Abstract
Alkalosis enhances human exercise performance, and reduces K+ loss in contracting rat muscle. We investigated alkalosis effects on K+ regulation, ionic regulation and fatigue during intense exercise in nine untrained volunteers. Concentric finger flexions were conducted at 75% peak work rate (∼3 W) until fatigue, under alkalosis (Alk, NaHCO3, 0.3 g kg−1) and control (Con, CaCO3) conditions, 1 month apart in a randomised, double-blind, crossover design. Deep antecubital venous (v) and radial arterial (a) blood was drawn at rest, during exercise and recovery, to determine arterio-venous differences for electrolytes, fluid shifts, acid–base and gas exchange. Finger flexion exercise barely perturbed arterial plasma ions and acid–base status, but induced marked arterio-venous changes. Alk elevated [HCO3−] and PCO2, and lowered [H+] (P < 0.05). Time to fatigue increased substantially during Alk (25 ± 8%, P < 0.05), whilst both [K+]a and [K+]v were reduced (P < 0.01) and [K+]a-v during exercise tended to be greater (P= 0.056, n = 8). Muscle K+ efflux at fatigue was greater in Alk (21.2 ± 7.6 µmol min−1, 32 ± 7%, P < 0.05, n = 6), but peak K+ uptake rate was elevated during recovery (15 ± 7%, P < 0.05) suggesting increased muscle Na+,K+-ATPase activity. Alk induced greater [Na+]a, [Cl−]v, muscle Cl− influx and muscle lactate concentration ([Lac−]) efflux during exercise and recovery (P < 0.05). The lower circulating [K+] and greater muscle K+ uptake, Na+ delivery and Cl− uptake with Alk, are all consistent with preservation of membrane excitability during exercise. This suggests that lesser exercise-induced membrane depolarization may be an important mechanism underlying enhanced exercise performance with Alk. Thus Alk was associated with improved regulation of K+, Na+, Cl− and Lac−.
Marked ionic disturbances occur within contracting skeletal muscle cells and the surrounding interstitium, and these most probably contribute to the complex, multifactorial phenomenon known as muscle fatigue. Muscle excitation elicits cellular potassium (K+) efflux and sodium (Na+) influx (for review, see Sejersted & Sjøgaard, 2000) and to counter these ion fluxes, a rapid and dramatic activation of the Na+,K+-ATPase enzyme (Clausen, 2003). However, the maximal Na+,K+-ATPase activity in muscle is acutely depressed during fatiguing exercise (Fraser et al. 2002; Leppik et al. 2004) and during intense muscle contractions is insufficient to match excitation-induced Na+–K+ fluxes (Sejersted & Sjøgaard, 2000; Clausen, 2003). These result in at least a doubling of muscle extracellular [K+] (Juel et al. 2000; Nielsen et al. 2004; Street et al. 2005) and of intracellular [Na+] (Sjøgaard et al. 1985; Juel, 1986; Balog & Fitts, 1996), and up to a 20% decline in intracellular [K+] (Sjøgaard et al. 1985; Lindinger et al. 1990). These changes may impair cell membrane excitability and thereby contribute to muscle fatigue (Fitts, 1994; Sejersted & Sjøgaard, 2000). Furthermore, muscle K+ efflux during intense muscle contractions is considerable, with arterial and venous potassium concentrations ([K+]) increasing to as much as 7 and 8 mm, respectively (Sejersted & Sjøgaard, 2000).
Whilst intense muscle contractions increase intracellular lactate ([Lac−]) and hydrogen ion concentrations ([H+]) (Fitts, 1994), these do not appear to be major factors contributing to muscle fatigue. Elevated lactate per se does not impair muscle force (Posterino et al. 2001) and intracellular acidosis may even exert a protective effect on muscle function, via higher muscle chloride (Cl−) conductance (Nielsen et al. 2001; Pedersen et al. 2004, 2005). Furthermore, maintenance of normal extracellular [Na+] and [Cl−] each counter the effects of elevated extracellular [K+] on membrane potential and fatigue and thereby also confer protective effects on skeletal muscle function (Cairns et al. 2004). Maintenance of muscle extracellular [Na+] is vital since a decline in extracellular [Na+] exacerbates the K+-induced decline in force (Renaud et al. 1996).
In apparent contrast to recent studies in isolated rat muscles, which indicate that acidosis may benefit muscle performance (Nielsen et al. 2001; Pedersen et al. 2004, 2005), metabolic alkalosis in humans typically enhances whole-body exercise performance, including during short-term intense (Sutton et al. 1981; Verbitsky et al. 1997) and endurance exercise (Potteiger et al. 1996). The suggested beneficial effects of alkalosis include an increase in extracellular proton buffer capacity (Costill et al. 1984; Bouissou et al. 1988; Kesl & Engen, 1998; Lindinger et al. 1999; Street et al. 2005), increased muscle phosphorylase, phosphofructokinase and pyruvate dehydrogenase activities (Hollidge-Horvat et al. 2000) and enhanced muscle Lac− and H+ release (Linderman & Fahey, 1991). Far less is known about possible effects of alkalosis on muscle K+, Na+ and Cl− fluxes, their regulation in blood, and whether improved regulation of these ions exerts any beneficial effects on human muscle performance.
Both respiratory and metabolic alkalosis increased muscle Lac− efflux, decreased K+ efflux, and reduced Na+, Cl− and water influx during stimulation of isolated rat hind limb muscle, but force output was unchanged (Lindinger et al. 1990). In resting humans, metabolic alkalosis decreased arterial and venous [K+], and increased the arterial plasma strong ion difference ([SID]); however, exercise effects with this lowered extracellular [K+] were not determined (Lindinger et al. 1999). The effects of alkalosis on venous [K+] ([K+]v) during exercise are unclear, with reports of no change during knee extension or cycling exercise (Stephens et al. 2002; Street et al. 2005), but a reduction in [K+]v during wrist flexion exercise (Raymer et al. 2004). Interestingly, the decreased [K+]v occurred together with improved exercise performance (Raymer et al. 2004). However, since none of arterial [K+], arterio-venous [K+] differences, muscle K+ efflux, or [K+] recovery data were reported in these studies, it is not possible to determine the overall effects of alkalosis on K+ regulation, or whether these changes were important in enhanced muscle performance. Recently, alkalosis was found to lower muscle interstitial [K+] during knee extensor contractions, but [K+]v was unchanged and muscle performance was not significantly improved (Street et al. 2005). Therefore studies investigating K+ regulation across contracting muscle and in plasma are required to determine the effects of alkalosis on K+ regulation and explore the role of improved K+ homeostasis in enhanced exercise performance in humans. No previous studies have investigated the effects of alkalosis on Na+ or Cl− exchange across muscle during fatiguing exercise in humans. The importance of such an investigation is evident since Na+ and Cl− can each affect muscle function (Renaud et al. 1996; Cairns et al. 2004) and can also modulate muscle [H+] via the [SID] (Lindinger et al. 1990). Investigation of Na+ or Cl− regulation in plasma and their exchange across muscle are clearly required to understand alkalosis effects on human muscle function.
The contracting muscle mass may be important in determining alkalosis effects on muscle function. During whole-body exercise, the arterio-venous [K+] difference ([K+]a-v) across contracting muscle typically declines to zero before the end of exercise (Vøllestad et al. 1994; Wasserman et al. 1997; Putman et al. 2003), whereas during knee extensions, the [K+]a-v remained negative (Rolett et al. 1990; Bangsbo et al. 1992, 1996). These differences may reflect a larger reduction in muscle K+ during smaller muscle mass exercise (Sejersted & Sjøgaard, 2000). Consequently, the effects of alkalosis on muscle K+ efflux, plasma K+, recovery K+ uptake and exercise performance may be more readily determined during exercise with a smaller muscle mass. Hence the effects of alkalosis on regulation of K+ and other strong ions were investigated during and following exercise performed by a small muscle group, the forearm finger flexors, which also allowed measurement of arterio-venous ion differences.
Finally, although numerous studies have investigated metabolic and cardiovascular responses to isometric muscle contractions of the forearm (Van Beekvelt et al. 2001; Binzoni et al. 2002; Hamann et al. 2004) very little is known about the effects of dynamic, concentric exercise in small muscle mass exercise. It is known that very high blood flows occur during small muscle mass exercise, with the potential for greater ion exchange across muscle. We therefore also determined the influence of both exercise and alkalosis on ionic, acid–base, metabolic and cardiovascular responses to finger flexor muscle contractions. This utilized a novel custom-designed ergometer which enabled dynamic, concentric contractions of the forearm finger flexor muscles.
This study investigated three hypotheses. First, that sodium bicarbonate-induced alkalosis would delay fatigue during intense, dynamic, concentric finger flexion exercise in humans. Second, that alkalosis would enhance muscle K+ handling, as measured by reductions during finger flexion exercise in each of arterial [K+], venous [K+] and the arterio-venous [K+] difference, as well as muscle K+ efflux at fatigue. Third, that alkalosis would elevate plasma [Cl−] and [Na+] and augment muscle Cl− and Na+ uptake during exercise.
Methods
Subjects
Nine healthy untrained volunteers, comprising five males and four females, gave written, informed consent and participated in the study. Ethical clearance was obtained from the Victoria University of Technology Human Research Ethics Committee, and conforms to the Declaration of Helsinki. Physical characteristics of the subjects were (mean ±s.d.): age, 22.7 ± 1.4 years; body mass, 70.6 ± 2.3 kg; and height, 172.8 ± 3.4 cm. In the 24 h prior to each visit, subjects refrained from vigorous activity and ingestion of caffeine and alcohol.
Overview of test procedures
Subjects attended the laboratory on five separate occasions. During their initial laboratory visit, anthropometric measurements of forearm length and circumferences were made in triplicate (coefficient of variation (CV) range, 1.5–4.4%) to determine forearm volume. Total forearm muscle mass was estimated at 414 ± 35g based on published MRI models (Jahn et al. 1999). Subjects were familiarized with finger flexion contractions, and was followed by an incremental finger flexion test. Incremental rhythmic finger flexion contractions were performed at a rate of 30 min−1, commencing at 2.64 ± 0.22 W. Resistance and thus power output were increased at the end of each minute, with power increased by an average of ∼0.17 W each minute. Contractions continued until volitional fatigue, to allow determination of their peak work rate (WRpeak). This allowed calculation of work rates at an intensity of 75% WRpeak, used in all subsequent finger flexion trials. Following a 30 min rest, subjects were familiarized with this experimental protocol.
To classify their general training status, subjects then underwent an incremental cycling test on an electrically braked ergometer (Excalibur Lode, Groningen, the Netherlands) to determine peak oxygen uptake (V̇O2peak). All methods, equipment and procedures for the V̇O2peak test were as previously described (Li et al. 2002). On all subsequent laboratory visits, subjects performed a finger flexion trial at 75% WRpeak continued to fatigue. The second and third pre-experimental trials determined intra-subject variability in finger flexion power output and time to fatigue. During two final visits, subjects performed finger flexion exercise to fatigue trials under randomised, cross-over, counterbalanced double-blind conditions of both alkalosis (Alk) and control (Con). These were conducted 1 month apart, and included arterial and venous blood sampling and measurements of forearm blood flow.
Finger flexion exercise tests
Finger flexion dynamometer
Subjects performed isotonic finger flexor contractions between pre-defined extension and flexion limits at a rate of 30 contractions min−1 on a custom-made finger flexion ergometer (Fig. 1). The unique ergometer design allowed concentric muscle contractions with negligible eccentric load, to minimize any eccentric-induced effects on muscle, including soreness or damage. The forearm of the non-dominant arm was used, with the principal muscles involved being the flexor digitorum profundus and flexor digitorum superficialis.
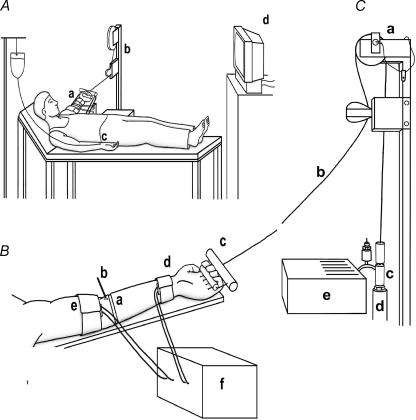
Figure 1. Detail of finger flexion ergometer and subject experimental set-up.
A, detail of subject position during finger flexion exercise. a, subjects lying supine with non-dominant exercising forearm elevated at an angle of ∼130 deg from the shoulder; b, finger flexion exercise ergometer; c, radial artery cannula; d, continuous real-time visual display of contraction rate and finger displacement on an elevated computer monitor. B, detail of forearm. a, extensometer strain gauge; b, antecubital venous cannula (retrograde); c, handgrip secured to fingers and connected to a multi-strand stainless steel cable; d, high pressure arterial occluding cuff; e, low pressure venous occluding cuff; f, plethysmograph. C, detail of finger flexion dynamometer. a, precision potentiometer fitted to pulley; b, multi-strand stainless steel cable; c, external plumbing; d, pneumatic piston; e, ergometer electronic controls.
