Abstract
Vascular endothelial growth factor (VEGF) is expressed by the podocytes of renal glomeruli, and has profound influences on systemic microvascular permeability and haemodynamics. We describe an extensive refinement of a model that permits evaluation of the ultrafiltration coefficient (LpA) of isolated mammalian glomeruli, in the absence of circulating and haemodynamic influences, and tested the hypothesis that VEGF influences glomerular LpA via an effect on endothelial cells. Glomeruli were isolated by sieving Wistar rat renal cortical tissue, and individually loaded onto a suction micropipette. Flowing perifusate containing 1% bovine serum albumin (BSA) was rapidly switched to an oncopressive perifusate containing 8% BSA, eliciting transglomerular fluid efflux. The rate of the resultant reduction in glomerular volume was used to calculate glomerular LpA (1.07 ± 0.53 nl min−1 mmHg−1 (mean ± s.d.), n = 51), which compares favourably with those reported in the same rat strain using different techniques. A significant relationship between LpA and initial glomerular volume (Vi) (r = 0.72, n = 41, P < 0.0001) necessitated correction of LpA for Vi. The initial rate of change of glomerular volume, normalized for Vi, showed a strong positive correlation with applied oncotic gradient (Pearson r = 0.59, n = 28, P < 0.001), as predicted by Starling's law of filtration. A 60 min exposure of glomeruli to 1 nm VEGF increased glomerular LpA/Vi (1.19 ± 0.19 (n = 10) to 2.23 ± 0.33 (n = 9) min−1 mmHg−1 (mean ± s.e.m.); P < 0.02). Time- and concentration-dependent relations between VEGF and LpA/Vi were observed. The VEGF-induced elevation of LpA/Vi was blocked by the selective VEGF-R2 inhibitor ZM323881. We suggest that glomerular VEGF contributes to the high physiological permeability of mammalian glomeruli to water through an action on endothelial cells.
Vascular endothelial growth factor (VEGF) was originally described by its ability to increase macromolecular extravasation in the systemic circulation (Senger et al. 1983), hence the initial term ‘vascular permeability factor’. Subsequent investigations have shown that VEGF increases systemic microvessel permeability to both water (Bates & Curry, 1996) and albumin (Wu et al. 1996) and alters vessel diameter and compliance (Bates, 1998). VEGF also plays a fundamental role in systemic endothelial cell maintenance and angiogenesis (Ferrara, 2004). VEGF and its associated receptors are strongly expressed in renal glomeruli, and are traditionally considered to exhibit a trans-basement membrane distribution. In situ hybridization reveals strong VEGF expression by podocytes (Bailey et al. 1999), and the cognate VEGF receptors VEGFR-1 and VEGFR-2 are found on glomerular endothelial cells (Simon et al. 1995). However, recent work suggests that podocytes also express VEGF receptors (namely VEGFR-1 and neuropilin-1, but not VEGFR-2), and that this coexpression of VEGF and VEGF receptors by podocytes permits podocyte-mediated alterations in podocyte behaviour, such as Ca2+ homeostasis (Foster et al. 2003) and nephrin phosphorylation (Foster et al. 2005), representing a functional autocrine VEGF effect. The components of the VEGF axis are disturbed in a wide variety of glomerular pathologies associated with altered glomerular permeability (such as proteinuria) (Schrijvers et al. 2004), and VEGF appears to have an important role in glomerulogenesis (Kitamoto et al. 1997) and glomerular repair from injury in some animal models of glomerular disease (e.g. Masuda et al. 2001). VEGF induces endothelial cell fenestrations in vitro (Esser et al. 1998b), binds molecules which form important components of the glomerular basement membrane, such as heparan sulphate (Soker et al. 1993), and induces phosphorylation of the podocyte slit-diaphragm protein nephrin in conditionally immortalized human podocytes in vitro (Foster et al. 2005). The anatomical arrangement of the VEGF axis, and the ability of VEGF to interact with the major components of the glomerular filtration barrier in vitro, have led to the suggestion that VEGF may contribute to the normal control of glomerular permeability (Schrijvers et al. 2004).
In a recent phase II study of metastatic colon cancer (Kabbinavar et al. 2003), a significant excess of patients treated with monoclonal antibody therapy to VEGF experienced proteinuria compared with those in the control group. Although the occurrence of proteinuria was not replicated in the phase III study (Hurwitz et al. 2004), the threshold value for proteinuria of 500 mg day−1 in the latter study significantly exceeds that considered pathological in the normal population (Johnson & Feehally, 2003). Furthermore, VEGF monoclonal antibody therapy abrogated glomerular hyperfiltration and albuminuria in diabetic rats, but did not affect these parameters in control rats (de Vriese et al. 2001). Application of VEGF to the noradrenaline-preconstricted renal vascular bed of isolated perfused rat kidneys led to an increase in renal perfusion flow rate. However, no change in glomerular filtration rate or glomerular permeability to albumin was noted (Klanke et al. 1998). The role of VEGF in the control of glomerular permeability therefore remains unclear (Schrijvers et al. 2004).
Traditional, but infrequently attempted, techniques for examining glomerular permeability at the single-nephron level include direct glomerular capillary micropuncture (Brenner et al. 1971) and tubular stop-flow studies (Gertz et al. 1966). The profound alterations in haemodynamics induced by VEGF (Bates & Harper, 2003) are likely to complicate the assessment of glomerular ultrafiltration coefficient (LpA) using these techniques. The model developed by Savin and colleagues (Savin & Terreros, 1981) permits the study of glomerular filtration characteristics in isolation from circulating and haemodynamic influences. The volumetric response of an isolated glomerulus to a transglomerular oncotic gradient allows determination of LpA. Values obtained using this technique (Savin & Terreros, 1981) have been noted to correspond well with LpA values obtained using in vivo techniques (Maddox et al. 1992). Using this model, Savin and coworkers have shown that various mediators possess the ability to alter LpA, including angiotensin II (Wiegmann et al. 1990). We have therefore refined the methods of Savin to permit examination of the influence of VEGF on glomerular LpA.
Methods
Glomerular isolation and solutions
All experiments were performed on adult male Wistar rats (weight 257–346 g), and conformed to UK animal legislation. Animals were anaesthetized with 5% halothane (Merial, Essex, UK), and killed by cervical dislocation. Laparotomy and bilateral nephrectomy were performed immediately post mortem, and kidneys were placed in mammalian Ringer solution (mm: 115 NaCl, 5 KCl, 10 sodium acetate, 1.2 Na2HPO4, 25 NaHCO3, 1.2 MgSO4, 1 CaCl2, 5.5 d(+)glucose (all from VWR, Leics, UK), bubbled with 95%O2–5%CO2 (BOC, Manchester, UK) until pH 7.40–7.45), containing 1% bovine serum albumin (BSA) (Sigma, MO, USA). The outer 1–2 mm of superficial renal cortex was dissected from both kidneys, and glomeruli were isolated using a standard sieving technique (Savin & Terreros, 1981). The concentration of residual plasma proteins within glomerular capillaries equilibrates with the surrounding 1% BSA during this time. The glomerular harvest retained by the 100 μm mesh was transferred to either control or test solution at 37°C.
Perifusate containing mammalian Ringer solution and varying concentrations of BSA was adjusted to pH 7.45 ± 0.02 with NaOH or HCl accordingly. In each experiment, one perifusate contained 1% BSA (generically termed dilute perifusate), and the other contained a higher concentration of BSA (concentration 8%, other than in experiments examining the relationship between the initial rate of fluid flux and the applied oncotic gradient, in which the concentration was between 4 and 10%, generically termed concentrated perifusate). Mammalian Ringer solution was re-bubbled with 95%O2–5%CO2 until pH 7.40–7.45, and BSA solutions were re-made for each rat studied. The oncotic pressure of each BSA solution was determined with a modified Hanson's osmometer, using a semi-permeable membrane with 10 kDa cut-off (Amicon, Lexington, MA, USA). There was no change in the oncotic pressure of the perifusate solutions during the course of each experiment.
