Abstract
Calcium (Ca2+) signals affect virtually every biological process, including both contraction and gene transcription in smooth muscle. Ca2+-regulated gene transcription is known to be important for both physiological and pathological responses in smooth muscle. The aim of this review is to discuss the current understanding of gene transcription regulated by excitation through Ca2+ signalling using a comparison of the two most characterized Ca2+-regulated transcription factors in smooth muscle, Ca2+–cyclic AMP response element binding protein (CREB) and nuclear factor of activated T-cells (NFAT). Recent studies have shown commonalities and differences in the regulation of CREB and NFAT through both voltage- and non-voltage-gated Ca2+ channels that lead to expression of smooth muscle cell specific differentiation markers as well as markers of proliferation. New insights into the regulation of specific genes through companion elements on the promoters of Ca2+-regulated genes have led to new models for transcriptional regulation by Ca2+ that are defined both by the source and duration of the Ca2+ signal and the composition of enhancer elements found within the regulatory regions of specific genes. Thus the combination of signalling pathways elicited by particular Ca2+ signals affect selective promoter elements that are key to the ultimate pattern of gene transcription.
Excitation–contraction coupling in smooth muscle cells (SMCs) is tightly controlled by spatio-temporal Ca2+ events that involve both influx of extracellular Ca2+ and release from selective intracellular Ca2+ stores (reviewed in Bolton et al. 2004). Recent findings have revealed that the Ca2+-regulated transcription factors CREB and NFAT have selective Ca2+ source requirements and are influenced by transcriptional coactivators and cofactors for CREB and NFAT. Excitation–transcription coupling is thus clearly relevant to both normal physiological responses and to the pathogenesis of vascular diseases that are known to include altered Ca2+ handling and changes in gene expression.
Ca2+ regulation of CREB and gene transcription in smooth muscle
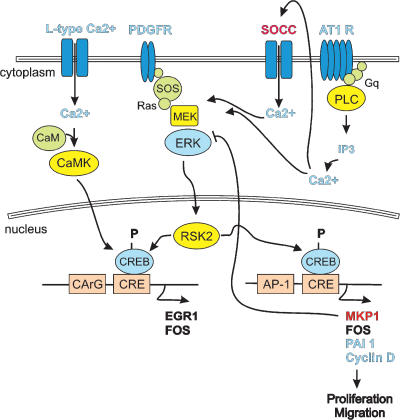
CREB (Ca2+–cyclic AMP response element binding protein) regulates transcription through recognition and binding to Ca2+–cyclic AMP (cAMP)-response elements (CREs) in the promoter of many genes (Shaywitz & Greenberg, 1999). Stimulus-induced CREB activation requires phosphorylation of 133serine to promote recruitment of CREB binding protein (CBP300) and other cofactors to form an active transcriptional complex (Gonzalez et al. 1989; Mayr & Montminy, 2001). Ca2+ from many sources can activate CREB-induced transcription through the Ca2+ calmodulin-dependent protein kinase (CaMK), growth factor/MAPK, and cAMP-dependent protein kinase pathways (Shaywitz & Greenberg, 1999) (Fig. 1). Inactivation of CREB through phosphatase activity can also be regulated by Ca2+ through Ca2+–CaM activation of the protein phosphatase calcineurin which indirectly leads to dephosphorylation of CREB (Alberts et al. 1994).
Figure 1. Regulation of CREB activation through multiple signalling cascades in SMCs.
Store-operated Ca2+ channel (SOCC), phospholipase C (PLC), son of sevenless (SOS), platelet-derived growth receptor (PDGFR), GTPase binding protein q (Gq), extracellular-signal regulated kinase (ERK), MAPK/ERK kinase (MEK), receptor signal kinase (RSK), angiotensin II type 1 receptor (AT1R), serum response element (CArG), plasminogen activator 1 inhibitor (PAI1).
CREB activation elicited by membrane depolarization and Ca2+ entry through voltage-dependent calcium channels (VDCCs) has been confirmed in both cultured SMCs and intact cerebral arteries (Cartin et al. 2000; Stevenson et al. 2001a). Depolarization-mediated CREB phosphorylation correlates with an increase in transcription of the CRE-containing immediate early gene, c-fos, and is sensitive to inhibitors of VDCCs.
