Abstract
Increased intrauterine prostaglandin (PG) production is crucial for the initiation of parturition. To investigate the mechanisms controlling intrauterine PG synthesis, we examined the expression of the key PG biosynthetic isoenzymes, PG-H2 synthase (PTGS)-1 and -2, in the amnion, visceral yolk sac (VYS), placenta and myo-endometrium of pregnant guinea pigs. This animal model was chosen because the hormonal milieu of pregnancy and the role of PGs in the hormonal control of parturition are similar to those in the human. PTGS1 mRNA abundance, measured by real-time RT-PCR, increased in the amnion and the placenta during the last third of gestation. During labour, PTGS1 mRNA levels decreased precipitously in all four tissues. PTGS1 protein abundance, assessed by immunoblotting, increased to high levels in the amnion and the placenta by the end of pregnancy and remained high during labour. PTGS2 mRNA expression was higher in the placenta than in the other tissues, but did not change before and during labour. PTGS2 protein expression decreased in the placenta and remained low in the other tissues during labour. Immunohistochemistry showed pervasive PTGS1 protein expression in the amnion and strong expression in the parietal yolk sac membrane (PYS) covering the placenta. PTGS2 was expressed in the PYS and the endometrium. The PTGS inhibitor piroxicam, administered in doses that inhibited PTGS1 but not PTGS2, significantly prolonged gestation. These data suggest that PGs generated by intrauterine PTGS1 are involved in the timing of birth in guinea pigs. The induction of PTGS1 in the amnion and the PYS is a critical event leading to labour in guinea pigs and models analogous changes in the human gestational tissues before labour.
Prostaglandins (PGs) produced by the intrauterine tissues (placenta, fetal membranes, decidua/endometrium and myometrium) play pivotal roles in the onset of labour in mammalian species (Challis et al. 2000). The involvement of PGs in parturition is best characterized in mice, where increased production of PGF2α by the endometrium causes luteolysis, which decreases circulating progesterone levels, triggering birth. Studies in knockout mice have shown that the induction of PG-H2 synthase-1 (PTGS1, other synonyms are COX-1 and PGHS-1), an enzyme that catalyses the committing and rate-limiting step in PG biosynthesis, is the critical event that leads to the increased endometrial PGF2α production at term (Reese et al. 2000; Tsuboi et al. 2000; Gupta et al. 2001). In women, the expression of the other isoenzyme, PTGS2 (COX-2, PGHS-2), increases in the fetal membranes (amnion and chorion laeve) before and during labour (Hirst et al. 1995; Mijovic et al. 1999; Slater et al. 1999). Furthermore, women give birth without a need for luteolysis and without a decrease in circulating progesterone levels. The differences between human and murine parturition suggest that the control of birth by PGs and progesterone is fundamentally different in the two species. For this reason, the utility of the mouse is limited in studies where new information on the hormonal mechanisms of birth is extrapolated from an animal model to humans.
Molecular phylogenetics has shown robustly that mouse and man belong to the same superorder of placental mammals (Euarchontoglires) (Carter, 2001; Springer et al. 2003). The cohort Euarchonta, which includes the Primates, and the cohort Glires, which includes the Rodenta and Lagomorpha (rabbit), separated approximately 87 million years ago, followed by the separation of the Rodent and Lagomorph orders. The caviomorph (guinea pig) and murid (rat, mouse) lineages of Rodenta diverged later (approx. 67 million years ago) according to the molecular data. In view of this timeline, it is remarkable that guinea pigs (Cavia porcellus) give birth in the presence of high circulating progesterone levels and without a requirement for prepartum luteolysis, like higher primates and unlike mice and rabbits (Challis et al. 1975; Thorburn & Challis, 1979). Moreover, circulating sex steroid levels in guinea pigs and women follow analogous patterns during pregnancy, and responses to progesterone antagonist treatments are similar (Challis et al. 1971; Tulchinsky et al. 1972; Elger et al. 1986). Guinea pigs are therefore considered the best non-primate species to model the steroid regulation of human pregnancy. The similarities extend to the role of PGs. PG administration induces labour and delivery in guinea pigs, like in women and other mammals (Elger & Hasan, 1985). The guinea pig gestational tissues (amnion, visceral yolk sac, placenta and myo-endometrium) produce labour-promoting PGs (PGE2 and PGF2α), and the PG output of the placenta and the amnion increases with advancing pregnancy (Moussard et al. 1986; Schellenberg & Kirkby, 1997). Schellenberg and colleagues have demonstrated that the predominant intrauterine source of PGs is the amnion membrane and that PTGS activity in the amnion rises before and during labour as a result of increased enzyme synthesis (Schellenberg & Kirkby, 1997; Schellenberg et al. 2003). They have detected both PTGS1 and -2 mRNAs in the guinea pig amnion, but found no change in PTGS2 mRNA abundance during the last third of gestation. In the present investigation we have characterized the expression of the other PTGS isoenzyme, PTGS1, in the guinea pig amnion, visceral yolk sac (VYS; the anatomical equivalent of the chorion membrane), placenta and myo-endometrium in late pregnancy. We have measured PTGS1 mRNA and protein levels and determined the cellular localization of PTGS1 protein by immunohistochemistry. We have also measured PTGS2 mRNA levels, protein abundance and protein localization in the same tissues to assess the relative importance of the two isoenzymes in intrauterine PG-production. Furthermore, we have tested the involvement of PTGS1 in the timing of labour by measuring the effect of selective PTGS1 inhibition on gestational length. The results show that PTGS1 is selectively induced in the guinea pig amnion and placenta at term and that PTGS1 activity is involved in the normal timing of birth. We suggest that PGs control parturition in guinea pigs in a manner that is analogous to humans, with amniotic PTGS1 performing a role corresponding to the role of amniotic PTGS2 in humans.