Apparatus
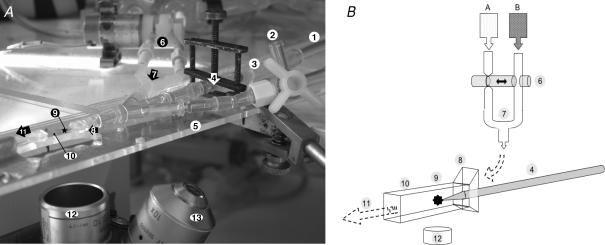
Micropipettes were pulled from glass capillary tubes (o.d. 1.2 mm; Clark Electromedical Instruments, Reading, UK), and the 15 μm aperture tip was placed within a flange-ended, rectangular cross-section glass microslide (i.d. 400 μm × 4 mm; Camlab, Cambridge, UK) above the ×10 objective of an inverted microscope (Leica DM IL HC Fluo) (Fig. 1). Magnification was further adjusted using a ×1/×1.5/×2 zoom set in the body of the microscope. A monochrome video camera (Hitachi KP-M3AP) was attached to the top of the microscope to permit binocular visualization and simultaneous recording of images of individual glomeruli loaded into the system, and it was held by gentle suction on the micropipette tip. The video camera was connected through a digital timer (FOR.A VT33) to a videocassette recorder (Panasonic AG7350) and monochrome monitor (Sony SSM-125CE). Perifusate was held in one of two heated glass reservoirs (Radnoti, CA, USA), placed at a height of between 40 and 140 cm above the microslide. The reservoirs were connected to the microslide via a series of heat-insulated Tygon tubes (Fisher, UK), which passed through either channel of an either/or rapid-response remote tap (075P3; Bio-Chem Valve, Inc.) placed as close as possible to the micropipette tip. Further tubing connected the exit of the microslide to a series of interchangeable collection chambers: one to waste, and one chamber per perifusate. Perifusate was thence recycled to the initial heated reservoirs by means of two peristaltic pumps. A water bath and pump (Techne, Cambridge, UK) were used to circulate warmed fluid through a series of tubes and jackets designed to heat regulate the entire pre-microslide apparatus. The bath temperature and flow rate were adjusted to achieve a flowing perifusate temperature (at the locus of the glomerulus) of 37 ± 0.5°C in all experiments. Flow rates (typically 1 ml s−1) for each perifusate were calculated and equalized before introduction of glomeruli. There was no significant relationship between LpA/Vi values (LpA values normalized for initial glomerular volume (Vi)) elicited with perifusate flow rates between 0.75 and 1.75 ml s−1, and the perifusate flow rate applied (r=−0.14, n = 51, P > 0.3).
Figure 1. Glomerular oncometry apparatus.
A, photograph of the apparatus (note that the insulating apparatus required for paired studies has been removed for clarity): 1, aspiration line; 2, three-way tap for injection of glomeruli; 3, distal tip of suction micropipette; 4, micropipette within holder, clamped onto mount for stability; 5, Perspex mount; 6, head of remote tap bearing two perifusate-containing lines, and permitting rapid perifusate exchange; 7, confluence of perifusate lines; 8, flanged end of rectangular glass microslide; 9, locus of isolated glomerulus; 10, body of microslide; 11, route of perifusate efflux; 12, ×4 objective; 13, ×10 objective. B, illustration of the apparatus, with the same reference numbers as for A. In addition: A, heated reservoir containing ‘dilute’ perifusate; B, heated reservoir containing ‘concentrated’ perifusate.
Glomerular volumetric change
Glomeruli that were free of Bowman's capsule and arteriolar or tubular fragments, and which were macroscopically intact, were chosen for study. Isolated glomeruli exhibited normal morphology, both at the light and electron microscope level (Fig. 2), in keeping with previous reports (Savin & Terreros, 1981). The mean ± s.e.m. volume of glomeruli harvested from the superficial cortex of rat kidneys (1.05 ± 0.04 nl, n = 51) was not significantly different from that reported in the literature (1.08 ± 0.06 nl, n = 20, P > 0.7, unpaired t test) (Pinnick & Savin, 1986). All glomerular observations were performed within 3 h of nephrectomy, as there was no significant decline in LpA/Vi values obtained from glomeruli incubated at 37°C for up to 3 h post mortem (r=−0.13, n = 41, P > 0.4). After a period of equilibration in flowing ‘dilute’ perifusate (approximately 2 min), the perifusate was rapidly changed to ‘concentrated BSA’ perifusate. Perifusate exiting the microslide and entering the collection chambers was diverted to waste for 2 s prior to, and 8 s after, the switch of perifusate, before perifusate recycling was re-started. Perifusate switch was confirmed by the appearance of lines of altered refraction around the glomerulus (the Schlieren phenomenon). Following perifusate exchange, erythrocytes began to move longitudinally within glomerular capillaries, providing a useful index of changes in glomerular volume elicited by trans-filtration barrier fluid movement between the perifusate and glomerular capillaries.
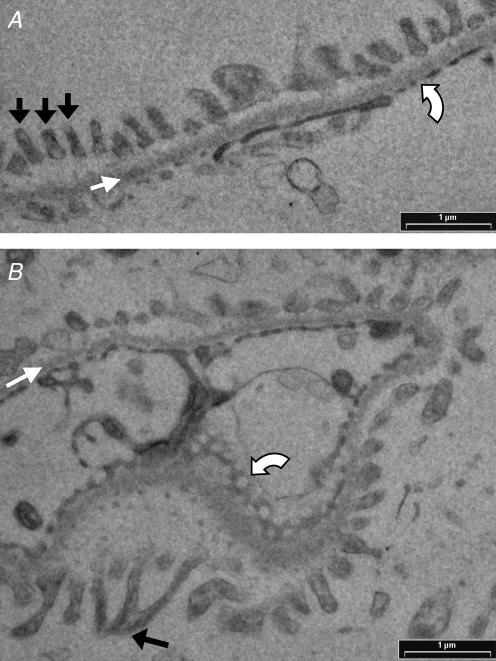
Figure 2. Transmission electron micrographs of isolated glomeruli.
Glomeruli were isolated from the superficial renal cortex of Wistar rat kidneys, and processed for transmission electron microscopy (TEM). A, normal glomerular filtration barrier, displaying evenly distributed podocyte foot processes (black arrows), uniform glomerular basement membrane (white arrow) and endothelial fenestrae (curved white arrow). B, micrograph displaying an enface view of the glomerular filtration barrier. The uniform filtration barrier (white arrow) remains apparent. Fenestrae appear as circular discontinuities in the endothelium (curved white arrow). Branching of podocyte processes to form interdigitating foot processes (black arrow) is also apparent.
Analysis of glomerular volumetric change
Perifusate switches were recorded on videotape at 50 fields s−1. Video sequences were reviewed (off-line) using an Apple Video Player (Apple, USA), and a sequence of still images, straddling the time point at which perifusate switch occurred, were created. The glomerular outline in each image was replicated using the magnetic lasso tool in Adobe Photoshop 7.0 (Adobe Systems, Inc., CA, USA), and the area (A, μm2) of the glomerular image calculated using Image J (US National Institutes of Health). Computer analysis of video images of volumetric changes of glomeruli enables measurement of the entire glomerular perimeter, rather than just four glomerular diameters at 45 deg intervals (Savin & Terreros, 1981). Glomerular volume (nl) was derived from these area measurements by substituting glomerular image area (A) into the formula
| (1) |
(where r= glomerular radius) to reveal:
| (2) |
Glomerular volume (nl) was plotted against time since first appearance of the Schlieren phenomenon (Fig. 3A). Two regression lines were then applied to these points (Fig. 3B). The slope of the first line was prescribed as zero, and this first line was applied to points covering the period before perifusate switch, when baseline glomerular volume was stable. The two lines were calculated to meet at their breakpoint. The breakpoint was determined iteratively, and not confined to the time of the video image at which the Schlieren phenomenon became evident. This defined the time point at which the glomerular volume starts to decline. The second line was applied to points covering a time period of at least 0.04 s from the breakpoint (i.e. at least three volume measurements), and points covering a time period of no more than 0.1 s (i.e. no more than six volume measurements). Within these confines, the points to which an applied regression line had the greatest slope were chosen. These time confines were applied to this line in order to minimize slope measurement error introduced by using too few time points, and in order that the slope should relate to the period of glomerular volume change before erythrocyte movement within glomerular capillaries began.
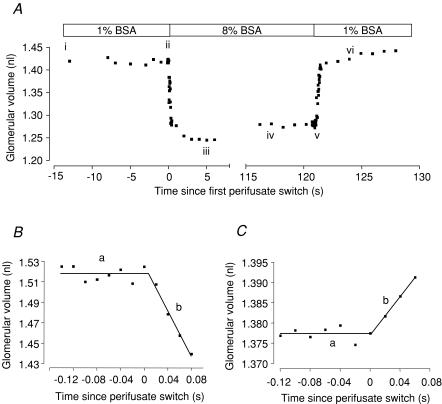
Figure 3. Representative plots of glomerular volume change versus time since perifusate switch.
A, volume change displayed by one glomerulus, in response to ‘dilute to concentrated’, then ‘concentrated to dilute’ switch of solutions. i–ii, glomerulus volume is stable when surrounded by dilute perifusate (1% BSA); ii, perifusate switch to concentrated BSA (8%) reduces glomerular volume by 10% (iii); glomerular volume then stabilizes (iv) (equilibration); v, glomerular swelling on re-introduction of ‘dilute’ perifusate (vi). B, fluid egress from isolated glomerulus (filtration): initial rate of reduction of glomerular volume when exposed to 8% BSA (oncotic gradient 29.2 mmHg). The slope of regression line is set at zero (a). The direction of fluid movement out of the glomerulus is in the same direction as glomerular filtration (slope of line b =−1.125 nl s−1). C, fluid ingress into isolated glomerulus (reabsorption): application of a 9.8 mmHg oncotic gradient induces glomerular swelling (slope of line b = 0.240 nl s−1).
