Abstract
Interaction between spontaneous and neurally mediated regulation of tone in the corpus cavernosum smooth muscle (CCSM) of the rabbit was investigated. Changes in isometric muscle tension, intracellular Ca2+ concentration ([Ca2+]i) and membrane potential were recorded. CCSM developed spontaneous contractions, transient increases in [Ca2+]i (Ca2+ transients) and depolarizations. This spontaneous activity was abolished by blocking L-type Ca2+ channels (nicardipine, 1 μm), sarcoplasmic reticulum Ca2+ pump activity (cyclopiazonic acid, 10 μm), Ca2+-activated Cl− channels (niflumic acid, 10 μm) or cyclooxygenase-2 (COX-2; NS-398, 1 μm). Transmural nerve stimulation initiated either α-adrenergic contractions or nitrergic relaxations of CCSM depending on the level of muscle tone. NS-398 suppressed nerve-evoked contractions by about 70% but caused only a 40% reduction in the corresponding Ca2+ transient. Blocking nitric oxide synthase with Nω-nitro-l-arginine (LNA, 100 μm) reinforced nerve-evoked Ca2+ transients by about 150%, whilst increasing the corresponding Ca2+ transients by only 20%. In CCSM preparations that had been pre-contracted with either noradrenaline (0.3 μm) or prostaglandin F2α (0.1 μm), nerve stimulation inhibited about 70% of the contraction and caused only a 20% decrease in [Ca2+]i. Fluorescent immunohistochemistry with COX-2 antibodies and the reverse transcriptase-polymerase chain reaction (RT-PCR) method showed that the enzyme and its mRNA were highly expressed in the CCSM. These results suggest that spontaneously produced prostaglandins (PGs) not only contribute to the generation of spontaneous contractions but also facilitate nerve-evoked contractions. Conversely, spontaneously released nitric oxide (NO) suppresses excitation. Thus, interaction between spontaneous and neurally mediated regulation of CCSM tone may be fundamental to maintaining the muscle contractility. In addition, both PGs and NO appear to alter CCSM tone with only small changes in [Ca2+]i.
Corpus cavernosum smooth muscle (CCSM) tone is controlled by the balance between contractile and relaxant factors to determine the contractile state of the penis. Relaxation of CCSM is principally mediated by nitric oxide (NO), which is released from both parasympathetic nerves and endothelium, and results in penile erection (Andersson, 2001). Neurally released NO is still considered to play a central role in CCSM relaxation (Holmquist et al. 1992), and may be particularly important in initiating erection (Hurt et al. 2002). Endothelial NO is produced continuously in response to shear stress and seems to play a dominant role in maintaining erection (Hurt et al. 2002). Unless CCSM receives parasympathetic neural input, it remains contracted for the majority of time to maintain penile detumescence. Contraction of CCSM is generally believed to depend on neurally released noradrenaline (NAd) which acts tonically on α-adrenoceptors on the CCSM membrane (Andersson, 2001). In addition, spontaneous contractions may contribute to the overall tone of CCSM, although mechanisms underlying these contractions still remain to be established.
Spontaneous contractions have been recorded from CCSM from various mammals, including man (Fovaeus et al. 1987; Christ et al. 1990). Since these contractions depend on the extracellular Ca2+, and are blocked by either L-type Ca2+ channel blockers or ATP-sensitive K+ channel openers, they probably result from the influx of Ca2+ through L-type Ca2+ channels (Fovaeus et al. 1987; Christ et al. 1990). Unfortunately, no reports of intracellular recordings in intact CCSM are known to us that show generation of spontaneous action potentials. However, spontaneous action potentials recorded from intact corpus spongiosum smooth muscle (CSSM) preparations of the rat and guinea-pig are abolished by L-type Ca2+ channel blockers (Hashitani, 2000; Hashitani et al. 2002). Furthermore, nifedipine-sensitive Ca2+ currents have been recorded from isolated rabbit CCSM cells (Craven et al. 2004), suggesting that the individual CCSM cells may be capable of generating action potentials by the opening of L-type Ca2+ channels.
Spontaneous contractions of CCSM are also inhibited by lowering the temperature, suggesting that some metabolic processes are involved in their generation (Christ et al. 1990). Spontaneous Ca2+ transients recorded from CSSM of the guinea-pig are readily blocked by CPA, ryanodine or 2-aminoethoxydriphenyl borate (2-APB), suggesting that Ca2+ release from intracellular Ca2+ stores via both InsP3- and ryanodine-receptors contributes to their generation (Hashitani & Suzuki, 2004). Consistently, Ca2+-activated Cl− currents have been recorded from isolated CCSM, indicating that activation of these channels by Ca2+ released from intracellular stores may underlie spontaneous depolarizations by triggering action potentials (Craven et al. 2004). In addition, spontaneous contractions of CCSM are known to be inhibited by indomethacin, and thus spontaneous formation of prostaglandins may be involved in their generation (Christ et al. 1990).
The α-adrenoceptor-mediated contractions of CCSM are thought to result from both calcium release from intracellular stores through InsP3 production and calcium influx through L-type calcium channels (Andersson, 2001). Besides these Ca2+-dependent contractile mechanisms, increasing the sensitivity of contractile protein to Ca2+ is now considered to play an essential role in α-adrenoceptor-mediated contractions in both CSSM (Hashitani et al. 2002) and CCSM (Takahashi et al. 2003). Since CCSM develop spontaneous activity, neurally released NAd may alter this activity. Conversely, spontaneously released substances may modulate neurally mediated contractions. However, the interaction between spontaneous activity and neurally released NAd has not been clarified.
In CSSM, NO/cGMP is believed to cause relaxation mainly by reducing the sensitivity of contractile proteins to Ca2+, although NO/cGMP are also capable of lowering [Ca2+]i by stimulating Ca2+-ATPase or by inhibiting L-type calcium channels (Hashitani et al. 2002). However, the detailed mechanisms underlying nitrergic relaxation of CCSM remain to be established. Furthermore, NO may be spontaneously released in CCSM to stabilize excitation of this muscle.
The purpose of the present study is firstly to understand the mechanisms for spontaneous regulation of CCSM tone, particularly focusing on the role of endogenous prostaglandins and NO, and secondly to investigate the interaction between spontaneous and neurally mediated regulation of CCSM excitation. Changes in muscle tension and [Ca2+]i in intact CCSM preparations of the rabbit were measured. Intracellular microelectrodes were also used to identify underlying membrane potential changes.