Methods
Materials
The sources and suppliers of the materials used are described in the Supplemental material.
Animals and tissue collection
Outbred tri-colour guinea pigs were time-mated at the Animal Services Unit of the University of Newcastle, Australia. The amnion and VYS membranes, placentae and the myo-endometria were collected at six consecutive stages of late gestation, listed in Table 1. These stages were based on the criteria described by Glasier & Hobkirk (1993). Tissues were collected from animals anaesthetized with xylazine hydrochloride (5–10 mg kg−1s.c.) and ketamine hydrochloride (50–100 mg kg−1s.c.). Anaesthesia was maintained with 2–4% halothane gas administered via a face-mask. Animals were killed immediately after tissue collection by an intravenous pentobarbitone overdose. The attachment of the VYS to the endometrium was determined during tissue collection to differentiate between Stages B and C. The separation of the pubic symphysis, which occurs 6–9 days before delivery in our colony, has been determined by gentle palpation. To determine the onset of labour, the pregnant dams were observed without disturbance using an infrared camera. Labouring guinea pigs were euthanized by CO2 inhalation for 5 min following the delivery of at least one pup and tissues were collected from the mother and the undelivered pups. The animals have been monitored during all procedures to ensure that no pain and suffering occurred at any time. Six guinea pigs have been assigned to each gestational stage group, and each pregnancy produced one to six pups. For this reason, the number of maternal and fetal tissues obtained at each gestational stage varied between 6 and 24. The details of animal husbandry, time-matings and the number of tissues processed for analysis at each stage are described in the online Supplemental material. All procedures were approved by the University of Newcastle Animal Care and Ethics Committee and were carried out in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Table 1.
Gestational stages at which guinea pig tissues were collected
| Group | Description |
|---|---|
| A | 44–47 days gestation; ‘early’ |
| B | 52–56 days; before attachment of visceral yolk sac (VYS) to the endometrium |
| C | 54–64 days; following attachment of VYS to the endometrium |
| D | 57–65 days; first day of palpable pubic symphysis (PS) separation |
| E | 62–66 days; fifth day following PS separation |
| L | During labour, following the delivery of at least one pup |
Each group comprised 6 pregnant animals.
RNA extraction and real-time RT-PCR
Total RNA was extracted with TRIZOL® reagent, column-purified and treated with DNase I. Complementary DNA (cDNA) was synthesized by reverse transcription using random hexamer primers.
The abundance of PTGS1 and PTGS2 mRNAs relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were determined by real-time PCR using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Primer sequences are listed in Supplemetary Table 1. The real-time PCR systems have been optimized to achieve equal efficiency of amplification of the target and reference mRNAs, and relative abundance was calculated after determining the threshold amplification cycle numbers (Livak & Schmittgen, 2001). The amplification products were characterized by dissociation curve analysis, agarose gel electrophoresis and nucleotide sequencing. Procedural details and the results of PCR optimization and PCR product analysis are described in the online Supplemental material.
Immunoblotting and immunohistochemistry
PTGS1 and PTGS2 protein abundance in the amnion, VYS, placenta and myo-endometrium of guinea pigs were determined by immunoblotting. The intra- and interassay coefficients of variation for the quantitative immunoblotting systems were between 7% and 10%. PTGS1 and PTGS2 proteins were localized in formalin-fixed, paraffin-embedded tissue sections by immunohistochemistry. The details of these procedures are described in the Supplemental material.