Calculation of LpA
The slope of the second regression line describes the greatest initial rate of change of glomerular volume. Starling's law of filtration dictates that:
| (3) |
where Jv is the rate of fluid flux, Lp is the hydraulic conductivity, Pc is the capillary hydrostatic pressure, Pi is the interstitial hydrostatic pressure, σ is the reflection coefficient, Πc is the capillary oncotic pressure, and Πi is the interstitial oncotic pressure. Glomerular volume change indicates fluid efflux from the glomerulus driven by the oncotic pressure gradient applied by introduction of the ‘concentrated’ perifusate in the vicinity of a glomerulus, the capillary contents of which have equilibrated with the initial ‘dilute’ perifusate. The second regression line, determined as outlined above, describes the greatest rate of fluid efflux from the glomerulus; this equates with the term Jv in Starling's equation. A net hydrostatic force (Pc−Pi) acting across the isolated glomerulus can be assumed to be negligible: the arteriolar remnants are open, there is an absence of flow within the glomerular capillaries (as indicated by the absence of movement of erythrocytes in the first 0.1 s after perifusate switch), and equilibration of the hydrostatic forces necessary to induce flow of each perifusate is rigorously ensured in each experiment. Previous work has demonstrated that σ of isolated glomeruli is not significantly different from 1 (Savin et al. 1992). One can therefore rearrange the Starling equation to show that:
| (4) |
(in nl min−1 mmHg−1) (where ΔΠ is the difference between capillary and interstitial oncotic pressure).
Determination of mean fractional filtration rate
As described above, a plot of glomerular volume against time was generated for each glomerulus studied. In addition to the calculation of LpA, these plots were also used to determine the mean fractional filtration rate (Jv(f)) from populations of glomeruli exposed to the same experimental conditions (Fig. 4). For each glomerulus, volumes calculated from each video frame were normalized to the ‘breakpoint’ volume (i.e. the volume at the time of initiation of glomerular shrinkage; see above), and time zero was prescribed as the ‘breakpoint’ time. Hence the fold change in glomerular volume could be calculated for all glomeruli exposed to the same experimental conditions, and at all time points between 0.1 s prior to, and at least 0.1 s after, the onset of glomerular shrinkage (Fig. 4A). This information was used to generate a plot of fold change in glomerular volume versus time. To calculate the mean Jv(f), a linear regression line was applied to the initial period of mean glomerular shrinkage (Fig. 4B). This is in contrast to the application of the initial slope of a single exponential curve fitted to the mean volumetric change (Savin, 1986). We have noted better indices of accordance (standard deviation of residuals) of glomerular volumetric change during this initial phase to linear (0.0017) rather than non-linear (0.0041) models. We also suggest that the use of linear modelling is more in keeping with the hypothesis that the rate of change of glomerular volume during this initial time period represents fluid efflux whilst capillary contents are conserved and luminal hydrostatic pressures remain negligible. Examination of control glomeruli revealed that application of the line to the first 0.08 s provided the most appropriately fitting line (as determined by a ‘runs test’) applied to the maximum number of volumetric points possible (thereby providing the best estimate of mean Jv(f)). The slopes of these lines can be used to compare mean fractional change in volume per unit time, or fractional rate of fluid efflux. This mean Jv(f) is therefore used to compare populations of glomeruli exposed to different experimental conditions.
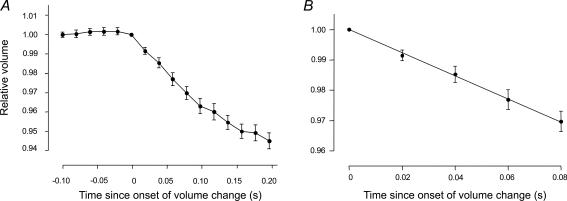
Figure 4. Calculation of mean fractional filtration rate (Jv(f)).
A, for each individual glomerulus, glomerular volume is normalized to that at the onset of the shrink phase of filtration, and time is normalized to the onset of the shrink phase of filtration. Relative glomerular volume can then be plotted against time for a population of glomeruli exposed to the same experimental conditions. B, a linear regression line is applied to the change in relative volume that occurs in the first 0.08 s after the onset of the shrink phase of glomerular filtration. The magnitude of the slope of this line (−0.38 ± 0.01 s−1) describes the mean filtration rate for this representative control population (n = 10).
Repetitive filtration (paired) experiments
The ability to compare sequential measurements of LpA in the same glomerulus, rather than comparing different populations of glomeruli, has been postulated to permit evaluation of small mediator-induced changes in LpA (Savin, 1986). Such repetitive evaluations also enable examination of LpA values immediately following short periods of mediator incubation (up to approximately 15 min, given the time constraints of the experimental procedure). To this end, the apparatus and methodology were adjusted to permit repetitive evaluation of LpA values obtained from one glomerulus.
The portion of the microslide containing the glomerulus was heat regulated with flow of fluid from the water bath through large-calibre (700 μm × 7 mm) rectangular cross-section glass microslides. The relative flow of fluid through these heat-regulating microslides, as compared with that through the remainder of the heat-regulating system, was adjusted to achieve a temperature of 36.5 ± 0.5°C for static perifusate solutions in the immediate vicinity of the glomerulus, whilst maintaining the temperature of flowing perifusate, at the locus of the glomerulus, at 37 ± 0.5°C.
Paired glomerular oncometry studies have been reported (Savin, 1986) in glomeruli in which afferent and efferent arteriolar remnants were crimped in the holding micropipette. That procedure was employed in order that intracapillary plasma proteins, which are critical in the establishment of a transglomerular oncotic gradient, are not lost during the expansion in glomerular volume, which occurs when ‘dilute’ perifusate is applied to a glomerulus that has been equilibrated in ‘concentrated’ perifusate. We elected to adopt two experimental protocols in the evaluation of repetitive filtration studies in the same glomerulus to test the hypothesis that conservation of capillary contents is an essential element of repetitive filtration studies. These two protocols differed in the duration of time between the first ‘dilute’ to ‘concentrated’ switch and restoration of the initial ‘dilute’ perifusate. The mean ± s.e.m. interval between application of the ‘concentrated’ perifusate and restoration of the initial ‘dilute’ perifusate was 40.6 ± 4.4 s in the ‘delayed replacement’ protocol; this interval was 1.2 ± 0.1 s in the ‘rapid replacement’ protocol. Evaluation of glomerular volume immediately prior to each switch, erythrocyte movement, and ultrafiltration coefficient corrected for glomerular volume (LpA/Vi) and mean Jv(f), was made for each repetition of filtration using both of the protocols.
VEGF experiments
Recombinant human VEGF (rhVEGF165; R & D Systems, MN, USA) was used. PTK787/ZK222584 was obtained from Novartis Pharma AG, Basel, Switzerland, and dissolved in N,N-dimethylformamide (Sigma, MO, USA) at a final concentration of 100 nm, which is a concentration previously shown to cause inhibition of VEGF-R1 and -R2 signalling (Whittles et al. 2002). ZM323881 was obtained from Calbiochem, CA, USA, and dissolved in N,N-dimethylformamide at a final concentration of 10 nm, which is a concentration previously shown to cause selective inhibition of VEGF-R2 signalling in systemic microvessels, thereby blocking VEGF-mediated increases in hydraulic conductivity in that model (Whittles et al. 2002).
Following isolation, glomeruli were incubated at 37°C in either 1% BSA solution, or 1% BSA solution containing test solutions, in unpaired experiments. Glomeruli from each solution were then individually loaded into the microslide observation chamber after the prescribed exposure period. In paired experiments, the ‘delayed replacement’ protocol was used. Flow was halted, and sufficient volume of VEGF-containing ‘dilute’ solution was injected into the microslide to ensure that the glomerulus was entirely bathed in VEGF-containing solution. Control paired studies performed at the same time employed BSA-containing ‘dilute’ solution for microslide injection. Subsequent ‘dilute’ to ‘concentrated’ perifusate switches were performed as soon as possible after re-initiation of flow (typically under 5 s). Blinding to the experimental condition was maintained from the time of solution creation until completion of regression line application.
Statistical analysis
The frequency distribution of LpA/Vi values was not significantly different from the normal distribution (mean ± s.e.m. 1.02 ± 0.06 min−1 mmHg−1; Kolmogorov-Smirnov distance 0.10, n = 51, P > 0.1). Two-sided parametric tests were therefore employed: Pearson's rank for examination of correlation (r), unpaired t test for unpaired experiments, paired t test for paired experiments, and repeated-measures ANOVA test for repeated paired observations. Unpaired t tests were used to compare mean Jv(f). All experiments were designed with sufficient power to detect a twofold change in both LpA and mean Jv(f) (assuming α= 0.05 and 1 − β= 0.8), using the statistical package G-Power (http://www.psychologie.uni-trier.de//pub/gpowermac/gpower212.sit.hqx) (unpaired experiments) or the formulae described by Machin & Campbell (1987) (paired experiments). A P value of less than 0.05 was considered statistically significant. Results are reported as means ± s.e.m. unless otherwise stated.