Methods
Tissue preparation
The procedures described have been approved by the animal experimentation ethics committee of the Physiological Society of Japan. Male rabbits, weighing 2–3 kg, were humanely killed by exsanguination under pentobarbitone (i.v.) anaesthesia. The penis was rapidly removed and placed in physiological saline. After removal of the bulbospongiosum muscle and the corpus spongiosum, the tunica albuginea, which covers the bottom of the penile shaft, was slit open with bilateral incisions. Both corpus cavernosa were then dissected from the tunica albuginea and were pinned out in a dissecting chamber. For tension recordings, each CCSM preparation was cut into strips approximately 1 mm × 1 mm × 5 mm. For Ca2+ measurements or intracellular recordings, small pieces of CCSM, approximately 2 mm long and 1 mm wide, were dissected and then several smooth muscle layers were removed leaving a single layer of smooth muscle.
Isometric tension recordings
For isometric tension recordings, CCSM preparations were transferred to 2 ml organ baths and were superfused with warmed (36°C) physiological saline at a constant flow rate (2 ml min−1). Silk threads were tied around both ends of a strip, one of them was fixed at the bottom of the organ bath and the other was connected to an isometric force transducer that was connected to a bridge amplifier. Isometric tension changes were digitized using a Digidata 1200 interface and stored on a personal computer for later analysis. After setting up, the preparations were allowed to equilibrate for 90–120 min. During this period the tension was adjusted several times until a final basal tension of approximately 4 mN was achieved.
Intramural nerves were selectively stimulated by passing brief pulses of constant current (duration 100 μs) between two parallel silver-plated electrodes placed in the organ bath. The neural selectivity was confirmed by sensitivity to tetrodotoxin (1 μm).
Intracellular calcium measurements
For the measurement of changes in [Ca2+]i, small CCSM preparations were pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corporation, Midland, MI, USA) at the bottom of a recording chamber (volume, approximately 1 ml) which was mounted on the stage of an inverted microscope. After 1 h incubation with warmed (35°C) physiological saline, spontaneous movements of the tissues were observed. Subsequently the preparations were loaded with a fluorescent dye, by incubation in nominally Ca2+-free solution containing fura-2 AM (10 μm; Molecular Probes) and cromphor EL (0.01%, Sigma) for 1 h at room temperature. After loading, preparations were superfused with dye-free warmed (35°C) physiological saline at a constant flow (about 2 ml min−1) for 30 min. Preparations loaded with fura-2 were illuminated with ultraviolet light, wavelengths 340 and 380 nm, alternating at a frequency higher than 40 Hz. The ratio of the emission fluorescence (R340/380) in a desired size of rectangular window was measured through a barrier filter (peak transmission, 510 nm; sampling time, 150–210 ms), using a microphotoluminescence measurement system (ARGUS/HiSCA, Hamamatsu Photonics, Hamamatsu, Japan), and was taken as an index of [Ca2+]i. In vitro calibration using calcium calibration buffer kits (Molecular Probes) determined that [Ca2+]i was 108.1 nm at R340/380= 0.8 and 348 nm at R340/380= 1.3, and that the relationship between [Ca2+]i and R340/380 was linear within this range.
In some experiments, isometric tension recordings were taken simultaneously with measurements of [Ca2+]i. To detect isometric tension changes of the preparations, an L-shaped fine needle connected to a force transducer was inserted into the cavernosal space, and was then carefully pulled towards surrounding muscle bundles.
For transmural nerve stimulation, the preparations were placed between a pair of platinum electrodes in the recording chamber, and were stimulated by passing brief pulses of constant current (duration, 50 μs). The neural selectivity was confirmed by sensitivity to tetrodotoxin (1 μm).
Intracellular recordings
For recording of the membrane potential, CCSM preparations were pinned out in the same type of recording chamber used for the calcium experiments and were superfused with warmed (35°C) physiological saline at a constant flow rate (2 ml min−1). After 1 h equilibration, individual CCSM cells were impaled with glass capillary microelectrodes, filled with 0.5 m KCl (tip resistance, 150–250 MΩ). Membrane potential changes were recorded using a high input impedance amplifier (Axoclamp-2B, Axon Instruments), and displayed on a cathode-ray oscilloscope (SS-9622, Iwatsu, Tokyo, Japan). After low-pass filtering (cut-off frequency, 1 kHz), membrane potential changes were digitized with a Digidata 1200 interface (Axon Instruments), and stored on a personal computer for later analysis.
Immunohistochemical studies
For the fluorescence immunohistochemistry, the penis was cut across into a few segments and fixed overnight at 4°C in Bouin's solution without acetic acid (formalin-picric acid = 3 = 1). Fixed tissues were processed routinely and embedded in Paraplast embedding media (Sigma). Round sections, 5 μm thick, were prepared and mounted onto poly-l-lysine-coated glass slides. After being dewaxed in xylene and rehydrated in ethanol to water, the sections were boiled using a microwave oven in 10 mm citric acid pH 6.0, for 5 min to retrieve the antigen. An affinity purified goat polyclonal antibody raised against a peptide mapping at the C-terminus of COX-2 (200 μg ml−1, Santa Cruz Biotech. Inc., Santa Cruz, CA, USA) was used at a concentration of 10–1000 ng ml−1. The sections were incubated overnight with the first antibody at 37°C, and then with the second antibody (FITC-conjugated bovine antigoat IgG, Santa Cruzn Biotech. Inc.). Immunostained cells were observed under a UV microscope equipped with a colour chilled 3 CCD camera (Nikon Eclipse E600).
To identify the morphological characteristics of cells with COX-2 immunoreactivity, conventional light microcopy was also carried out. Paraffin-embedded cross sections of formalin-fixed rabbit penis (3 μm thick) were examined after haematoxylin and eosin (HE) staining.
Detection of COX-2 messenger RNA by the RT- PCR method
Two parts of the penis, the corpus cavernosum and the corpus spongiosum, were separated under a stereoscopic microscope, quickly frozen in liquid nitrogen, and stored at −80°C until the total RNA was extracted. Total RNAs were prepared by ISOGEN (Nippon Gene, Tokyo, Japan) from the two parts of the penis. Expression of mRNAs encoding cyclooxygenase-2, cyclooxygenase-1 and β-actin as controls was determined by RT-PCR (n = 3). Total RNA (1 μg) was converted into cDNA by reverse transcription using poly (dN)6 and poly (dT)12–18 primers (Amersham Pharmacia Biotech, Buckinghamshire, UK) and Moloney murine leukaemia virus reverse transcriptase (Life Technology Inc., Rockville, MD, USA) in a total reaction volume of 50 μl. The primer sets of COX-2 (forward: 5′- AATCGTTCGAGGAACTTACAGGGGAGAAGG-3′; reverse: 5′- CGTCTACAGTTCAGTGGAGCGGCCTTTCAG-3′), COX-1 (forward: 5′- GTTCTTGTTCAACACCTCCATGCTGGTGGA-3′; reverse: 5′- CCCAAAGATGGAGTTTGGCTGGCACTTTTC-3′) and β-actin (forward: 5′-TTCTACAATGAGCTGCGTGTGG-3′; reverse: 5′-GTTTCATGGATGCCACAGGATTCC-3′) were made using sequences based on the nucleotide sequences in the rabbit, and their product sizes were 434, 340 and 564 base pairs, respectively. The amplification protocol consisted of 25 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 55°C and extension for a min at 72°C. The PCR products were electrophoresed on 2% agarose gel stained with ethidium bromide and visualized by ultraviolet illumination.
