ± 1.8 |
| All controls | 23 | 92 ± 4 | 62 ± 3††† | −3.3 ± 0.3 |
| Control | 13 | 89 ± 5 | 60 ± 5 | −3.5 ± 0.4 |
| All l-NAME | 13 | 99 ± 5* | 83 ± 6*** | −6.9 ± 1.5 |
| Control | 9 | 101 ± 6 | 63 ± 5 | −3.1 ± 0.4 |
| All l-NAME + Furosemide | 9 | 48 ± 8***, 2++ | 44 ± 9*, 2+ | −3.0 ± 0.8 + |
P < 0.05,
P < 0.01,
P < 0.001 versus respective control;
P < 0.05,
P < 0.01,
###P < 0.001 versus preceding group;
P < 0.01,
P < 0.001 hindlimb versus kidney;
P < 0.001 versus step-increase of RAP (Table 3);
P < 0.05,
P < 0.01,
P < 0.001 ‘All l-NAME’versus‘All l-NAME + Furosemide’; ‘units s−1’=ΔRVR/baseline RVR/time = (mmHg (ml min−1)−1) (mmHg (ml min−1)−1)−1 s−1.
Figure 5. Comparison of the dynamics of the myogenic response to a step increase and step decrease of renal artery pressure (RAP).
Time course of RAP (A) and of the autoregulatory response of renal vascular resistance (B) during the initial 10 s after step increase (•) and step decrease (○) of RAP, averaged from the control periods of all rats. Mean ± s.e.m., n = 23.
l-NAME inhibition of NOS produced vasoconstriction, reducing RBF by 50% and increasing RAP by 25% (Table 1). A major finding was that the renal myogenic response was enhanced to provide an autoregulatory efficiency of 79% (Figs 1A and 4, Table 2). In addition, the myogenic response was accelerated; the slope of the initial RVR change was enhanced 2- to 3-fold (Fig. 1A, Table 2). Thus, removing the attenuating effects of NO affected both the speed and the strength of the myogenic response. Accordingly, the myogenic response accounted for a larger fraction of autoregulation in a shorter period of time: 79% was achieved within 4 s (Fig. 1A, Table 2). Total autoregulatory efficiency increased slightly. It is noteworthy that NOS inhibition led to the emergence of oscillations of RVR with a cycle length of 4–5 s, i.e. 0.20–0.25 Hz, an observation compatible with reduced restraint of a well-damped second-order control system or transition from a first- to a second-order system. The consistency of such oscillations averaged for eight animals is remarkable, indicating that they are highly synchronized and reproducible.
Normalizing the elevated RAP during NOS inhibition to the control level of 102 mmHg attenuated the enhanced myogenic response (52 versus 79%, P < 0.05, Figs 1A and 4, Table 2). Complete restoration was seen in some of the animals. In contrast, acceleration of the myogenic adaptation was not affected by the RAP reduction, remaining 2- to 3-fold greater than control (Table 2).
The effects of NOS inhibition on the RVR responses to a step decrease of RAP were similar in general to values recorded when RAP was increased. The strength of the myogenic response was enhanced by NOS inhibition (84 versus 59%, P < 0.05, Figs 1B and 4, Table 3). Enhancement of the speed, which doubled numerically (Table 3), is apparent in the time course (Fig. 1B).
Since NOS inhibition exerted potent systemic and renal vascular effects, control experiments were conducted in which similar amounts of renal vasoconstriction and hypertension were produced by Ang II. The responses are shown in Fig. 1C, and numerical means are given in Table 2 and Fig. 4. Ang II affected neither the strength nor the speed of the myogenic response (32%, 2.8 U s−1, respectively). Subsequent RAP reduction to the basal level had no influence on the myogenic response, as was the case for total autoregulatory efficiency. Likewise, Ang II had no effect on RVR responses to the RAP step reduction (Fig. 1D, Table 3).
To test whether the effects of NO are intrinsic to the renal myogenic response or depend on TGF, l-NAME was given after inhibition of TGF by furosemide. As is shown in Fig. 2A and summarized in Table 2, furosemide alone reduced overall autoregulation by 43% and tended to augment the myogenic response, indicating effective inhibition of TGF and its contribution to RBF autoregulation. An important observation was that l-NAME failed to augment the renal myogenic response when TGF was inhibited. The strength of the myogenic response remained constant (44 versus 45%, Figs 2A and 4, Table 2) while NO inhibition produced the same systemic pressor effect as noted earlier in Group A (+25 versus+26%, Table 1). Likewise, NOS inhibition failed to affect the strength and rate of the myogenic response during furosemide administration in the response to step reduction of RAP (Fig. 2B, Table 3).
A potentially important result is that a slow component of RVR adaptation persisted, participating in the secondary rise of RVR after 10 s and accounting for 15–20% of total autoregulation during furosemide inhibition of TGF (Table 2). These findings provide evidence for a contribution of a third mechanism, a system that is independent of TGF and slower than the myogenic response. Interestingly, NOS inhibition eliminated this third component of RVR adaptation, reducing its participation from 21 to 8% of total autoregulation (P < 0.05, Fig. 2A), indicating that this sluggish mechanism is mediated by or dependent on NO. A similar trend was seen after a step reduction of RAP (Table 3).
In other animals we tested whether inhibition of TGF would reverse the augmenting effect of NOS inhibition on the myogenic response. Figures 3C and 4 show that NOS inhibition by l-NAME reproduced the results seen earlier (Figs 1A and 4) as the myogenic response was markedly enhanced (93 versus 41%, Table 2), accelerated 4-fold, and oscillations were unmasked. The same tendencies were seen following a step reduction of RAP (Fig. 3D) as l-NAME impacted on the myogenic response in terms of oscillations (Fig. 3D), strength (81 versus 62%) and acceleration (6.8 versus 3.3 U s−1) (Table 3).