In order to facilitate comparison of the results in this paper with those reported in the literature using Wistar rats, direct glomerular capillary micropuncture (Aukland et al. 1977) and stop-flow studies (Arendshorst & Gottschalk, 1974) were reviewed. In order to calculate LpA values from direct glomerular capillary micropuncture reports, glomerular capillary pressure (Pgc) measurements (Aukland et al. 1977) have been combined with the most concordant results reported in the literature, predominantly comparing rat strain and volume status of the animal. Single-nephron glomerular filtration rate (SNGFR) values are from Table 3 of Davis et al. (1988), in which the loop of Henle inflow rate was within the ‘normal range’, i.e. 10–25 nl min−1 (Davis et al. 1988), and Πa (afferent arteriolar oncotic pressure) is from Table 2 of the same paper. Πe (efferent arteriolar oncotic pressure) has been calculated from Table 2 of the review by Maddox et al. (1992). <Πgc> (mean glomerular oncotic pressure) is calculated using the formula <Πgc> = (Πa+Πe)/2, according to the convention of Ott et al. (1976). LpA has been calculated from the quotient of SNGFR and (Pgc − <Πgc>).
Table 2.
Summary of the major differences between the work of Savin et al. and the current study
| Savin et al.* | Current study | |
|---|---|---|
| Apparatus | Room temperature, open bath | 37°C, closed system |
| Application of oncotic gradient | Gravity-fed replacement of static solution at uncontrolled rate | Controlled, continuous flow; rapid/reproducible switch |
| Direction of transglomerular fluid flux | Into glomerulus – non-physiological | Leaving glomerulus – physiological |
| Repetitive filtration studies | Crimped arterioles (rarely published) | Double perifusate switch |
| Estimation of glomerular volume | Four equally spaced glomerular diameters | Glomerular perimeter |
| Estimation of initial rate of change of glomerular volume | Greatest difference in glomerular volume occurring in 0.017 s | Actual rate of change in glomerular volume occurring in 0.04–0.1 s |
| Units of measurement | LpA | LpA/Vi, mean Jv(f) |
Results
Validation of method
Control glomeruli
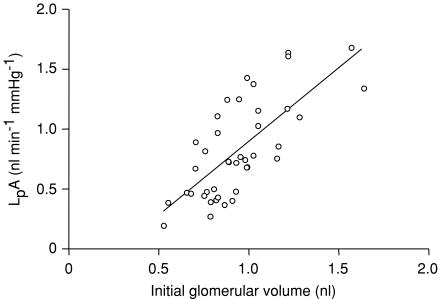
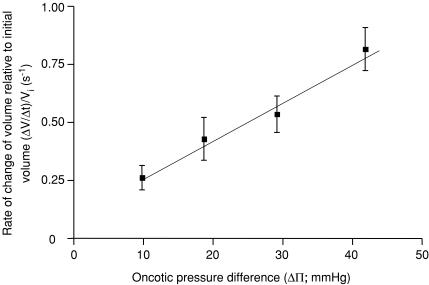
Mean ± s.e.m.LpA values from control glomeruli were 1.07 ± 0.07 nl min−1 mmHg−1 (n = 51). Mean Jv(f) was −0.41 ± 0.02 s−1 (n = 51). LpA values demonstrated a significant correlation with initial glomerular volume (r= 0.72, n = 41, P < 0.0001, LpA= 1.23−0.33 nl min−1 mmHg−1, Fig. 5). Henceforth, LpA values are corrected for initial glomerular volume (Vi): LpA/Vi (min−1 mmHg−1). The initial rate of change of glomerular volume (ΔV/Δt), corrected for Vi ((ΔV/Δt)/Vi; s−1), significantly and linearly increased with the oncotic pressure difference (r= 0.59, n = 28, P < 0.001, (ΔV/Δt)/Vi= 0.02ΔΠ+ 0.1 s−1; Table 1 and Fig. 6).
Figure 5. Relationship between LpA and initial glomerular volume.
The ultrafiltration coefficient (LpA) was calculated for glomeruli of different sizes under control conditions. A strong positive linear correlation was observed between LpA and initial glomerular volume (Vi) (r= 0.72, n = 41, P < 0.0001, regression line LpA= 1.23−0.33 nl min−1 mmHg−1) .
Table 1.
Effect of oncotic pressure on glomerular ultrafiltration rate
| BSA concentration (mg ml−1) | Oncotic pressure difference (mmHg) | Rate of change of volume ((ΔV/Δt)/Vi) (s−1) | Number of glomeruli examined |
|---|---|---|---|
| 40 | 9.8 | 0.26 ± 0.05 | 6 |
| 60 | 18.7 | 0.43 ± 0.09 | 4 |
| 80 | 29.2 | 0.53 ± 0.08 | 14 |
| 100 | 41.8 | 0.81 ± 0.09 | 4 |
Figure 6. Relationship between initial rate of change of glomerular volume per unit initial glomerular volume and oncotic gradient applied.
Populations of glomeruli were exposed to different oncotic gradients, by altering the BSA concentration of the ‘concentrated’ perifusate. As predicted by Starling's equation, the magnitude of the rate of change of initial glomerular volume per unit initial glomerular volume was linearly related to the magnitude of the oncotic gradient applied. (Linear regression (ΔV/Δt)/Vi= 0.02 mmHg + 0.1 s−1; r = 0.59, n = 28, P < 0.001).
Repetitive filtration (paired) control studies
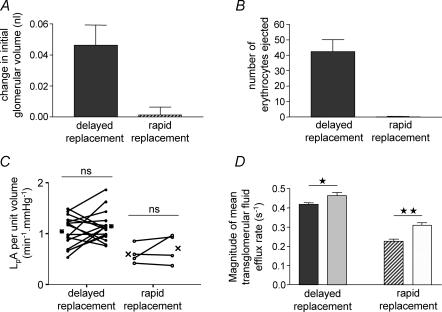
Glomerular volume before the first repetition of filtration (0.95 ± 0.06 nl; n = 16) was slightly, but significantly greater than glomerular volume before the second repetition (0.90 ± 0.06; n = 16) (P < 0.005, paired t test) using the ‘delayed replacement’ protocol (Fig. 7A). In addition, the number of erythrocytes ejected on re-application of the initial ‘dilute’ perifusate using the ‘delayed replacement’ protocol was 42.4 ± 7.8 (n = 5), a figure significantly different from zero (P < 0.01, one-sample t test versus zero) (Fig. 7B). In contrast, using the ‘rapid replacement’ protocol, glomerular volume was unchanged prior to the first (0.97 ± 0.16; n = 4) and second (0.97 ± 0.16; n = 4) repetition of filtration (P > 0.8; paired t test) (Fig. 7A), and the number of erythrocytes ejected (0.2 ± 0.2; n = 5) was not significantly different from zero (P > 0.35, one-sample t test versus zero) (Fig. 7B).
Figure 7. Comparison of ‘delayed replacement’ (40 s delay) and ‘rapid replacement’ (1.2 s delay) paired filtration study protocols.
A, rapid replacement resulted in no significant change of initial glomerular volume (P > 0.8, one-sample t test versus 0), in contrast to delayed replacement (filled column). B, rapid replacement, in contrast to delayed replacement, resulted in no ejection of erythrocytes (P > 0.35, one-sample t test versus 0). C, there was no significant change in LpA per unit volume (LpA/Vi, min−1 mmHg−1) between the first and second filtrations using both the ‘delayed replacement’ and ‘rapid replacement’ protocols. D, there was a significant change in the mean rate of transglomerular fluid efflux between the first (dark filled column) and second (light filled column) repetition of filtration in populations of glomeruli subjected to both the ‘delayed replacement’ (*P < 0.05, unpaired t test, n = 5) and ‘rapid replacement’ (**P < 0.005, unpaired t test, n = 5) protocols.
LpA/Vi, examined repetitively in the same glomerulus using the ‘delayed replacement’ protocol, was unaltered between the first (1.05 ± 0.08 min−1 mmHg−1) and second (1.15 ± 0.07 min−1 mmHg−1) repetition of filtration (P > 0.25, paired t test; n = 16) (Fig. 7C). This was also true when the ‘rapid replacement’ protocol was employed (first LpA/Vi 0.6 ± 0.09 min−1 mmHg−1; second LpA/Vi 0.71 ± 0.14 min−1 mmHg−1; P > 0.35, paired t test; n = 4). However, there was a statistically significant 11% increase in the magnitude of mean Jv(f) for the second repetition of filtration as compared with the first repetition (first −0.42 ± 0.01; second −0.46 ± 0.02; n = 5 pairs, P < 0.05, unpaired t test) using the ‘delayed replacement’ protocol (Fig. 7D), and a 37% elevation using the ‘rapid replacement’ protocol (first −0.23 ± 0.01; second −0.31 ± 0.01; n = 5 pairs, P < 0.005, unpaired t test). Therefore paired analysis of mean Jv(f) can only determine agonist-induced increases in filtration rate greater than that induced by the experimental protocol alone, or reductions in filtration rate.