Inhibition of PTGS1 in vivo
Pregnant guinea pigs were injected with the preferential PTGS1 inhibitor piroxicam (Meade et al. 1993) or vehicle every day from the first day of pubic symphysis separation until delivery. Animals were randomly assigned to the piroxicam and vehicle treatment groups on the first day of pubic symphysis separation. The guinea pigs were killed following delivery, and blood was collected by cardiac puncture. PTGS1 and PTGS2 activity was measured in the collected blood by established procedures to verify the selective inhibition of PTGS1 by the piroxicam treatment protocol employed (Smith et al. 1998; Chan et al. 1999). Technical details are described in the Supplemental material.
Statistical analysis
The Skewness and Kurtosis Test for normality was used to assess the distribution of data (Intercooled Stata v 8.0 software; Stata Corporation, College Station, TX, USA). Where appropriate, data were transformed to normal equivalent deviates (NED) in order to achieve normal distribution (Rowe, 2002). Analysis of variance (ANOVA) using general linear models (GLM), with individual pregnancies nested within their gestational stage, was performed to assess gestational stage-dependent differences in mRNA or protein levels (Minitab release 12.1 software; Minitab Inc., State College, PA, USA). Differences between the gestational stage groups were resolved by Tukey's multiple comparison test if significant F-values were found with ANOVA. The number of tissues analysed by real time RT-PCR and immunoblotting at the various gestational stages were used as the n-values in the statistical analyses. The n-values for each group are listed in the Supplemental material. Student's t test was used to compare gestational length, PTGS1 activity and PTGS2 activity between vehicle- and piroxicam-treated animals. For all analyses, P < 0.05 was considered statistically significant.
Results
PTGS1 expression
PTGS1 messenger RNA
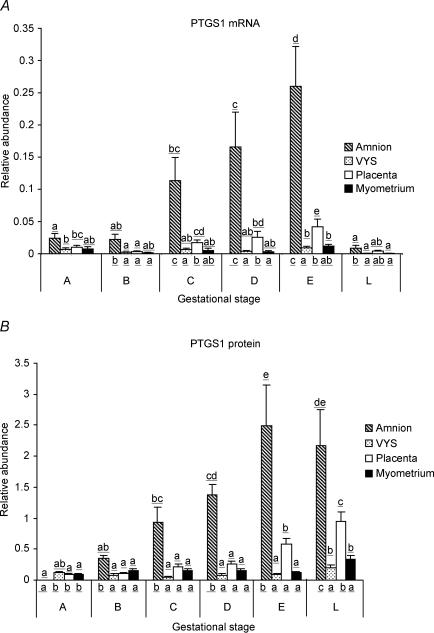
PTGS1 mRNA was detected by real time RT-PCR in all tissue types (Fig. 1A). At the earliest gestational stage studied (44–47 days, Stage A), PTGS1 mRNA relative abundance was not different among the tissues. As pregnancy advanced, PTGS1 mRNA abundance increased sharply in the amnion and reached significantly higher levels than in the VYS, placenta and the myo-endometrium. In labour, however, amniotic PTGS1 mRNA levels decreased precipitously (Stage L). Placental expression of PTGS1 mRNA also increased with advancing pregnancy, while the increase of PTGS1 mRNA levels in the VYS and the myo-endometrium was marginal. PTGS1 mRNA levels were significantly lower in these tissues during labour than at late gestation (Stage E), like in the amnion.
Figure 1. PTGS1 mRNA (A) and protein (B) relative abundance in guinea pig amnion, visceral yolk sac (VYS), placenta and myo-endometrium throughout late gestation and in labour.
Means ± s.e.m. are presented, n = 6–24 per group as detailed in the online Supplemental material. Letters above the bars indicate significance levels between gestational stages for each tissue type, while letters under the bars indicate significance levels between tissue types at each gestational stage (P < 0.05, nested ANOVA using general linear models, with Tukey's multiple comparison test). Gestational stages are described in Table 1.
PTGS1 protein
PTGS1 protein abundance was measured in the particulate (microsomal) fractions by immunoblotting. The results are shown in Fig. 1B. At Stage A, PTGS1 protein levels were low in the VYS, placenta and myo-endometrium and were undetectable in the amnion. As gestation advanced, PTGS1 protein expression increased steadily in the amnion, reaching significantly higher levels than in the other tissues. Furthermore, PTGS1 protein levels remained high in the amnion during labour, in contrast with the cognate mRNA. Placental PTGS1 protein abundance also increased by late pregnancy (Stage E) and rose further in labour, despite the fall of PTGS1 mRNA levels in labouring animals. A small, but significant, increase of PTGS1 protein expression occurred during labour in the VYS and in the myo-endometrium.