In this same group we also tested the effects of NOS inhibition on myogenic autoregulation in the hindlimb vascular bed. Iliac blood flow (IBF) displayed autoregulatory responses to a 20 mmHg step increase in AP that was weaker than for the renal circulation (35 versus 95%, P < 0.01, Figs 3A and 4, Table 2). Over the initial 10 s, IBF autoregulation was similar to that of the kidney, except for a more pronounced overshoot in IBF. Importantly, there was no secondary rise of hindlimb resistance, and the final level of IBF autoregulation (35%) was similar to the myogenic response of the hindlimb and the kidney (30 and 41%, respectively, Fig. 4, Table 2). Collectively, our results suggest that small, brief step changes of perfusion pressure elicit autoregulation in the hindlimb that is due predominately, if not entirely, to the myogenic response. Moreover, the myogenic response in the hindlimb has the same strength and speed as in the renal circulation. In contrast, the myogenic mechanism in the kidney is supported by TGF and probably a third regulator. Another difference between vascular beds is the impact of NOS inhibition on the myogenic responses.
As noted earlier in Group A, inhibition of NOS augmented and accelerated renal autoregulation. IBF autoregulation, however, was not affected by l-NAME, even though absolute IBF was reduced (Figs 3A and 4, Table 4). The speed of the adaptation was also not altered (Table 2). Similar IBF results were obtained when perfusion pressure was reduced (Fig. 3B, Table 3). For unknown reasons, the myogenic response and total autoregulation in the hindlimb tended to increase during furosemide administration (Figs 3B and 4, Table 2).
Table 4.
Absolute values of baseline blood flow in the left renal (RBF) and iliac artery (IBF) in protocol D
| Experimental period | n | RBF (ml min−1) | IBF (ml min−1) |
|---|---|---|---|
| Control | 5 | 8.9 ± 0.8 | 2.9 ± 0.2 |
| l-NAME | 5 | 3.9 ± 0.6*** | 1.8 ± 0.1*** |
| l-NAME + Furosemide | 5 | 6.4 ± 1.0**, ## | 1.6 ± 0.1*** |
P < 0.01,
P < 0.001 versus respective control;
P < 0.01 versus preceding group.
Discussion
The present study provides new information that the balance between the regulating mechanisms of RBF autoregulation is dynamic not static and that NO is an important modulating factor capable of changing the balance in a healthy kidney. NO markedly attenuates the strength, speed and oscillations of the myogenic response to changes in renal perfusion pressure in the kidney. NO normally restrains the contribution of the myogenic response to overall autoregulation of RBF, thereby slowing the speed of adaptive changes in preglomerular vascular resistance and allowing more and slower fluctuations of RAP to reach glomerular and postglomerular capillaries. The responses to 20 mmHg increases and decreases in RAP, which were intentionally kept within the autoregulatory range, are symmetrical, indicating that RBF autoregulation is designed to buffer changes in mean RAP and respond similarly to systolic and diastolic RAP. Further insight into this interaction derives from a dependency of NO dampening on the level of RAP and a functional TGF. In contrast, the hindlimb circulation devoid of TGF has a myogenic response that is similar in strength and speed as that in the kidney and is unaffected by the absence of NO.
The characteristics of the myogenic response are derived from the autoregulatory adaptation of RVR occurring within the initial 7–10 s after a rapid step change of RAP as previously employed (Just & Arendshorst, 2003). This analysis is based on the known response time of the renal myogenic response (Young & Marsh, 1981; Clausen et al. 1992) and initial 10 s delay of TGF (Daniels & Arendshorst, 1990). Such a time lag between mechanisms is evident in the transition between the two distinct phases of RVR between 7 and 10 s in the control curves. This conclusion is reinforced by the finding that the secondary rise of RVR is almost abolished by inhibition of TGF with furosemide (Just et al. 2001; Just & Arendshorst, 2003; Wronski et al. 2003), whereas the rapid myogenic response is enhanced, not reduced (Just & Arendshorst, 2003; Wronski et al. 2003). Collectively, these points support the view that the rise of RVR within the first 7–10 s reflects the myogenic response without an obvious contribution from TGF. The step changes in RAP were intentionally kept small (20 mmHg) to assure testing within the autoregulatory pressure range. Our results for control conditions confirm our previous observations during euvolaemia that the myogenic response provides an efficiency of 30–40% of perfect autoregulation, corresponding to ∼50% of the observed autoregulatory strength of the kidney (Just & Arendshorst, 2003).
The major new finding is that NOS inhibition markedly augments and accelerates the contribution of the myogenic response to overall blood flow autoregulation in the kidney, while causing minor to no improvements in total autoregulatory efficiency. That the improved autoregulation within the first 7–10 s was due to an acceleration of TGF rather than an augmentation of the myogenic response seems unlikely, because there are no indications that the dynamics of TGF change during NOS inhibition; TGF-associated oscillations in the transfer function remain clearly visible around 0.03 Hz at the same centre frequency as during control conditions. Furthermore, the effects of NOS inhibition on the myogenic response cannot be ascribed to the vasoconstrictor effects of l-NAME because no such modulation was observed during infusion of Ang II that produced identical effects on baseline RAP and RBF, in agreement with a previous report (Just et al. 2002).