Mode of operation
Subjects lay supine on a couch with the arm extended and placed perpendicular to the body in a frontal plane and elevated at an angle of ∼130 deg from the shoulder (Fig. 1). The arm was elevated above the heart, and also fully supported. Subjects flexed the fingers in an arc from the distal 3rd row phalanges to the proximal end of the metacarpals, ensuring the finger tips made contact with the palm of the hand. The torso was secured to the couch by velcro straps to minimize lateral body movement, and involvement of other muscle groups. A handgrip was secured to the subject's fingers at the distal third row phalanges (exclusive of thumb) by adjusting a cushioned bar securely against the fingers. The handgrip was connected by a multi-strand stainless-steel cable (19 gauge, 90 lb breaking strain) to a pneumatic piston (Bimba Manufacturing, Melbourne, Australia), with an intervening pulley, ensuring that the piston was lifted vertically. A load cell (Xtran 200N, Applied Measurement, Sydney, Australia) and a precision potentiometer (RS Components, Sydney, Australia) were fitted to the piston and the pulley, to record force and displacement, respectively, thus enabling calculation of power output during finger flexion contractions. The load cell and potentiometer were calibrated prior to, and at the completion of, each test by hanging precision weights from the pulley hand piece and by displacing the piston through a series of precise distances, respectively, using custom software (Labview 3.1, National Instruments, Austin, TX, USA). Subjects viewed a continuous real-time visual display of contraction rate and finger displacement on an elevated computer monitor. A personal computer fitted with an analog-to-digital converter card (Computer Boards multichannel DAS16), enabled simultaneous acquisition of contraction force, piston displacement, as well as extensometer strain gauge displacement (blood flow) and arterial and venous blood pressure data.
Forearm blood flow
Forearm blood flow (FBF) was measured using venous occlusion plethysmography. Laboratory environmental conditions were maintained constant to minimize fluctuations in skin blood flow between visits. A venous occlusion cuff was placed just proximal to the olecranon process of the exercising forearm and periodically inflated to 55 mmHg, to occlude venous outflow. An extensometer strain gauge (Brimacombe et al. 1991) was placed around the widest circumference of the forearm to detect circumference changes. The extensometer dimensions were 4 mm diameter, 120 mm flaccid length, with resolution noise-limited to 0.1 µm. The extensometer was calibrated prior to each test by manually stretching the extensometer through a range of precise distances (Linear Tools digital caliper) via custom-made software (Labview 3.1, National Instruments). The hand circulation was occluded during all FBF measurements using a wrist cuff inflated to 200 mmHg. Deep antecubital venous blood pressure measurements were recorded simultaneously with forearm circumference measurements using a pressure transducer (Abbott Critical Care Systems, Chicago, IL, USA) attached to the catheter via a saline-filled extension set. Pressure transducers were calibrated following each test against a range of pressures measured by a mercury sphygmomanometer. Three consecutive FBF measurements were taken in rapid succession at each time point and data averaged. The duration of venous occlusion depended on the rate of flow on each occasion, being 10–20 s for low flows under resting conditions, but only 1–3 s for high flows immediately after contractions (Fig. 2). FBF measurements were terminated when venous pressure exceeded 55 mmHg. FBF were calculated as a change in forearm circumference (mm s−1), then corrected for any change in forearm baseline due to the forearm swelling effects of finger flexion contractions, and substituted with the subject's forearm anthropometric data. A double truncated cone forearm geometric model (not shown) was used to determine forearm volume changes and express FBF in millilitres per minute.
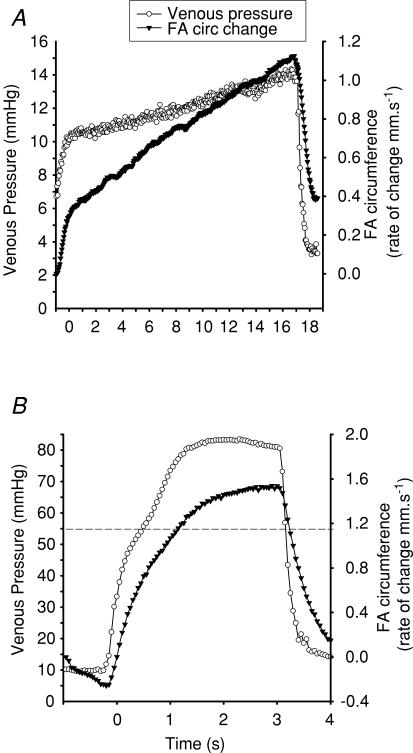
Figure 2. Typical forearm circumference change and venous pressure.
Venous blood pressure (○, primary y-axis) and simultaneous rate of change in forearm (FA) circumference (▾, secondary y-axis), used to calculated forearm blood flow at rest (A) and at fatigue (B). Dashed line represents the venous cut-off pressure of 55 mmHg, at which point the flow measurement was discontinued. Note plateau effects above 55 mmHg.
Experimental trials
All subjects completed one trial under NaHCO3 (Alk) and Con (Con) conditions, 1 month apart. Subjects reported to the laboratory after consuming 0.3 g−1 kg−1 of either encapsulated NaHCO3 or CaCO3 (AnalaR BDH, UK) together with 1 l of water, in five equal doses at 15 min intervals, starting 3 h prior to exercise. Heart rate and rhythm were monitored by electrocardiogram (Mortara, Boston, MA, USA). Catheters (20 or 22G, Jelco) were inserted retrogradely into the deep antecubital vein (v) of the contracting forearm and anterograde in the radial artery (a) of the non-contracting arm, under local anaesthesia (2% lignocaine injection). Subjects then rested for ∼30 min prior to the commencement of each trial. Intra-arterial and intravenous pressures were continually monitored (Marquette 710, Milwaukee, WI, USA) by electronic pressure transducers (Abbott Critical care Systems, Chicago IL, USA) connected to saline-filled cannulae via an extension line. Blood pressure signals were then interfaced with the finger flexion exercise computer system, enabling continual integration between power output, forearm circumference and blood pressure data. Arterial lines were kept patent by a slow, sterile, isotonic saline infusion under pressure. Subjects then performed finger flexion contractions at 75% WRpeak at a rate of 30 contractions min−1 and continued until fatigue. Fatigue for all trials was defined as a failure to maintain power output and/or cadence for eight consecutive contractions. Arterial and venous blood samples (each 5 ml) were taken simultaneously at rest, during 1 min intervals until fatigue, and at 1, 2, 5 and 10 min post-exercise. Hand blood flow was occluded for 10 s prior to and during venous blood sampling by a high-pressure wrist cuff. Forearm circumference measurements were made for FBF calculations immediately following blood sampling at rest, fatigue, 1, 2, 5, and 10 min post-exercise.
Blood sampling and analyses
Blood samples were immediately analysed in duplicate for plasma electrolytes (K+, Na+, Cl−), acid–base status (HCO3−, pH), gas tensions (PO2 and PCO2), O2 saturation (SO2), haemoglobin (Hb) and haematocrit (Hct), using automated blood gas and haematology analysers and plasma and whole blood lactate concentration ([Lac−]) spectrophotometrically, as previously described in detail (Fraser et al. 2002; Sangkabutra et al. 2003). All analysers were calibrated immediately before, during and after measurements with precision standards in the range of the measurements. Plasma protein, and hence total weak acid concentration [ATot] was not measured due to freezer failure and loss of plasma samples. An additional 5 ml of blood was taken at rest and at fatigue for plasma catecholamine analyses using the following method. Blood was immediately placed in lithium heparin tubes (125 IU) and 0.1 m sodium metabisulphite (125 µl), mixed and centrifuged at 4000 r.p.m. for 10 min at 4°C (3K 15 refrigerated centrifuge; Sigma, Laborzentrifugen, Germany). Plasma was stored at −80°C until assayed for adrenaline ([Adr]) and noradrenaline ([Nor]) concentrations by high-performance liquid chromatography with electrochemical detection (Sangkabutra et al. 2003).
Calculations
Plasma hydrogen concentration (nmol l−1) was derived from measured pH. Changes from resting levels in plasma volume (ΔPVa) and blood volume (ΔBVa) and changes in venous compared with arterial plasma (ΔPVa-v) and blood volume (ΔBVa-v) across the forearm were calculated during and following exercise, from changes in [Hb] and Hct, as previously described (McKenna et al. 1997a). These calculations enabled corrections to be made for effects of fluid shifts on ion concentrations in plasma and blood during and following exercise. Plasma and blood ion efflux data were corrected for fluid shifts. Plasma [ion]a-v (mmol l−1) were corrected for ΔPVa-v using the equation: [ion]a-v= ([ion]a/(1 +ΔPVa-v)) −[ion]v (McKenna et al. 1997a). Net ion fluxes across the forearm were calculated as the product of corrected [ion]a-v and plasma blood flow, and expressed in µmol min−1. A similar correction was made for whole-blood [ion]a-v but using ΔBVa-v. Plasma strong ion difference ([SID], mmol l−1) was calculated as ([K+]+[Na+]) − ([Lac−]+[Cl−]) (McKenna et al. 1997a) and calculations of whole-blood CO2 and O2 content, muscle V̇CO2, V̇O2 and respiratory exchange ratio (RER) were as previously described (McKenna et al. 1997b). Plasma flow was calculated as FBF × Hct (expressed as a fraction).
Statistical analysis
Results are expressed as mean ± s.e.m. unless otherwise stated. A two-way ANOVA with repeated measures was employed for all blood variables to assess treatment (Alk or Con) or time (rest, exercise, recovery) main effects. Treatment–time interactions were not significant unless stated. Post hoc analyses used the least significant difference test. Time to fatigue was analysed using a paired Student's t test. Statistical significance was accepted at P < 0.05. Calculated effect size using Cohen's d are presented when a variable was close to significantly different between treatments.
Results
Exercise tests
Pre-experiment peak exercise performance tests
Leg cycling incremental exercise V̇Ocpeak was 3.52 ± 0.30 l min−1 (49.9 ± 4.2 ml kg −1min−1), and peak work rate was 269 ± 25 W. In contrast, incremental finger flexion exercise test peak work rate (WRpeak) was only 4.06 ± 0.34 W, and the peak force was 19.22 ± 1.27 N. During incremental tests, the average finger power output and force production at each work rate were each linear over time (both R2= 0.99). Mean force, power output and time to fatigue during finger flexion were each highly reproducible during variability trials, with a CV = 3.3% (Table 1).
Table 1.
Performance characteristics during finger flexion exercise at 75% work rate peak to fatigue for control (Con, CaCO3, 0.3 g kg−1) and alkalosis (Alk, NaHCO3, 0.3 g kg−1)
| Trial | Mean force (N) | Mean power output (W) | Fatigue time (min) |
|---|---|---|---|
| Variability 1 | 16.6 ± 0.9 | 2.86 ± 0.16 | 10.1 ± 0.8 |
| Variability 2 | 16.9 ± 0.8 | 2.89 ± 0.14 | 10.2 ± 0.8 |
| CV (%) | 3.3 ± 0.6 | 3.1 ± 0.6 | 2.1 ± 0.5 |
| Con | 16.5 ± 0.9 | 2.95 ± 0.12 | 10.2 ± 0.8 |
| Alk | 16.8 ± 0.8 | 2.98 ± 0.16 | 12.7 ± 1.2† |
Mean ± s.e.m., n = 9 for Con and Alk, and for pre-experiment variability trials, n = 12. Coefficient of variation was calculated from variability trials 1 and 2.
Alk greater than Con (P < 0.05).
Alkalosis enhanced finger flexion exercise performance
Time to fatigue at 75% WRpeak was increased by 25.4 ± 8.1% during Alk compared with Con (P < 0.05). This was not due to differences between trials in mean power output or force as these were well matched, with no differences between Alk and Con (Table 1). No trial order effects were found.
Cardiovascular changes during finger flexion exercise
Forearm blood flow
Exercise
FBF increased ∼20-fold from rest to immediately post-exercise (P < 0.001), then decreased by ∼50% within the first minute of recovery, and remained ∼3.5-fold higher than rest at 10 min post-exercise (P < 0.05, Table 2).
Table 2.
Rate of forearm blood flow (ml min−1) measured at rest, immediately post-fatigue, and during recovery, under Con and Alk conditions
| Rest | Fatigue | Recovery (min) | ||||
|---|---|---|---|---|---|---|
| +1 | +2 | +5 | +10 | |||
| Con | 7.6 ± 1.0 | 162.4 ± 30.9 ** | 83.8 ± 5.1* | 55.9 ± 9.5* | 34.3 ± 5.3* | 25.7 ± 2.8* |
| Alk | 8.5 ± 0.9 | 162.2 ± 32.4 ** | 82.4 ± 14.7* | 61.5 ± 13.1* | 37.6 ± 8.3* | 30.5 ± 6.2* |
Greater than rest (P < 0.001);
greater than all other time points (P < 0.001). Mean ± s.e.m., n = 6.