Although CREB is predominantly nuclear, it has been shown to accumulate in the cytoplasm of vascular SMCs when nuclear import is blocked prior to membrane depolarization (Stevenson et al. 2001a). While the relevance of nuclear shuttling of CREB has not been established, it could explain CREB interactions with multiple kinases that do not cross the nuclear membrane and the perceived loss of CREB from the nucleus following ischaemia (Klemm et al. 2001).
A role for SR Ca2+ in signalling to CREB in SMCs has been demonstrated both in response to Ca2+ release from inositol 1,4,5-trisphosphate (IP3) receptors and through store-operated Ca2+ entry. Endothelin-1, platelet-derived growth factor (PDGF), sphingosine-1-phosphate, aldosterone, low-density lipoprotein (LDL) and ischaemia have all been shown to induce CREB phosphorylation in SMCs through an IP3-dependent mechanism (Christ et al. 1999; Stevenson et al. 2001a; Coussin et al. 2003; Egan & Nixon, 2004; Rius et al. 2004; Meller et al. 2005). The majority of these pathways are also influenced by MAPK signalling, and a link between Ca2+ and MAPK signalling to CREB has been observed for noradrenaline (norepinephrine)-induced CREB signalling (Hu et al. 1999). Store-operated Ca2+ entry (SOCE), in response to SR Ca2+ depletion, has also been shown to stimulate CREB phosphorylation in both cultured and intact vascular SMCs (Fig. 2), and a role for SOCE has been reported in CREB activation stimulated by angiotensin II (Pulver et al. 2004). Determinations of the relevance of SOCE activation by other IP3 mediators and its importance in MAPK signalling are important targets of future investigations related to CREB signalling.
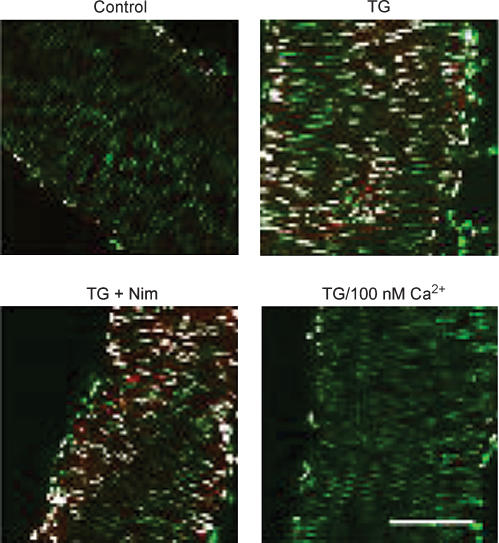
Figure 2. CREB is activated by SOCE in intact vascular smooth muscle.
From Pulver et al. (2004), used with permission. Rat cerebral arteries were isolated and incubated in HEPES-buffered saline (HBS) with normal Ca2+ (2 mmol l−1), 100 nmol l−1 Ca2+, or 100 nmol l−1 nimodipine (Nim) for 15 min. Arteries were then exposed to 100 nmol l−1 thapsigargin (TG) for 15 min or 60 mm K+ for 10 min. CREB phosphorylation was detected by anti-P-CREB immunofluorescence. Shown are confocal images representing P-CREB (red), YOYO-1 DNA nuclear stain (green) and overlap of P-CREB and YOYO-1 DNA (white). Bar represents 100 μm.
Not all Ca2+ signals lead to activation of CREB. Release of Ca2+ from ryanodine receptors (RyR) in the form of Ca2+ sparks has been shown to have an inhibitory effect on CREB activation, probably through membrane hyperpolarization (Cartin et al. 2000). These results stress the importance that the nature of the Ca2+ signal has on the downstream changes in gene transcription mediated by CREB.