Our quantitative studies extend earlier reports of NOS inhibition augmenting the strength of the myogenic response based on transfer function analysis (Wang et al. 1999; Wang & Cupples, 2001). The improved autoregulatory efficiency of the myogenic response in our RAP step responses corresponds well with the fractional compensation calculated from transfer gain at 0.06–1.0 Hz. However, a major change in transfer gain during NOS inhibition was not found in dogs (Just et al. 1999). The reasons for the variable results are not clear but may derive from species differences, from imposed forced (Wang et al. 1999; Wang & Cupples, 2001) versus spontaneous fluctuations of RAP (Just et al. 1999), or from using shorter (Wang et al. 1999; Wang & Cupples, 2001) or longer (Just et al. 1999) time segments for spectral calculations. Another possibility is a masking of improved myogenic autoregulation by superimposition of feedback oscillations of TGF, which seem to be more prominent in dogs (Just et al. 1998, 1999) than in rats (Wang et al. 1999; Wang & Cupples, 2001). Moreover, it should be appreciated that there are concerns about the reliability of the absolute level of the transfer gain in the low frequency range as an accurate measure of autoregulatory efficiency (Bidani et al. 2003). These reservations highlight the importance of using more quantitative, direct methods such as that used in the present study.
Our data clearly show that NOS inhibition increased the speed of the myogenic response and also led to strong and reproducible and highly synchronized oscillations of RVR. The accelerated myogenic response agrees with a slight shift of the corner frequency associated with the myogenic response in the transfer function (Just et al. 1999; Wang & Cupples, 2001). Transfer function analysis also shows an elevated transfer gain at the same frequency in the absence of NO, consistent with enhanced feedback oscillations (Just et al. 1999; Wang et al. 1999; Wang & Cupples, 2001). However, from transfer functions alone, it cannot be decided whether the elevated gain might also be caused by enhanced vascular compliance. In this regard, the present results in the time domain clearly demonstrate the emergence of strong and synchronized oscillations in the absence of NO.
The negligible effect of NOS inhibition on total autoregulatory efficiency fits nicely with classical RBF studies reporting unaltered steady-state autoregulatory efficiency in the absence of NO (Majid & Navar, 1992; Beierwaltes et al. 1992; Baumann et al. 1992).
The absence of direct modulation of the myogenic response by NO in the absence of TGF activity is congruent with studies on isolated afferent arterioles (Yip & Marsh, 1996) or the hydronephrotic kidney (Hayashi et al. 1995). At variance, however, are other studies on isolated vessels (Juncos et al. 1995), the hydronephrotic kidney (Hoffend et al. 1993) or interlobular arteries in the juxtamedullary nephron preparation (Imig et al. 1993; Bouriquet & Casellas, 1995). Although the reasons for the discordant results are not clear, it should be appreciated that when an effect of NO was detected, NO levels were either raised experimentally (Bouriquet & Casellas, 1995) or may have been artificially elevated due to the use of erythrocyte-free perfusates that lack the NO scavenging effect of haemoglobin (Imig et al. 1993; Juncos et al. 1995). In fact, the effect of NOS inhibition could be mimicked by adding erythrocytes to the perfusate in one study (Imig et al. 1993). A mitigating effect of NO when elevated but not when reduced is consistent with the observation that steady-state RBF autoregulation of the intact kidney is impaired by infusion of acetylcholine (Baer et al. 1970), but well maintained during NOS inhibition (Majid & Navar, 1992; Beierwaltes et al. 1992; Baumann et al. 1992).
Furthermore, we investigated the influence of the ambient RAP level on NO effects and found that mechanical restoration of RAP to normotensive levels during NOS inhibition attenuated the increase in the strength of the myogenic response. The reason for this pressure dependency of NO effects is not clear, but may be secondary to reduced TGF activity at the reduced RAP. TGF is known to be stronger at higher levels of RAP (Schnermann & Briggs, 1989). Even though the myogenic response was numerically larger during NOS inhibition with servo-controlled RAP than during control conditions, it did not reach statistical significance. However, transfer function studies reported a significant enhancement of the myogenic response in the absence of NO even when the concomitant hypertension was prevented by servo-control of RAP (Wang & Cupples, 2001). Pressure dependency was not addressed in that study as observations were not made at elevated RAP. In contrast to our finding that the myogenic strength is pressure dependent, the enhancement of the speed of the myogenic response during NOS inhibition was not affected by restoration of the RAP. This is in line with findings of transfer function analysis of an augmented slope of gain reduction between 0.2 and 0.06 Hz during NOS inhibition with servo-controlled RAP (Wang & Cupples, 2001).
A surprising finding was that NOS inhibition abolished the slow adaptation of RVR occurring after 10 s during furosemide application. The fact that this slow adaptation was present after the myogenic response was complete and during furosemide inhibition of TGF confirms previous findings suggesting participation of a third mechanism to RBF autoregulation (Just et al. 2001; Just & Arendshorst, 2003). The elimination of this response by NOS inhibition indicates mediation by NO. This is particularly intriguing with regard to previous observations in dogs indicating a regulatory function of NO in a frequency range lower than TGF (Just et al. 1999). Future studies are necessary to further characterize this regulatory component.
Although we cannot exclude that the slow component of autoregulation observed during furosemide treatment between 10 and 30 s reflects remnant TGF function due to incomplete blockade of TGF, this seems unlikely for two reasons. First, doubling the dose of 10 mg kg−1 furosemide did not induce additional effects in a previous study (Just & Arendshorst, 2003). Second, given the well-known attenuating effect of NO on TGF (Wilcox et al. 1992; Thorup et al. 1993), it is difficult to understand why NOS inhibition would eliminate rather than enhance a remnant activity of TGF.