Alkalosis
No significant differences were found in FBF between Con and Alk (Table 2).
Heart rate
Heart rate increased above rest (Con, 55 ± 3 beats min−1; Alk, 67 ± 4 beats min−1) to fatigue during finger flexion exercise (Con, 81 ± 5 beats min−1; Alk, 82 ± 7 beats min−1), and then fell to below rest by 10 min recovery (Con, 54 ± 2 beats min−1; Alk, 60 ± 5 beats min−1; P < 0.01). Although the pre-exercise heart rate was lower in Con (P < 0.01), there were no differences in heart rate during exercise or recovery between Con and Alk.
Plasma catecholamines
Exercise
Arterial plasma noradrenaline increased from rest to fatigue for Con (0.72 ± 0.12 and 1.24 ± 0.2 nmol l−1, respectively, P < 0.01), whilst arterial plasma adrenaline tended to increase at fatigue for Con (0.49 ± 0.08 and 0.74 ± 0.15 nmol l−1, respectively, P= 0.08, Cohen's d= 0.36).
Alkalosis
Arterial plasma noradrenaline increased from rest to fatigue also for Alk (0.78 ± 0.09 and 1.20 ± 0.3 nmol l−1, respectively, P < 0.01). Arterial plasma adrenaline similarly tended to increase at fatigue in Alk (0.37 ± 0.06 and 0.58 ± 0.08 nmol l−1). However, no significant effects were found between trials for noradrenaline or adrenaline.
Acid–base balance and metabolism during finger flexion exercise
Plasma [H+]
Exercise
Arterial plasma [H+] ([H+]a) did not differ from rest during exercise or recovery (Fig. 3A). In contrast, antecubital venous plasma [H+] ([H+]v) increased rapidly to a plateau during the first 3 min of exercise (P < 0.05), then decreased rapidly throughout recovery (P < 0.05), reaching rest levels within 5 min (Fig. 3B). The plasma [H+]a-v became increasingly negative during the first 4 min of exercise (P < 0.05) and increased throughout recovery, but remained negative at 10 min recovery (P < 0.05, Fig. 3C). Muscle H+ efflux (plasma [H+]a-v× plasma flow) increased from rest to fatigue by ∼58-fold (Con) and 64-fold (Alk), decreased by ∼50% at 1 min recovery, and continued to decline until 10 min of recovery (P < 0.001, Table 3).
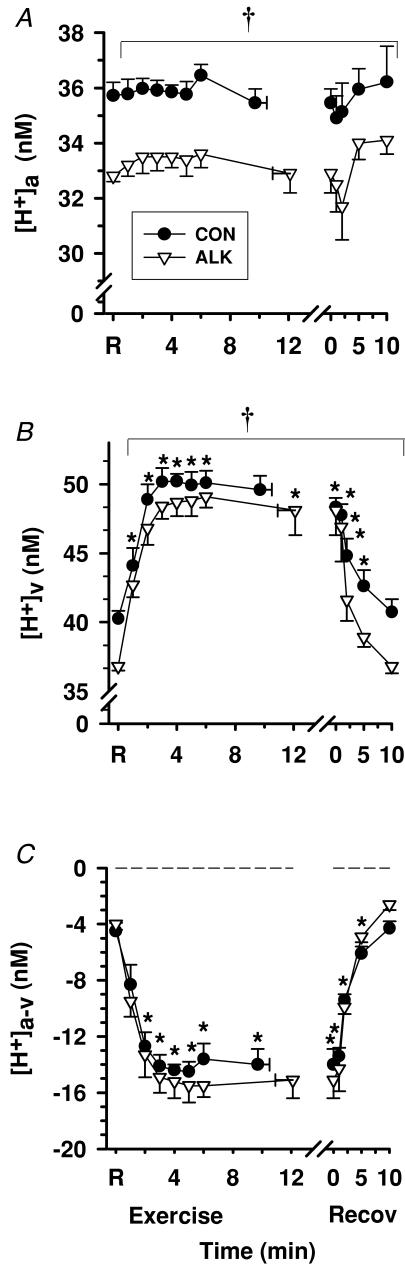
Figure 3. Effects of alkalosis on plasma [H+] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [H+], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.01, time main effect). †Con > Alk (P < 0.05, treatment main effect). Data expressed as mean ± s.e.m., n = 8. The fatigue point during exercise has been replotted as time zero in recovery. Dashed line represents zero arterio-venous difference.
Table 3.
Net ion fluxes into or out of plasma across the forearm musculature measured at rest, immediately post-fatigue, and during recovery, under Con and Alk conditions
| Rest | Fatigue | Recovery (min) | ||||
|---|---|---|---|---|---|---|
| +1 | +2 | +5 | +10 | |||
| Con | ||||||
| H+ efflux | −21 ± 4 | −1233 ± 181* | −628 ± 61* | −320 ± 41* | −144 ± 6* | −77 ± 13* |
| HCO3− efflux | −10.3 ± 4.2 | −456.3 ± 131* | −101.8 ± 43.5* | −40.8 ± 7.3* | −26.6 ± 8.4 | −31.2 ± 9.7* |
| K+ fluxes | 0.1 ± 0.3 | −42.5 ± 10.7* | 30.9 ± 7.8* | 20.1 ± 7.5* | 10.3 ± 4.2* | 7.0 ± 2.8* |
| Na+ fluxes | −7.7 ± 5.7 | −276.1 ± 190.8* | −47.5 ± 152* | 25.6 ± 65.3 | −69.1 ± 27.3* | −68.7 ± 31.5* |
| Cl− fluxes | 2.6 ± 3.9 | 407.1 ± 101.9* | 204.2 ± 28.5 | 46.2 ± 47.2 | −48.3 ± 30.5 | −59 ± 19.0 |
| Lac− efflux | −0.3 ± 0.5 | −148.8 ± 37.8* | −111.5 ± 30.3* | −78.6 ± 24.2* | −37.2 ± 11.4* | −10.6 ± 3.3* |
| Alk | ||||||
| H+ efflux | −21 ± 1 | −1364 ± 248* | −754 ± 174* | −394 ± 82* | −135 ± 22* | −69 ± 20* |
| HCO3− efflux | −13.9 ± 3.2 | −459 ± 94.9* | −99.0 ± 39.3* | −44.4 ± 19.7* | −12.9 ± 20.1 | −32.5 ± 8.9* |
| K+ fluxes † | 0.5 ± 0.5 | −63.7 ± 13.5* | 22.4 ± 6.3* | 22.8 ± 10.7* | 12.6 ± 3.5 | 7.3 ± 1.7 |
| Na+ fluxes | 18.1 ± 8.0 | −78.6 ± 121.3* | −114.6 ± 73.9* | −2.3 ± 34.5 | −87.1 ± 26.1* | −75.9 ± 36.1* |
| Cl− fluxes † | 2.4 ± 5.2 | 558.2 ± 162.8* | 8.9 ± 72.1 | 194.1 ± 132.2 | −24 ± 21.6 | −61.8 ± 22.4 |
| Lac− efflux † | −0.3 ± 0.6 | −295.5 ± 62* | −173.4 ± 45.4* | −124.7 ± 35.5* | −39.3 ± 7.6* | −15.5 ± 3* |
All units are µmol min−1, except for H+ which is pmol min−1. Mean ± s.e.m., n = 6.
Different to rest (P < 0.001).
Alk > Con (P < 0.05).
A negative value denotes net ion flux into plasma across forearm musculature, and positive values denote net flux from plasma across musculature. Fluxes calculated from (a–v [ion] difference × forearm plasma flow). Fluxes for K+, Na+, Cl− and Lac− corrected for arterio-venous ΔPV.
Alkalosis
Both [H+]a and [H+]v were lower in Alk (P < 0.05), whereas [H+]a-v (Fig. 3C) and H+ efflux (Table 3) were unchanged by Alk. Resting plasma [H+]a and [H+]v were 2.9 ± 0.2 and 3.5 ± 0.4 nmol l−1 lower during Alk than Con, respectively, whilst the peak rise in [H+]a or [H+]v above rest did not differ between trials.
Plasma [HCO3−]
Exercise
Plasma [HCO3−]a was unchanged during exercise or recovery (Fig. 4A), whereas [HCO3−]v increased with exercise and then fell below resting levels during recovery (P < 0.05, Fig. 4B). Plasma [HCO3−]a-v remained negative throughout exercise and recovery (except at 5 min recovery in Alk), indicating a net gain in plasma HCO3− across forearm muscles (P < 0.05, Fig. 4C). Muscle HCO3−‘apparent efflux’ increased from rest to fatigue by ∼43-fold (Con), decreasing substantially by 1 min recovery, continued to decline for the remainder of recovery, but remained ∼3-fold greater at 10 min post-exercise than at rest (P < 0.001, Table 3).
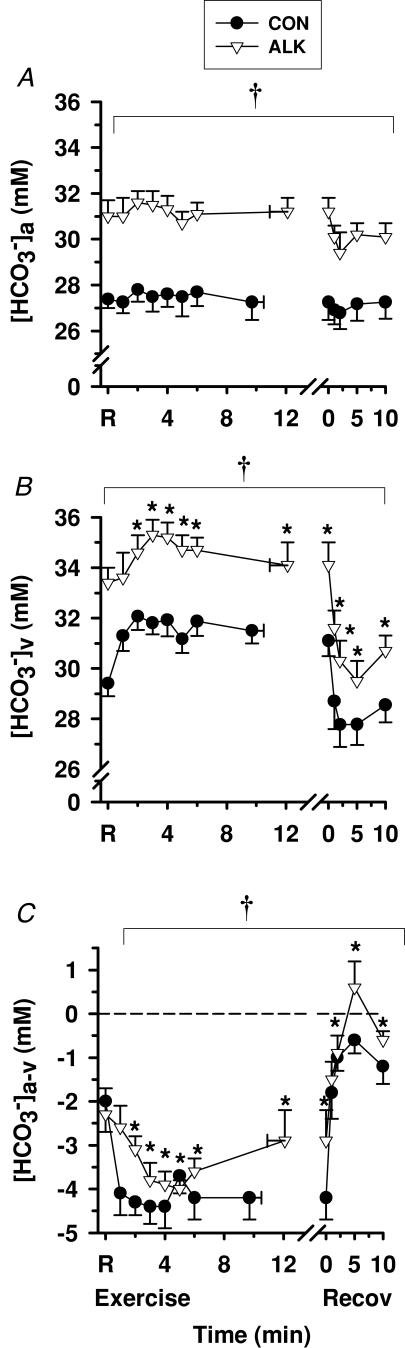
Figure 4. Effects of alkalosis on plasma [HCO3−] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [HCO3−], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.05 time main effect). †Alk different to Con (P < 0.05, treatment main effect). Sample size and data presentation as in Fig. 3.
Alkalosis
Both [HCO3−]a and [HCO3−]v were greater during Alk than Con (P < 0.05), and plasma [HCO3−]a-v was less negative during Alk than Con (P < 0.05, Fig. 4C). There was no difference in muscle HCO3− efflux between Alk and Con (Table 3).
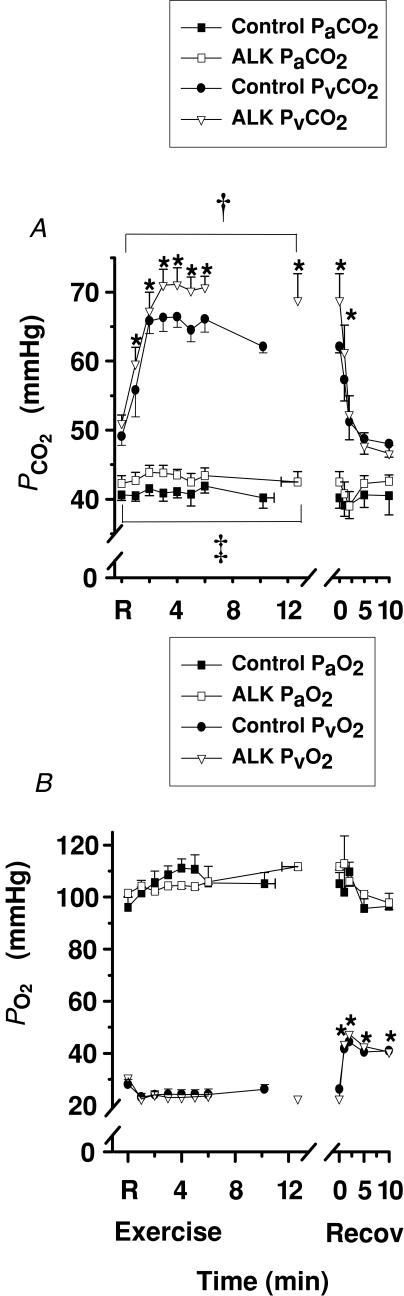
Plasma PCO2
Exercise
Plasma Pa,CO2 did not differ from rest during exercise or recovery (Fig. 5A). In contrast, plasma Pv,CO2 increased sharply during the first 3 min of exercise, then plateaued, and fell rapidly in recovery (P < 0.05, Fig. 5A).