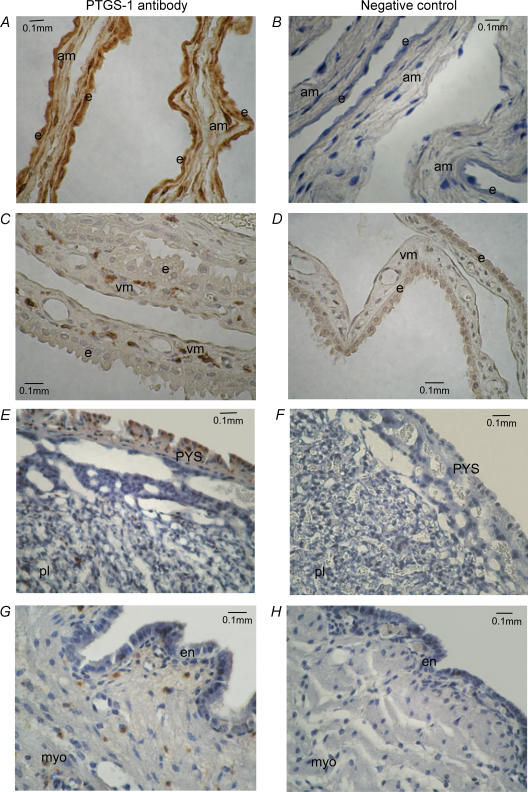
PTGS1 protein localization
Representative immunohistochemical pictures showing PTGS1 protein localization in amnion, VYS, placenta and myo-endo-metrium are presented in Fig. 2. PTGS1 protein was pervasively expressed in the epithelium and mesoderm of the amnion membrane (Fig. 2A). In the VYS, PTGS1 protein was present in sporadic cells within the mesoderm, but the epithelium was devoid of positive staining (Fig. 2C). PTGS1 protein was abundantly expressed in the parietal yolk sac membrane (PYS) overlaying the placenta and in sporadic cells within the placental labyrinth (Fig. 2E), but not in other placental cells (trophoblast and endothelium). The endometrium and myometrium expressed PTGS1 only in isolated sporadic cells (Fig. 2G).
Figure 2. PTGS1 protein localization in guinea pig gestational tissues by immunohistochemistry.
Tissue sections incubated with PTGS1 antibody are shown in panels A, C, E and G; negative controls (PTGS1 antibody preabsorbed with immunizing peptide) are shown in panels B, D, F and G. PTGS1 protein is indicated by brown staining; counterstaining is with Scott's blue. The tissues were obtained from animals at term prior to labour onset (Gestational Stage E). A and B, amnion; C and D, visceral yolk sac; E and F, placenta; and G and H, myo-endometrium. e, epithelium; am, amnion mesoderm; vm, visceral yolk sac mesoderm; PYS, parietal yolk sac; pl, placental labyrinthe; en, endometrium; myo, myometrium.
PTGS2 expression
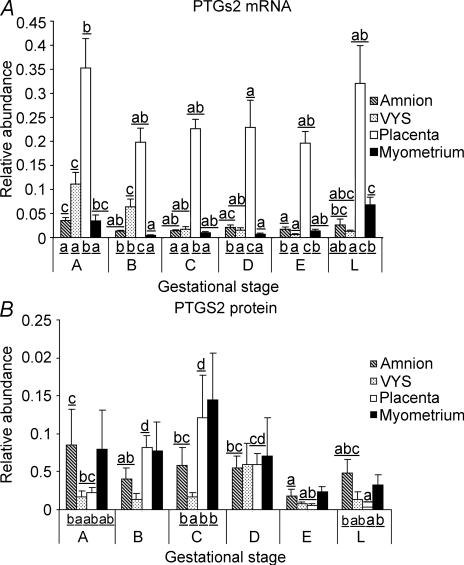
PTGS2 mRNA
PTGS2 mRNA was present in all tissues examined, with the highest relative abundance in the placenta (Fig. 3A). There was a significant decrease in placental PTGS2 mRNA level in the late group (Stage D) compared to the earliest group (Stage A), but there was no difference in PTGS2 mRNA abundance between placentas collected at Stages B, C, D, E and L. PTGS2 mRNA levels were relatively low in the amnion and the myo-endometrium at Stage A and decreased further with advancing pregnancy. A slight, but significant, increase was observed, however, in the two tissues with labour. PTGS2 mRNA expression decreased in the VYS as gestation progressed and remained low during labour.
Figure 3. PTGS2 mRNA (A) and protein (B) relative abundance in amnion, VYS, placenta and myo-endometrium of guinea pigs throughout late gestation and in labour.
Details are described in the legend to Fig. 1. Multiple comparisons are presented where a significant effect was found by ANOVA.
