Abstract
Smooth muscles from the urethra and bladder display characteristic patterns of spontaneous contractile activity in the filling phase of the micturition cycle. Tonic contractions are seen in the urethral smooth muscles, and phasic contractions occur in the detrusor. Overactivity in the detrusor is a common clinical problem. The ion channels in the smooth muscle membranes play an important role in determining the functional properties, and are obvious targets for treatment of the overactive bladder. Recent evidence suggests that interstitial cells may also play a role in determining the pattern of spontaneous activity, although their precise role is less well established in the urinary tract than in the gut. The ion channels involved in these cells are also of interest. This review discusses what is known of ion channels in these tissues, and their implications for function.
In this brief review, the intention is to discuss the relationship between the types and properties of the ion channels that are present in the lower urinary tract smooth muscles, and the overall functions of the organs in which the smooth muscles occur. Particular attention will be paid to the activity in the filling phase of the micturition cycle. Any attempt to link mechanisms and function will need to take into account differences between the species that are used for the experimental work.
The common function of the bladder in mammals is to store and expel urine; there should therefore be underlying similarities in the properties of the detrusor in all species. However, there will be an important functional difference in those mammals that use urine as a territorial scent marker, since this requires a mechanism to produce small spurts of urine in addition to a mechanism that will empty the bladder. Evidence suggests that the purinergic and cholinergic functions of the parasympathetic innervation have evolved to achieve this distinction. Thus one might expect the properties of the unstimulated detrusor to be similar in the different species.
In contrast the properties of the urethral smooth muscles may be quite different. Although the underlying functions of all urethras are to prevent leakage of urine during filling by generating a urethral closure pressure, and to allow voiding at micturition, the physical constraints will vary between the species. Variable factors will be the size, the geometry of the lower urinary tract, the pressures that are likely to arise in the bladder during filling and how these are transferred to the urethra (including gravitational forces), and the role of the striated muscles that are also involved in controlling the urethral pressures. In small mammals the resting urethral pressures are quite low, and in large mammals and man they can be high. The involvement of the autonomic innervation and the contribution of the urethral striated muscles to urethral closure pressure varies widely, and spontaneous oscillations in urethral pressure occur in some species during micturition and appear to play a role in expelling urine. Significant differences may thus be expected between the species.
Spontaneous activity
Smooth muscles in both bladder (trigone and detrusor) and urethra show spontaneous contractile activity during the filling phase. In the human urethra, tonic spontaneous contractile activity in the smooth muscles generates a significant proportion of the closure pressure which allows urine to remain in the bladder against the forces of gravity. Additional force applied to the bladder wall by abdominal pressure generated during normal human activity, is counteracted by reflexes enhancing smooth muscle contractile activity through the sympathetic nervous system, and enlisting urethral striated muscle activation through the somatic nerves. L-type Ca2+ channels are required to maintain the smooth muscle tone which falls with Ca2+ channel blockers. Relaxation of the urethral smooth muscle to allow micturition requires activation of inhibitory nitrergic nerves to cause a rapid drop in urethral pressure. It is not clear how this is achieved, but the myocytes respond to nitric oxide with a rise in cGMP (Smet et al. 1996).
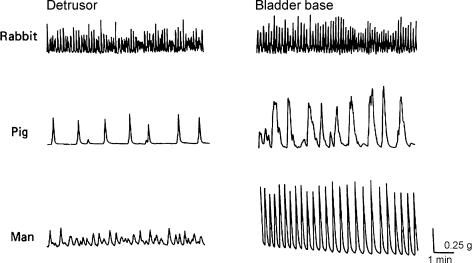
The type of spontaneous mechanical activity in the detrusor is more unusual, and fairly unique for a smooth muscle. Isolated detrusor strips often generate phasic contractions, with frequencies that are species specific, but usually of the order of tens of contractions each minute (Sibley, 1984; Fig. 1). The contractions are small compared with the contraction that can be evoked by stimuli; they rise and fall from a low resting tone, and do not normally show the type of tetanic fusion that is seen in other electrically excitable smooth muscles. This spontaneous activity can be seen in the whole bladder as relatively isolated contractions of parts of the bladder wall. These contractions have been called ‘micromotions’ (Coolsaet, 1985; Coolsaet et al. 1993) and have been studied in isolated guinea-pig and mouse bladders (Drake et al. 2003; Gillespie, 2004) where they may, in the full bladder, occupy sufficient of the detrusor to be associated with very small changes in intravesical pressure. In larger bladders the detrusor is so compliant that small contractions of part of the wall are unlikely to raise the pressure significantly.