It should be noted that there is another, even slower, component of autoregulation seen during control, l-NAME, and Ang II, reflected by the rise of RVR between 40 and 120 s. This putative fourth component is clearly TGF dependent, since it is abolished by furosemide (Fig. 2; Just & Arendshorst, 2003). A possible explanation is a progressive reduction of proximal tubular reabsorption occurring with a rise of RAP (Walstead & Yip, 2004). This might lead to a slowly increasing tubular load and macula densa signal, thus inducing a progressive secondary TGF-mediated vasoconstriction. However, it seems unlikely that this slow rise of RVR from ∼80–95% of perfect autoregulation between 40 and 120 s can explain the increase in RVR between 10 and 30 s in the absence of TGF.
Another important observation concerns our analysis of the dynamics of the myogenic response of RBF in the whole kidney in vivo (Fig. 5) as compared with those reported for changes in afferent arteriolar diameter in the saline-perfused chronically hydronephrotic kidney (Loutzenhiser et al. 2002). Loutzenhiser et al. found a more rapid myogenic response to an 80 mmHg increase in RAP than an 80 mmHg decrease due to a shorter delay in diameter change (0.3 versus 1 s) and differing time constants: mono-exponential (4 s) for constriction and bi-exponential (1 s and 14 s) for dilatation. As a result, they concluded that afferent arteriolar autoregulation is more responsive to systolic than to mean RAP. In contrast, our data show that the dilator response was stronger overall with a shorter time constant (2.6 s) than the constrictor response (5.1 s). If these dynamics are the only determinants for the responsiveness to systolic versus mean pressure, then our results would speak against a preferential response to systolic pressure, congruent with earlier studies that did not detect an influence of pulse pressure on RBF autoregulation (Selkurt, 1951; Ritter, 1952). However, the initial delay time was slightly longer for the dilator versus the constrictor response (0.53 versus 0.39 s), a difference considerably smaller than that reported for the hydronephrotic kidney (1.0 versus 0.3 s) (Loutzenhiser et al. 2002). Loutzenhiser et al. believe the difference in delay time is the major determinant for the preferential response to systolic pressure, although it was not tested whether their model retains the same characteristics when only different delay times are included and time constants are set equal. Taken together, our data confirm a longer delay time for the dilator than for the constrictor action of the myogenic response in the renal vasculature for integrated RBF in the intact kidney. However, the difference in delay times is rather small and further investigation is required to determine if it is responsible for any preferential response to systolic versus mean pressure.
With regard to the conclusion that the NO action on the myogenic response is entirely dependent on an active TGF, one would expect that the myogenic response in an extrarenal vascular bed devoid of TGF would not be affected by NOS inhibition. Previous studies of the hindlimb circulation found no enhancement of myogenic responsiveness during NOS inhibition in a blood-perfused preparation (Ekelund et al. 1992). This is also the case for isolated skeletal muscle arterioles after removal of the endothelium (Falcone et al. 1991; Sun et al. 1994). On the other hand, many studies have reported attenuating effects of NO on myogenic responsiveness and autoregulation in vascular beds of skin (Griffith & Edwards, 1990), coronary (Rubanyi et al. 1986; Ueeda et al. 1992; Pohl et al. 1994), mesenteric (Pohl et al. 1991; Brookes & Kaufman, 2003), and skeletal muscle (Rubanyi et al. 1986; Johnsson et al. 1991; de Wit et al. 1998; Nurkiewicz & Boegehold, 1999). It is of interest that NOS inhibition enhanced myogenic responsiveness of large-diameter arteries in the cremaster circulation, whereas myogenic tone of small arterioles was unaffected (de Wit et al. 1998). In view of the conflicting results for the skeletal muscle circulation, we investigated the autoregulatory responses in the hindlimb simultaneously with those in the kidney.
Our results demonstrate that autoregulation of the hindlimb circulation displays a similar response time and strength to the myogenic response in the kidney. It seems very likely therefore that the responses in this non-renal vascular bed to small, brief RAP changes reflect mainly, if not exclusively, the myogenic response. Importantly, these regulatory responses were not affected by NOS inhibition, in marked contrast to the enhancement of the renal myogenic response in the same animals. This occurred while both IBF and RBF were reduced by NO inhibition. Accordingly, we conclude that NO, presumably endothelial in origin, does not affect the myogenic response in the hindlimb circulation. Our results are consistent with the lack of effect of NOS inhibition on the myogenic response in blood-perfused skeletal muscle preparation (Ekelund et al. 1992), in contrast to an effect reported for a cell-free perfusate (Johnsson et al. 1991). The finding that the reduction of baseline flow was less pronounced in the hindlimb than in the kidney agrees with the literature (Sonntag et al. 1992; Sigmon et al. 1993).
Collectively, our results indicate that NO attenuates the myogenic response in renal autoregulation but not in skeletal muscle. This effect is largely dependent on TGF and is more prominent at higher levels of RAP. Signalling pathways of this effect of NO may be the same as those known to mediate the general vasodilator effects of NO, i.e. activation of soluble guanlylate cyclase, cGMP and cGMP-dependent kinase (Carvajal et al. 2000; Schlossmann et al. 2003), which have been shown to affect Ca2+-entry, -release and -sensitivity, as well as modulating factors of smooth muscle contraction such as cAMP, phospholipase C, protein kinase C and large conductance K+ (BK) channels (Carvajal et al. 2000; Schlossmann et al. 2003). In addition, cGMP-independent, direct inhibition of cytochrome P450-induced production of 20-hydroxyeicosatrienoic acid (20-HETE) and subsequent reduced inhibition of BK channels (Zou et al. 1996) or reduced activation of rho kinase (Randriamboavonjy et al. 2003) could also play a role. However, studies in the hydronephrotic kidney suggest that cGMP-independent pathways may be of minor importance for the effects of endothelial-derived NO in the afferent arteriole (Trottier et al. 1998).