PTGS2 protein
PTGS2 protein levels, determined by immunoblotting, were variable between individual tissues, especially in the amnion and the myo-endometrium (Fig. 3B). In the placenta, statistical analysis showed a significant increase of PTGS2 protein abundance from Stage A to Stage C, followed by a decrease to low levels before labour (Stage E) and during labour (Stage L). PTGS2 protein was expressed in low and unchanging levels in the VYS. PTGS2 protein abundance in the amnion followed the trend of mRNA expression, exhibiting a significant decrease between Stages A and E. Variable expression in the myo-endometrium thwarted the statistical demonstration of differences during pregnancy, but a tendency for decreasing PTGS2 levels by term and in labour was observed. Between-tissue variance was significant in groups A, C and L, showing relatively low PTGS2 protein expression in the VYS at Stages A and C and diminished placental expression of enzyme protein during labour (L).
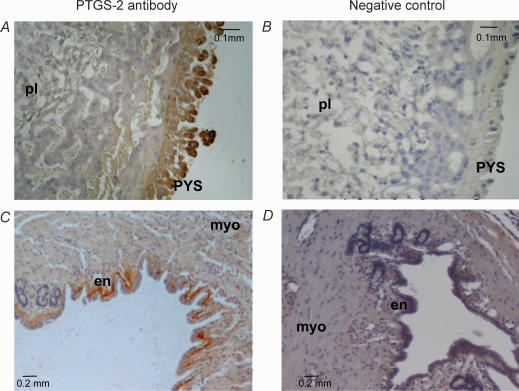
PTGS2 protein localization
Representative pictures showing the immunohistochemical localization of PTGS2 protein are presented in Fig. 4. In the placenta, PTGS2 expression was limited to the PYS membrane covering the placental surface (Fig. 4A). PTGS2 protein was also expressed in the endometrial layer of the uterus, showing stronger staining on the basal side (Fig. 4C). No staining of PTGS2 protein was seen in the amnion and the VYS (not shown).
Figure 4. PTGS2 protein localization in guinea pig placenta and myo-endometrium by immunohistochemistry.
Details are described in the legend to Fig. 2. A and B, placenta; C and D, myo-endometrium. Tissues were obtained from animals at Gestational Stage A. PYS, parietal yolk sac; pl, placental labyrinthe; en, endometrium; myo, myometrium.
PTGS inhibition in vivo
Selective inhibition of PTGS isoenzymes in pregnant animals may provide information about the involvement of PTGS1 and -2 in the control of gestational length. We have tested several PTGS inhibitors (nimesulide, valeryl salicylate, niflumic acid) for isoenzyme selectivity in vitro, but none of them was sufficiently selective towards guinea pig PTGS1 or -2. The preferential PTGS1 inhibitor piroxicam (Meade et al. 1993), however, showed good selectivity towards guinea pig PTGS1 in the preliminary in vitro experiments. We have injected pregnant guinea pigs with 5 mg kg−1 day−1 piroxicam starting on the first day of pubic symphysis separation, which is a gestational stage (Stage D) when PTGS1 is significantly induced in the amnion (Fig. 1). Piroxicam treatment resulted in a significant increase of the interval between pubic symphysis separation and delivery (Table 2). To verify the selective inhibition of PTGS1 in vivo by our piroxicam dose regimen, we have measured the activity of PTGS1 and PTGS2 in whole blood collected from the piroxicam-treated and vehicle-treated animals. The results in Table 2 show that PTGS1 activity was significantly reduced in the piroxicam-treated guinea pigs while PTGS2 activity was not significantly different between the two groups. These data suggest that PTGS1 activity is required for timely onset of labour in guinea pigs. The length of labour and the well-being of the mother and the newborns were not affected by the piroxicam or vehicle (canola oil : DMSO 3 : 1) treatment.
Table 2.
The effects of piroxicam treatment on the interval between symphysis separation and birth and on the activity of PGHS1 and PGHS2
| Parameter | Vehicle | Piroxicam | P |
|---|---|---|---|
| Days between symphysis separation and birth | 8.5 ± 0.34 (6) | 10.8 ± 0.95 (6) | 0.043* |
| PGHS1 activity (ng TXB2 ml−1 (10 min)−1) | 211.33 ± 50.49 (3) | 36.22 ± 18.77 (4) | 0.014* |
| PGHS2 activity (ng PGE2 ml−1 (24 h)−1) | 4.489 ± 1.02 (4) | 3.67 ± 0.65 (4) | 0.522 |
Values are means ± s.e.m., with number of animals shown in parentheses. Guinea pigs were treated with vehicle (canola oil : DMSO 3 : 1) or piroxicam (5 mg kg−1 day−1) s.c. from the first day of pubic symphysis separation until delivery.
Significant difference calculated by the two-tailed t test.