Figure 1. Examples of spontaneous mechanical activity in strips dissected from the bladders of rabbit, pig and man.
From Sibley (1984).
The role of the spontaneous activity seems to be to allow the individual muscle bundles to adjust their length in response to filling. It would be impractical for the bladder simply to remain relaxed and floppy during filling, since contraction at micturition can only raise intravesical pressure once the bladder has taken a shape with the minimum surface area available to it. The normal bladder appears to maintain this shape throughout filling, allowing micturition to be started rapidly whenever it is convenient for the bladder to be emptied. Synchronization of contraction is achieved through the dense parasympathetic innervation (Daniel et al. 1983; Gabella, 1995), and nerve-evoked contraction is achieved largely through intracellular mechanisms (inositol 1,4,5-trisphosphate (IP3) production and Ca2+ sensitization) (Iacovou et al. 1990; Fry et al. 2004; Takahashi et al. 2004).
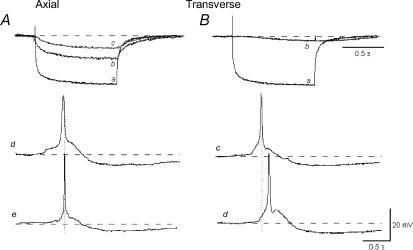
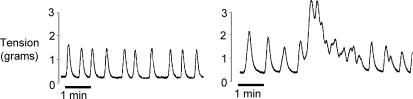
The fact that small areas of the detrusor contract in isolation is unusual for smooth muscles and suggests that the overall coupling between the myocytes is relatively poor. Detailed studies of coupling using microelectrodes and current injection indeed show that, although current spreads well in a muscle bundle in the axial direction, coupling in the transverse direction is poor (Fig. 2) (Bramich & Brading, 1996; Hashitani & Brading, 2003a). Indeed Tomita has shown, using extracellular polarization, that bladder is less well coupled than most other smooth muscles (Tomita, 1990; Parekh et al. 1990). Intuitively this seems a sensible property for the bladder – if activity were well coupled, spontaneous pressure changes would be expected, and this is undesirable for the storage of urine. However, in strips of detrusor obtained from overactive bladders, tetanic contractions more typical of well-coupled smooth muscles are often seen (Fig. 3) and give rise to the undesirable symptoms of the overactive bladder syndrome.
Figure 2. Electrical coupling between cells in a muscle bundle of pig detrusor.
A, axial direction. Intracellular current injected through the microelectrode hyperpolarized the cell (a). Simultaneous recording of the electrotonic potential change in a cell 200 μm (b) and 400 μm away (c) in the axial direction. Action potentials recorded simultaneously in two cells separated by 400 μm occur at the same time (d and e). B, transverse direction. Intracellular current injected through the microelectrode hyperpolarized the cell (a). Simultaneous recording of the electrotonic potential change in a cell 50 μm away (b). Action potentials recorded simultaneously in two cells separated by 100 μm occur with a delay (c and d). The resting potential was –40 mV. Adapted from Hashitani & Brading (2003a).
Figure 3. Spontaneous mechanical activity recorded from strips of human detrusor.
Left: from a normal bladder. Right: from the bladder of a patient with overactive bladder syndrome, showing tetanic contractions. Figure courtesy of I. Mills.