Solutions and drugs
The ionic composition of physiological saline was as follows (mm): NaCl, 119; KCl, 5.0; CaCl2, 2.5; MgCl2, 2.0; NaHCO3; 25.0; NaH2PO4; 1.0 and glucose, 11.0. The solution was bubbled with 95% O2 and 5% CO2 to maintain pH 7.4.
Drugs used were charybdotoxin (CTX), iberiotoxin (IbTX), 3-morpholino-sydnonimine (SIN-1) hydrochloride, Nω-nitro-l-arginine (LNA), l-arginine, cyclopiazonic acid (CPA), nifedipine, nicardipine, niflumic acid, noradrenaline hydrochloride, ouabain, NS-398, prostaglandin F2α, SC-560 (all from Sigma). These drugs were dissolved in distilled water except nifedipine and niflumic acid, which were dissolved in 100% ethanol, and CPA, nicardipine, NS-398 and SC-560, which were dissolved in dimethyl sulphoxide (DMSO). The final concentration of these solvents in physiological saline did not exceed 1 : 1000.
Calculations and statistics
Measured values are expressed as mean ±s.d. Statistical significance was tested using Student's t test, and probabilities of less than 5% were considered significant.
Results
General observations
Spontaneous contractions developed in about 70% of CCSM preparations. However, the frequency and amplitude of the contractions varied substantially between tissues, and thus quantitative characteristics of spontaneous contractions for entire experiments were not obtained. Typically, spontaneous contractions consisted of a rapid initial phase which was followed by a sustained phase with oscillatory contractions (bursting contractions; Fig. 1Aa). In 10 preparations, each burst lasted between 130–1420 s (mean 511.7 ± 249 s) and after various quiescent periods, bursts of contractions were generated repeatedly throughout the experiments. Thus, the preparations were contracted for about 70% of examined time (72.7 ± 12.5%, n = 10). In preparations which generated continuous spontaneous contractions, their frequency was 25.4 ± 9.5 min−1 (Fig. 1Ab, n = 7). Individual preparations exhibited one particular type of spontaneous contractions, i.e. bursting or continuous, throughout the experiments. Regardless of the pattern, spontaneous contractions were not blocked by phentolamine (1 μm, n = 5), guanethidine (10 μm, n = 3), atropine (1 μm, n = 3), or tetrodotoxin (1 μm, n = 4), indicating that their generation does not rely on neural activity.
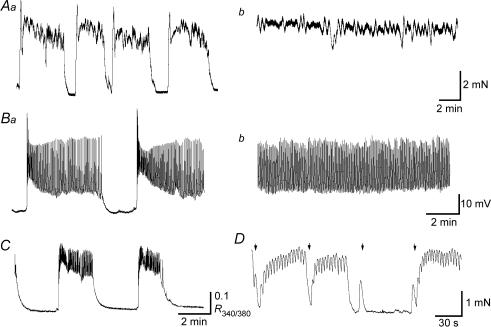
Figure 1. Spontaneous excitations in corpus cavernosum smooth muscles of the rabbit.
A large CCSM preparation exhibited bursting spontaneous contractions (Aa). Another preparation developed continuous contractions (Ab). In a small CCSM preparation, bursts of spontaneous depolarizations were generated (Ba). In a different preparation, continuous spontaneous depolarizations were generated (Bb). A small CCSM preparation exhibited spontaneous increases in [Ca2+]i (C). In a smaller CCSM preparation which developed spontaneous contractions, transmural stimulation applied during a contracting phase initiated phasic relaxations (1st and 2nd stimuli, D). In contrast, stimulation applied during a period of relaxation evoked a phasic contraction (3rd stimulus) or initiated spontaneous contractions (4th stimulus, D). The scale bar on the right of Ab applies to both traces in A and the scale bar on the right of Bb refers to both traces in B.
In a separate series of experiments using small CCSM preparations, spontaneous contractions, which were similar to those in larger preparations, were exhibited by about 80% of preparations, whilst the remaining 20% of preparations were quiescent. Intracellular recordings from a cell in a multicellular CCSM preparation demonstrated that the smooth muscle itself could generate bursts of spontaneous depolarizations consisting of both a rapid initial phase and a following oscillatory depolarization (Fig. 1Ba). However, unlike contractile activity, continuous depolarization was more commonly seen (Fig. 1Bb). When changes in [Ca2+]i were measured, CCSM developed spontaneous Ca2+ transients which had a rapid rising phase and subsequent oscillatory changes in [Ca2+]i (Fig. 1C).
During the relaxed phases, brief transmural stimulation (1 s, 20 Hz) either evoked phasic contractions or initiated prolonged contractions which were very similar to spontaneous contractions (Fig. 1D). These responses were blocked by either phentolamine (1 μm, n = 4) or guanethidine (10 μm, n = 3), indicating that they result from the activation of α-adrenoceptors by neurally released NAd. In contrast, nerve stimulation, applied during the contracting phase, either initiated phasic relaxations or terminated spontaneous contractions (Fig. 1D). These relaxant effects were abolished by LNA (100 μm, n = 7), suggesting that they are mediated by neurally released NO.
Properties of spontaneous depolarizations
To further investigate the properties of the spontaneous depolarizations, intracellular recordings were made from CCSM. Spontaneous depolarizations were characterized in 17 preparations; they occurred either continuously (n = 14) or in bursting patterns (n = 2). During the period of action potential generation, they occurred with frequencies between 11 and 30 min−1 (mean 22.6 ± 6.1 min−1, n = 17), had peak amplitudes between 23 and 34 mV (mean 27.0 ± 3.8 mV, n = 17) and half-widths ranging from 134 to 451 ms (mean 283.6 ± 112.5 ms n = 17). The resting membrane potential, which was defined as the most negative potential level during spontaneous depolarizations, varied between −50 and −41 mV (mean −46.4 ± 3.2 mV, n = 17).