Effect of VEGF on ultrafiltration coefficient
Time course
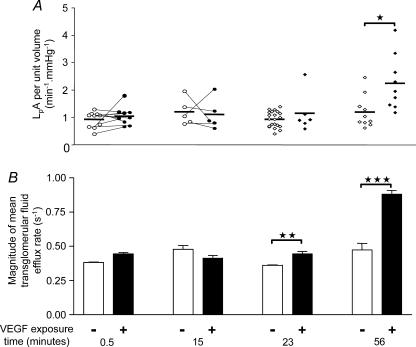
VEGF has been shown to alter systemic microvascular hydraulic conductivity in a biphasic manner – acutely over a few seconds, and chronically over many minutes up to 24 h (Bates & Curry, 1996). To determine whether VEGF increased hydraulic permeability in glomeruli, glomerular filtration was examined after four different durations of VEGF exposure: 30 s, 15 min, 23 ± 1.8 min and 56 ± 5.4 min (Fig. 8). A 30 s exposure to VEGF did not increase LpA/Vi (0.92 ± 0.09 to 1.04 ± 0.10 min−1 mmHg−1; n = 10 pairs; P > 0.35, paired t test) (Fig. 8A). The 17% increase in mean Jv(f) after exposure to VEGF for 30 s (from −0.38 ± 0.005 to 0.44 ± 0.01 s−1; n = 5) (Fig. 8B) was no different from the 15% increase seen after exposure to control solution for 30 s (from −0.38 ± 0.01 to −0.44 ± 0.02 s−1; n = 5). Similarly, 15 min exposure to VEGF did not increase LpA/Vi (1.20 ± 0.22 to 1.10 ± 0.25 min−1 mmHg−1; n = 5 pairs; P > 0.75, paired t test). There was also no difference in mean Jv(f) after exposure to VEGF for 15 min (−0.48 ± 0.03 compared with 0.41 ± 0.02 s−1; n = 5; P > 0.1, unpaired t test) or after exposure to control solution for 15 min (−0.50 ± 0.03 to −0.52 ± 0.02 s−1; n = 5; P > 0.5, unpaired t test).
Figure 8. Effect of VEGF on glomerular hydraulic permeability.
Glomeruli were exposed to VEGF (1 nm) (filled symbols/bars) or control solution (open symbols/bars) for between 0.5 and 56 min. A, effect of VEGF on LpA/Vi. Circles connected by lines represent LpA/Vi values from the same glomerulus before and after exposure to VEGF. Diamond-shaped symbols represent single LpA/Vi measurements in unpaired studies. Horizontal bars represent the mean of each group. VEGF exposure for 56 min elicited an approximate doubling of LpA/Vi, *P < 0.02, unpaired t test. B, bars represent mean ± s.e.m. transglomerular fluid efflux rates (Jv(f)) from populations of glomeruli exposed to control or VEGF (1 nm) solution. VEGF exposure for 30 s or 15 min increased mean Jv(f) by no more than that seen in control-exposed glomeruli. VEGF exposure for 23 min (**P < 0.005, unpaired t test) and 56 min (***P < 0.0001, unpaired t test) elicited a significant increase in mean Jv(f).
LpA/Vi values after exposure to VEGF for 23 min (1.14 ± 0.29 min−1 mmHg−1; n = 6) were not significantly greater than control (0.92 ± 0.06 min−1mmHg−1; n = 21; P > 0.25, unpaired t test); however, the mean Jv(f) after 23 min VEGF exposure (−0.44 ± 0.02 s−1) was significantly greater than that observed after exposure to control solution (−0.36 ± 0.003 s−1, n = 5, P < 0.005, unpaired t test). When compared with controls, 56 min exposure to VEGF elicited a significant, approximately twofold, elevation in both LpA/Vi (1.19 ± 0.19 (n = 10) to 2.24 ± 0.33 (n = 9) min−1 mmHg−1, P < 0.02, unpaired t test) and mean Jv(f) (−0.47 ± 0 to −0.88 ± 0.03 s−1; n = 5, P < 0.0001, unpaired t test).
Concentration–response effect of VEGF
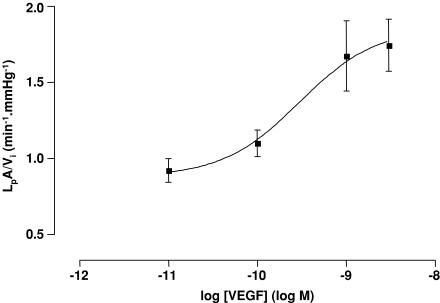
To determine whether a concentration–response effect of VEGF on glomerular hydraulic permeability could be discerned, glomerular filtration was examined after 60 min exposure to four different concentrations of VEGF. Exposure to 10 pm VEGF and 100 pm VEGF elicited non-significant elevations in LpA/Vi and mean Jv(f) when compared with control glomeruli (control glomeruli, LpA/Vi 0.85 ± 0.05 min−1 mmHg−1, mean Jv(f) −0.32 ± 0.01 s−1; 10 pm VEGF, LpA/Vi 0.92 ± 0.08 min−1 mmHg−1, mean Jv(f) −0.42 ± 0.03 s−1; 100 pm VEGF, LpA/Vi 1.10 ± 0.09 min−1 mmHg−1, mean Jv(f) −0.46 ± 0.02 s−1; all P > 0.05 versus control, one-way ANOVA, Bonferroni correction). Exposure to 1 nm VEGF and 3 nm VEGF elicited significant elevations in LpA/Vi and mean Jv(f) when compared with control glomeruli (1 nm VEGF, LpA/Vi 1.67 ± 0.23 min−1 mmHg−1, mean Jv(f) −0.66 ± 0.04 s−1; 3 nm VEGF, LpA/Vi 1.74 ± 0.17 min−1 mmHg−1, mean Jv(f) −0.66 ± 0.07 s−1; all P < 0.001 versus control, one-way ANOVA, Bonferroni correction). A plot of LpA/Vi against the logarithm of the concentration of VEGF is shown in Fig. 9; this plot reveals an EC50 of VEGF on LpA/Vi of 296 pm. An EC50 of 299 pm is obtained when mean Jv(f) is plotted against the logarithm of the concentration of VEGF.
Figure 9. Dose-related elevation of LpA/Vi by VEGF.
Populations of glomeruli were exposed to different conentrations of VEGF for 60 min. Concentrations of VEGF between 10 pm and 3 nm elicited dose-dependent elevations of LpA/Vi, revealing an EC50 of 295 pm.
Blockade of VEGF effect with selective receptor inhibitors
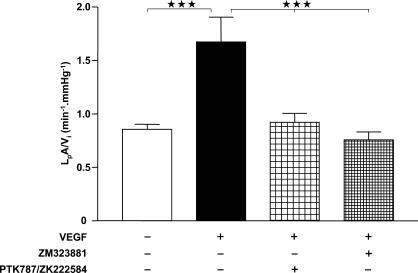
In an attempt to discern the VEGF receptor through which the VEGF-induced elevation of LpA/Vi is mediated, we co-incubated glomeruli for 60 min with 1 nm VEGF with either of the VEGF receptor inhibitors PTK787/ZK222584 (at 100 nm, a non-selective inhibitor of VEGF receptors R1 and R2) or ZM323881 (at 10 nm, a selective inhibitor of VEGF-R2) (Fig. 10). Application of 1 nm VEGF for 60 min again approximately doubled LpA/Vi as compared with control (control, 0.85 ± 0.05 min−1 mmHg−1, n = 41; VEGF, 1.67 ± 0.23 min−1 mmHg−1, n = 16; one-way ANOVA with Bonferroni correction P < 0.001). This effect was eliminated by co-application of the pan-VEGF receptor inhibitor 100 nm PTK787/ZK222584 (0.92 ± 0.08 min−1 mmHg−1, n = 20; one-way ANOVA with Bonferroni correction P < 0.001 versus VEGF alone, P > 0.05 versus control), and by co-application of the VEGF-R2 inhibitor, 10 nm ZM323881 (0.76 ± 0.07 min−1 mmHg−1, n = 24; one-way ANOVA with Bonferroni correction P < 0.001 versus VEGF alone, P > 0.05 versus control).
Figure 10. Blockade of VEGF-induced elevation of LpA/Vi by VEGF-R2 inhibition.
Populations of glomeruli were exposed for 60 min to control solution (open bar), 1 nm VEGF alone (filled bar), 1 nm VEGF plus the pan-VEGF receptor inhibitor (100 nm PTK787/ZK222584) (loosely cross-hatched bar), or 1 nm VEGF plus the selective VEGF-R2 inhibitor (10 nm ZM323881) (tightly cross-hatched bar). ⋆⋆⋆P < 0.001, one-way ANOVA with Bonferroni correction.