Ion channels and spontaneous activity in the urethra
Species studied
Probably because of the small size of the urethras in rats, guinea-pigs and mice, there are no current reports of the ionic channels present and their function in the urethral smooth muscles of these species, although microelectrode recordings in guinea-pig urethral smooth muscle has shown the presence of slow waves (Hashitani & Edwards, 1999). Structural and histological data are available for some of these small mammals, a few measurements of the urethral pressure have been published and some studies have attempted to assess the role of the smooth muscles in urethral function. It is, however, easier to obtain urethral smooth muscles for electrophysiological and functional evaluation from larger mammals, and most studies using strips or isolated myocytes have been carried out on urethral smooth muscles from rabbits and pigs. In the pig, which is thought to be the most suitable animal model for the human (Crowe & Burnstock, 1989; Greenland et al. 1996), detailed studies are available on smooth muscle structure, contractile activity and innervation (e.g. Bridgewater et al. 1993, 1995). On the electrophysiological side, however, membrane potential and ion channels have only been studied to any extent on isolated urethral myocytes using patch clamp techniques (Brading et al. 1996; Teramoto & Brading, 1996), and although the myocytes seem to be electrically inexcitable, there is no information available about the spontaneous electrical properties of strips of pig urethral smooth muscle. In the rabbit, several studies on the properties of strips are available (e.g. Mattiasson et al. 1990; Andersson et al. 1991; Hashitani et al. 1996), and it is clear that slow waves are present and the strips electrically excitable, but almost nothing is known about the functional characteristics of the urethra in vivo, and relatively little about individual ion channels in the smooth muscles. This is a great pity because there seem to be considerable differences between the two species, and the excellent studies by the Belfast group (e.g. Sergeant et al. 2000; Hollywood et al. 2003a; Johnston et al. 2005) into one of the most exciting areas currently under investigation, that is the role of the interstitial cells in these tissues, began and has continued in rabbit urethra. However, it may not be possible to extrapolate from the rabbit to the pig and human – more studies on these three species are badly needed.
Potassium channels in the urethra
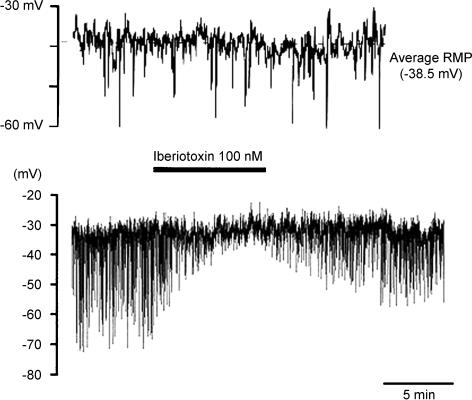
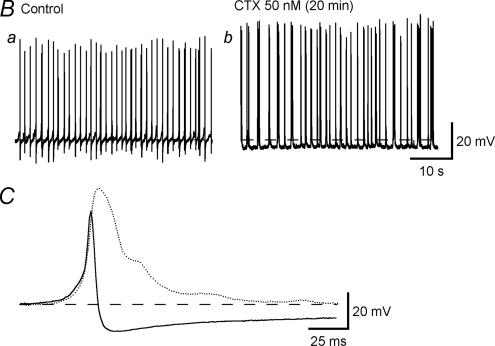
Extensive patch clamp experiments examining K+ channel properties in isolated pig urethral myocytes have been undertaken by Teramoto (e.g. Brading et al. 1996; Teramoto & Brading, 1996). Under current clamp conditions with relatively low intracellular Ca2+ buffering, the myocytes do not show any excitable behaviour, but spontaneous transient hyperpolarizations are seen that are blocked by iberiotoxin (Fig. 4). The use of various K+ channel blockers leads to the conclusion that the myocytes have predominantly three types of K+ channel: small and large Ca2+-activated K+ channels, and KATP channels. Isolated strips generate continuous spontaneous tone (Fig. 5) which is dependent on Ca2+ entry and is reduced by L-type Ca2+ channel blockers (which also slightly depolarize the cells) and also by NO donors and KATP openers. Whereas depolarizing voltage steps in most myocytes initiate sustained outward currents, a few cells show an initial transient component suggesting that some A-type K+ currents may also be present.
Figure 4. Membrane potential of pig urethral myocytes under current clamp conditions.