Ca2+ regulation of NFAT in smooth muscle
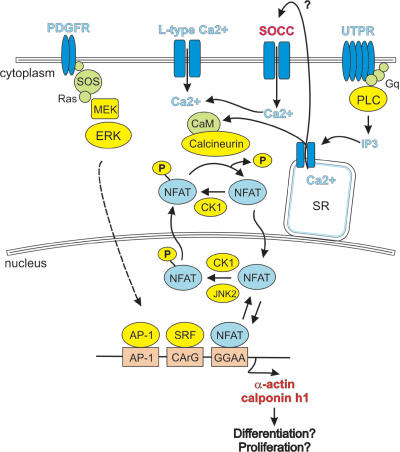
Nuclear factor of activated T cells (NFAT) has recently been shown to play an important role in regulating Ca2+-dependent gene transcription in SMCs. The activation of NFAT is regulated through its subcellular localization, which reflects the intensity of Ca2+–calcineurin signalling and the activities of several nuclear protein kinases (Fig. 3) (Hogan et al. 2003). Upon elevation of intracellular Ca2+ levels, the Ca2+–CaM-dependent phosphatase, calcineurin, dephosphorylates NFAT, allowing for the translocation of the NFAT–calcineurin complex into the nucleus. Because of its dependence on Ca2+–calcineurin signalling, NFAT has the ability to sense dynamic changes in [Ca2+]i and frequencies of Ca2+ oscillations (Dolmetsch et al. 1997; Li et al. 1998).
Figure 3. Regulation of NFAT via nuclear translocation in SMCs.
NFAT translocation and transcriptional activity has been clearly demonstrated in both cultured and native SMCs (Boss et al. 1998; Stevenson et al. 2001b). In cerebral SMCs, the vasoconstrictor UTP, and other Gq/11-coupled receptor agonists induce NFAT4 nuclear accumulation. This induction is dependent on release of Ca2+ from intracellular stores and requires function of VDCCs (Stevenson et al. 2001b; Gomez et al. 2002). However, counter to expectations, a sustained increase in [Ca2+]i induced by membrane depolarization is not sufficient to achieve NFAT4 nuclear accumulation in vascular SMCs (Stevenson et al. 2001b).
One possibility for this finding is that NFAT nuclear accumulation is further regulated by serine/threonine protein kinases, which promote the nuclear export of NFAT. Recent work examining the role of c-Jun amino-terminal kinase (JNK) using JNK knockout animals suggest that although elevated Ca2+ levels are sufficient to promote NFAT nuclear import, suppression of NFAT nuclear export activity is also required. Specifically, nuclear JNK2 has been shown to selectively promote the nuclear export of NFAT4 in both transfection studies (Chow et al. 1997) and native isolated cerebral arteries (Gomez et al. 2003). These data suggest that although Ca2+ elevation is necessary, it may not be the rate-limiting step in NFAT nuclear accumulation in SMCs.
In intact arteries, NFAT translocates to the nucleus in response to physiological intraluminal pressure. Translocation is dependent on Ca2+ influx through VDCCs, requires the nitric oxide/protein kinase G pathway, and correlates with an inhibition of JNK-dependent nuclear export (Gonzalez Bosc et al. 2004). These results implicate a potential role for endothelial-derived nitric oxide in the regulation of NFAT activity and suggest that physiological pressure changes are sufficient to induce transcriptional activity through NFAT.
Ca2+ and smooth muscle-specific gene expression
Many vascular disease states are characterized by changes in gene expression that inhibit differentiation and promote the proliferative phenotype. The serum response factor (SRF) DNA binding site or CArG box (CC[A/T]6GG) plays an important role in regulating SMC-specific genes (Kumar & Owens, 2003). Evidence is now emerging that suggests a complex interaction between Ca2+ signalling, regulatory elements and cofactors that affect SRF binding and/or transcriptional activity. Wamhoff et al. (2004) found that Ca2+ influx through VDCCs stimulates expression of SRF-dependent SMC differentiation markers through a mechanism requiring Rho kinase and the SRF coactivator myocardin. In addition, Ca2+-dependent CRE elements have been found adjacent to CArG elements in several SRF-regulated SMC genes (Sekiguchi et al. 2001; Najwer & Lilly, 2005). Furthermore, Ca2+-dependent NFAT activation has been shown to cooperatively regulate the activity of an intronic CArG enhancer of smooth muscle α-actin (Gonzalez Bosc et al. 2005). Thus, co-activators as well as CREB and NFAT have the capacity to direct expression of SRF-regulated genes in response to Ca2+ signalling in SMCs.