Discussion
Here we describe an extensive refinement of a very powerful, yet not widely used, method for measurement of glomerular ultrafiltration coefficient. There are very few techniques with which it is possible to study the filtration characteristics of renal glomeruli at the single-nephron level. The use of an oncometric technique in isolated glomeruli offers the possibility of examining the filtration characteristics of glomeruli, independent of circulating and haemodynamic influences, from a wide variety of species, including man (Savin & Terreros, 1981). We have refined the model of Savin and coworkers in an attempt to improve approximation with the in vivo situation, and to address some of the potential sources of error in the model. The major differences between the two models are summarized in Table 2.
Limitations and advantages of this refinement of the Savin oncometric method for measurement of ultrafiltration coefficient
Experimental methods
Fluid egress from glomeruli has been studied here, rather than fluid ingress as in Savin's method (Savin & Terreros, 1981), as the former is the direction of fluid movement in the production of the primary glomerular ultrafiltrate in vivo. The similarity of LpA values for fluid movement in either direction under control conditions (Savin & Terreros, 1981) does not necessarily imply that both will be altered in the same way by mediators that affect LpA. Thermoregulation of the apparatus permits evaluation of LpA in glomeruli incubated and examined at 37°C. Tissue compliance, fluid viscosity and the cellular effects of mediators are all more likely to be in keeping with the in vivo situation at this temperature. Pre-determination of the flow rates of both perifusate solutions optimizes the repeatability of the characteristics of new perifusate introduction. In addition, the use of two flowing perifusate solutions with equal flow rates eliminates the potential influence of shear rate introduced by injecting new perifusate in the vicinity of the isolated glomerulus at an indeterminable and non-reproducible rate (Savin & Terreros, 1981). The only force driving transglomerular fluid flux in this model will be the oncotic pressure difference between the two perifusate solutions.
Analysis methods
The two methods for determination of LpA also differ considerably in the manner of estimation of the greatest initial rate of change in glomerular volume. We have elected to apply a regression line to glomerular volume measurements from at least three, and no more than six, time points. This substantially reduces the calculated slope when compared to the use of the greatest difference between two points within the same time frame, as employed by Savin et al. (see Fig. 11) (Savin & Terreros, 1981). Nevertheless, the use of multiple time points reduces error attributable to single-point variation. We have also chosen to limit the period of examination to the first 0.1 s after introduction of an oncotic gradient, as we have replicated the finding of Savin et al. that this is the earliest time at which erythrocytes are observed to move within capillaries. It is important to limit the period of examination to that prior to longitudinal movement of intracapillary contents, since this is the only period of the examination during which the capillary contents can be assumed not to have been affected by changes induced by fluid movement across the open ends of the afferent and efferent arterioles.
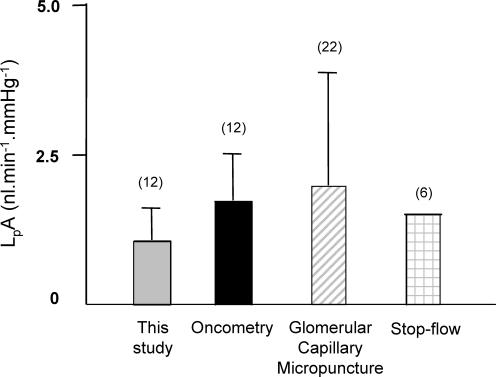
Figure 11. Comparison of ultrafiltration coefficient (LpA) values obtained in the current study with those reported in the literature for Wistar rats.
LpA values from this report (grey bar) have been compared with the results of this paper, but analysed according to Savin's ‘greatest difference’ method (black bar; see Discussion; Savin & Terreros, 1981), with results from direct glomerular micropuncture studies (hatched bar; see Methods; Aukland et al. 1977), and with results from stop-flow studies (Arendshorst & Gottschalk, 1974). Numbers in parentheses represent number of animals studied. Error bars represent standard deviation (where reported). LpA values were similar to those published in the literature.
Determination of the mean Jv(f) for populations of glomeruli provides an alternative index of mean glomerular permeability to water. We have elected to examine fold changes in glomerular volume. This is in contrast to the use of absolute changes in glomerular volume employed in the only previous report of population mean glomerular volumetric change (Savin, 1986). We believe the former to be more appropriate given the strong positive correlation between LpA and Vi.
The coefficient of variation for LpA values derived from 10 repetitive evaluations of the shrink phase of two glomeruli were 21.2 and 16.1% (mean 18.7%). This is similar but lower than the coefficient of variation for LpA obtained by Savin & Terreros (1981) (24.86%, n = 17 rats). The finding that the coefficient of variation for LpA measurements is in the order of 20% identifies this as the lower limit of resolution of this technique. The coefficient of variation for mean Jv(f) for five independent control populations was 12.7%. This lower coefficient of variation for mean Jv(f) (as compared with those for LpA or LpA/Vi) suggests that more subtle changes in glomerular hydraulic permeability characteristics can be discerned with this analysis method.
Other possible confounding errors in the system
Assumptions inherent to any oncometric method have been extensively discussed elsewhere in the literature (Savin & Terreros, 1981). However, one principal assumption is that the reflection coefficient (σ) ‘is not significantly different from one’ (Savin et al. 1992). Any change in σ, particularly that induced by mediators such as VEGF, would change the applied oncotic pressure gradient. Future studies regarding the potential influence of VEGF on σ will address this assumption.
The characteristics of the perifusate switch will have a significant influence on the extent to which the glomerulus is exposed to the intended oncotic gradient. It is likely that less than 100% of the glomerular filtration barrier is exposed to the oncotic gradient immediately. The LpA/Vi value obtained using this technique may therefore not include a filtration contribution from the deepest portions of the glomerulus, leading to further underestimation of the true LpA. However, recent studies have shown that it is unlikely that all parts of the glomerulus contribute equally to the filtration of plasma across the basement membrane, due to the three-dimensional relationship between the glomerular filtration barrier and the position of surrounding podocyte cell bodies (Neal et al. 2005).
Single glomerulus repetitive filtration studies
In one previous report (Savin, 1986), paired glomerular experiments have been possible, but only in glomeruli in which afferent and efferent arteriolar fragments have been crimped in the holding micropipette. We have employed a different strategy to ensure that capillary contents are conserved between repetitions of filtration. The replacement of the initial ‘dilute’ perifusate very shortly after application of the initial ‘dilute’ to ‘concentrated’ switch (‘rapid replacement’ protocol) provides sufficient video frames for the analysis of the initial phase of reduction in glomerular volume, whilst still permitting conservation of capillary contents during the subsequent increase in glomerular volume (as evinced by the absence of change in glomerular volume, and absence of capillary content expulsion). Our apparatus permits this protocol because the use of two flowing perifusate solutions ensures complete solution exchange around the glomerulus, thereby allowing repetitive application of a new perifusate of known oncotic composition under identical repetitive conditions. We concur with Savin in finding that ultrafiltration coefficient values do not alter between repetitions of filtration in the same glomerulus (Savin, 1986). However, this is true whether a protocol that does (‘rapid replacement’) or does not (‘delayed replacement’) conserve capillary contents is employed. More striking is the finding that a non-significant, mean 19%, elevation in LpA/Vi is accompanied by a significant, mean 16%, elevation in mean Jv(f) in consecutive repetitions of filtration, suggesting that this more discriminatory test is able to discern subtle changes in glomerular hydraulic permeability which occur as a consequence of repetitive filtration studies.
Comparison of results obtained using this modification with those in the literature
This modification of the Savin method results in measurements of ultrafiltration coefficient that conform to two principal requirements that indicate a relatively high degree of confidence in the results. The first is that Fig. 6 demonstrates that the initial rate of change of glomerular volume per unit volume conforms to Starling's prediction of filtration, being linearly dependent on the applied pressure difference. This is only likely to be the case if the glomerular swelling rate is proportional to the ultrafiltration coefficient. Secondly, we report LpA values that are broadly comparable with those derived using the same technique and with those derived using other techniques in this strain of rats (Fig. 11).
Influence of VEGF on glomerular ultrafiltration coefficient
Comparison with previous reports
This is the first report to suggest that VEGF may influence glomerular ultrafiltration characteristics from normal animals. Previous reports using isolated kidney preparations have demonstrated no effect of VEGF on surrogate markers of glomerular permeability to water (Klanke et al. 1998). It is notable that the concentration of VEGF used in that study (6.6 ng ml−1; 165 pm) is a concentration similar to that which revealed no significant change in LpA/Vi in the current study (100 pm). Unfortunately concentration–response information in that system was not possible because of limited availability of VEGF. In addition, the use of whole-kidney glomerular filtration rate as the primary outcome measure of glomerular permeability to water would have added further difficulties when attempting to discern any VEGF-induced elevation in LpA in that study. Not only does this term encompass a number of variables, such as the number of glomeruli recruited and the net ultrafiltration pressure in addition to the glomerular ultrafiltration coefficient, but most significantly it has been noted that, in many circumstances, ‘increases in Kf [LpA] above normal will have no effect on single-nephron glomerular filtration rate’ (Maddox et al. 1992). Similar comments also apply to other investigations of the role of VEGF on glomerular permeability in whole animals (de Vriese et al. 2001). Whilst the absence of transcapillary hydrostatic pressure in our system undoubtedly misrepresents the in vivo situation, and therefore does not incorporate any alterations in ultrafiltration coefficient that may be brought about by VEGF-induced haemodynamic alterations in vivo, this model does permit selective examination of the role of mediators on glomerular ultrafiltration coefficient without masking or alteration by haemodynamic or circulating influences. As discussed above, this is both a strength and limitation of this model.