Whole cell recordings with a pipette solution containing 140 mm KCl, 2 mm Mg2+, 2 mm ATP and 50 μm EGTA. Bath solution contained 140 mm NaCl, 2 mm Ca2+, 5 mm KCl and 1.2 mm Mg2+. Upper trace, under normal depolarizations. Lower trace more compressed, showing that the maxi-K channel blocker iberiotoxin blocks the large spontaneous hyperpolarizations. Figure courtesy of N. Teramoto.
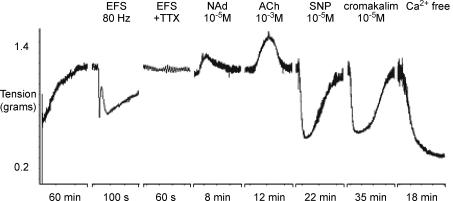
Figure 5. Contractile activity in a strip of smooth muscle dissected from a pig urethra.
The tissue develops spontaneous tone after an initial tensioning to 1 g. It responds to stimulation of its intrinsic nerves (EFS) with a biphasic relaxation which is blocked by TTX. Applications of noradrenaline (NAd) and acetylcholine (ACh) contract the tissue further, but the nitric oxide donor sodium nitroprusside (SNP) and the KATP channel opener cromakalim both relax the tissue, as does removal of extracellular calcium. Adapted from Greenland et al. (1996).
Calcium channels in the urethra
Although the isolated myocytes of the pig urethra are not electrically excitable, the ability of L-type Ca2+ channels blockers to relax spontaneous tone shows that these channels are clearly present and open in the normal unstimulated strips. When the K+ channels are blocked with Cs+ in the pipette, small transient inward currents can be evoked with a window current in a range that would encompass the normal resting potential of the cells (unpublished observations). In the rabbit and human urethra the myocytes have been shown to possess both L- and T-type Ca2+ channels (Hollywood et al. 2003b; Bradley et al. 2004).
Interstitial cells in the urethra
The rabbit isolated urethral strips do not develop spontaneous tone, but microelectrode studies (Hashitani et al. 1996) show that the tissue develops regular slow waves and presumably under the right conditions, these slow waves may give rise to action potentials and phasic contractions. Similar slow waves have been recorded in guinea-pig urethra (Hashitani & Edwards, 1999). In 2000, the Belfast group (Sergeant et al. 2000) published striking evidence that the rabbit urethra possessed interstitial cells with structural and morphological properties similar to those found in the interstitial cells of Cajal in the gut. They isolated and recorded from these cells and showed that they generated slow waves very similar to the slow waves recorded in the urethral smooth muscles with microelectrodes (Hashitani et al. 1996). Ca2+ imaging demonstrated that the majority of these interstitial cells show spontaneous oscillations in [Ca2+]i. Evidence now strongly suggests that these slow waves are in fact generated by interstitial cells through a combination of Ca2+ release from the internal stores and the opening of Ca2+-activated Cl− channels (Hollywood et al. 2003a). Depolarizing current is then injected into the neighbouring smooth muscle cells to produce slow waves. The interstitial cells respond to transmitters, and the complex excitatory and inhibitory innervation that is found in the urethra may have input onto both cell types.
The presence of pacemaker cells in the urethra introduces another order of complexity into relating ion channels to function, and makes it difficult to investigate their role in determining the contractile activity of strips of tissue and of whole organs. The two types of cell have several mechanisms in common, and in particular Ca2+ release from the stores through IP3 and ryanodine receptors may occur in both types, but have different functions. L-type Ca2+ channels do not seem to be important in the interstitial cells, but Ca2+ entry is necessary, since slow waves cease rapidly in Ca2+-free solutions (Johnston et al. 2005). The interstitial cells and smooth muscle cells may also overlap in the other types of ion channels they possess, and also in their innervation by autonomic nerves. It thus seems that the situation in the urethra will turn out to be rather similar to that of the gastrointestinal tract, although the evidence to support this assumption is, at present, still being accumulated.