Discussion
Increased PG synthesis in the uterus is critical for labour onset in guinea pigs, as in other mammalian species (Elger & Hasan, 1985). It has been shown that PGE2 and PGF2α production increases in the amnion and the placenta of guinea pigs by late gestation, and the activity of PTGS, the committing and rate limiting enzyme of the PG biosynthetic pathway, rises dramatically in guinea pig amnion before and during labour (Moussard et al. 1986; Moussard et al. 1987; Schellenberg & Kirkby, 1997). PTGS2 mRNA has been detected in the amnion, but its level did not change at term (Schellenberg et al. 2003). Our results are in agreement with these findings and extend them to PTGS2 protein abundance, which was either unchanged or decreased to low levels in the four gestational tissues by late pregnancy and labour. We have shown, however, that the expression of the other PTGS isoenzyme, PTGS1, increases in guinea pig amnion, VYS, placenta and myo-endometrium by term and, at the protein level, during labour. Moreover, selective inhibition of PTGS1 activity in vivo significantly delayed delivery. Collectively, our data demonstrate that PTGS1 expression rises in the guinea pig uterus during late pregnancy and this enzyme generates PGs that control the timing of birth in this species.
PTGS1 mRNA and protein are expressed at higher levels in the amnion and the placenta than in the VYS and the myo-endometrium. This suggests that the former two tissues are the principal sources of labour-promoting prostaglandins. In the placenta, strong PTGS1 protein expression has been localized to the PYS membrane, which constitutes the surface layer of the placenta and faces the uterine wall. Increased PTGS1 expression in the amnion and the PYS ensures that the whole surface of the feto-placental unit increases its PG-producing capacity by the end of gestation. PTGS1 protein has also been localized to sporadic single cells in the VYS, placenta and myo-endometrium. Although we have not characterized these cells in the present investigation, the possibility exists that they correspond to resident or migrating inflammatory cells. Further work is needed to determine whether inflammatory mechanisms are involved in guinea pig parturition similarly to other mammalian species including humans (Dudley, 1999).
Figure 1 shows that PTGS1 mRNA abundance exhibited a similar overall trend of change in all four tissues towards term: major up-regulation in the amnion and a significant increase in the placenta, VYS and myo-endometrium. Increasing mRNA expression was accompanied by increased protein abundance in the amnion and the placenta, suggesting that PTGS1 protein expression was influenced by mRNA levels in late gestation. During labour, however, mRNA abundance decreased precipitously in all four tissues, while protein abundance either increased (VYS, placenta, myo-endometrium) or remained unchanged (amnion). Thus, protein turnover plays a critical role maintaining high PTGS1 protein levels during labour. Protein-level regulation of PTGS2 expression occurs in the placenta and the VYS too, as indicated by the disparate changes of PTGS2 mRNA and protein abundance in these tissues (Fig. 3). PTGS regulation at the protein synthesis level has been suggested in a former study by Schellenberg et al. (2003), who assessed de novo PTGS synthesis rates in amnion explants pretreated with aspirin and concluded that the PTGS enzyme accumulates in the amnion in vivo during late gestation. Thus, regulation of mRNA and protein synthesis collectively leads to increased PTGS1 and decreased PTGS2 abundance in the gestational tissues by term, resulting in the dominance of PTGS1 over PTGS2 around the time of labour.
Pregnancy maintenance in guinea pigs does not require a functioning corpus luteum during the second half of gestation, because a luteo-placental shift of progesterone production occurs around the 4th week of pregnancy (Csapo et al. 1981). This is analogous to the luteo-placental shift of progesterone production in early human pregnancy and suggests that the endocrine mechanisms that maintain and terminate pregnancy are similar in the two species. Intrauterine PGs, for example, should promote parturition by a mechanism that does not involve luteolysis and systemic progesterone withdrawal, which are essential actions of labour-promoting PGs in mice. Interestingly, the amnion membrane is the major intrauterine source of PGs in guinea pigs as well as in humans. The exact role of these PGs is unknown, but recent experiments suggest that PGs may decrease progesterone responsiveness in cultured human myometrial cells (Madsen et al. 2004) and decidua explants (Goldman et al. 2005) by modulating progesterone receptor isoform expression. Decreasing progesterone responsiveness of target tissues may cause ‘functional’ progesterone withdrawal even when circulating progesterone levels are high. The guinea pig can be an ideal animal model to test the possibility that PGs contribute to functional progesterone withdrawal by down-regulating progesterone responsiveness in vivo.
The mechanisms that stimulate PTGS expression in the human or guinea pig amnion are unknown. It has to be noted, though, that in the human amnion, the inducible PTGS isoenzyme, PTGS2, is up-regulated at labour (Hirst et al. 1995; Mijovic et al. 1999; Slater et al. 1999), while in the guinea pig gestational tissues, PTGS1 is induced. This may be a case of evolutionary convergence where two different isoforms of an enzyme have been recruited to perform homologous functions in two species. PTGS1 is generally considered a constitutive enzyme; however, up-regulated PTGS1 mRNA expression is not unique to guinea pig gestational tissues; it occurs in murine endo-metrium between 15 and 17 days of pregnancy (Tsuboi et al. 2000).