Time course of VEGF effect
Exposure of systemic microvessels to VEGF for 30 s elicits a median elevation of Lp by eightfold, followed by a secondary increase in Lp that is present 24 h after 10 min exposure to VEGF (Bates & Curry, 1996). This secondary increase in Lp begins to appear about 1 h after initial VEGF exposure (D. O. Bates, unpublished observations), as is seen in culture (Cohen et al. 1999). The absence of a VEGF-induced elevation of glomerular LpA/Vi when exposure times are at or below 15 min may reflect differences between the two microvascular beds. For instance, the constitutive fenestration of glomerular endothelial cells, and the 50-fold higher resting permeability of glomerular, as compared with systemic, capillaries (Deen et al. 1973) may mean that very brief exposure to VEGF elicits no further elevation in the hydraulic permeability of glomerular capillaries. The time course of our results are more consistent with those seen in other glomerular permeability examinations, such as the reduction in transendothelial electrical resistance (a marker of glomerular permeability to water and small solutes) of monolayers of human glomerular endothelial cells in response to VEGF, which occurs over 30–60 min (Chen et al. 2002; Satchell et al. 2004), and the similar time course of increases in the number of vesicular organelles in VEGF-treated immortalized rat glomerular endothelial cells (Chen et al. 2002). The fact that LpA/Vi values of control glomeruli do not alter for three hours after glomerular isolation suggests that the elevation of LpA/Vi values induced by longer (>20 min) exposure to VEGF is not mediated by a survival-related permeability effect on glomerular cells. In addition, given the observed time course, it is unlikely that the alterations in endothelial cell behaviour which are important in angiogenesis (e.g. proliferation; Ferrara, 2004) will be involved in this system. The signalling pathways involved in the acute VEGF-induced alterations in permeability are still undergoing elucidation, but appear to involve phospholipase C, diacylglycerol, store-independent elevations in intracellular Ca2+, nitric oxide and cyclic GMP (Bates & Harper, 2003). Increased endothelial nitric oxide synthase (eNOS) phosphorylation and consequently elevated nitric oxide levels have recently been demonstrated to be induced by 30 min exposure of isolated glomeruli to 100 ng ml−1 VEGF (Wang et al. 2004), and this may represent an important pathway for VEGF-mediated alterations in glomerular permeability to water.
Concentration-dependent effect of VEGF
In this study, we report that the VEGF-induced alterations in glomerular permeability are concentration dependent. This is in keeping with the findings of Eremina and colleagues, who have elegantly demonstrated a VEGF dose-sensitivity of the glomerulus with regard to glomerular development and pathology using podocyte-specific VEGF genetic manipulation studies (Eremina et al. 2003), and also in keeping with the concentration-dependent increase in eNOS expression following 24 h exposure of isolated glomeruli to VEGF (Wang et al. 2004).
VEGF, VEGF-R2 and glomerular endothelium
We have demonstrated that the VEGF-mediated elevations in glomerular permeability to water are mediated via VEGF-R2, which is in keeping with the majority of actions of VEGF (Bernatchez et al. 1999), including VEGF-induced elevations in systemic microvessel permeability to water (Whittles et al. 2002). A role for VEGF-R2-mediated signalling in glomerular pathology is suggested by the repeated demonstration of alterations in VEGF-R2 levels in animal models (Horita et al. 1998; Cooper et al. 1999; Kanellis et al. 2004) and human disease (Thomas et al. 2000; Bortoloso et al. 2004), but direct examination of a functional effect of VEGF-R2 on the glomerular filtration barrier has not previously been demonstrated.
In addition, a number of reports from different laboratories have repeatedly demonstrated the selective location of VEGF-R2 on glomerular endothelial cells (Simon et al. 1995; Cooper et al. 1999; Kitamoto et al. 2001; Foster et al. 2003). This identifies glomerular endothelial cells as the location (within the tri-layered glomerular filtration barrier) for the VEGF-induced alteration in permeability seen in this system. VEGF alters the transendothelial electrical resistance, a marker of permeability to water and small solutes, of monolayers of glomerular endothelial cells in vitro (Satchell et al. 2004). Genetic alteration of VEGF expression in podocytes leads to ultrastructural alterations in glomerular endothelial cells (Eremina et al. 2003). Renal lesions in pre-eclampsia are almost entirely confined to the glomerular endothelium, and are associated with elevated levels of the VEGF-antagonist soluble fms-like tyrosine kinase 1 (sFlt1) (Karumanchi et al. 2005), and administration of this protein to pregnant rats recapitulates the glomerular lesions of pre-eclampsia (Maynard et al. 2003). It is noteworthy that pre-eclampsia is associated with a reduction in glomerular filtration rate (Karumanchi et al. 2005). These studies provide supporting evidence for the hypothesis that VEGF may alter glomerular permeability to water via an action on glomerular endothelial cells.
VEGF causes the appearance of vesiculo-vacuolar organelles in glomerular endothelial cells in culture (Chen et al. 2002), with a concurrent increase in surrogate markers of monolayer permeability to water. VEGF can elicit the appearance of fenestrations in endothelial cells derived from non-fenestrated microvascular beds in vitro (Esser et al. 1998b) and in vivo (Roberts & Palade, 1995), but whether antagonism of VEGF alters glomerular endothelial fenestration remains debated (Gerber et al. 1999; Ostendorf et al. 1999). VEGF has also been shown to precipitate the formation of transcellular gaps (Michel & Neal, 1999) and induce alterations in adherens junction proteins, an effect likely to be mediated via VEGF-R2 (Esser et al. 1998a). Mathematical models predict a ‘negligible’ contribution of endothelial cells to overall glomerular hydraulic resistance (Drumond & Deen, 1994), unless endothelial cell fenestrae are modelled as containing gel-like glycocalyx, for which improvements in fixation technique are providing increasing evidence (Rostgaard & Qvortrup, 1997; Hjalmarsson et al. 2004). Under these circumstances, models suggest that the endothelial cell layer may contribute up to 24% of the overall glomerular hydraulic resistance (Drumond & Deen, 1994). The significant endothelial-cell-mediated, VEGF-induced elevation in LpA/Vi observed in this model therefore supports the glycocalyx-filled fenestral model postulated by Drumond & Deen (1994). Rapid VEGF-induced changes in glycocalyx have previously been postulated (Fu & Shen, 2003), and alterations in endothelial-cell glycocalyx have been shown to effect a 2.5-fold change in hydraulic conductivity in other microvascular beds (Adamson, 1990) and postulated to alter glomerular permeability (Ciarimboli et al. 2003). Alternatively, VEGF may modulate an alternative characteristic of endothelial fenestrae (e.g. fenestral density) (Gerber et al. 1999), thus eliciting an alteration in normalized ultrafiltration coefficient. The overwhelming contribution of endothelial fenestrae to the hydraulic permeability of the glomerular endothelial cell layer makes it unlikely that changes in intercellular junctions would be of sufficient magnitude to double the overall ultrafiltration coefficient of the filtration barrier.
A doubling of the hydraulic conductivity of the glomerular filtration barrier, due solely to an increase in endothelial-mediated VEGF-R2 signalling, would result in a doubling of protein clearance purely by convective means, independently of GFR changes, reflection coefficient reductions (which cannot be ruled out in these experiments), or alterations in slit diaphragm permeability. Therefore these results suggest that changes in glomerular endothelial permeability to water could contribute significantly to proteinuria when VEGF concentrations are raised. Future investigations into the effects of VEGF on glomerular ultrastructure and signalling pathways should yield further insights into the contributions of VEGF and endothelial cells to the regulation of glomerular permeability.
In summary, we report refinement of a model that permits examination of the permeability characteristics of glomeruli from a wide variety of animals, including humans. LpA values obtained in the present study compare favourably with those observed using a variety of other in vivo techniques. Whilst a plethora of information supports the hypothesis that VEGF in the glomerulus promotes endothelial cell proliferation and maintenance (Schrijvers et al. 2004), and the increase in prevalence of proteinuria seen in patients treated with anti-VEGF monoclonal antibody (bevacizumab) for disseminated colorectal carcinoma (Kabbinavar et al. 2003), has already highlighted the possibility that interference with the VEGF axis may perturb glomerular selective-permeability characteristics, this is the first report to confirm the speculation that VEGF may regulate glomerular permeability to water (Schrijvers et al. 2004). Our observations that this effect is mediated via VEGF-R2, selectively expressed by glomerular endothelial cells, highlights important issues regarding contributions of the individual components of the glomerular filtration barrier to water permeability.