Relationship between ion channels and function in the urethra
L-type Ca2+ channels are important for the tone of the urethral smooth muscles during the filling stage of the micturition cycle. Overlapping activity of Ca2+-activated K+ channels probably prevents net inward currents and regenerative activity during sustained tone, but in species in which slow waves are generated by interstitial cells, oscillating membrane potentials and Ca2+ currents may allow the membranes to become excitable. Quite how this is linked with function is not known. The enhancement and switch-off of tone through the autonomic innervation may also function both directly on the smooth muscle and indirectly through input to the interstitial cells, and in both cases involve modulation of K+ channel function.
Ion channels and spontaneous activity in the detrusor
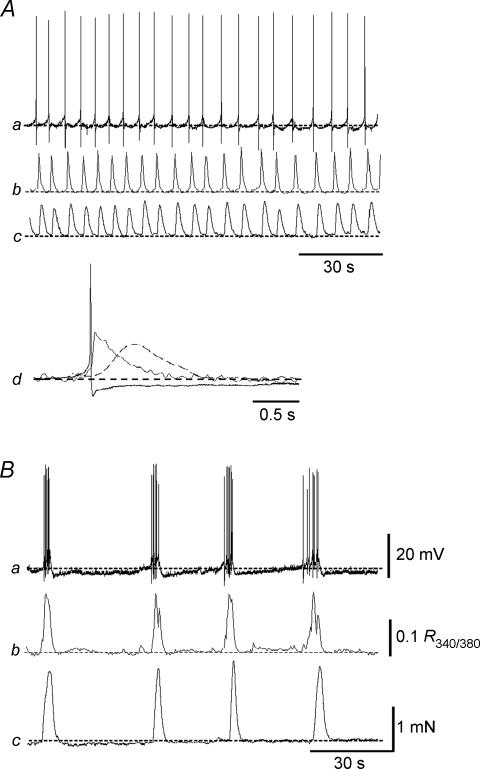
Detrusor myocytes are electrically excitable but do not show slow wave activity even when intact strip preparations are used. Microelectrode recordings of detrusor in various species show similar activity, and that the overall frequency of the action potentials is normally much higher than the spontaneous contractions seen in mechanical recordings from muscle strips. Careful examination of the spontaneous activity in detrusor preparations containing only one or a few muscle bundles has been undertaken by Hashitani (Hashitani et al. 2000, 2001, 2004a, b; Hashitani & Brading, 2003a, b), who used simultaneous recording of membrane potential and tension, and also fluorescent Ca2+ imaging to characterize the activity. In all species so far studied, two patterns of spike activity are seen – either continuous repetitive firing of single action potentials, or bursting activity. In small strips showing continuous firing, it is clear that rises in [Ca2+]i and contraction are correlated with individual action potentials. In those showing bursting activity the contractions and rises in [Ca2+]i occur synchronously with the bursts of action potentials (Fig. 6). The poor electrical coupling between bundles and the likelihood that they are contracting independently probably accounts for the relatively small and low frequency contractions seen in the size of strips (a few milligrams) normally used for tension recording.
Figure 6. Correlation between electrical and mechanical activity and intracellular calcium simultaneously recorded from two strips of guinea-pig detrusor.
In A spontaneous action potentials were generated individually, and in B the action potentials occurred in bursts. Aa and Ba, membrane potential. Ab and Bb, calcium transient. Ac and Bc, tension. Ad, on a faster time scale the three signals are superimposed. The action potential and calcium transient were followed by the contraction. From Hashitani et al. (2004a).
There is considerable interest in the ion channels involved in generating the spontaneous activity in the detrusor. Much of this interest is driven by the need to develop drugs to treat the symptoms of bladder overactivity, which include urgency and urge incontinence. It is known that in humans with overactive bladders and in animal models generating bladder overactivity, there is an increase in the spontaneous activity and also a change in the pattern of contractions suggesting that the smooth muscles are better synchronized in this condition (Brading, 1997; Turner & Brading, 1997). The overactive bladder syndrome (Abrams, 2000) is very widespread in the population, with a prevalence of about 16%, and there is a potentially enormous market for drugs to control the condition, since the current treatment, anti-muscarinic therapy, is less than ideal. Hence the interest in the potential benefit of modifying the ion channels involved.