The paradox that remains is the existence of CArG elements in genes that promote de-differentiation as well as differentiation. What mechanisms underlie this distinction? New evidence supports the hypothesis that growth and developmental signals modulate SRF-dependent gene expression by regulating repressive cofactors that compete for SRF binding (Wamhoff et al. 2004; Wang et al. 2004). In light of the complexities regulating SRF function, future experiments are warranted to explore these and other potential requirements for SRF-dependent progression to distinct SMC phenotypes.
CREB and NFAT in smooth muscle pathologies
The pathogenesis of vascular diseases such as hypertension includes altered Ca2+ handling that triggers changes in gene expression, and these changes are probably attributed to the ability of mature smooth muscle cells to de-differentiate and proliferate (Somlyo & Somlyo, 1994). Although proliferation is important in recovering from vascular injury, arterial intervention procedures such as angioplasty result in abnormal proliferation and restenosis.
Cerebral arteries from hypertensive animals exhibit elevated SMC Ca2+, phospho-CREB and c-fos mRNA (Wellman et al. 2001). Interestingly, these effects are readily reversed by in situ inhibition of VDCCs, suggesting a defect in the ‘off’ mechanism of CREB activation. These findings provide an important link between altered gene regulation through CREB and de-regulation of Ca2+ signalling in a disease state. In pulmonary artery SMCs CREB is necessary and sufficient for induction of transient receptor potential cation channel 4 (TRPC4), that has been linked to the development of pulmonary hypertension (Landsberg & Yuan, 2004; Zhang et al. 2004).
Increased levels of phospho-CREB have also been correlated with the proliferative response associated with arteriolar injury including angiotensin II-induced hypertension, chronic nicotine administration and oxidative endothelial injury (Gerzanich et al. 2003). In addition, expression of dominant negative CREB constructs suppresses neointimal formation and increases apoptosis following balloon injury (Tokunou et al. 2003). Transient ischaemia also leads to CREB phosphorylation and increased CRE-mediated Bcl-2 expression (Meller et al. 2005). Together, these results suggest a role for CREB in both survival and proliferation of SMCs following injury. The role of CREB in the proliferative response to disease is clearly complex, however, in that SMC proliferation induced by in vivo hypoxia correlates with a reduction in CREB content (Klemm et al. 2001).
Although NFAT has also been shown to regulate genes related to cell cycle progression and cell differentiation in T cells and neurones, the functions of NFAT in SMCs are largely unknown. In aortic SMCs, NFAT2 nuclear translocation has been correlated with differentiation and found to be important for driving transcription of the smooth muscle-specific Sm-myosin heavy chain (MHC) promoter in a Ca2+- and calcineurin-dependent manner (Wada et al. 2002). However, NFAT also plays a role in vascular SMC (VSMC) proliferation and motility induced by both receptor tyrosine kinase (RTK) and G protein-coupled receptor agonists (GPCR), PDGF and thrombin, respectively (Yellaturu et al. 2002; Liu et al. 2004). Taken together these findings reveal that NFAT, like CREB, is a likely candidate for mediating both differentiation and mitogenic effects in SMCs.
Few studies have directly compared NFAT and CREB signalling in SMCs. In a model of VLDL-induced SMC proliferation, NFAT activation correlates with a decrease in phospho-CREB, suggesting that the proliferative effect of VLDL increases NFAT activity while reducing CREB activity (Lipskaia et al. 2003). One caveat to these experiments is that in this model CREB is phosphorylated under basal conditions, an observation that has not been detected in other SMC culture cell systems or in intact arteries (Cartin et al. 2000; Pulver et al. 2004; Meller et al. 2005).
Overall, the evidence suggests that physiological or pathological alterations in Ca2+ signalling pathways are likely to have effects on both CREB and NFAT function and have the potential to disrupt normal patterns of gene transcription in SMCs. Future studies focused on CREB- and/or NFAT-dependent transcription patterns hold the promise of better understanding the role of excitation–transcription coupling as it relates to genomic effects on smooth muscle phenotype.