Figure 5. Effects of alkalosis on plasmaPCO2andPO2at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
A, arterial Con (▪), Alk (□) and venous Con (•), Alk (▿) PCO2. B, arterial Con (▪), Alk (□) and venous Con (•), Alk (▿) PO2. *Different from rest (P < 0.05, main effect for time). †Treatment main effect for Pv,CO2 during exercise (P < 0.05). ‡Treatment effect for Pa,CO2 during exercise (P= 0.068, Cohen's d= 0.4). Sample size and data presentation as in Fig. 3.
Alkalosis
Pv,CO2 was greater at rest and throughout exercise during Alk (P < 0.01), with a similar trend in Pa,CO2 (P= 0.068, Cohen's d= 0.4). No difference was evident between treatments in recovery for either Pv,CO2 or Pa,CO2.
Plasma PO2
Exercise
Pa,O2 was not significantly changed during exercise and recovery (Fig. 5B). Pv,O2 was unchanged during exercise, but rose sharply during the first 2 min of recovery and remained above rest (P < 0.05, Fig. 5B).
Alkalosis. No effect of Alk was found on either arterial or venous PO2.
Blood CO2 content (CCO2) and forearm V̇CO2 (V̇m,CO2)
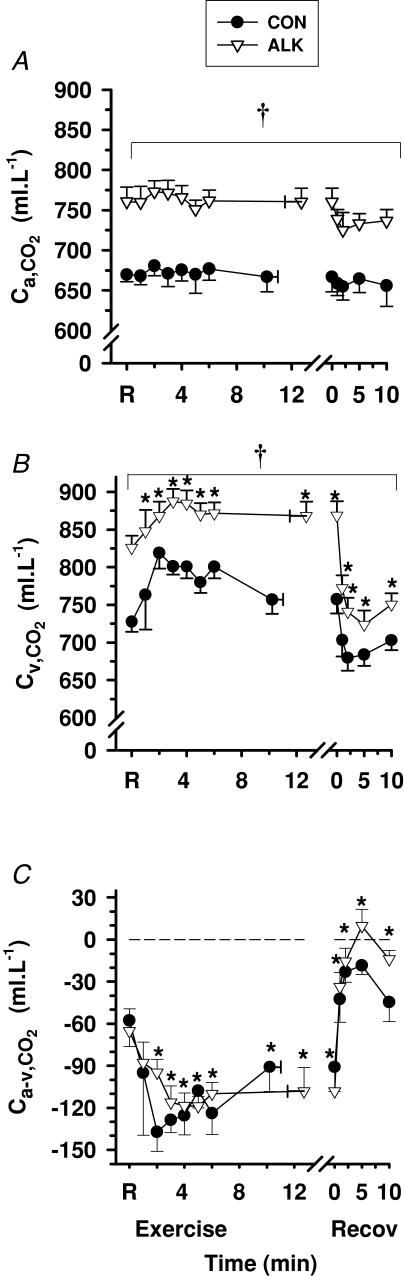
Exercise
Ca,CO2 did not vary from rest during exercise or recovery (Fig. 6A), whilst Cv,CO2 increased during early exercise (P < 0.01) and decreased rapidly during the first 2 min of recovery (P < 0.01, Fig. 6B). Ca-v,CO2 decreased during exercise (P < 0.01) and except for 5 min recovery in Alk, remained negative throughout recovery (Fig. 6C). Forearm muscle CO2 output (V̇m,CO2) increased 35-fold from rest to fatigue (P < 0.001, Table 4), before declining to near pre-exercise resting values after 1 min recovery.
Figure 6. Effects of alkalosis on CO2 content at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in CO2 content, under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.01, time main effect). †Alk > Con (P < 0.05, treatment main effect). Sample size and data presentation as in Fig. 3.
Table 4.
Forearm muscle lactate efflux, V̇O2, V̇CO2 and RER at rest, immediately post-fatigue and during recovery, under Con and Alk conditions
| Rest | Fatigue | Recovery (min) | ||||
|---|---|---|---|---|---|---|
| +1 | +2 | +5 | +10 | |||
| Con | ||||||
| Lac−m efflux | −0.2 ± 0.6 | −182.3 ± 50.8* | −134.8 ± 36.4* | −88.0 ± 24.8* | −46.8 ± 14.2* | −18.3 ± 6.2* |
| V̇m,O2 | 0.6 ± 0.1 | 22.2 ± 4.0* | 4.5 ± 0.5 | 1.8 ± 0.2 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| V̇m,CO2 | 0.5 ± 0.2 | 17.7 ± 7.3* | 4.9 ± 1.5 | 1.1 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.3 |
| RERm | 0.71 ± 0.2 | 0.98 ± 0.06* | 0.86 ± 0.19* | 0.59 ± 0.08* | 0.56 ± 0.09* | 0.56 ± 0.25* |
| Alk | ||||||
| Lac−m efflux‡ | −1.0 ± 1.4 | −353.7 ± 110.4* | −159.0 ± 45.9* | −144.9 ± 52.7* | −57.6 ± 21.0* | −24.8 ± 11.6* |
| V̇m,O2 | 0.6 ± 0.1 | 20.4 ± 4.1* | 4.4 ± 1.1 | 2.1 ± 0.5 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| V̇m,CO2 | 1.6 ± 0.9 | 18.1 ± 4.3* | 9.9 ± 3.2 | 7.7 ± 3.3 | 4.8 ± 2.1 | 3.8 ± 1.3 |
| RERm | 0.94 ± 0.2 | 0.90 ± 0.08* | 0.92 ± 0.17* | 0.70 ± 0.57* | 0.60 ± 0.46* | 0.54 ± 0.08* |
Net Lac− flux expressed in µmol min−1 and V̇O2, in ml min−1. Mean ± s.e.m., n = 6;
Different from rest (P < 0.001).
Alk > Con during recovery (P < 0.05). A negative value denotes net efflux from contracting musculature, and positive values denote net influx. Muscle lactate efflux calculated from ([BLac−]a-v× forearm blood flow), with [BLac−]a-v corrected for arterio-venous ΔBV.
Alkalosis
Ca,CO2 and Cv,CO2 were higher during Alk, by 85.5 ± 6.2 ml l−1 and 115 ± 13.2 ml l−1 compared with control (P < 0.05), respectively, whereas no differences were found between trials for Ca-v,CO2 (Fig. 6) or V̇m,CO2 (Table 4).
Blood O2 content (CO2) and forearm muscle V̇O2 (V̇m,O2)
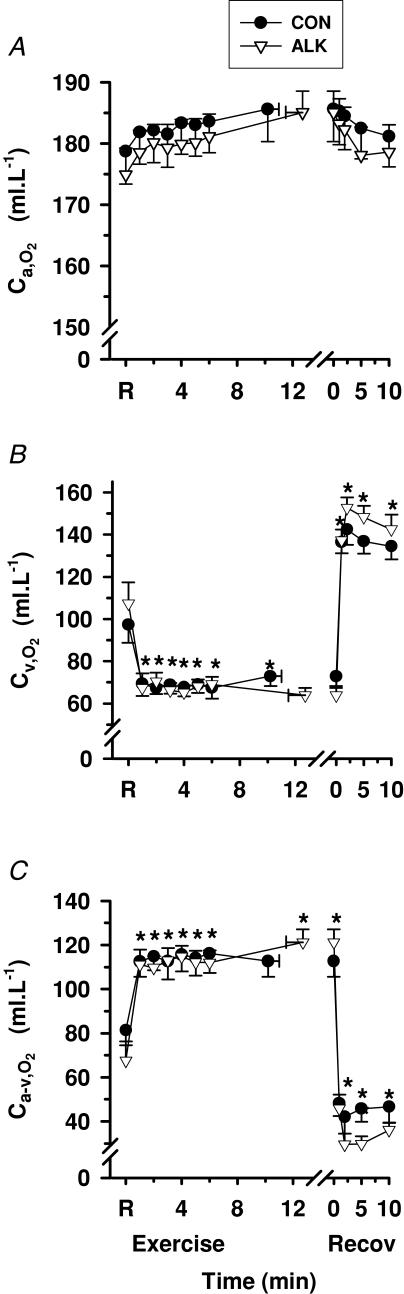
Exercise
Ca,O2 did not change significantly from rest during exercise or recovery (Fig. 7A), whereas Cv,O2 decreased during exercise (P < 0.01), and increased rapidly during recovery (P < 0.01, Fig. 7B). Ca-v,O2 increased rapidly during exercise (P < 0.01), and decreased rapidly during recovery (P < 0.01, Fig. 7C). Forearm muscle O2 uptake V̇m,O2 increased rapidly from rest to fatigue by 37-fold (P < 0.001, Table 4), before decreasing towards pre-exercise values after 1 min recovery.
Figure 7. Effects of alkalosis on O2 content at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in O2 content, under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.01, time main effect). Sample size and data presentation as in Fig. 3.
Alkalosis
There was no effect of Alk on Ca,O2, Cv,O2, Ca-v,O2 or V̇m,O2.
Forearm muscle RER
Exercise
Forearm respiratory exchange ratio (RERm) increased at fatigue from rest, and fell to below rest in recovery (P < 0.05; Table 4).
Alkalosis
There were no effects of Alk on RERm.
Blood [Lac−]
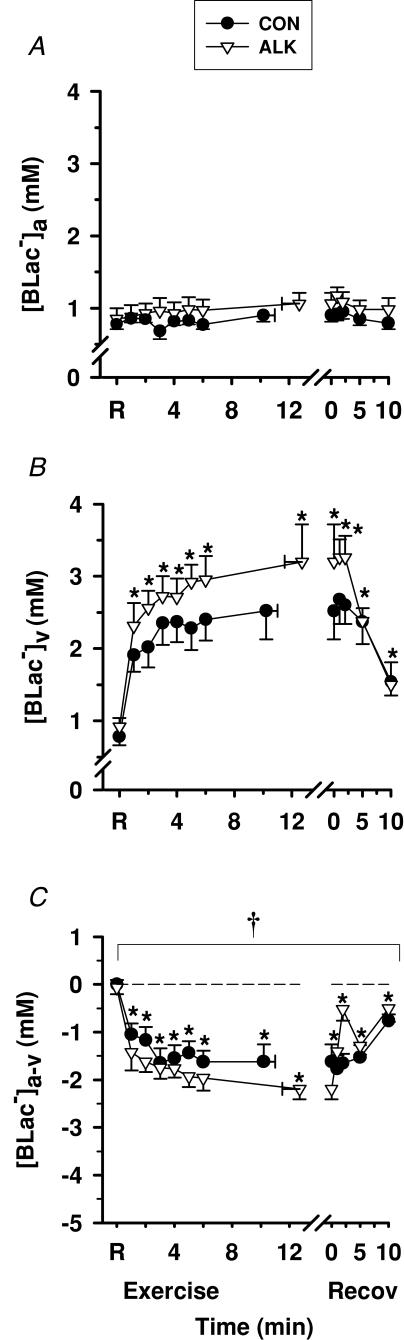
Exercise
Whole-blood [Lac−]a was unchanged, whilst [Lac−]v increased above resting levels during exercise and decreased during recovery (P < 0.05, Fig. 8). Blood [Lac−]a-v decreased during exercise and increased during recovery, but remained negative throughout, reflecting a continued net release of lactate from muscle into blood across the forearm musculature (P < 0.05, Fig. 8C). Muscle lactate efflux increased from rest to fatigue by ∼900-fold in control (P < 0.01, Table 4).
Figure 8. Effects of alkalosis on whole-blood [Lac−] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in whole-blood [Lac−], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.05, time main effect). †Alk > Con (P < 0.05, treatment main effect). Data and presentation as in Fig. 3. Arterio-venous whole-blood differences are corrected for the a–v decline in blood volume.
Alkalosis
Blood [Lac−]a and [Lac−]v were not significantly different between Con and Alk. Blood [Lac−]a-v was more negative in Alk during exercise (P < 0.05), with the greatest difference at fatigue (Con, −1.62 ± 0.36 mmol l−1; Alk, −2.19 ± 0.22 mmol l−1, P < 0.05). Muscle lactate efflux increased from rest to fatigue by ∼350-fold in Alk (P < 0.01, Table 4) and was ∼2-fold greater at fatigue during Alk compared with Con (P < 0.05).