Potassium channels in the detrusor
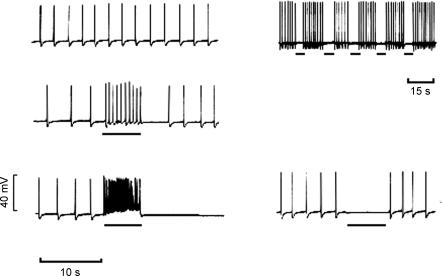
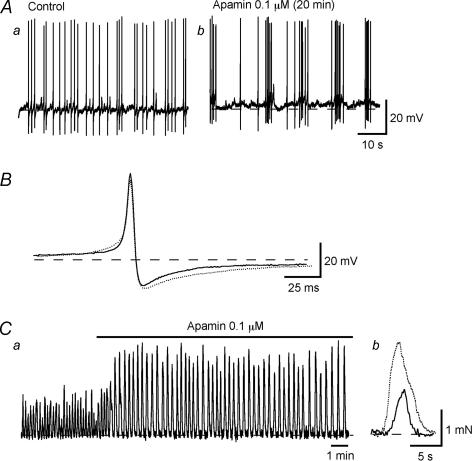
Initial studies on isolated cells using patch clamp techniques (Klockner & Isenberg, 1985a, b) suggested that the upstroke of the action potential was carried by Ca2+ and the repolarization involved activation of a voltage-sensitive K+ channel. Further work has been carried out using microelectrodes to determine the involvement of K+ channels in action potential generation (Fujii et al. 1990; Heppner et al. 1997; Hashitani & Brading, 2003a, b). It seems clear that detrusor myocytes possess several types of K+ channel, including large and small Ca2+-activated channels as well as voltage-sensitive K+ and KATP channels. The frequency of the action potentials is also very voltage sensitive: small changes in potential cause large changes in frequency (Fig. 7). In early work action potentials were recorded in hypertonic solution to suppress spontaneous mechanical activity, and slightly different effects are seen under isotonic conditions. This suggests that the spatial arrangement of the sarcoplasmic reticulum and plasma membrane, which are likely to be abnormal in the hypertonic medium, may be important. Results with various K+ channel blockers suggest that the resting membrane potential may have a contribution from voltage-sensitive K+ channels and small conductance Ca2+-activated K+ channels, and be sensitive to the global [Ca2+]i. Hence 4AP and apamin, which show selective blockade of these channels, respectively, have little effect on action potential shape, but slightly depolarize and increase spike frequency (Fig. 8). In contrast altering the Ca2+-activated Ca2+ release or blocking the maxi-K channels with iberiotoxin or charybdotoxin (Fig. 9) have major effects on the repolarization of the spikes. KATP channel openers hyperpolarize the membrane and block spike production.
Figure 7. Microelectrode recordings from guinea-pig detrusor: effects of depolarizing and hyperpolarizing current injection on action potential frequency.
Horizontal bars indicate extracellular polarization applied through partition electrodes. Figure courtesy of J. Mostwin.
Figure 8. Effects of apamin on action potentials recorded from strips of guinea-pig detrusor.
A, action potentials before (a) and after apamin (b). B, superimposed action potentials from the two conditions. Ca, spontaneous contractile activity from a strip and the effects of apamin. Cb, expanded view of a single contraction before and after apamin. From Hashitani & Brading (2003b).
Figure 9. Effects of Ca2+-activated K+ channel blocker charybdotoxin on action potentials in guinea-pig detrusor.
The lower trace is an overlay of an action potential before and after charybdotoxin. From Hashitani & Brading (2003b).
As expected, drugs that block K+ channels tend to increase the spontaneous mechanical activity of detrusor strips. Also, mice genetically modified to not express the maxi-K channels have overactive bladders (Meredith et al. 2004). KATP channel openers abolish spontaneous mechanical activity and eliminate unstable contractions in a pig model (Foster et al. 1989; Buckner et al. 2002). Unfortunately they tend to have widespread effects and also markedly reduce blood pressure, which render them too non-selective for clinical use (Fabiyi et al. 2003). The potential for modulating the other K+ channels or the L-type Ca2+ channels is being explored (Gopalakrishnan & Shieh, 2004).