An important further example of evolutionary convergence relates to the function of the fetal membrane that is positioned between the amnion and the uterine wall. In humans, this membrane is the chorion leave, which is of trophoblastic origin, while in guinea pigs, the corresponding membrane is the VYS, which is derived from the endoderm of the embryo. PG-metabolic activity and enzyme expression are high and PTGS expression is relatively low in both tissues (Keirse et al. 1978). Apparently, the chorionic and VYS membranes perform a homologous function in the two species, which is to control the transfer of uterotonic PGs from the amnion to the myometrium. The expression of the PG-inactivating enzyme prostaglandin dehydrogenase (PGDH) has been reported to decrease in the human chorion and the guinea pig VYS before labour (Van Meir et al. 1997; Welsh et al. 2002).
In conclusion, our study reinforces the view that gestational length is controlled in an analogous fashion by PGs in guinea pigs and humans. The similarities, together with the similarities of progesterone action, indicate that guinea pigs can serve as a relevant non-primate model for studies where conclusions and inferences are extrapolated to the physiology and pathophysiology human birth.
Acknowledgments
The authors wish to thank Mrs Gemma Madsen, Mothers and Babies Research Centre, for sharing guinea pig tissue samples, and Dr Andrew Bisits, Discipline of Reproductive Medicine, John Hunter Hospital, for advice on statistical analysis. This work was supported by grants provided by the University of Newcastle Research Grants Committee and by the Mothers and Babies Research Centre.
Supplemental material
The online version of this paper can be accessed at:10.1113/jphysiol.2005.098129 http://jp.physoc.org/cgi/content/full/jphysiol.2005.098129/DC1 and consists of a detailed description of the materials and methods used, and the procedures and the data validating the real-time RT-PCR and immunoblotting procedures are presented. There are also four figures:
Supplementary Figure 1. Representative dissociation curves for PCR amplification products for PTGS1 (A), PTGS2 (B) and GAPDH (C) mRNAs.
Supplementary Figure 2. PCR amplification efficiency plot of PTGS1 (A) and PTGS2 (B) cDNA, relative to GAPDH cDNA
Supplementary Figure 3. Immunoblot detection of PTGS1 protein in guinea pig myo-endometrium
Supplementary Figure 4. Immunoblot detection of PTGS2 protein in guinea pig placenta
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Carter AM. Evolution of the placenta and fetal membranes seen in the light of molecular phylogenetics. Placenta. 2001;22:800–807. doi: 10.1053/plac.2001.0739. [DOI] [PubMed] [Google Scholar]
- Challis JR, Davies IJ, Ryan KJ. The effects of dexamethasone and indomethacin on the outcome of pregnancy in the rabbit. J Endocrinol. 1975;64:363–370. doi: 10.1677/joe.0.0640363. [DOI] [PubMed] [Google Scholar]
- Challis JR, Heap RB, Illingworth DV. Concentrations of oestrogen and progesterone in the plasma of non-pregnant, pregnant and lactating guinea-pigs. J Endocrinol. 1971;51:333–345. doi: 10.1677/joe.0.0510333. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, et al. Rofecoxib [Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl) -3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999;290:551–560. [PubMed] [Google Scholar]
- Csapo AI, Puri CP, Tarro S. Relationship between timing of ovariectomy and maintenance of pregnancy in the guinea-pig. Prostaglandins. 1981;22:131–140. doi: 10.1016/0090-6980(81)90060-5. [DOI] [PubMed] [Google Scholar]
- Dudley DJ. Immunoendocrinology of preterm labor: the link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol. 1999;180:S251–S256. doi: 10.1016/s0002-9378(99)70711-8. [DOI] [PubMed] [Google Scholar]
- Elger W, Beier S, Chwalisz K, Fahnrich M, Hasan SH, Henderson D, Neef G, Rohde R. Studies on the mechanisms of action of progesterone antagonists. J Steroid Biochem. 1986;25:835–845. doi: 10.1016/0022-4731(86)90314-6. [DOI] [PubMed] [Google Scholar]
- Elger W, Hasan SG. Studies on the mechanism of action of antifertile PG in animal models. Acta Physiol Hung. 1985;65:415–432. [PubMed] [Google Scholar]
- Glasier MA, Hobkirk R. Nuclear receptors for progesterone and estradiol in the guinea pig uterine compartment during gestation. Steroids. 1993;58:478–483. doi: 10.1016/0039-128x(93)90006-9. [DOI] [PubMed] [Google Scholar]