Haematology and fluid shifts
Haemoglobin and haematocrit
Exercise
Arterial and venous [Hb] increased from rest (Con, 13.6 ± 0.4 g dl−1; Alk, 13.4 ± 0.4 g dl−1) to fatigue (Con, 14.1 ± 0.4 g dl−1; Alk, 14.0 ± 0.4 g dl−1; P < 0.001). Arterial and venous Hct also increased from rest (Con, 38.5 ± 1.5%; Alk, 37.6 ± 0.9%) to fatigue (Con, 39.9 ± 1.5%; Alk, 38.9 ± 1.1%; P < 0.001).
Alkalosis
There were no differences between Con and Alk for either [Hb] or Hct.
Plasma and blood volume changes
Exercise
Plasma volume (PV) declined from rest during exercise when calculated for both arterial and venous blood, by ∼5% at fatigue (P < 0.05, data not shown). Whilst ΔPVa remained negative throughout recovery, ΔPVv increased above rest at 10 min recovery (P < 0.05). A negative ΔPVa-v, indicating a small net loss in PV, occurred across the exercising forearm (P < 0.05), which was reversed to a net PV gain during recovery (P < 0.05, data not shown). Similar exercise effects were found for ΔBVa and for ΔBVv (data not shown).
Alkalosis
There was no effect of Alk on ΔPVa, ΔPVv or ΔPVa-v. The nadir ΔPVa (Con, −4.8 ± 0.9%versus Alk, −6.6 ± 1.1%) and ΔPVv (Con, −6.4 ± 1.9%versus Alk, −7.0 ± 0.8%) were not significantly different between treatments. No Alk main effects were found on BVa, BVv or BVa-v (data not shown).
Plasma electrolytes
Plasma [K+]
Exercise
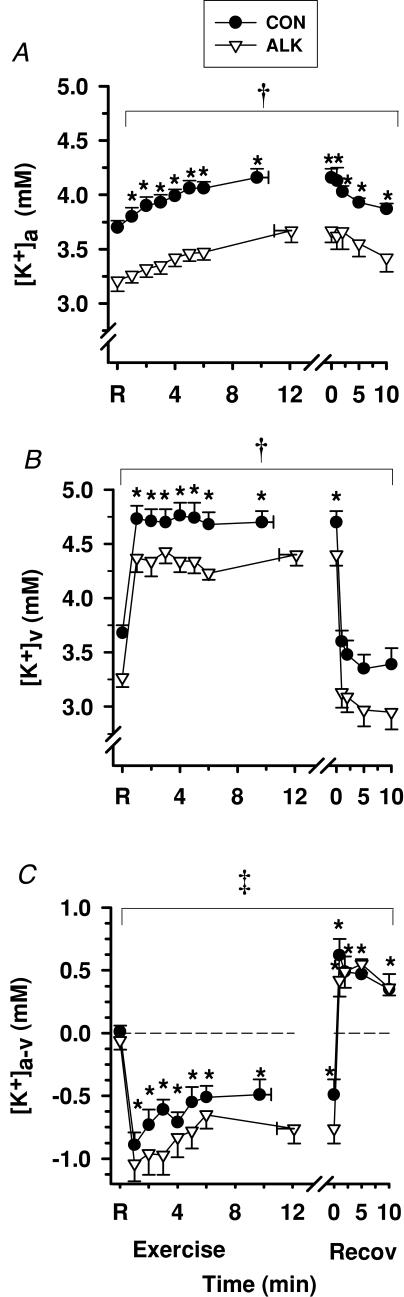
Plasma [K+]a increased above rest by 1 min exercise, continued to increase throughout exercise, decreased slowly in recovery, but remained above rest after 10 min (P < 0.01; Fig. 9A). In contrast, [K+]v rose sharply by 1 min of exercise, plateaued until fatigue and then declined rapidly during recovery (P < 0.01, Fig. 9B). The [K+]a-v decreased by 1 min of exercise (− 0.90 ± 0.10 mmol l−1 during Con), representing net K+ entry into plasma traversing forearm muscle. An immediate reversal of Δ[K+]a-v to positive values occurred within the first 1 min of recovery, reflecting K+ loss from plasma (P < 0.01, Fig. 9C). K+ efflux from muscle into plasma increased dramatically from 0.1 ± 0.3 µmol min−1 at rest, to 42.5 ± 10.7 µmol min−1 at fatigue (P < 0.001, Table 3), with net K+ removal from plasma evident throughout recovery (Table 3).
Figure 9. Effects of alkalosis on plasma [K+] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [K+], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.01, time main effect). †Alk < Con (P < 0.05, treatment main effect). ‡Alk > Con (P= 0.056, Cohen's d= 0.44, treatment main effect). Sample size and data presentation as in Fig. 3. Arterio-venous [K+] differences are corrected for the a–v decline in plasma volume.
Alkalosis
Both [K+]a and [K+]v were less in Alk than in Con (P < 0.01), being lower at rest by 0.49 ± 0.08 and 0.41 ± 0.08 mmol l−1, respectively (P < 0.05). The peak rise in [K+]a during exercise (Δ[K+]a 0.46 ± 0.02 and 0.46 ± 0.01 mmol l−1) and in Δ[K+]v (1.02 ± 0.04 and 1.13 ± 0.01 mmol l−1) were similar during Con and Alk, respectively. Similar to Con, [K+]a-v decreased rapidly by 1 min of exercise (−1.04 ± 0.14 mmol l−1) and a tendency was observed for a wider (more negative) [K+]a-v during exercise in Alk (P= 0.056; Cohen's d= 0.44, Fig. 9C). At fatigue, muscle K+ efflux was greater during Alk by 21.2 ± 7.6 µmol min−1 (32 ± 7%, P < 0.05, Table 3). In contrast, the subsequent K+ uptake from fatigue to 10 min recovery was greater during Alk, with the estimated peak at 86 ± 19 µmol min−1 compared with 73 ± 14 µmol min−1 in Con (15 ± 7%, P < 0.05). This probably reflects greater Na+,K+-ATPase-mediated K+ uptake into forearm muscle with Alk.
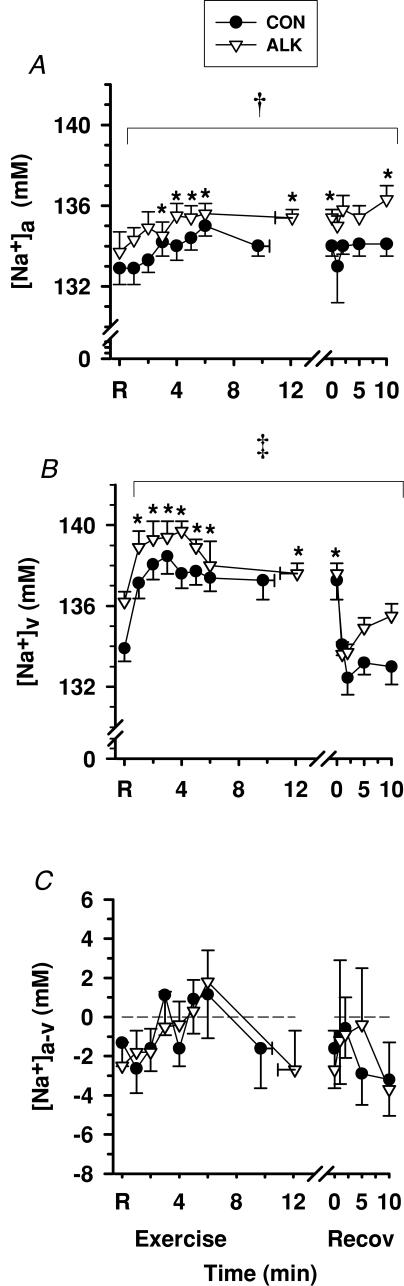
Plasma [Na+]
Exercise
Plasma [Na+]a did differ significantly from rest during exercise or recovery (Fig. 10A), whilst [Na+]v increased during exercise (P < 0.05, Fig. 10B) and declined to rest levels during recovery. Plasma [Na+]a-v fluctuated considerably and did not differ significantly from rest during exercise or in recovery. Despite considerable variability, for Con, an apparent muscle Na+ flux was greater at fatigue than rest, and decreased in recovery (P < 0.05, Table 3).
Figure 10. Effects of alkalosis on plasma [Na+] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [Na+] differences, under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.05, time main effect). †Alk > Con (P < 0.05, treatment main effect). ‡Alk tended to be > Con (P= 0.066, Cohen's d= 0.46, treatment main effect). Sample size and data presentation as in Fig. 3. Arterio-venous [Na+] differences are corrected for the a–v decline in plasma volume.
Alkalosis
[Na+]a was greater (P < 0.05) and [Na+]v tended to be greater during Alk (P= 0.066, Cohen's d= 0.46). There was no effect of Alk on [Na+]a-v and there were no treatment main effects detected for muscle Na+ flux.
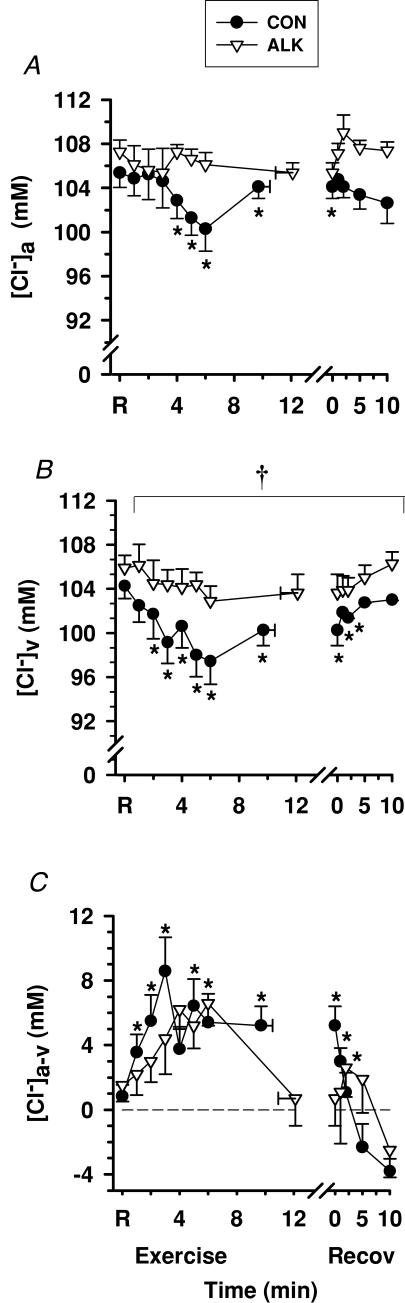
Plasma [Cl−]
Exercise
Both [Cl−]a and [Cl−]v decreased slightly from rest during exercise (P < 0.05, Fig. 11A and B). Plasma [Cl−]a-v was positive throughout rest, exercise, and up to 5 min recovery during Con, reflecting net Cl− movement out of plasma traversing the forearm (P < 0.01; Fig. 11C). By 10 min recovery, the [Cl−]a-v was negative, indicating a reversal to a net Cl− movement from muscle into plasma. Influx of Cl− from plasma to muscle from rest to fatigue increased substantially during Con (P < 0.001, Table 3).
Figure 11. Effects of alkalosis on plasma [Cl−] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [Cl−], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.01, time main effect). †Alk > Con (P < 0.05, treatment main effect). Sample size and data presentation as in Fig. 3. Arterio-venous [Cl−] differences are corrected for the a–v decline in plasma volume.
Alkalosis
Alk did not significantly affect plasma [Cl−]a or [Cl−]a-v, but [Cl−]v was greater during Alk (P < 0.05). However, Cl− flux was 27% greater during Alk (151.1 ± 178.0 µmol min−1, P < 0.05, Table 3), indicating greater Cl− flux from plasma across the forearm muscles.
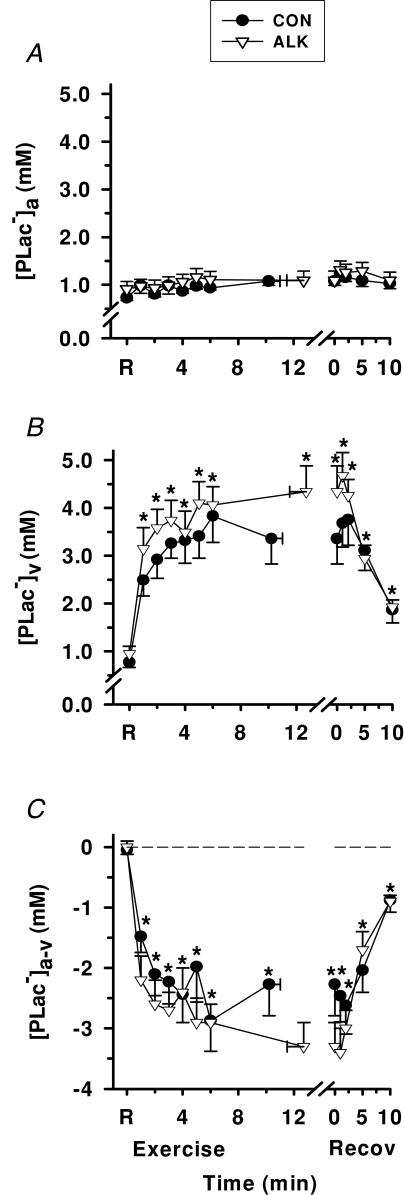
Plasma [Lac−>]
Exercise
Plasma [Lac−]a did not differ from rest during exercise or recovery. Plasma [Lac−]v increased during exercise (P < 0.05), peaked at 5 min exercise, and decreased after 2 min recovery (P < 0.05, Fig. 12B). Plasma [Lac−]a-v decreased from rest during exercise to a nadir of −2.3 ± 0.5 mmol l−1 during Con, reflecting net lactate release into plasma across the contracting forearm muscles. Following ∼2 min of recovery, [Lac−]a-v increased (P < 0.05) but remained negative at 10 min recovery (Fig. 12C). Lac− flux into plasma increased from 0.3 ± 0.5 µmol min−1 at rest, to 148.8 ± 37.8 µmol min−1 at fatigue, and decreased during recovery to remain elevated at 10.6 ± 6.6 µmol min−1 by 10 min recovery, during Con trials (P < 0.001, Table 3).