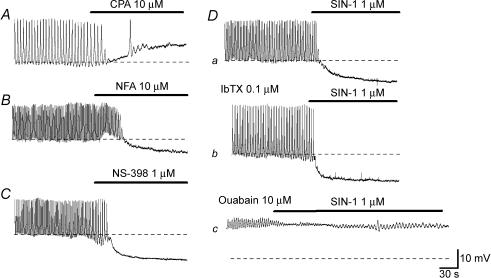
Spontaneous depolarizations were abolished by either nifedipine (1 μm, n = 3) or nicardipine (1 μm, n = 5) without changing the membrane potential, indicating that they result from the activation of L-type Ca2+ channels. CPA (10 μm, n = 4) depolarized the membrane by about 15 mV (mean 15.3 ± 3.0 mV) and prevented the generation of spontaneous depolarizations (Fig. 2A). Niflumic acid (10 μm, n = 4) hyperpolarized the membrane by about 5 mV (mean 5.7 ± 2.1 mV) and abolished spontaneous depolarizations (Fig. 2B). These results suggested that the opening of Ca2+-activated Cl− channels by the release of Ca2+ from intracellular stores was involved in their generation. In addition, spontaneous depolarizations were also abolished by NS-398 (1 μm, n = 5; Fig. 2C), an inhibitor of cyclo-oxygenase 2, suggesting that spontaneously produced prostaglandins contribute to their generation. NS-398 (1 μm) hyperpolarized the membrane by about 8 mV (mean 8.0 ± 3.6 mV, n = 5).
Figure 2. Pharmacological properties of spontaneous depolarizations in CCSM cells.
In a CCSM preparation, CPA (10 μm) depolarized the membrane by about 10 mV and prevented the generation of spontaneous action potentials (A). In a different preparation, niflumic acid (NFA, 10 μm) hyperpolarized the membrane by about 5 mV and abolished spontaneous action potentials (B). NS-398 (1 μm) hyperpolarized the membrane by about 10 mV and prevented action potential generation (C). In another preparation, SIN-1 (1 μm) hyperpolarized the membrane by about 10 mV and abolished action potentials (Da). In the same preparation which had been exposed to IbTX (0.1 μm), SIN-1 (1 μm) hyperpolarized the membrane by about 10 mV and prevented action potential generation (Db). In the presence of ouabain (10 μm), SIN-1 (1 μm) hyperpolarized the membrane by a few mV and failed to abolish membrane oscillations (Dc). The scale bar on the right of Dc applies to all traces. Resting membrane potentials were −48 mV for A, −45 mV for B, −46 mV for C and −48 mV for D.
SIN-1 (1 μm, n = 5) hyperpolarized the membrane by about 10 mV (mean 9.5 ± 1.6 mV) and prevented the generation of spontaneous depolarizations (Fig. 2Da). In preparations which had been exposed to either iberiotoxin (IbTX; 0.1 μm) or charybdotoxin (CTX; 0.1 μm), SIN-1-induced hyperpolarizations had a similar amplitude to those in control conditions (9.5 ± 2.6 mV in IbTX, n = 4, P > 0.05; 8.8 ± 1.7 mV in CTX, n = 4, P > 0.05) and abolished spontaneous depolarizations (Fig. 2Db). Ouabain (10 μm) depolarized the membrane to around −25 mV (−27.3 ± 3.9 mV, n = 5) and suppressed spontaneous depolarizations. In the presence of ouabain (10 μm), the amplitude of SIN-1 (1 μm) -induced hyperpolarizations were greatly suppressed (1.9 ± 0.7 mV, n = 5, P < 0.05) and failed to prevent the generation of spontaneous depolarizations (Fig. 2Dc).
Effects of NS-398 on spontaneous and neurally evoked changes in [Ca2+]i and tension
Since spontaneous contractions recorded from CCSM have been reported to be inhibited by indomethacin (Christ et al. 1990), the role of prostaglandins in regulating CCSM excitation was further investigated.
During preliminary experiments, SC-560, an inhibitor for COX-1, had much less inhibitory effect on spontaneous contractions than did NS-398, an inhibitor for COX-2. Briefly, SC-560 did not suppress spontaneous contractions at 10 nm which is considered to selectively inhibit COX-1 (see Potenza et al. 2002). Therefore, the following investigations focused on the role of COX-2 in regulating CCSM tone.
NS-398 (1 μm) prevented the generation of spontaneous contractions (n = 6), suggesting that activation of COX-2 is involved in their generation (Fig. 3A).
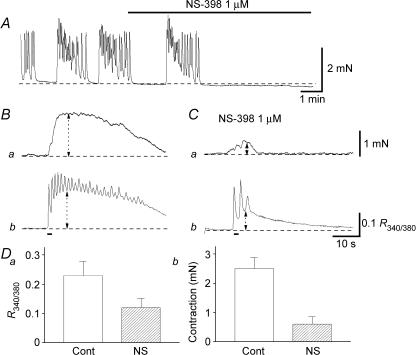
Figure 3. Effects of NS-398 on spontaneous and nerve-evoked excitation.
In a CCSM preparation that generated spontaneous contractions, NS-398 (1 μm) abolished the contractions (A). When changes in tension were measured simultaneously with changes in [Ca2+]i in another preparation, transmural stimulation evoked phasic contractions (Ba) and corresponding increases in [Ca2+]i (Bb) which lasted for some 30 s. NS-398 (1 μm) strongly suppressed nerve-evoked contractions (Ca), and also reduced the amplitude of the initial phase of Ca2+ transients by about 50% (Cb). These results are summarized in D. The scale bar on the right of Ca applies to both tension traces and the scale bar on the right of Cb applies to both [Ca2+]i traces.
To examine the effects of COX-2 inhibition on nerve-evoked contractions, isometric tension changes were recorded simultaneously with changes in [Ca2+]i. In control conditions, a brief stimulation (1 s, 20 Hz) initiated a transient contraction (Fig. 3Ba) and a corresponding Ca2+ transient which lasted for some 30 s (Fig. 3Bb). NS-398 (1 μm) inhibited the initial component of contraction from 2.5 ± 0.38 to 0.61 ± 0.24 mN (P < 0.05, 22.1 ± 10.8% of control, n = 6; Fig. 3Ca), whilst reducing the associated Ca2+ transients from 0.23 ± 0.02 R340/380 to 0.12 ± 0.01 R340/380 (P < 0.05, 53.3 ± 4.5% of control, n = 6; Fig. 3Cb). These results are summarized in Fig. 3D. NS-398 also strongly suppressed the sustained phase of Ca2+ transients and contractions. These results indicate that spontaneously produced prostaglandins via COX-2 contribute not only to the generation of spontaneous contractions but also to reinforcement of nerve-evoked contractions.