What is not clear is how NO affects myogenic responsiveness in conjunction with TGF. One intriguing hypothesis is that NO derived from macula densa cells rather than endothelial cells is responsible for the effects of NO. The reason why macula densa-derived NO might have more impact than endothelial-derived NO might involve the microenvironment of the juxtaglomerular apparatus and more ready accessibility from basolateral versus luminal aspects of the afferent arteriole. Another possibility that might contribute to the putative preferential influence of NO from the macula densa is that NO may not affect the myogenic response directly but rather may act on a target that is more easily accessible by NO from the macula densa than from the endothelium. Such a target, e.g. macula densa cells themselves (Ren et al. 2000) or mesangial cells, may then release a non-NO mediator. Future studies are required to test the relative potency of NO derived from neuronal NOS in macula densa cells on the renal myogenic response as compared with endothelial NOS. Another explanation for the TGF dependency of the NO effect might be that TGF signals during resting or elevated tubular NaCl concentrations condition the myogenic response to be more susceptible to the effects of NO, irrespective of NO origin.
In view of the ability of NO to attenuate TGF, at least at high tubular flow rates (Wilcox et al. 1992; Thorup et al. 1993), it may seem surprising that the balance between the autoregulating mechanisms is shifted towards the myogenic response rather than TGF during NOS inhibition. A possible explanation is that the myogenic response is faster and upstream of TGF, so it effectively buffers a pressure perturbation and thus minimizes an error signal reaching TGF. Accordingly, the myogenic response would dominate autoregulation, unless it is restrained by processes such as interactions with TGF (Schnermann & Briggs, 1989; Kallskog & Marsh, 1990; Just & Arendshorst, 2003). NO might act to increase the strength of these interactions and thereby attenuate the dominance of the myogenic response. One possibility of such NO-modulated coupling is by gap junctions. However, NO appears to suppress rather than enhance gap junction communication (Bolanos & Medina, 1996; Kameritsch et al. 2005). It should also be kept in mind that enhanced TGF tone does not necessarily imply large autoregulatory efficiency of TGF, if the operating point is at one of the saturation ends of TGF (Thomson et al. 2004). Another possible explanation that could contribute to our results is interaction between TGF-mediated vasoconstriction of the terminal afferent arteriole and myogenic responses at more upstream sites as proposed in the concept of ascending myogenic response (Moore et al. 1994). According to this view, a localized vasoconstriction not only reduces intraluminal pressure distal from that site but also flattens the profile of intravascular pressure upstream. Thus, any change in RAP would cause a larger initial change in intraluminal pressure in the proximal vascular segments and subsequently elicit an enhanced myogenic response. If NOS inhibition were to induce localized vasoconstriction of the terminal afferent arteriole, the ascending myogenic response could explain or contribute to the enhanced myogenic autoregulation during NOS inhibition in our results. Although there is little information on the distribution of the effects of NOS inhibition along the renal vascular tree, available in vitro data are compatible with localized constriction at the end of the afferent arteriole (Imig & Roman, 1992). Interestingly, the constrictor effects of NOS inhibition are more uniform along the preglomerular vasculature in preparations devoid of TGF (Hoffend et al. 1993). Although we have no measure of TGF tone in our in vivo setting, the finding of a vasodilator response to TGF inhibition during l-NAME addition as compared with the lack of such effect in the normal animal suggests enhanced TGF tone in the absence of NO. Since the effect of TGF is confined to the most distal part of the afferent arteriole (Casellas & Moore, 1990), and NO is known to attenuate TGF (Wilcox et al. 1992; Thorup et al. 1993), NOS inhibition via activation of TGF may cause localized vasoconstriction and thereby enhanced myogenic autoregulation via the ascending myogenic response. However, our observation that infusion of Ang II failed to affect myogenic autoregulation despite an identical degree of overall renal vasoconstriction as l-NAME, speaks against this hypothesis, unless it is postulated that Ang II causes more uniform vasoconstriction along the vascular tree, even though it is also known to enhance TGF (Huang & Navar, 1988). To date, reports on the effects of Ang II are variable, favouring either homogenous distribution along the preglomerular vasculature (Carmines et al. 1986) or a preferential action on the distal afferent arteriole (Casellas et al. 1985). Clearly, more work needs to be done to more completely understand the intriguing effects of NO on RBF autoregulation in the whole kidney described in the present study.
Perspectives
As a whole, our data provide new information about NO attenuating the myogenic response in the normal kidney with an intact TGF and possibly mediating a third mechanism distinct from myogenic and TGF. The ability of NO to dampen myogenic tone is dependent on an active TGF. By restraining the myogenic response to changes in RAP, TGF and the third mechanism assume larger roles at the expense of decelerating overall autoregulation. The slower autoregulation allows a greater amount and a slower type of RAP fluctuations to reach glomerular and postglomerular capillaries. The ability of NO to attenuate the strength and the speed of the myogenic response impacts on glomerular filtration, pressure natriuresis and hypertensive renal damage.
It is worth noting that a role of NO in pressure natriuresis is well established (Evans et al. 2005) and the modulation of the balance between autoregulating mechanisms might be a contributing mechanism. Furthermore, RBF autoregulation in Brown-Norway rats, a rat strain highly susceptible to hypertensive renal damage (Churchill et al. 1997), has a weak, attenuated myogenic response, a defect that can be corrected by NOS inhibition (Wang & Cupples, 2001). Similar effects could play a role in Fawn-hooded rats, that are genetically predisposed to glomerulosclerosis and show a weak myogenic response and slow autoregulation (van Dokkum et al. 1999), or early in diabetes mellitus, which is accompanied by an increased risk for glomerulosclerosis and evidence for elevated renal levels of NO (Komers & Anderson, 2003).