Figure 12. Effects of alkalosis on plasma [Lac−] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [Lac−], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.001, time main effect). Data and presentation as in Fig. 3. Arterio-venous [Lac−] differences are corrected for the a–v decline in plasma volume.
Alkalosis
Plasma [Lac−]a, [Lac−]v and [Lac−]a-v did not differ significantly between Alk and Con. However, at fatigue, lactate flux at fatigue was 50% greater during Alk, and remained greater throughout recovery (P < 0.01, Table 3).
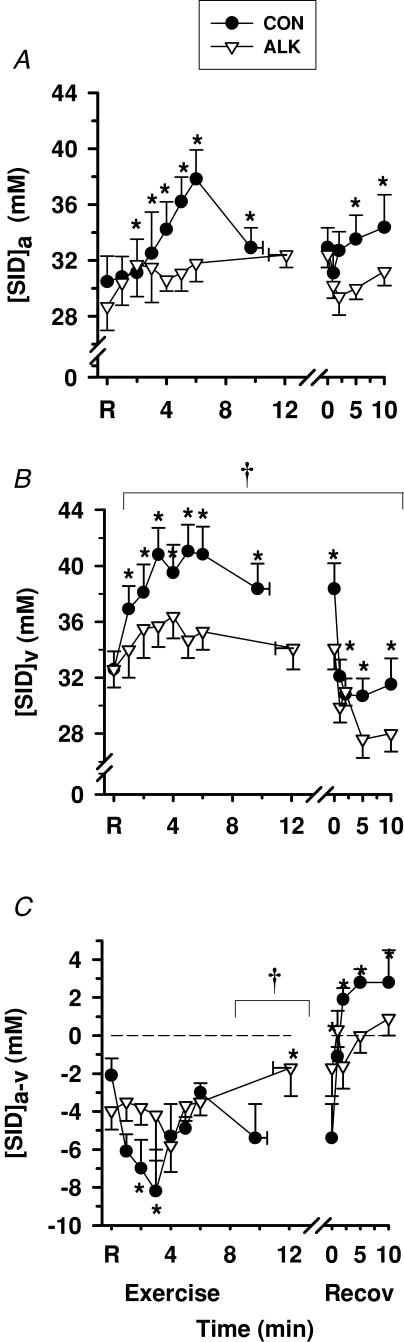
Strong ion difference ([SID])
Exercise
Plasma [SID]a increased above rest during exercise (P < 0.01) and remained slightly elevated during recovery (P < 0.01, Fig. 13A). Plasma [SID]v also increased during exercise, but in contrast, decreased rapidly during recovery (P < 0.001; Fig. 13B). Plasma [SID]a-v was negative throughout exercise, and became positive after 1 min recovery (Fig. 13B).
Figure 13. Effects of alkalosis on plasma [SID] at rest, during finger flexion exercise at 75% WRpeak continued to fatigue, and recovery.
Arterial (A), venous (B) and calculated arterio-venous differences (C) in plasma [SID], under Con (•) and Alk (▿) conditions. *Different from rest (P < 0.001, main effect for time). §Time main effect where post hoc analysis could not detect the location of differences (P < 0.01). Con > Alk (P < 0.05, treatment main effect). Data and presentation as in Fig. 3. Dashed line represents zero arterio-venous difference.
Alkalosis
Plasma [SID]a did not differ significantly between trials, whilst [SID]v was lower in Alk than in Con during exercise and recovery (P < 0.05). Plasma [SID]a-v was less negative at fatigue during Alk than Con (P < 0.05, Fig. 13C).
Discussion
This study examined the effects of alkalosis during and following exercise on ionic and acid–base status, metabolism and blood flow during fatiguing contractions of the finger flexor muscles performed using a unique custom-designed ergometer. The acute effects of exercise on these variables were firstly comprehensively described, since little is known about these during dynamic, concentric exercise with such a small muscle mass. Furthermore, this is important to allow understanding of the responses to alkalosis. Despite fatiguing exercise barely perturbing arterial plasma ionic and acid–base variables, large arterio-venous differences across the contracting forearm muscles occurred. These, together with a dramatic increase in blood flow, indicated substantial ionic fluxes and acid–base disturbances with small muscle mass exercise.
Sodium bicarbonate-induced alkalosis markedly affected these muscle ionic exchanges and enhanced muscle performance, with a ∼25% increase in time to fatigue during finger flexion exercise. The major findings were that whilst Alk lowered arterial and venous plasma [K+] compared with Con, K+ efflux into plasma at fatigue was greater, indicating greater muscle K+ release during fatiguing exercise. Conversely, a greater muscle K+ uptake was found in recovery with Alk, which indicates increased muscle Na+,K+-ATPase activity and possible increased Na+–K+–2Cl− co-transporter (NKCC) activity. These together suggest that Alk increased muscle K+ loss during exercise despite increased muscle Na+,K+-ATPase activity. We also demonstrate for the first time that arterial [Na+] was higher and Cl− influx into muscle greater in Alk; this together with lower [K+] and increased muscle Na+,K+-ATPase activity, may have contributed to muscle performance enhancement via a membrane stabilizing effect. We confirm in this exercising model that muscle Lac− efflux was greater during alkalosis, indicating enhanced Lac− transport from the muscle and most likely, an increased glycolytic rate. Finally, Alk had no effect on fluid shifts, forearm blood flow, muscle V̇O2 or RER during exercise or recovery.
Physiological responses to dynamic exercise of the finger flexor muscles
To understand alkalosis effects in the contractile model used, it was first essential to determine the ionic, acid–base, metabolic and cardiovascular responses during and after dynamic exercise of the finger flexor muscles. Although numerous studies have investigated metabolic and cardiovascular responses to isometric muscle contractions of the forearm (Van Beekvelt et al. 2001 Hamann et al. 2004), relatively little is known about these effects using such a small muscle mass performing dynamic, concentric exercise. This investigation firstly necessitated development of a unique ergometer capable of enabling dynamic forearm flexor contractions, with precise measurements of muscle power and work, but which also restricted exercise to concentric contractions, to avoid eccentric contraction-induced muscle soreness and damage. The ‘eccentric unloading’ of the ergometer during the relaxation part of the duty cycle of this model means it is highly likely that the forearm extensor muscles would contribute very little to contractions, in contrast to what would occur in handgrip or wrist flexion exercise models.
The extremely low peak power output of ∼3 W represented only ∼1.5% of that achieved during two-legged incremental cycling exercise and verifies the small magnitude of contracting muscle mass that was utilized. Consequently, disturbances in arterial plasma ionic and acid–base status were minimal or absent, as expected. No changes were found with exercise in any of arterial plasma [H+], [HCO3−], PCO2, PO2, [Lac−] or [Na+], with only a small increase in arterial plasma [K+] (∼0.5 mmol l−1) and small declines in plasma volume (∼5%), [Cl−] (∼5 mmol l−1) and [SID] (∼7 mmol l−1). Additionally, only small increases in heart rate, blood pressure and adrenaline were observed, which are all in keeping with activation of only a small muscle mass. In contrast, dramatic increases were observed in forearm blood flow, O2 uptake and CO2 output during dynamic exercise to fatigue, by ∼20-, 35- and 37-fold, respectively. Furthermore, relatively large ionic and acid–base changes were observed in blood draining the active muscles, with increases in deep antecubital venous plasma [H+] (∼10 nmol l−1), [HCO3−] (∼3 mmol l−1), PCO2 (∼17 mmHg), [Lac−] (∼3 mmol l−1), [Na+] (∼4 mmol l−1), [K+] (∼1 mmol l−1) and [SID] (∼8 mmol l−1), and with declines in plasma volume (∼5%) and [Cl−] (∼7 mmol l−1). These changes are much more dramatic when considered in the context of the large increase observed in forearm blood flows, indicating large ionic and metabolic fluxes across the forearm muscles.
Forearm blood flow
Whilst venous occlusion plethysmography is a well established non-invasive method of estimating forearm blood flow, there are important limitations. This includes an inability to collect measurements during forearm exercise due to movement artefacts, resulting in a time lag of ∼2–5 s between the end of exercise and the initiation of blood flow measurements. Therefore, absolute blood flow measurements at fatigue could be slightly underestimated due to a possible immediate decrement in blood flow. Consequently ionic and metabolic fluxes across the forearm muscles would also be slightly underestimated. However, since FBF timing was consistent between trials and FBF was unaffected by Alk, this is therefore not a major limitation. Furthermore, the peak blood flow measured here was ∼162 ml min−1, very similar to the ∼175 ml min−1 measured by Doppler ultrasound after 5 min dynamic handgrip exercise (Perrey et al. 2001). No recovery flow data were provided in that study, which would also have utilized a higher muscle mass (Perrey et al. 2001). In this study, increases in blood flow with exercise are assumed to primarily reflect skeletal muscle blood flow, as arterial inflow to and venous outflow from the hand was occluded ∼10 s prior to blood sampling, which immediately preceded forearm circumference measures. Also, the contribution of forearm skin blood flow is assumed minimal and constant between trials, due to a constant cool laboratory temperature of 21°C. Finally, it is likely that blood flow was at about steady state for the majority of exercise (between ∼2–6 min), since each of Ca-v2, Ca-v,CO2, [BLac−]a-v and [K+]v were relatively unchanged after the first minute during exercise, and each of these would be sensitive to altered blood flow. Furthermore tight coupling also exists between power output, blood flow, contracting musculature and peripheral V̇m,O2 (Saltin et al. 1998), and power output was maintained constant during the exercise protocol. At fatigue V̇m,O2 was ∼21 ml min−1 and both FBF and V̇m,O2 decreased rapidly within the first minute of recovery, similar to other reports (Perrey et al. 2001; MacDonald et al. 2001).
Marked ion fluxes during finger flexion exercise
To our knowledge, no previous studies have published fluid shifts during dynamic forearm exercise. Interestingly, the arterio-venous fluid loss during exercise for ΔPVa-v was only ∼3% and for ΔBVa-v∼2%. These were minor compared with the corresponding ∼14% and ∼9% losses during high-intensity sprint cycling exercise (McKenna et al. 1997a), reflecting the far smaller size of contracting muscle and also the high blood flow observed. Consequently, dynamic finger flexor exercise invoked considerable fluxes of strong ions across the contracting finger flexor muscles, with dramatic increases at fatigue in plasma fluxes of, K+ and Lac−, and decline in plasma Cl− flux, together with large increases in arterio-venous H+ and HCO3− differences. Thus, despite the small contracting muscle mass, dramatic increases in ionic exchanges were observed.
The small peak rise in [K+]v during finger flexion exercise (∼1 mmol l−1) was similar to that observed during 5 min of dynamic handgrip exercise at a similar relative work rate (MacDonald et al. 2001) and also during incremental wrist flexion exercise (Raymer et al. 2004). However, these [K+]v increases were small compared with 30 s sprint exercise with a large muscle mass, where the peak increases in plasma [K+]a and of [K+]v were ∼2.6 and 3.8 mmol l−1, respectively, at a far higher power output (McKenna et al. 1997b). An undershoot of [K+]v below initial resting values was observed during recovery, consistent with observations in most other forms of exercise, most probably due to the concomitantly decreased muscle interstitial [K+] (Sejersted & Sjøgaard, 2000). Due to the movement of K+ and water in opposite directions across the contracting muscle membrane, the [K+]a-v and K+ fluxes were corrected for ΔPVa-v (McKenna et al. 1997a). An important finding was that no widening of the [K+]a-v was apparent at fatigue in the control trial, which contrasts with previous reports during isometric quadriceps contractions (Verburg et al. 1999) and cycling (Sahlin & Broberg, 1989). This suggests that under these conditions there was not a sudden rise in muscle K+ loss that precipitated muscle fatigue.