- Goldman S, Weiss A, Almalah I, Shalev E. Progesterone receptor expression in human decidua and fetal membranes before and after contractions: possible mechanism for functional progesterone withdrawal. Mol Hum Reprod. 2005;11:269–277. doi: 10.1093/molehr/gah161. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Sato TA, Keelan JA, Marvin KW, Mitchell MD. Expression of prostaglandin H synthase-1 and -2 in murine intrauterine and gestational tissues from mid pregnancy until term. Prostaglandins Other Lipid Med. 2001;66:17–25. doi: 10.1016/s0090-6980(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Hirst JJ, Teixeira FJ, Zakar T, Olson DM. Prostaglandin endoperoxide-H synthase-1 and -2 messenger ribonucleic acid levels in human amnion with spontaneous labor onset. J Clin Endocrinol Metab. 1995;80:517–523. doi: 10.1210/jcem.80.2.7852513. [DOI] [PubMed] [Google Scholar]
- Keirse MJ, Hicks BR, Kendall JZ, Mitchell MD. Comparison of intrauterine prostaglandin metabolism during pregnancy in man, sheep and guinea pig. Eur J Obstet Gynecol Reprod Biol. 1978;8:195–203. doi: 10.1016/0028-2243(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔ CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89:1010–1013. doi: 10.1210/jc.2003-031037. [DOI] [PubMed] [Google Scholar]
- Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- Mijovic JE, Zakar T, Angelova J, Olson DM. Prostaglandin endoperoxide H synthase mRNA expression in the human amnion and decidua during pregnancy and in the amnion at preterm labour. Mol Hum Reprod. 1999;5:182–187. [Google Scholar]
- Moussard C, Alber D, Henry JC. 14C-labeled arachidonic acid bioconversion in guinea pig placenta during the last third of gestation. Prostaglandins. 1987;34:79–90. doi: 10.1016/0090-6980(87)90265-6. [DOI] [PubMed] [Google Scholar]
- Moussard C, Alber D, Remy-Martin JP, Henry JC. Placental biosynthesis and metabolism of prostanoids: special reference to guinea-pig during the last third of gestation. Prostaglandins Leukot Med. 1986;21:37–49. doi: 10.1016/0262-1746(86)90161-7. [DOI] [PubMed] [Google Scholar]
- Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci U S A. 2000;97:9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe K. Background Paper to Keynote Address Presented at the RMIT Statistics Seminar Series, October 11, 2002. Camberwell, Victoria, Australia: Australian Council for Educational Research; 2002. The measurement of latent and composite variables from multiple items or indicators: Applications in performance indicator systems; pp. 1–23. available at: http://www.acer.edu.au. [Google Scholar]
- Schellenberg JC, Kirkby W. Production of prostaglandin F2 alpha and E2 in explants of intrauterine tissues of guinea pigs during late pregnancy and labor. Prostaglandins. 1997;54:625–638. doi: 10.1016/s0090-6980(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Schellenberg JC, Shelling AN, Van Ee CC. Activity, synthesis, storage, and messenger RNA of cyclooxygenase in intrauterine tissues of guinea pigs near term and during labor. Prostaglandins Leukot Essent Fatty Acids. 2003;68:291–298. doi: 10.1016/s0952-3278(03)00009-7. [DOI] [PubMed] [Google Scholar]
- Slater D, Dennes W, Sawdy R, Allport V, Bennett P. Expression of cyclo-oxygenase types-1 and -2 in human fetal membranes throughout pregnancy. J Mol Endocrinol. 1999;22:125–130. doi: 10.1677/jme.0.0220125. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Placental mammal diversification and the Cretaceous–Tertiary boundary. Proc Natl Acad Sci U S A. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn GD, Challis JR. Endocrine control of parturition. Physiol Rev. 1979;59:863–918. doi: 10.1152/physrev.1979.59.4.863. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sugimoto Y, Iwane A, Yamamoto K, Yamamoto S, Ichikawa A. Uterine expression of prostaglandin H2 synthase in late pregnancy and during parturition in prostaglandin F receptor-deficient mice. Endocrinology. 2000;141:315–324. doi: 10.1210/endo.141.1.7236. [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- Van Meir CA, Ramirez MM, Matthews SG, Calder AA, Keirse MJ, Challis JR. Chorionic prostaglandin catabolism is decreased in the lower uterine segment with term labour. Placenta. 1997;18:109–114. doi: 10.1016/s0143-4004(97)90081-3. [DOI] [PubMed] [Google Scholar]
- Welsh T, Mesiano S, Walters W, Zakar T. Expression of prostaglandin H synthase (PGHS)-1 and -2 and prostaglandin dehydrogenase type-1 (PGDH) in guinea pig intrauterine tissues during late gestation. J Soc for Gynecologic Investigation. 2002;9(Suppl) Abstract no. 230 (Scientific Program and Abstracts of the 49th Annual Meeting of the SGI, Los Angeles, California) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.