Effects of prostaglandin F2a on NAd-induced contractions
Since the inhibition of COX-2 suppressed neurally mediated contractions, a possible synergism between prostaglandins and NAd was investigated. Prior to this the mechanisms of PG- and NAd-induced contractions were examined. Both NAd (0.3 μm) and prostaglandin F2α (0.1 μm) contracted CSM. Nicardipine (1 μm) reduced the amplitude of NAd-induced contractions from 5.6 ± 1.2 to 2.7 ± 0.6 mN (P < 0.05, 49.1 ± 11.5% of control, n = 8) and reduced PGF2α-induced contractions from 3.3 ± 0.7 to 0.31 ± 0.11 mN (P < 0.05, 9.6 ± 3.3% of control, n = 6). Nicardipine (1 μm) also abolished spontaneous contractions (n = 7). Y-27632 (1 μm), an inhibitor for Rho kinase reduced NAd-induced contractions from 6.3 ± 1.5 to 0.65 ± 0.18 mN (P < 0.05, 10.3 ± 2.4% of control, n = 3) and reduced the amplitude of PGF2α-induced contractions from 3.4 ± 1.1 to 0.22 ± 0.07 mN (P < 0.05, 6.4 ± 4.4% of control, n = 5). Y-27632 (1 μm) also greatly attenuated spontaneous contractions (n = 5). These results suggest that both NAd- and PGF2α-induced contractions rely mainly on Ca2+ influx through L-type Ca2+ channels and increasing Ca2+ sensitivity presumably through Rho kinase activation.
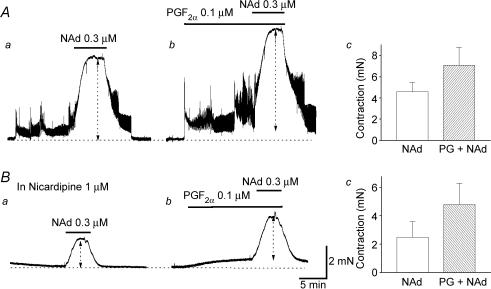
In preparations pre-contracted with PGF2α (0.1 μm), the amplitude of NAd-induced contractions was increased from 4.6 ± 0.4 to 7.1 ± 0.9 mN (P < 0.05, 152 ± 17.2% of control responses, n = 4; Fig. 4A). In preparations which had been exposed to nicardipine (1 μm), pre-treatment with PGF2α (0.1 μm) increased the amplitude of NAd-induced contractions from 2.4 ± 0.4 to 4.8 ± 0.6 mN (P < 0.05, 202 ± 23.1% of control responses, n = 5; Fig. 4B). These results indicate that PGF2α, reinforces NAd-induced contractions by mechanisms which do not depend on Ca2+ influx through L-type Ca2+ channels.
Figure 4. Effects of PGF2α on NAd-induced contractions in CCSM.
In a spontaneously active CCSM preparation, NAd (0.3 μm) initiated sustained contractions (Aa). In the same preparation, PGF2α (0.1 μm) caused sustained contractions, and a subsequent application of NAd (0.3 μm) initiated larger contractions than those in control conditions (Ab and c). In another preparation, which had been exposed to nicardipine (1 μm), NAd (0.3 μm) initiated sustained contractions (Ba). PGF2α (0.1 μm) caused a small sustained contraction, and a subsequent application of NAd (0.3 μm) initiated a larger contraction than that in control conditions (Bb and c). The scale bar on the right of Bb applies to all traces.
Effects of LNA on spontaneous and neurally evoked [Ca2+]i and tension
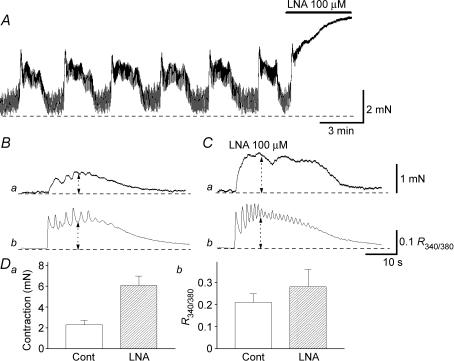
To investigate a possible role of spontaneously released NO in CCSM, the effects of LNA on spontaneous and nerve-evoked contractions were examined.
In spontaneously active preparations (n = 5), LNA (100 μm) initiated sustained contractions (Fig. 5A). Subsequent application of either l-arginine (1 mm, n = 3) or NS-398 (1 μm, n = 4) inhibited the sustained contraction to return the muscle tone to the resting level. In quiescent preparations, LNA was either without effect (n = 3) or induced spontaneous contractions (n = 3).
Figure 5. Effects of LNA on spontaneous and nerve-evoked excitation in CCSM.
In a CCSM preparation that exhibited spontaneous contractions, LNA (100 μm) caused sustained increases in tension (A). In another preparation, transmural nerve stimulation initiated phasic contraction (Ba) and corresponding increases in [Ca2+]i (Bb). In the same preparation that had been exposed to LNA (100 μm), transmural stimulation initiated contractions that were about two times larger than those in control condition (Ca). LNA also increased the amplitude of nerve-evoked Ca2+ transients by about 20% (Cb). These results are summarized in D. The scale bar on the right of Ca applies to both tension traces and the scale bar on the right of Cb applies to both [Ca2+]i traces.
LNA (100 μm) also increased the amplitude of nerve-evoked contractions from 2.3 ± 0.4 to 6.1 ± 0.9 mN (P < 0.05, 262.8 ± 46.3% of control, n = 6; Fig. 5Ba and Ca) and increased the amplitude of corresponding Ca2+ transients from 0.23 ± 0.04 R340/380–0.26 ± 0.08 R340/380(P < 0.05, 123.8 ± 8.6% of control, n = 6; Fig. 5Bb and Cb). These results are summarized in Fig. 5D, and suggest that spontaneously released NO contributes to the suppression not only of spontaneous but also nerve-evoked contractions.
The relationship between neurally released NO and PG- and NAd-induced contractions
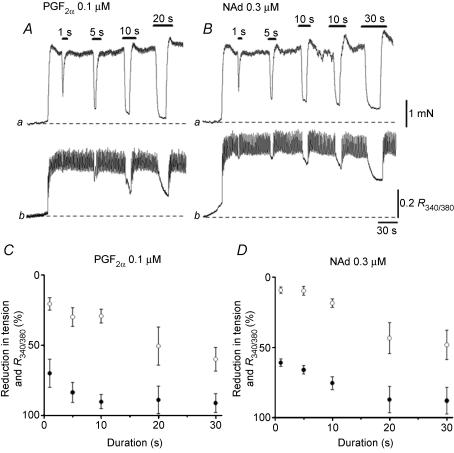
To clarify the interaction between neurally released NO and NAd- or PGF2α-induced excitation in CCSM, the effects of nerve stimulation on muscle tension and [Ca2+]i in preparations pre-contracted with either NAd or PGF2α were investigated.