References
- Alberts AS, Montminy M, Shenolikar S, Feramisco JR. Expression of a peptide inhibitor of protein phosphatase 1 increases phosphorylation and activity of CREB in NIH 3T3 fibroblasts. Mol Cell Biol. 1994;14:4398–4407. doi: 10.1128/mcb.14.7.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Gordienko DV, Povstyan OV, Harhun MI, Pucovsky V. Smooth muscle cells and interstitial cells of blood vessels. Cell Calcium. 2004;35:643–657. doi: 10.1016/j.ceca.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Boss V, Abbott KL, Wang XF, Pavlath GK, Murphy TJ. The cyclosporin A-sensitive nuclear factor of activated T cells (NFAT) proteins are expressed in vascular smooth muscle cells. Differential localization of NFAT isoforms and induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors. J Biol Chem. 1998;273:19664–19671. doi: 10.1074/jbc.273.31.19664. [DOI] [PubMed] [Google Scholar]
- Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca2+ to CREB activation and gene expression in intact cerebral arteries from mouse: roles of ryanodine receptors and voltage-dependent Ca2+ channels. Circ Res. 2000;86:760–767. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- Christ M, Gunther A, Heck M, Schmidt BMW, Falkenstein E, Wehling M. Aldosterone, not estradiol, is the physiological agonist for rapid increases in camp in vascular smooth muscle cells. Circulation. 1999;99:1485–1491. doi: 10.1161/01.cir.99.11.1485. [DOI] [PubMed] [Google Scholar]
- Coussin F, Scott RH, Nixon GF. Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels. Biochem Pharmacol. 2003;66:1861–1870. doi: 10.1016/s0006-2952(03)00546-x. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Egan CG, Nixon GF. Endothelin-1- and depolarization-induced differential regulation of cAMP response element-binding protein in proliferating and developed vascular smooth muscle. Cellular Signalling. 2004;16:1387–1396. doi: 10.1016/j.cellsig.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Ivanova S, Simard JM. Early pathophysiological changes in cerebral vessels predisposing to stroke. Clin Hemorheol Microcirc. 2003;29:291–294. [PubMed] [Google Scholar]
- Gomez MF, Bosc LV, Stevenson AS, Wilkerson MK, Hill-Eubanks DC, Nelson MT. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: role of JNK2 and Crm-1. J Biol Chem. 2003;278:46847–46853. doi: 10.1074/jbc.M304765200. [DOI] [PubMed] [Google Scholar]
- Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem. 2002;277:37756–37764. doi: 10.1074/jbc.M203596200. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Yamamoto KK, Fischer WH, Karr D, Menzel P, Biggs W, Vale WW, Montminy MR. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989;337:749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez Bosc LV, Layne JJ, Nelson MT, Hill-Eubanks DC. Nuclear factor of activated T cells and serum response factor cooperatively regulate the activity of an alpha-actin intronic enhancer. J Biol Chem. 2005;280:26113–26120. doi: 10.1074/jbc.M411972200. [DOI] [PubMed] [Google Scholar]
- Gonzalez Bosc LV, Wilkerson MK, Bradley KN, Eckman DM, Hill-Eubanks DC, Nelson MT. Intraluminal pressure is a stimulus for NFATc3 nuclear accumulation: role of calcium, endothelium-derived nitric oxide, and cGMP-dependent protein kinase. J Biol Chem. 2004;279:10702–10709. doi: 10.1074/jbc.M312920200. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Hu Z-W, Shi X-Y, Lin RZ, Chen J, Hoffman BB. α1-Adrenergic receptor stimulation of mitogenesis in human vascular smooth muscle cells: role of tyrosine protein kinases and calcium in activation of mitogen-activated protein kinase. J Pharmacol Exp Ther. 1999;290:28–37. [PubMed] [Google Scholar]
- Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE-B. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Owens GK. Combinatorial control of smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2003;23:737–747. doi: 10.1161/01.ATV.0000065197.07635.BA. [DOI] [PubMed] [Google Scholar]