Our hindlimb data clearly show the absence of any modulating influence of physiological levels of NO on myogenic autoregulation in the skeletal muscle circulation. This agrees with the only other in vivo study investigating the effect of NO on myogenic responsiveness on total organ blood flow (Ekelund et al. 1992). On the contrary, many studies detecting an enhancement of myogenic responsiveness during NOS inhibition were performed in vitro using cell-free perfusion media, which may have caused supranormal levels of NO both in skeletal muscle (Johnsson et al. 1991) and in other organs (Griffith & Edwards, 1990; Ueeda et al. 1992; Pohl et al. 1994; Imig et al. 1993). It appears that an augmentation of myogenic responsiveness in response to NOS inhibition particularly occurs in those tissues and vessel segments in which myogenic responses are weak or absent normally (Griffith & Edwards, 1990; de Wit et al. 1998; Brookes & Kaufman, 2003). This emphasizes the importance of validating in vitro findings of single vessels by in vivo blood flow studies.
Acknowledgments
This work was supported by NIH research grant HL-02334 from the Heart, Blood and Lung Institute and by the Guyton Award for Excellence in Integrative Physiology from the American Physiological Society.
References
- Ajikobi DO, Novak P, Salevsky FC, Cupples WA. Pharmacological modulation of spontaneous renal blood flow dynamics. Can J Physiol Pharmacol. 1996;74:964–972. [PubMed] [Google Scholar]
- Baer PG, Navar LG, Guyton AC. Renal autoregulation, filtration rate, and electrolyte excretion during vasodilation. Am J Physiol. 1970;219:619–625. doi: 10.1152/ajplegacy.1970.219.3.619. [DOI] [PubMed] [Google Scholar]
- Baumann JE, Persson PB, Ehmke H, Nafz B, Kirchheim HR. Role of endothelium-derived relaxing factor in renal autoregulation in conscious dogs. Am J Physiol. 1992;263:F208–F213. doi: 10.1152/ajprenal.1992.263.2.F208. [DOI] [PubMed] [Google Scholar]
- Beierwaltes WH, Sigmon DH, Carretero OA. Endothelium modulates renal blood flow but not autoregulation. Am J Physiol. 1992;262:F943–F949. doi: 10.1152/ajprenal.1992.262.6.F943. [DOI] [PubMed] [Google Scholar]
- Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. ‘Step’ vs. ‘dynamic’ autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol. 2003;285:F113–F120. doi: 10.1152/ajprenal.00012.2003. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Medina JM. Induction of nitric oxide synthase inhibits gap junction permeability in cultured rat astrocytes. J Neurochem. 1996;66:2091–2099. doi: 10.1046/j.1471-4159.1996.66052091.x. [DOI] [PubMed] [Google Scholar]
- Bouriquet N, Casellas D. Interaction between cGMP-dependent dilators and autoregulation in rat preglomerular vasculature. Am J Physiol. 1995;268:F338–F346. doi: 10.1152/ajprenal.1995.268.2.F338. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, Kaufman S. Myogenic responses and compliance of mesenteric and splenic vasculature in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1604–R1610. doi: 10.1152/ajpregu.00411.2002. [DOI] [PubMed] [Google Scholar]
- Carmines PK, Morrison TK, Navar LG. Angiotensin II effects on microvascular diameters of in vitro blood-perfused juxtamedullary nephrons. Am J Physiol. 1986;251:F610–F618. doi: 10.1152/ajprenal.1986.251.4.F610. [DOI] [PubMed] [Google Scholar]
- Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Casellas D, Carmines PK, Navar LG. Microvascular reactivity of in vitro blood perfused juxtamedullary nephrons from rats. Kidney Int. 1985;28:752–759. doi: 10.1038/ki.1985.194. [DOI] [PubMed] [Google Scholar]
- Casellas D, Moore LC. Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol. 1990;258:F660–F669. doi: 10.1152/ajprenal.1990.258.3.F660. [DOI] [PubMed] [Google Scholar]
- Churchill PC, Churchill MC, Bidani AK, Griffin KA, Picken M, Pravenec M, Kren V, St Lezin E, Wang JM, Wang N, Kurtz TW. Genetic susceptibility to hypertension-induced renal damage in the rat. Evidence based on kidney-specific genome transfer. J Clin Invest. 1997;100:1373–1382. doi: 10.1172/JCI119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen G, Oien AH, Aukland K. Myogenic vasoconstriction in the rat kidney elicited by reducing perirenal pressure. Acta Physiol Scand. 1992;144:277–290. doi: 10.1111/j.1748-1716.1992.tb09297.x. [DOI] [PubMed] [Google Scholar]
- Daniels FH, Arendshorst WJ. Tubuloglomerular feedback kinetics in spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol. 1990;259:F529–F534. doi: 10.1152/ajprenal.1990.259.3.F529. [DOI] [PubMed] [Google Scholar]
- de Wit C, Jahrbeck B, Schafer C, Bolz SS, Pohl U. Nitric oxide opposes myogenic pressure responses predominantly in large arterioles in vivo. Hypertension. 1998;31:787–794. doi: 10.1161/01.hyp.31.3.787. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Bjornberg J, Grande PO, Albert U, Mellander S. Myogenic vascular regulation in skeletal muscle in vivo is not dependent of endothelium-derived nitric oxide. Acta Physiol Scand. 1992;144:199–207. doi: 10.1111/j.1748-1716.1992.tb09286.x. [DOI] [PubMed] [Google Scholar]
- Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- Falcone JC, Davis MJ, Meininger GA. Endothelial independence of myogenic response in isolated skeletal muscle arterioles. Am J Physiol. 1991;260:H130–H135. doi: 10.1152/ajpheart.1991.260.1.H130. [DOI] [PubMed] [Google Scholar]
- Griffith TM, Edwards DH. Myogenic autoregulation of flow may be inversely related to endothelium-derived relaxing factor activity. Am J Physiol. 1990;258:H1171–H1180. doi: 10.1152/ajpheart.1990.258.4.H1171. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Suzuki H, Saruta T. Nitric oxide modulates but does not impair myogenic vasoconstriction of the afferent arteriole in spontaneously hypertensive rats. Studies in the isolated perfused hydronephrotic kidney. Hypertension. 1995;25:1212–1219. doi: 10.1161/01.hyp.25.6.1212. [DOI] [PubMed] [Google Scholar]
- Hoffend J, Cavarape A, Endlich K, Steinhausen M. Influence of endothelium-derived relaxing factor on renal microvessels and pressure-dependent vasodilation. Am J Physiol. 1993;265:F285–F292. doi: 10.1152/ajprenal.1993.265.2.F285. [DOI] [PubMed] [Google Scholar]
- Huang WC, Navar LG. Tubuloglomerular feedback-dependent influence of angiotensin II on the kidney in rats. Proc Natl Sci Counc Repub China B. 1988;12:180–185. [PubMed] [Google Scholar]
- Imig JD, Gebremedhin D, Harder DR, Roman RJ. Modulation of vascular tone in renal microcirculation by erythrocytes: role of EDRF. Am J Physiol. 1993;264:H190–H195. doi: 10.1152/ajpheart.1993.264.1.H190. [DOI] [PubMed] [Google Scholar]
- Imig JD, Roman RJ. Nitric oxide modulates vascular tone in preglomerular arterioles. Hypertension. 1992;19:770–774. doi: 10.1161/01.hyp.19.6.770. [DOI] [PubMed] [Google Scholar]
- Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- Johnsson E, Folkow B, Karlstrom G. Myogenic responsiveness in rat hindquarter vessels during constant-flow and constant-pressure perfusion in vitro; effects of various potassium concentrations and of endothelial nitrous oxide blockade. Acta Physiol Scand. 1991;142:319–328. doi: 10.1111/j.1748-1716.1991.tb09164.x. [DOI] [PubMed] [Google Scholar]
- Juncos LA, Garvin J, Carretero OA, Ito S. Flow modulates myogenic responses in isolated microperfused rabbit afferent arterioles via endothelium derived nitric oxide. J Clin Invest. 1995;95:2741–2748. doi: 10.1172/JCI117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R619–R631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- Just A, Ehmke H, Toktomambetova L, Kirchheim HR. Dynamic characteristics and underlying mechanisms of renal blood flow autoregulation in the conscious dog. Am J Physiol Renal Physiol. 2001;280:F1062–F1071. doi: 10.1152/ajprenal.2001.280.6.F1062. [DOI] [PubMed] [Google Scholar]
- Just A, Ehmke H, Wittmann U, Kirchheim HR. Tonic and phasic influences of nitric oxide on renal blood flow autoregulation in conscious dogs. Am J Physiol. 1999;276:F442–F449. doi: 10.1152/ajprenal.1999.276.3.F442. [DOI] [PubMed] [Google Scholar]
- Just A, Ehmke H, Wittmann U, Kirchheim HR. Role of angiotensin II in dynamic renal blood flow autoregulation of the conscious dog. J Physiol. 2002;538:167–177. doi: 10.1113/jphysiol.2001.012593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just A, Wittmann U, Ehmke H, Kirchheim HR. Autoregulation of renal blood flow in the conscious dog and the contribution of the tubuloglomerular feedback. J Physiol. 1998;506:275–290. doi: 10.1111/j.1469-7793.1998.275bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallskog O, Marsh DJ. TGF-initiated vascular interactions between adjacent nephrons in the rat kidney. Am J Physiol. 1990;259:F60–F64. doi: 10.1152/ajprenal.1990.259.1.F60. [DOI] [PubMed] [Google Scholar]
- Kameritsch P, Khandoga N, Nagel W, Hundhausen C, Lidington D, Pohl U. Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J Cell Physiol. 2005;203:233–242. doi: 10.1002/jcp.20218. [DOI] [PubMed] [Google Scholar]
- Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol. 2003;284:F1121–F1137. doi: 10.1152/ajprenal.00265.2002. [DOI] [PubMed] [Google Scholar]
- Kramp R, Fourmanoir P, Caron N. Endothelin resets renal blood flow autoregulatory efficiency during acute blockade of NO in the rat. Am J Physiol Renal Physiol. 2001;281:F1132–F1140. doi: 10.1152/ajprenal.0078.2001. [DOI] [PubMed] [Google Scholar]
- Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res. 2002;90:1316–1324. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- Majid DS, Navar LG. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol. 1992;262:F40–F46. doi: 10.1152/ajprenal.1992.262.1.F40. [DOI] [PubMed] [Google Scholar]
- Moore LC, Rich A, Casellas D. Ascending myogenic autoregulation: interactions between tubuloglomerular feedback and myogenic mechanisms. Bull Math Biol. 1994;56:391–410. doi: 10.1007/BF02460464. [DOI] [PubMed] [Google Scholar]
- Moore LC, Schnermann J, Yarimizu S. Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am J Physiol. 1979;237:F63–F74. doi: 10.1152/ajprenal.1979.237.1.F63. [DOI] [PubMed] [Google Scholar]
- Navar LG, Inscho EW, Majid DSA, Imig JD, Harrison Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Boegehold MA. Limitation of arteriolar myogenic activity by local nitric oxide: segment-specific effect of dietary salt. Am J Physiol. 1999;277:H1946–H1955. doi: 10.1152/ajpheart.1999.277.5.H1946. [DOI] [PubMed] [Google Scholar]