In tissues pre-contracted with PGF2α (0.1 μm), brief nerve stimulation (1–10 s at 10 Hz) initiated phasic relaxation and reduced the contractions by 70–90% in a train duration-dependent manner. Nerve stimulation also reduced [Ca2+]i by about 20–30% (Fig. 6A). For example, a 1 s stimulation inhibited the contractions by 70.7 ± 10.0% and reduced [Ca2+]i by 20.1 ± 4.5% (n = 4). Both the relaxation and reduction in [Ca2+]i were abolished with LNA (100 μm). Prolonged stimulation (20–30 s at 2 Hz) caused about 90% relaxation and reduced Ca2+ by about 50%. These results are summarized in Fig. 6C.
Figure 6. Effects of transmural nerve stimulation on PGF2α- and NAd-induced excitations.
In a CCSM preparation, PGF2α (0.1 μm) initiated sustained contraction (Aa) and an associated increase in [Ca2+]i (Ab). In the presence of PGF2α (0.1 μm), brief transmural stimulation initiated phasic relaxation (Aa) and reduced [Ca2+]i by about 30% (Ab). Prolonged stimulation inhibited PGF2α-induced contraction by about 90% (Aa) and reduced [Ca2+]i by about 50% (Ab). In the same preparations, NAd (0.3 μm) caused sustained contraction (Ba) and a corresponding increase in [Ca2+]i (Bb). Brief transmural stimulations initiated phasic relaxations (Ba) and reduced [Ca2+]i by about 20% (Bb). Prolonged stimulation inhibited the contraction by about 90% (Ba) and reduced [Ca2+]i by about 50% (Bb). These results are summarized in C and D where open circles (○) indicate changes in [Ca2+]i and filled circles (•) indicate changes in tension. The scale bar on the right of Ba applies to both tension traces and the scale bar on the right of Bb refers to both Ca2+ traces.
Similarly, in CCSM pre-contracted with NAd, brief transmural stimuli inhibited contraction by about 60–70%, whilst reducing [Ca2+]i by some 10% (Fig. 6A). Prolonged stimulation caused about 90% of relaxation, and reduced [Ca2+]i by about 40%. These results are summarized in Fig. 6D.
Immunohistochemistry of COX-2-positive cells and the expression of mRNAs encoding COX-2 in the corpus cavernosum and the corpus spongiosum
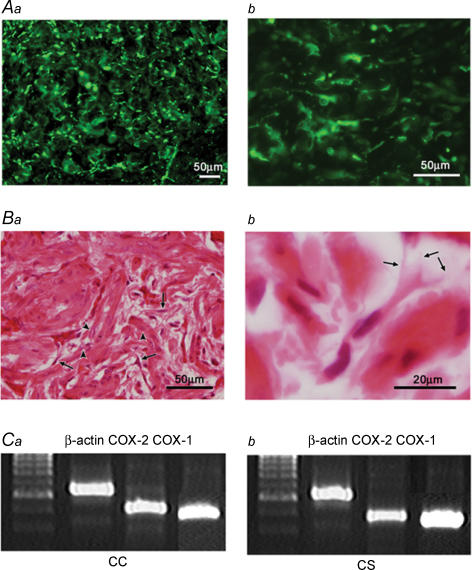
Immunostained COX-2 cells were scattered throughout cross sections of the penis, with a large population in the corpus cavernosum (Fig. 7Aa) and the corpus spongiosum. The urethral epithelium was also stained with COX-2 antibody. COX-2 cells in the corpus cavernosum were spindle- or star-shaped cells and often had some branches (Fig. 7Ab). Background CCSM cells also expressed a weak immunoreactivity for COX-2.
Figure 7. Immunohistochemistry of COX-2-positive cells and the expression of mRNAs encoding COX-1 and COX-2 in the corpus cavernosum and the corpus spongiosum.
COX-2 immunoreactive cells were widely distributed throughout the corpus cavernosum (Aa). COX-2 immunoreactivity was found in a population of cells which had spindle- or star-shaped cells with some branches (Ab). HE staining of the smooth muscle cells and interstitial cells in corpus cavernosum (Ba). Smooth muscle cells had a large, clear nucleus (arrow heads), while interstitial cells were characterized by their smaller, darker nucleus (arrows). Interstitial cells typically had spindle- or star-shaped cell bodies and had some branches (arrows) which connected with neighbouring cells (Bb). RT-PCR showed that the mRNAs encoding COX-2, 434 base-pair products, were expressed in the corpus cavernosum (CC, Ca) and the corpus spongiosum (CS, Cb) with small variations. Under the same experimental conditions the amount of the gene expression of COX-1, 340 base-pair products, and β-actin, 564 base-pair products, were the same in the two parts of the tissue. The left-hand lanes are for molecular markers, 100 base-pair ladder.
HE staining was used to examine the smooth muscle cells and interstitial cells in corpus cavernosum (Fig. 7Ba) and corpus spongiosum. Smooth muscle cells had a large, clear nucleus, while interstitial cells were characterized by their smaller, darker nuclei. Note that the perikarya of the interstitial cells were much smaller than those of smooth muscle cells. Interstitial cells typically had spindle- or star-shaped cell bodies and had some branches which connect with neighbouring cells (Fig. 7Bb).
The gene expression of COX-2 was observed by RT-PCR. It showed that the mRNAs encoding COX-2, 434 base-pair products, were expressed in the corpus cavernosum and the corpus spongiosum with small variations (n = 3, Fig. 7C). Interestingly, mRNAs encoding COX-1, 340 base-pairs products, were also expressed in both corpus cavernosum and corpus spongiosum. The immunohistochemical and biochemical results indicate that COX-2 is highly expressed in the corpus cavernosum and the corpus spongiosum of the penis.
Discussion
In the rabbit corpus cavernosum, smooth muscle cells are spontaneously active and endogenous prostaglandins may contribute to the generation of this activity through the activation of COX-2. Spontaneously produced prostaglandins may also reinforce contractions that are mediated by neurally released NAd, probably by increasing the sensitivity of the contractile apparatus to Ca2+ ions. Conversely, spontaneously released NO suppresses both spontaneous and NAd-mediated contractions. Neurally released NO initiates CCSM relaxation with only a small reduction in [Ca2+]i.