Loss of Cl− but not Na+ from plasma during finger flexion exercise
There was a clear net Cl− efflux from plasma throughout exercise, with a 156-fold increase in efflux observed from rest to fatigue. This is consistent with muscle Cl− uptake during intense large muscle mass exercise (McKenna et al. 1997a). Presumably most of this Cl− entered the forearm muscle with increases in both the interstitial and intracellular spaces (Sjøgaard et al. 1985), although some Cl− shift into the red blood cells may also have occurred (Prange et al. 2001). In isolated rat muscle, low extracellular [Cl−] reduced force and high Cl− conductance may counter, in part, a hyperkalaemic-induced reduction in membrane potential (Cairns et al. 2004). Thus it is possible that muscle Cl− influx during exercise in human muscle contractions may similarly act to preserve muscle function and delay fatigue.
The Na+ fluxes during exercise and at fatigue were inconsistent and differed to large muscle mass exercise. Whilst the fatigue data indicate an apparent Na+ influx into plasma, suggesting Na+ release by the contracting muscles, this is quite unlikely. First, the arterio-venous Na+ difference fluctuated throughout exercise, without a clear direction of net Na+ movement. Second, there was considerable between-subject variability in the calculated muscle Na+ fluxes during exercise and early recovery (note high s.e.m. for [Na+]a-v). The lack of consistent Na+ fluxes observed also match with reports of no net muscle Na+ uptake during moderate intensity arm cranking exercise (Volianitis & Secher, 2002), or during low and moderate intensity cycling (Wasserman et al. 1997). The lack of net Na+ exit from plasma appears consistent with the very small arterio-venous fluid shifts observed during finger flexion exercise, in contrast to a clear Na+ loss from the circulating plasma across the muscle seen in high-intensity cycling exercise (McKenna et al. 1997a).
Acid–base changes with forearm exercise
The small contracting muscle mass was insufficient to perturb arterial acid–base status, whereas an ∼20% increase in venous [H+] during exercise was observed. The origin of this venous acidosis can be ascertained through analysis of the independent variables determining [H+], namely PCO2, [SID] and [ATot] (Johnson et al. 1996). The rise in venous [H+] is consistent with the sharp increase in PCO2, similar to observations made during intense leg cycling exercise (Kowalchuk et al. 1988; McKenna et al. 1997a), being attenuated by the corresponding ∼8 mmol l−1 rise in venous [SID] during exercise in the control trial. The increased [SID]v was mainly influenced by an ∼7 mmol l−1 decrease in [Cl−]v with similar, opposing increases in [Na+] and [Lac−], and an ∼1 mmol l−1 rise in [K+]. The direction and magnitude of venous strong ion and [SID] changes in the circulation was similar to those described during handgrip exercise (Raymer et al. 2004) and during low-intensity cycling exercise (Miller et al. 2005), but clearly differ from the decreased plasma SID observed at the end of high-intensity cycling exercise (McKenna et al. 1997b). In recovery, whilst [SID] declined, the sharp decline in PCO2 allowed a decline in [H+]v. Increased plasma [HCO3−]v and thus a more negative [HCO3−]a-v during exercise are consistent with the effects of increases in Pv,CO2 and [SID]v (Johnson et al. 1996). Whilst plasma [ATot] was not assessed in this study, this reflects plasma protein concentration, which was probably slightly increased with exercise due to the small decline in plasma volume. The resultant increase in [ATot] would therefore tend to increase [H+]v during finger flexion exercise.
Alkalosis enhanced finger flexion exercise performance
An important finding was that Alk clearly enhanced performance during small muscle group contractions, with time to fatigue delayed by 25%. Our exercise findings are in broad agreement with numerous human exercise studies utilizing a large muscle mass, that demonstrated 19–49% increases in performance with alkalosis (Sutton et al. 1981; Costill et al. 1984; Iwaoka et al. 1989). This study advances a previous finding of performance enhancement in a small exercising muscle mass with Alk (Raymer et al. 2004), through analysis of arterio-venous ion fluxes across contracting muscle.
Alkalosis effects on K+ during exercise
Following alkalosis, subjects were mildly hypokalaemic at rest, consistent with previous findings in humans (Lindinger et al. 1999; Raymer et al. 2004) and in dogs (Suzuki et al. 1990). A possible mechanism is Na+–H+ antiporter-mediated Na+–H+ exchange, with consequent increased intracellular [Na+] (Lindinger et al. 1990), driving increased Na+,K+-ATPase activity (Sabatini, 1996; Weinman & Shenolikar, 1993) and lowering extracellular [K+]. With alkalosis, both [K+]a and [K+]v remained systematically lower throughout exercise and recovery. Although we did not measure muscle interstitial [K+], it is likely that this was also lower during Alk (Street et al. 2005). Muscle force is substantially depressed by a reduced intracellular-to-extracellular [K+] ratio (Cairns et al. 1995; Nielsen et al. 1998). It is therefore conceivable that reduced plasma [K+] contributes to lower muscle extracellular [K+] with Alk, maintaining a high intracellular-to-extracellular [K+] ratio and thus muscle membrane excitability during exercise, thereby contributing to the observed improvement in muscle performance.
We hypothesized that Alk would reduce peak muscle K+ release, based on findings in isolated contracting rat muscles (Lindinger et al. 1990). In contrast, muscle K+ release at fatigue was actually 49% greater in Alk compared with Con (∼64 versus ∼43 µmol min−1, respectively). This magnitude of K+ release was similar to that observed (∼69 µmol min−1) across the gastrocnemius muscle during 3 min of plantar flexion, at a similar low work rate of 2.6 W (Green et al. 2000). The greater K+ release was not influenced by differences in fluid shifts, as the arterio-venous decline in plasma volume was small and unchanged by Alk. The increased K+ release at fatigue with Alk must be considered in the context of the ∼17% greater K+ uptake rates into previously contracting muscles during recovery in Alk. Greater muscle K+ re-uptake most probably reflects an increased muscle Na+,K+-ATPase activity and possibly Na+–K+–2Cl− co-transporter activity (Gosmanov et al. 2003). This was unlikely to be mediated by catecholamines as these were barely elevated and did not differ between trials. Thus increased K+ release at fatigue in Alk probably occurred in the face of augmented muscle Na+,K+-ATPase activity, suggesting increased opening of some K+ channels. It is unlikely that this greater K+ leak occurred via K+ATP channels as numerous studies show that intracellular [H+] is reduced with Alk at rest and/or during exercise (Neilsen et al. 2002; Stephens et al. 2002; Raymer et al. 2004). It is possible that voltage-sensitive K+ channels are responsible for the majority of K+ leak across the sarcolemma during Alk. Increased Na+,K+-ATPase activity would probably exert a greater electrogenic effect, which together with lower extracellular fluid [K+], would act to preserve muscle membrane potential and excitability with Alk. This suggests that maintenance of a [K+] gradient for K+ dissipation during exercise is important for muscle performance (Lindinger et al. 1995).
Alkalosis affects plasma Na+ and Cl− regulation
Arterial plasma [Na+] was higher and [Na+]v tended to be higher with Alk, consistent with previous reports of increased [Na+]v (Lindinger et al. 1999; Raymer et al. 2004; Street et al. 2005) and [Na+]a with oral NaHCO3 intake (Lindinger et al. 1999). Alk did not modulate either the arterio-venous Na+ difference or the calculated muscle Na+ fluxes during exercise and early recovery and these measures were quite variable. However, elevated systemic [Na+] and thus Na+ delivery to muscles may be important in enhancing muscle function with Alk by providing a higher plasma–interstitium–intracellular gradient and thereby preserving t-tubular [Na+]. Similarly, Alk effects on Cl− may also be important to delayed muscle fatigue. Both [Cl−]v and muscle Cl− fluxes during exercise were higher with Alk, which together with elevated muscle Cl− conductance, may stabilize the cell membrane during contractions, as demonstrated under non-physiological conditions in mice muscles (Cairns et al. 2004).
Alkalosis interpreted via a physicochemical approach
Alkalosis increased venous PCO2 (∼5 mmHg), arterial CO2 content (∼90 ml l−1), venous CO2 content (∼70 ml l−1), and decreased venous [SID] (∼7 mmol l−1). No difference was found between trials for arterial PCO2 and [SID]. Few studies have examined the effects of alkalosis during exercise using the Stewart physico-chemical approach to acid–base balance. The lower [SID]v with Alk was the result of systematic ionic changes during exercise, predominantly contributed to by a greater [Cl−] (∼80% of lower [SID] in Alk) and [Lac−] (∼1%) and by a decrease in [K+] (∼3%), partially countered by a greater [Na+] (∼16%). These findings differ from estimates of a greater increase in [SID]v during alkalosis, where [Lac−]v and [Cl−]v were less than in the control trial (Raymer et al. 2004). The lower [SID]v during recovery in Alk was mainly due to the higher [Lac−]v. The small increments in [Na+]v of ∼2–3 mmol l−1 in control and Alk trials were balanced by ∼2–3 mmol l−1 increments in [Lac−]v, which were almost identical to the changes observed in stimulated rat muscle following alkalosis (Lindinger et al. 1990). The effects of Alk on increasing arterial and venous [HCO3−] can be mainly attributed to the increased PCO2, although the lesser decline in [HCO3−]a-v during exercise in Alk may be attributed to a smaller rise in [SID]v. The lower [H+] with Alk cannot be explained by either [SID], which was lower, or the PCO2, which was higher with Alk.
During Alk, Lac− flux into plasma was greater at fatigue, probably reflecting both enhanced glycolytic ATP production and augmented Lac− transport from exercising muscle (Sutton et al. 1981; Juel, 1997). However, it is unlikely that lactate played a significant fatiguing role in the present study (Posterino et al. 2001). Increased acidosis was found to have little effect on glycolysis or fatigue in humans (Bangsbo et al. 1996) or in isolated muscle preparations (Westerblad & Länergren, 1988). Indeed, in isolated rat muscle preparations, intracellular acidosis had a protective effect on excitability, and performance (Nielsen et al. 2001; Pedersen et al. 2004), although this has been recently challenged (Kristensen et al. 2004). Interestingly, a number of in situ and in vivo31P-MRS studies have demonstrated a strong correlation between a decline in intracellular pH and force production (Wilson et al. 1988; Miller et al. 1988; Raymer et al. 2004). Greater blood lactate during alkalosis may in addition have been due to reduced uptake by non-contracting tissue (Hollidge-Horvat et al. 2000).
Summary
Dynamic, concentric contractions of the finger flexor muscles barely perturbed systemic acid–base and ionic status but induced marked ionic exchanges across the contracting muscle, along with a dramatic increase in blood flow and metabolism. Muscle function was substantially improved with alkalosis. Responses of each of the strong ions K+, Na+, Cl− and Lac− during exercise were modified by alkalosis and their combined effects probably contributed to the improvement in muscle performance. Whilst increased muscle K+ efflux was observed at fatigue in alkalosis, the lower arterial and venous [K+], and presumably lower interstitial [K+], suggests preservation of the intracellular-to-extracellular [K+] gradient with alkalosis. Furthermore, the greater K+ reuptake in recovery suggests an increase in muscle Na+,K+-ATPase activity. The higher Na+ delivery to muscle and enhanced Cl− uptake by muscle would probably act in concert to further stabilize the muscle membrane in the face of exercise-induced hyperkalaemia. Together these suggest preservation of membrane excitability with alkalosis. Finally, enhanced Lac− release with alkalosis would act to reduce intracellular [H+], but whether this affects cellular excitability is unclear. Thus a major beneficial effect of alkalosis on muscle performance might be consequent to the integrated effects on K+, Na+, Cl− and Lac− homeostasis.
Acknowledgments
The authors thank Dr Al Cimmino for his technical assistance. We thank Dr Steve Fraser for assistance on some trial days; Dr Andrew Bjorksten for conducting the catecholamine assays; and the many selfless volunteers who dedicated their time to these trials. The paper is dedicated to the memory of our wonderful, inspiring colleague and mentor, Dr Sandford L. Skinner, who passed away on May 29th, 2005.