References
- Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol. 1990;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendshorst WJ, Gottschalk CW. Apparent filtration pressure disequilibrium and filtration coefficient in the rat kidney (abstract) Kidney Int. 1974;6:18A. [Google Scholar]
- Aukland K, Heyeraas Tonder K, Naess G. Capillary pressure in deep and superficial glomeruli of the rat kidney. Acta Physiol Scand. 1977;101:418–427. doi: 10.1111/j.1748-1716.1977.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Bailey E, Bottomley MJ, Westwell S, Pringle JH, Furness PN, Feehally J, Brenchley PE, Harper SJ. Vascular endothelial growth factor mRNA expression in minimal change, membranous, and diabetic nephropathy demonstrated by non-isotopic in situ hybridisation. J Clin Pathol. 1999;52:735–738. doi: 10.1136/jcp.52.10.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO. The chronic effect of vascular endothelial growth factor on individually perfused frog mesenteric microvessels. J Physiol. 1998;513:225–233. doi: 10.1111/j.1469-7793.1998.225by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol. 1996;271:H2520–H2528. doi: 10.1152/ajpheart.1996.271.6.H2520. [DOI] [PubMed] [Google Scholar]
- Bates D, Harper S. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2003;39:225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- Bortoloso E, Del Prete D, Dalla Vestra M, Gambaro G, Saller A, Antonucci F, Baggio B, Anglani F, Fioretto P. Quantitave and qualitative changes in vascular endothelial growth factor gene expression in glomeruli of patients with type 2 diabetes. Eur J Endocrinol. 2004;150:799–807. doi: 10.1530/eje.0.1500799. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971;50:1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Braet F, Brodsky S, Weinstein T, Romanov V, Noiri E, Goligorsky MS. VEGF-induced mobilization of caveolae and increase in permeability of endothelial cells. Am J Physiol Cell Physiol. 2002;282:C1053–C1063. doi: 10.1152/ajpcell.00292.2001. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Hjalmarsson C, Bokenkamp A, Schurek HJ, Haraldsson B. Dynamic alterations of glomerular charge density in fixed rat kidneys suggest involvement of endothelial cell coat. Am J Physiol Renal Physiol. 2003;285:F722–F730. doi: 10.1152/ajprenal.00227.2001. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Carbajal JM, Schaeffer RC., Jr VEGF stimulates tyrosine phosphorylation of beta-catenin and small-pore endothelial barrier dysfunction. Am J Physiol. 1999;277:H2038–H2049. doi: 10.1152/ajpheart.1999.277.5.H2038. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- Davis JM, Haberle DA, Kawata T, Schmitt E, Takabatake T, Wohlfeil S. Increased tubuloglomerular feed-back mediated suppression of glomerular filtration during acute volume expansion in rats. J Physiol. 1988;395:553–576. doi: 10.1113/jphysiol.1988.sp016934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen WM, Troy JL, Robertson CR, Brenner BM. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973;52:1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- Drumond MC, Deen WM. Structural determinants of glomerular hydraulic permeability. Am J Physiol. 1994;266:F1–F12. doi: 10.1152/ajprenal.1994.266.1.F1. [DOI] [PubMed] [Google Scholar]
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998a;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998b;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Foster RR, Hole R, Anderson K, Satchell SC, Coward RJ, Mathieson PW, Gillatt DA, Saleem MA, Bates DO, Harper SJ. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol. 2003;284:F1263–F1273. doi: 10.1152/ajprenal.00276.2002. [DOI] [PubMed] [Google Scholar]
- Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am J Physiol Renal Physiol. 2005;288:F48–F57. doi: 10.1152/ajprenal.00146.2004. [DOI] [PubMed] [Google Scholar]
- Fu BM, Shen S. Structural mechanisms of acute VEGF effect on microvessel permeability. Am J Physiol Heart Circ Physiol. 2003;284:H2124–H2135. doi: 10.1152/ajpheart.00894.2002. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Gertz KH, Mangos JA, Braun G, Pagel HD. Pressure in the glomerular capillaries of the rat kidney and its relation to arterial blood pressure. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;288:369–374. doi: 10.1007/BF00362581. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson C, Johansson BR, Haraldsson B. Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvasc Res. 2004;67:9–17. doi: 10.1016/j.mvr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Horita Y, Miyazaki M, Koji T, Kobayashi N, Shibuya M, Razzaque MS, Cheng M, Ozono Y, Kohno S, Taguchi T. Expression of vascular endothelial growth factor and its receptors in rats with protein-overload nephrosis. Nephrol Dial Transplant. 1998;13:2519–2528. doi: 10.1093/ndt/13.10.2519. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Feehally J. Comprehensive Clinical Nephrology. Edinburgh: Mosby; 2003. [Google Scholar]
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Kanellis J, Levidiotis V, Khong T, Cox AJ, Stacker SA, Gilbert RE, Cooper ME, Power DA. A study of VEGF and its receptors in two rat models of proteinuria. Nephron Physiol. 2004;96:P26–36. doi: 10.1159/000075577. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: a renal perspective. Kidney Int. 2005;67:2101–2113. doi: 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- Kitamoto Y, Takeya M, Tokunaga H, Tomita K. Glomerular endothelial cells are maintained by vascular endothelial growth factor in the adult kidney. Tohoku J Exp Med. 2001;195:43–54. doi: 10.1620/tjem.195.43. [DOI] [PubMed] [Google Scholar]
- Kitamoto Y, Tokunaga H, Tomita K. Vascular endothelial growth factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis. J Clin Invest. 1997;99:2351–2357. doi: 10.1172/JCI119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanke B, Simon M, Rockl W, Weich HA, Stolte H, Grone HJ. Effects of vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) on haemodynamics and permselectivity of the isolated perfused rat kidney. Nephrol Dial Transplant. 1998;13:875–885. doi: 10.1093/ndt/13.4.875. [DOI] [PubMed] [Google Scholar]
- Machin D, Campbell MJ. Statistical Tables for the Design of Clinical Trials. Oxford: Blackwell Scientific; 1987. [Google Scholar]
- Maddox DA, Deen WH, Brenner BM. Glomerular Filtration. In: Windhager EE, editor. Handbook of Physiology, Renal Physiology. New York: American Physiological Society (Oxford University Press); 1992. pp. 545–638. [Google Scholar]
- Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CC, Neal CR. Openings through endothelial cells associated with increased microvascular permeability. Microcirculation. 1999;6:45–54. [PubMed] [Google Scholar]
- Neal CR, Crook H, Bell E, Harper SJ, Bates DO. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol. 2005;16:1223–1235. doi: 10.1681/ASN.2004100822. [DOI] [PubMed] [Google Scholar]
- Ostendorf T, Kunter U, Eitner F, Loos A, Regele H, Kerjaschki D, Henninger DD, Janjic N, Floege J. VEGF (165) mediates glomerular endothelial repair. J Clin Invest. 1999;104:913–923. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott CE, Marchand GR, Diaz-Buxo JA, Knox FG. Determinants of glomerular filtration rate in the dog. Am J Physiol. 1976;231:235–239. doi: 10.1152/ajplegacy.1976.231.1.235. [DOI] [PubMed] [Google Scholar]
- Pinnick RV, Savin VJ. Filtration by superficial and deep glomeruli of normovolemic and volume-depleted rats. Am J Physiol. 1986;250:F86–F91. doi: 10.1152/ajprenal.1986.250.1.F86. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- Savin VJ. In vitro effects of angiotensin II on glomerular function. Am J Physiol. 1986;251:F627–F634. doi: 10.1152/ajprenal.1986.251.4.F627. [DOI] [PubMed] [Google Scholar]
- Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol. 1992;3:1260–1269. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- Savin VJ, Terreros DA. Filtration in single isolated mammalian glomeruli. Kidney Int. 1981;20:188–197. doi: 10.1038/ki.1981.121. [DOI] [PubMed] [Google Scholar]
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Simon M, Grone HJ, Johren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- Soker S, Svahn CM, Neufeld G. Vascular endothelial growth factor is inactivated by binding to alpha 2-macroglobulin and the binding is inhibited by heparin. J Biol Chem. 1993;268:7685–7691. [PubMed] [Google Scholar]
- Thomas S, Vanuystel J, Gruden G, Rodriguez V, Burt D, Gnudi L, Hartley B, Viberti G. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol. 2000;11:1236–1243. doi: 10.1681/ASN.V1171236. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nagase S, Koyama A. Stimulatory effect of IGF I and VEGF on eNOS message, protein expression, eNOS phosphorylation and nitric oxide production in rat glomeruli, and the involvement of PI3 K signaling pathway. Nitric Oxide. 2004;10:25–35. doi: 10.1016/j.niox.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Whittles CE, Pocock TM, Wedge SR, Kendrew J, Hennequin LF, Harper SJ, Bates DO. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002;9:513–522. doi: 10.1038/sj.mn.7800164. [DOI] [PubMed] [Google Scholar]
- Wiegmann TB, MacDougall ML, Savin VJ. Glomerular effects of angiotensin II require intrarenal factors. Am J Physiol. 1990;258:F717–F721. doi: 10.1152/ajprenal.1990.258.3.F717. [DOI] [PubMed] [Google Scholar]
- Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–H2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]