Spontaneous contractions have been recorded from CCSM obtained from various mammals, including humans, and are considered to contribute to maintaining detumescence (Andersson, 2001). In the present study, spontaneous contractions, Ca2+ transients and action potentials in the smooth muscle of the rabbit corpus cavernosum were abolished by L-type Ca2+ channel blockers. This was consistent with previous reports in corpus spongiosum smooth muscles (Hashitani, 2000; Hashitani et al. 2002), and indicates that the activation of L-type Ca2+ channels may be a fundamental process of spontaneous activity in CCSM. CCSM-generated spontaneous action potentials were very similar to those recorded from corpus spongiosum smooth muscles. Spontaneous action potentials were also abolished by either CPA or niflumic acid, suggesting that the activation of Ca2+-activated Cl− channels through Ca2+ release from intracellular stores may be involved in their generation. Since CCSM has a relatively depolarized resting membrane potential, i.e. about −45 mV, even a small depolarization may be sufficient to increase L-type Ca2+ channel activation. In isolated rabbit CCSM, the generation of spontaneous inward currents relies on the opening of Ca2+-activated Cl− channels by the release of Ca2+ from intracellular stores (Craven et al. 2004), suggesting that spontaneous action potentials in intact tissue are initiated by the CCSM themselves.
Besides these changes in membrane ionic conductance, spontaneous production of prostaglandins via COX-2 may contribute to the generation of spontaneous action potentials. This is consistent with a previous report that indomethacin inhibited spontaneous contractions in CCSM of men (Christ et al. 1990). Since COX-2 activity is thought to be induced upon inflammatory stimulation, while COX-1 is constitutively expressed, it was somewhat surprising that NS-398 suppressed spontaneous activity. Indeed, mRNAs encoding COX-1 were expressed in both corpus cavernosum and corpus spongiosum. However, COX-2 but not COX-1 appears to work as a primary enzyme in synthesizing prostaglandins to contribute to spontaneous contractions in the CCSM of the rabbit, as also shown in the guinea-pig upper urinary tract (Davidson & Lang, 2000), and thus COX-2 may be constitutively expressed at least in some tissues. The immunohistochemical and biochemical findings showed that COX-2 was highly expressed in a population of cells in the corpus cavernosum and the corpus spongiosum. The intact CCSM preparations employed in the present study are of heterogeneous morphology, including mainly smooth muscle cells, but also endothelium, autonomic nerves and interstitial cells. Interstitial cells are abundantly distributed in corpus spongiosum of the guinea-pig (Hashitani & Suzuki, 2004), and could be a source of prostaglandins. Indeed, it has been reported that interstitial cells in murine stomach and proximal colon express COX-2 activity and COX-2-dependent prostaglandins dampen spontaneous phasic contractions (Porcher et al. 2002, 2004). In the present study, HE staining revealed that cells with COX-2 immunoreactivity were spindle- or star-shaped with some branches and were connected with each other, suggesting that they were interstitial cells. It is also likely that prostaglandins are spontaneously released from the endothelium, which also releases nitric oxide, and may concurrently modulate spontaneous activity (Azadzoi et al. 1992).
Since LNA initiated sustained contractions and reinforced neurally mediated contractions, spontaneously released NO may suppress CCSM excitation–contraction coupling. In gastric smooth muscles, spontaneous release of NO has been reported to inhibit excitability of these muscles (Ozaki et al. 1992). It is most likely that NO is spontaneously released from the endothelium, although autonomic nerves may also be capable of releasing NO by tetrodotoxin-insensitive mechanisms. As regards NO-mediated inhibition of nerve-evoked contractions, both pre- and postsynaptic inhibition may be involved. In the rabbit renal artery, both a presynaptic inhibitory action of NO, presumably released from nitrergic nerves, and a postsynaptic inhibition by endothelium-derived NO on adrenergic excitation have been suggested (Vials et al. 1997). Nevertheless, NO seems to inhibit neurally mediated contractions with only a small reduction in [Ca2+]i. This is in good agreement with a previous study which revealed that the NO donor SIN-1 relaxes NAd-contracted corpus spongiosum smooth muscle with a small reduction in [Ca2+]i (Hashitani et al. 2002). However, since SIN-1 hyperpolarized the membrane of CCSM and prevented the generation of spontaneous action potentials, this Ca2+-dependent mechanism may also account for a part of NO-induced relaxation of CCSM. Since neither iberiotoxin nor charybdotoxin inhibited SIN-1-induced hyperpolarizations, large conductance Ca2+-activated potassium channels may not contribute to the hyperpolarizations. Consistent with previous studies, SIN-1-induced hyperpolarizations were largely attenuated by ouabain, suggesting that the sodium pump may be involved in generating the hyperpolarizations (Gupta et al. 1995; Hashitani, 2000).
NAd-induced contractions of CCSM are considered to result from both calcium release from intracellular stores through InsP3 production and calcium influx through L-type calcium channels (Andersson, 2001). In the present study, the blockade of L-type Ca2+ channels reduced NAd-induced contractions by about 50%, while 90% of the contraction was suppressed by inhibiting Rho kinase with Y-27632. These results suggest that NAd increases the sensitivity of contractile proteins to Ca2+ as previously reported for smooth muscles in both corpus spongiosum (Hashitani et al. 2002) and corpus cavernosum (Takahashi et al. 2003), and that extracellular Ca2+ contributes about a half of the increase in [Ca2+]i. Contractions induced by PGF2α were inhibited by about 90% with nicardipine, and were suppressed by over 90% with Y-27632. These results indicate that PGF2α-induced contractions largely depend on extracellular Ca2+, and that Ca2+ sensitization via Rho kinase dominantly contributes to the contractions. In the rabbit aorta, PGF2α-induced contractions depend on Rho-kinase-induced Ca2+ sensitization of contractile protein (Ito et al. 2003), and thus this mechanism may play a dominant role in generating PGF2α-induced contractions of vascular smooth muscles. In the present study, the pre-application of PGF2α reinforced NAd-induced contractions, regardless of the presence of blockers of L-type Ca2+ channels. Thus, PGF2α may potentiate the contractions through Ca2+-independent mechanisms, presumably by the activation of Rho kinase.
In the present study, brief nerve stimulation inhibited about 80% of the NAd- or PGF2α-induced contractions with only a 20% reduction in [Ca2+]i, indicating that neurally released NO relaxes pre-contracted CSM mainly by reducing the Ca2+ sensitivity of contractile protein. However, prolonged nerve stimulation relaxed pre-contracted CCSM with a substantial reduction in [Ca2+]i. Since SIN-1 hyperpolarized the membrane presumably by activating the sodium pump and prevented the generation of spontaneous action potentials, this mechanism may account for the reduction in [Ca2+]i. Indeed, neurally released NO hyperpolarized the membrane of CSSM of the rat by this mechanism and may account for a part of nerve-evoked relaxation (Hashitani, 2000). Alternatively, it might be possible that neurally released NO initiates relaxation of CCSM by reducing the Ca2+ sensitivity of contractile protein, and subsequently stimulates the production of endothelial NO which may reduce [Ca2+]i. It has been demonstrated that neurally released NO plays a principal role in the initiation of erection, while endothelial NO contributes to maintain the erection (Hurt et al. 2002). Nerve-evoked relaxation may stimulate the production of other endothelial relaxing substances, such as endothelium-derived hyperpolarising factor to hyperpolarize CCSM membrane (Angulo et al. 2003). Nevertheless, this secondary pathway may depend on the initial relaxation that is mediated by neurally released NO, as nerve-evoked relaxation was abolished by LNA.