- Landsberg JW, Yuan JX-J. Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci. 2004;19:44–50. doi: 10.1152/nips.01457.2003. [DOI] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Pourci M-L, Delomenie C, Combettes L, Goudouneche D, Paul J-L, Capiod T, Lompre A-M. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res. 2003;92:1115–1122. doi: 10.1161/01.RES.0000074880.25540.D0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Dronadula N, Rao GN. A novel role for nuclear factor of activated T cells in receptor tyrosine kinase and G protein-coupled receptor agonist-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:41218–41226. doi: 10.1074/jbc.M406917200. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan J-Q, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Najwer I, Lilly BJ. Calcium/calmodulin-dependent protein kinase IV activates cysteine-rich protein 1 through adjacent CRE and CArG elements. Am J Physiol Cell Physiol. 2005;289:C785–93. doi: 10.1152/ajpcell.00098.2005. [DOI] [PubMed] [Google Scholar]
- Pulver RA, Rose-Curtis P, Roe MW, Wellman GC, Lounsbury KM. Store-operated Ca2+ entry activates the CREB transcription factor in vascular smooth muscle. Circ Res. 2004;94:1351–1358. doi: 10.1161/01.RES.0000127618.34500.FD. [DOI] [PubMed] [Google Scholar]
- Rius J, Martinez-Gonzalez J, Crespo J, Badimon L. Involvement of neuron-derived orphan receptor-1 (NOR-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: role of CREB. Arterioscler Thromb Vasc Biol. 2004;24:697–702. doi: 10.1161/01.ATV.0000121570.00515.dc. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K, Kurabayashi M, Oyama Y, Aihara Y, Tanaka T, Sakamoto H, Hoshino Y, Kanda T, Yokoyama T, Shimomura Y, Iijima H, Ohyama Y, Nagai R. Homeobox protein Hex induces SMemb/nonmuscle myosin heavy chain-B gene expression through the cAMP-responsive element. Circ Res. 2001;88:52–58. doi: 10.1161/01.res.88.1.52. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Smooth muscle: excitation-contraction coupling, contractile regulation, and the cross-bridge cycle. Alcohol Clin Exp Res. 1994;18:138–143. doi: 10.1111/j.1530-0277.1994.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Stevenson AS, Cartin L, Wellman TL, Dick MH, Nelson MT, Lounsbury KM. Membrane depolarization mediates phosphorylation and nuclear translocation of CREB in vascular smooth muscle cells. Exp Cell Res. 2001a;263:118–130. doi: 10.1006/excr.2000.5107. [DOI] [PubMed] [Google Scholar]
- Stevenson AS, Gomez MF, Hill-Eubanks DC, Nelson MT. NFAT4 movement in native smooth muscle. A role for differential Ca2+ signaling. J Biol Chem. 2001b;276:15018–15024. doi: 10.1074/jbc.M011684200. [DOI] [PubMed] [Google Scholar]
- Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, Fukuyama K, Ono H, Iino N, Masuda S, Shimokawa H, Egashira K, Imaizumi T, Takeshita A. Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation. 2003;108:1246–1252. doi: 10.1161/01.CIR.0000085164.13439.89. [DOI] [PubMed] [Google Scholar]
- Wada H, Hasegawa K, Morimoto T, Kakita T, Yanazume T, Abe M, Sasayama S. Calcineurin-GATA-6 pathway is involved in smooth muscle-specific transcription. J Cell Biol. 2002;156:983–991. doi: 10.1083/jcb.200106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang D-Z, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Cartin L, Eckman DM, Stevenson AS, Saundry CM, Lederer WJ, Nelson MT. Membrane depolarization, elevated Ca2+ entry, and gene expression in cerebral arteries of hypertensive rats. Am J Physiol Heart Circ Physiol. 2001;281:H2559–H2567. doi: 10.1152/ajpheart.2001.281.6.H2559. [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Ghosh SK, Rao RK, Jennings LK, Hassid A, Rao GN. A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation. Biochem J. 2002;368:183–190. doi: 10.1042/BJ20020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Remillard CV, Fantozzi I, Yuan JX-J. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C1192–C1201. doi: 10.1152/ajpcell.00158.2004. [DOI] [PubMed] [Google Scholar]