- Pohl U, Herlan K, Huang A, Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol. 1991;261:H2016–H2023. doi: 10.1152/ajpheart.1991.261.6.H2016. [DOI] [PubMed] [Google Scholar]
- Pohl U, Lamontagne D, Bassenge E, Busse R. Attenuation of coronary autoregulation in the isolated rabbit heart by endothelium derived nitric oxide. Cardiovasc Res. 1994;28:414–419. doi: 10.1093/cvr/28.3.414. [DOI] [PubMed] [Google Scholar]
- Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- Ren YL, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int. 2000;58:2053–2060. doi: 10.1111/j.1523-1755.2000.00377.x. [DOI] [PubMed] [Google Scholar]
- Ritter ER. Pressure/flow relations in the kidney: Alleged effects of pulse pressure. Am J Physiol. 1952;168:480–489. doi: 10.1152/ajplegacy.1952.168.2.480. [DOI] [PubMed] [Google Scholar]
- Rubanyi G, Romero JC, Vanhoutte P. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36:1627–1639. [Google Scholar]
- Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35:21–27. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Briggs JP. Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol. 1989;256:F421–F429. doi: 10.1152/ajprenal.1989.256.3.F421. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Briggs JP. Restoration of tubuloglomerular feedback in volume-expanded rats by angiotensin II. Am J Physiol. 1990;259:F565–F572. doi: 10.1152/ajprenal.1990.259.4.F565. [DOI] [PubMed] [Google Scholar]
- Selkurt EE. Effect of pulse pressure and mean arterial pressure modification on renal hemodynamics and electrolyte and water excretion. Circ Res. 1951;4:541. doi: 10.1161/01.cir.4.4.541. [DOI] [PubMed] [Google Scholar]
- Sigmon DH, Carretero OA, Beierwaltes WH. Renal versus femoral hemodynamic response to endothelium-derived relaxing factor synthesis inhibition. J Vasc Res. 1993;30:218–223. doi: 10.1159/000158997. [DOI] [PubMed] [Google Scholar]
- Sonntag M, Deussen A, Schrader J. Role of nitric oxide in local blood flow control in the anesthetized dog. Pflugers Arch. 1992;420:194–199. doi: 10.1007/BF00374990. [DOI] [PubMed] [Google Scholar]
- Sun D, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated gracilis muscle arterioles. Am J Physiol. 1994;266:H1177–H1183. doi: 10.1152/ajpheart.1994.266.3.H1177. [DOI] [PubMed] [Google Scholar]
- Thomson SC, Deng A, Komine N, Hammes JS, Blantz RC, Gabbai FB. Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am J Physiol Renal Physiol. 2004;287:F732–F738. doi: 10.1152/ajprenal.00340.2003. [DOI] [PubMed] [Google Scholar]
- Thorup C, Sundler F, Ekblad E, Persson AE. Resetting of the tubuloglomerular feedback mechanism by blockade of NO-synthase. Acta Physiol Scand. 1993;148:359–360. doi: 10.1111/j.1748-1716.1993.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Trottier G, Triggle CR, O'Neill SK, Loutzenhiser R. Cyclic GMP-dependent and cyclic GMP-independent actions of nitric oxide on the renal afferent arteriole. Br J Pharmacol. 1998;125:563–569. doi: 10.1038/sj.bjp.0702090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkstra E, Braam B, Koomans HA. Impaired renal blood flow autoregulation in two-kidney, one-clip hypertensive rats is caused by enhanced activity of nitric oxide. J Am Soc Nephrol. 2000;11:847–855. doi: 10.1681/ASN.V115847. [DOI] [PubMed] [Google Scholar]
- Ueeda M, Silvia SK, Olsson RA. Nitric oxide modulates coronary autoregulation in the guinea pig. Circ Res. 1992;70:1296–1303. doi: 10.1161/01.res.70.6.1296. [DOI] [PubMed] [Google Scholar]
- van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol. 1999;276:R855–R863. doi: 10.1152/ajpregu.1999.276.3.R855. [DOI] [PubMed] [Google Scholar]
- Walstead C, Yip KP. Acute arterial hypertension inhibits proximal tubular fluid reabsorption in normotensive rat but not in SHR. Am J Physiol Regul Integr Comp Physiol. 2004;286:R726–R733. doi: 10.1152/ajpregu.00352.2003. [DOI] [PubMed] [Google Scholar]
- Wang X, Cupples WA. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can J Physiol Pharmacol. 2001;79:238–245. [PubMed] [Google Scholar]
- Wang X, Salevsky FC, Cupples WA. Nitric oxide, atrial natriuretic factor, and dynamic renal autoregulation. Can J Physiol Pharmacol. 1999;77:777–786. [PubMed] [Google Scholar]
- Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wronski T, Seeliger E, Persson PB, Forner C, Fichtner C, Scheller J, Flemming B. The step response: a method to characterize mechanisms of renal blood flow autoregulation. Am J Physiol Renal Physiol. 2003;285:F758–F764. doi: 10.1152/ajprenal.00420.2002. [DOI] [PubMed] [Google Scholar]
- Yip KP, Marsh DJ. [Ca2+]i in rat afferent arteriole during constriction measured with confocal fluorescence microscopy. Am J Physiol. 1996;271:F1004–F1011. doi: 10.1152/ajprenal.1996.271.5.F1004. [DOI] [PubMed] [Google Scholar]
- Young DK, Marsh DJ. Pulse wave propagation in rat renal tubules: implications for GFR autoregulation. Am J Physiol. 1981;240:F446–F458. doi: 10.1152/ajprenal.1981.240.5.F446. [DOI] [PubMed] [Google Scholar]
- Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]