Since the CCSM tone is determined by the balance between contracting and relaxing factors, the balance between spontaneously released NO and prostaglandins is important for the understanding of the pathophysiology of erectile dysfunction. Indeed, the blockade of guanylate cyclase in CCSM has been reported to increase both resting tension and NAd-induced contraction, and diminish ACh-induced relaxation, suggesting that the tonic production of cGMP inhibits CCSM excitability (Minhas et al. 2000). Since the effects of guanylate cyclase inhibition was reversed by indomethacin, an interaction and functional antagonism between cGMP and prostaglandins was also suggested. It has been reported that diminished NO production in diabetic CCSM is associated with increased Rho kinase activity (Bivalacqua et al. 2004). Conversely, increased Rho kinase activity may suppress the production of NO (Bivalacqua et al. 2004). In the present study, PGF2α -induced contractions were largely suppressed by a Rho kinase inhibitor, suggesting that increased cyclo-oxegenase activity might stimulate Rho kinase activity and then suppress NO production. Therefore, local delivery of a cyclo-oxygenase inhibitor in conjunction with oral phosphodiesterase type 5 (PDE5) inhibitors may be optimal for pharmacological treatment of erectile dysfunction.
In conclusion, spontaneously released prostaglandins reinforce spontaneous and neurally mediated contractions, while spontaneously released NO suppresses the excitability of CCSM. In both cases, modulation of calcium sensitivity seems to be a key mechanism for determining the tone of CCSM. Neurally released NO initiates CCSM relaxations mainly by a Ca2+-independent mechanism but may be associated with a reduction in Ca2+ to maintain penile erection.
Acknowledgments
The authors wish to thank Professor A. F. Brading and Dr F. R. Edwards for their critical reading of the manuscript. This project was supported by research grants from the Japan Society for the Promotion of Science (No. 15591704 and No. 17390443), Suzuken Memorial Foundation and Ichihara International Scholarship Foundation to H.H.
References
- Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernandez A, Gabancho S, Videla S, Saenz De Tejada I. Calcium dobesilate potentiates endothelium-derived hyperpolarizing factor-mediated relaxation of human penile resistance arteries. Br J Pharmacol. 2003;139:854–862. doi: 10.1038/sj.bjp.0705293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadzoi KM, Kim N, Brown ML, Goldstein I, Cohen RA, Saenz De Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Maayani S, Valcic M, Melman A. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101:375–381. doi: 10.1111/j.1476-5381.1990.tb12717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven M, Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide–cGMP pathway. J Physiol. 2004;556:495–506. doi: 10.1113/jphysiol.2003.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson ME, Lang RJ. Effects of selective inhibitors of cyclo-oxygenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2) on the spontaneous myogenic contractions in the upper urinary tract of the guinea-pig and rat. Br J Pharmacol. 2000;129:661–670. doi: 10.1038/sj.bjp.0703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fovaeus M, Andersson KE, Hedlund H. Effects of some calcium channel blockers on isolated human penile erectile tissues. J Urol. 1987;138:1267–1272. doi: 10.1016/s0022-5347(17)43582-8. [DOI] [PubMed] [Google Scholar]
- Gupta S, Moreland RB, Munarriz R, Daley J, Goldstein I, Saenz De Tejada I. Possible role of Na+, K+-ATPase in the regulation of human corpus cavernosum smooth muscle contractility by nitric oxide. Br J Pharmacol. 1995;116:2201–2206. doi: 10.1111/j.1476-5381.1995.tb15054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H. Neuroeffector transmission to different layers of smooth muscle in the rat penile bulb. J Physiol. 2000;524:549–563. doi: 10.1111/j.1469-7793.2000.t01-2-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Dickens EJ, Suzuki H. Cellular mechanisms of nitric oxide-induced relaxation of corporeal smooth muscle in the guinea-pig. J Physiol. 2002;538:573–581. doi: 10.1113/jphysiol.2001.013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Br J Pharmacol. 2004;141:199–204. doi: 10.1038/sj.bjp.0705622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist H, Hedlund H, Andersson KE. Characterization of inhibitory neurotransmission in the isolated corpus cavernosum from rabbit and man. J Physiol. 1992;449:295–311. doi: 10.1113/jphysiol.1992.sp019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Shimomura E, Iwanaga T, Shiraishi M, Shindo K, Nakamura J, Nagumo H, Seto M, Sasaki Y, Takuwa Y. Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F2α-induced contraction of rabbit aorta. J Physiol. 2003;546:823–836. doi: 10.1113/jphysiol.2002.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas S, Eardley I, Joyce AD, Morrison JB. The effect of cyclic GMP on rabbit corporal smooth muscle tone and its modulation by cyclo-oxygenase products. Prostaglandins Leukot Essent Fatty Acids. 2000;62:153–160. doi: 10.1054/plef.2000.0135. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Blondfield DP, Hori M, Publicover NG, Kato I, Sanders KM. Spontaneous release of nitric oxide inhibits electrical, Ca2+ and mechanical transients in canine gastric smooth muscle. J Physiol. 1992;445:231–247. doi: 10.1113/jphysiol.1992.sp018921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C, Horowitz B, Bayguinov O, Ward SM, Sanders KM. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology. 2002;122:1442–1454. doi: 10.1053/gast.2002.33065. [DOI] [PubMed] [Google Scholar]
- Porcher C, Horowitz B, Ward SM, Sanders KM. Constitutive and functional expression of cyclooxygenase 2 in the murine proximal colon. Neurogastroenterol Motil. 2004;16:785–799. doi: 10.1111/j.1365-2982.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D. Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G587–G594. doi: 10.1152/ajpgi.00391.2001. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Nishimura J, Hirano K, Naito S, Kanaide H. Modulation of Ca2+ sensitivity regulates contractility of rabbit corpus cavernosum smooth muscle. J Urol. 2003;169:2412–2416. doi: 10.1097/01.ju.0000065808.45445.a1. [DOI] [PubMed] [Google Scholar]
- Vials AJ, Crowe R, Burnstock G. A neuromodulatory role for neuronal nitric oxide in the rabbit renal artery. Br J Pharmacol. 1997;121:213–220. doi: 10.1038/sj.bjp.0701141. [DOI] [PMC free article] [PubMed] [Google Scholar]