References
- Balog EM, Fitts RH. Effects of fatiguing stimulation on intracellular Na+ and K+ in frog skeletal muscle. J Appl Physiol. 1996;81:679–685. doi: 10.1152/jappl.1996.81.2.679. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Graham T, Johansen L, Strange S, Christensen C, Saltin B. Elevated muscle acidity and energy production during exhaustive exercise in humans. Am J Physiol Regul Integr Comp Physiol. 1992;263:R891–R899. doi: 10.1152/ajpregu.1992.263.4.R891. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzoni T, Ngo L, Hiltbrand E, Springett R, Deply D. Non-standard O2 consumption-temperature curves during rest and isometric exercise in human skeletal muscle. Comp Biochem Physiol a Mol Integr Physiol. 2002;132:27–32. doi: 10.1016/s1095-6433(01)00525-6. [DOI] [PubMed] [Google Scholar]
- Bouissou P, Defer G, Guezennec CY, Estrade PY, Serrurier B. Metabolic and blood catecholamine responses to exercise during alkalosis. Med Sci Sports Exerc. 1988;20:228–232. doi: 10.1249/00005768-198806000-00003. [DOI] [PubMed] [Google Scholar]
- Brimacombe JR, MaCfie AG, McCrirrick A. The extensometer. Potential applications in anaesthesia and intensive care. Anaesthesia. 1991;46:756–761. doi: 10.1111/j.1365-2044.1991.tb09773.x. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relationship between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Ruzhynsky V, Renaud JM. Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C762–C770. doi: 10.1152/ajpcell.00589.2003. [DOI] [PubMed] [Google Scholar]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Costill DL, Verstappen F, Kuipers H, Janssen E, Fink W. Acid-base balance during repeated bouts of exercise: influence of HCO3. Int J Sports Med. 1984;5:228–231. doi: 10.1055/s-2008-1025910. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fraser SF, Li JL, Carey MF, Wang XN, Sangkabutra T, Sostaric S, Selig SE, Kjeldsen K, McKenna MJ. Fatigue depresses maximal in vitro skeletal muscle Na+-K+-ATPase activity in untrained and trained individuals. J Appl Physiol. 2002;93:1650–1659. doi: 10.1152/japplphysiol.01247.2001. [DOI] [PubMed] [Google Scholar]
- Gosmanov AR, Lindinger MI, Thomason DB. Riding the tides: K+ concentration and volume regulation by muscle Na+-K+-2Cl− cotransport activity. News Physiol Sci. 2003;18:196–200. doi: 10.1152/nips.01446.2003. [DOI] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bulow J, Kjar M. Interstitial and arterial–venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol. 2000;529:849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS, Shoemaker JK. Is the blood flow response to a single contraction determined by work performed? J Appl Physiol. 2004;96:2146–2152. doi: 10.1152/japplphysiol.00779.2003. [DOI] [PubMed] [Google Scholar]
- Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJF. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab. 2000;278:E316–E329. doi: 10.1152/ajpendo.2000.278.2.E316. [DOI] [PubMed] [Google Scholar]
- Iwaoka K, Okagawa S, Mutoh Y, Miyashita M. Effects of bicarbonate ingestion on the respiratory compensation threshold and maximal exercise performance. Jpn J Physiol. 1989;39:255–265. doi: 10.2170/jjphysiol.39.255. [DOI] [PubMed] [Google Scholar]
- Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999;84:1007–1010. doi: 10.1210/jcem.84.3.5522. [DOI] [PubMed] [Google Scholar]
- Johnson LJ, Heigenhauser GJF, Hsia CCW, Jones NL, Wagner PD. Determinants of gas exchange and acid-base balance during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. American Physiological Society: 1996. pp. 515–584. [Google Scholar]
- Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflugers Arch. 1986;406:458–463. doi: 10.1007/BF00583367. [DOI] [PubMed] [Google Scholar]
- Juel C. Lactate-proton cotransport in skeletal muscle. Physiol Rev. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kesl LD, Engen RL. Effects of NaHCO3 loading on acid-base balance, lactate concentration, and performance in racing greyhounds. J Appl Physiol. 1998;85:1037–1043. doi: 10.1152/jappl.1998.85.3.1037. [DOI] [PubMed] [Google Scholar]
- Kowalchuk JM, Heigenhauser GJ, Lindinger MI, Sutton JR, Jones NL. Factors influencing hydrogen ion concentration in muscle after intense exercise. J Appl Physiol. 1988;65:2080–2089. doi: 10.1152/jappl.1988.65.5.2080. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Albertsen J, Rentsch M, Juel C. Lactate and force production in skeletal muscle. J Physiol. 2004;562:521–526. doi: 10.1113/jphysiol.2004.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Li JL, Wang XN, Carey MF, Wrigley TV, McKenna MJ. Effects of fatigue and training on sarcoplasmic reticulum Ca2+ regulation in human skeletal muscle. J Appl Physiol. 2002;92:912–922. doi: 10.1152/japplphysiol.00643.2000. [DOI] [PubMed] [Google Scholar]
- Linderman J, Fahey TD. Sodium bicarbonate ingestion and exercise performance. An update. Sports Med. 1991;11:71–77. doi: 10.2165/00007256-199111020-00001. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG, Heigenhauser GJ. Role of skeletal muscle in plasma ion and acid-base regulation after NaHCO3 and KHCO3 loading in humans. Am J Physiol. 1999;276:R32–R43. doi: 10.1152/ajpregu.1999.276.1.R32. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Heigenhauser GJF, Spriet LL. Effects of alkalosis on muscle ions at rest and with intense exercise. Can J Physiol Pharmacol. 1990;68:820–829. doi: 10.1139/y90-125. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, McKelvie RS, Heigenhauser GJF. K+ and Lac− distribution in humans during and after high-intensity exercise: role in muscle fatigue attenuation? J Appl Physiol. 1995;78:765–777. doi: 10.1152/jappl.1995.78.3.765. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Naylor HL, Tschakovsky ME, Hughson RL. Peripheral circulatory factors limit rate of increase in muscle O2 uptake at onset of heavy exercise. J Appl Physiol. 2001;90:83–89. doi: 10.1152/jappl.2001.90.1.83. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJF, McKelvie RS, MacDougall JD, Jones NL. Sprint training enhances ionic regulation during intense exercise in men. J Physiol. 1997a;501:687–702. doi: 10.1111/j.1469-7793.1997.687bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJF, McKelvie RS, Obminski G, MacDougall JD, Jones NL. Enhanced pulmonary and active skeletal muscle gas exchange during intense exercise after sprint training in men. J Physiol. 1997b;501:703–716. doi: 10.1111/j.1469-7793.1997.703bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, Boska MD, Moussavi RS, Carson PJ, Weiner MW. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J Clin Invest. 1988;81:1190–1196. doi: 10.1172/JCI113434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Lindinger MI, Fattor JA, Jacobs KA, Leblanc PJ, Duong M, Heigenhauser GJF, Brooks GA. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J Appl Physiol. 2005;98:856–865. doi: 10.1152/japplphysiol.00753.2004. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Hein L, Svendsen LB, Secher NH, Quistorff B. Bicarbonate attenuates intracellular acidosis. Acta Anaesthesiol Scand. 2002;46:579–584. doi: 10.1034/j.1399-6576.2002.460516.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C, Bangsbo J. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol. 2004;554:857–870. doi: 10.1113/jphysiol.2003.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Hilsted L, Clausen T. Excitation-induced force recovery in potassium-inhibited rat soleus muscle. J Physiol. 1998;512:819–829. doi: 10.1111/j.1469-7793.1998.819bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, De Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J General Physiol. 2005;125:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Perrey S, Tschakovsky ME, Hughson RL. Muscle chemoreflex elevates muscle blood flow and O2 uptake at exercise onset in nonischemic human forearm. J Appl Physiol. 2001;91:2010–2016. doi: 10.1152/jappl.2001.91.5.2010. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Dutka TL, Lamb GD. L+-lactate does not affect twitch and tetanic responses in mechanically skinned mammalian muscle fibres. Pflugers Arch. 2001;442:197–203. doi: 10.1007/s004240100528. [DOI] [PubMed] [Google Scholar]
- Potteiger JA, Webster MJ, Nickel GL, Haub MD, Palmer RJ. The effects of buffer ingestion on metabolic factors related to distance running performance. Eur J Appl Physiol Occup Physiol. 1996;72:365–371. doi: 10.1007/BF00599698. [DOI] [PubMed] [Google Scholar]
- Prange HD, Shoemaker JL, Jr, Westen EA, Horstkotte DG, Pinshow B. Physiological consequences of oxygen dependent chloride binding to hemoglobin. J Appl Physiol. 2001;91:33–38. doi: 10.1152/jappl.2001.91.1.33. [DOI] [PubMed] [Google Scholar]
- Putman CT, Jones NL, Heigenhauser GJF. Effects of short-term training on plasma acid–ase balance during incremental exercise in man. J Physiol. 2003;550:585–603. doi: 10.1113/jphysiol.2003.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymer GH, Marsh GD, Kowalchuk JM, Thompson RT. Metabolic effects of induced alkalosis during progressive forearm exercise to fatigue. J Appl Physiol. 2004;96:2050–2056. doi: 10.1152/japplphysiol.01261.2003. [DOI] [PubMed] [Google Scholar]
- Renaud JM, Gramolini A, Light P, Comtois A. Modulation of muscle contractility during fatigue and recovery by ATP sensitive potassium channel. Acta Physiol Scand. 1996;156:2003–2012. doi: 10.1046/j.1365-201X.1996.210000.x. [DOI] [PubMed] [Google Scholar]
- Rolett EL, Strange S, Sjøgaard G, Kiens B, Saltin B. B2-drenergic stimulation does not prevent potassium loss from exercising quadriceps muscle. Am J Physiol Reg Int Comp Physiol. 1990;258:R1192–R1200. doi: 10.1152/ajpregu.1990.258.5.R1192. [DOI] [PubMed] [Google Scholar]
- Sabatini S. The cellular basis of metabolic alkalosis. Kidney Int. 1996;49:906–917. doi: 10.1038/ki.1996.125. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Broberg S. Release of K+ from muscle during prolonged dynamic exercise. Acta Physiol Scand. 1989;136:293–294. doi: 10.1111/j.1748-1716.1989.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Sangkabutra T, Crankshaw DP, Schneider C, Fraser SF, Sostaric S, Mason K, Burge CM, Skinner SL, McMahon LP, McKenna MJ. Impaired K+ rgulation contributes to exercise limitation in end-stage renal failure. Kidney Int. 2003;63:283–290. doi: 10.1046/j.1523-1755.2003.00739.x. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol Regul Integr Comp Physiol. 1985;248:R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, McKenna MJ, Canny BJ, Snow RJ, McConell GK. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Ex. 2002;34:614–621. doi: 10.1097/00005768-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Street D, Nielsen JJ, Bangsbo J, Juel C. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol. 2005;566:481–489. doi: 10.1113/jphysiol.2005.086801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JR, Jones NL, Toews CJ. Effect of pH on muscle glycolysis during exercise. Clin Sci. 1981;61:331–338. doi: 10.1042/cs0610331. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hishida A, Ohishi K, Kimura M, Honda N. Role of hormonal factors in plasma K+ alterations in acute respiratory and metabolic alkalosis in dogs. Am J Physiol. 1990;258:F305–F310. doi: 10.1152/ajprenal.1990.258.2.F305. [DOI] [PubMed] [Google Scholar]
- Van Beekvelt MCP, Colier WNJM, Wevers RA, Van Engelen BGM. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol. 2001a;90:511–519. doi: 10.1152/jappl.2001.90.2.511. [DOI] [PubMed] [Google Scholar]
- Van Beekvelt MCP, Shoemaker JK, Tschakovsky ME, Hopman MTE, Hughson RL. Blood flow and muscle oxygen uptake at the onset and end of moderate and heavy dynamic forearm exercise. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R1741–R1747. doi: 10.1152/ajpregu.2001.280.6.R1741. [DOI] [PubMed] [Google Scholar]
- Verbitsky O, Mizrahi J, Levin M, Isakov E. Effect of ingested sodium bicarbonate on muscle force, fatigue, and recovery. J Appl Physiol. 1997;83:333–337. doi: 10.1152/jappl.1997.83.2.333. [DOI] [PubMed] [Google Scholar]
- Verburg E, Hallen J, Sejersted OM, Vollestad NK. Loss of potassium from muscle during moderate exercise in humans: a result of insufficient activation of the Na+-K+-pump? Acta Physiol Scand. 1999;165:357–367. doi: 10.1046/j.1365-201X.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vøllestad NK, Hallen J, Sejersted OM. Effect of exercise intensity on potassium balance in muscle and blood of man. J Physiol. 1994;475:359–368. doi: 10.1113/jphysiol.1994.sp020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K, Stringer WW, Casaburi R, Zhang YY. Mechanism of the exercise hyperkalemia: an alternate hypothesis. J Appl Physiol. 1997;83:631–643. doi: 10.1152/jappl.1997.83.2.631. [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Shenolikar S. Regulation of the renal brush border membrane Na+-H+ exchanger. Annu Rev Physiol. 1993;55:289–304. doi: 10.1146/annurev.ph.55.030193.001445. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand. 1988;133:83–89. doi: 10.1111/j.1748-1716.1988.tb08383.x. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Mccully KK, Mancini DM, Boden B, Chance B. Relationship of muscular fatigue to pH and diprotonated Pi in humans: a 31P-NMR study. J Appl Physiol. 1988;64:2333–2339. doi: 10.1152/jappl.1988.64.6.2333. [DOI] [PubMed] [Google Scholar]