Of considerable interest is the effect of drugs on the pattern of the electrical activity. In tissues in which continuous action potentials are occurring, apamin, which blocks small conductance calcium-activated K+ channels, generates bursts of action potentials (Fig. 8), and 4AP, which blocks the voltage-sensitive K+ channels, in some tissues also may generate short bursts (Hashitani & Brading, 2003b). Both drugs increase the size of the spontaneous contractions (Fujii et al. 1990; Herrera et al. 2000; Hashitani & Brading, 2003a, b). Mice in which the expression of the SK3 gene has been manipulated (over-expressed or inhibited) show characteristic changes in bladder activity in vivo and in vitro, particularly by the presence of non-voiding contractions, but normal micturition was not affected (Herrera et al. 2003), again suggesting a role for small conductance K+ channels in modulating spontaneous activity.
Calcium channels in the detrusor
The L-type channels present in the detrusor are important for mediating the upstroke of the actions potentials, but the cells also express T-type channels. The L-type channels in guinea-pig detrusor display interesting behaviour in that they can switch into a long channel open mode in response to large depolarization (Nakayama & Brading, 1993a, b, 1995). They also trigger release of Ca2+ from adjacent sarcoplasmic reticulum by activation of ryanodine receptors (Herrera et al. 2000). Ca2+ can both inactivate the L-type channels, and also open Ca2+-activated K+ channels (Herrera & Nelson, 2002), thus causing rapid repolarization and the action potential after-hyperpolarization. Ryanodine (50 μm) and cyclopiazonic acid increase the amplitude of the action potentials and abolish the after-hyperpolarization as expected (Hashitani & Brading, 2003b). The effects of ryanodine on the spontaneous contractions in the guinea-pig seem to depend on the dose given – it can enhance the amplitude and reduce the frequency (50 μm) and can cause a transient increase in frequency followed by a decrease in frequency with little change in amplitude (10 μm;Herrera et al. 2000). The T-type channels activate at more negative potentials and may well play a role in generating the spontaneous activity (Sui et al. 2001, 2003; Chow et al. 2003), and Ni2+, which selectively blocks these channels attenuates the spontaneous mechanical activity to a greater extent than evoked activity.
Interstitial cells in the detrusor
In spite of this knowledge of the role of ion channels, it is still not clear what exactly underlies the bursting activity in the bladder strips. Single myocytes can generate action potentials, but tend not to do so spontaneously. Calcium imaging in single muscle bundles (Hashitani et al. 2001) shows that Ca2+ waves, presumably associated with action potentials, can arise in the centre of muscle bundles, but more often arise at the edges of a bundle and spread across it. Single muscle bundles show burst-type action potential activity relatively rarely, and although when this occurs the bursts of activity occur in all muscle fibres recorded from, the action potentials themselves are not well correlated when recorded from two sites simultaneously.
The detrusor also contains interstitial cells (McCloskey & Gurney, 2002; Davidson & McCloskey, 2005) and it is possible that these cells play some role. In the guinea-pig bladder these cells in the smooth muscle layers are mainly located at the edges of muscle bundles. Ca2+ imaging has demonstrated that unlike in the rabbit urethra, only a small percentage of the interstitial cells show spontaneous Ca2+ transients (McCloskey & Gurney, 2002), and the frequency and duration of these transients is quite different to the Ca2+ transients generated by the smooth muscle cells (Hashitani et al. 2004b). Slow waves are not seen in the smooth muscles cells in intact strips, and it has been proposed that the interstitial cells may be more important in mediating the propagation of action potentials along the bundles than in actually generating them. The finding in human bladder that the Kit-positive cells are increased in number in samples of bladder taken from patients with overactive bladders (Fry et al. 2005), may support this suggestion, since in these tissues the contractions appear better co-ordinated across the strips.
Conclusions
It seems clear that ion channels do play an important role in determining the properties of the spontaneous contractile activity in the urethra and detrusor, and that altering their function can have profound effects on this activity. However, there are still many areas of uncertainty. It would seem particularly important for those studying the ion channels in a particular species to extend their studies to correlate channel properties with function, and there are still far too few basic studies on human material. Two particular areas of uncertainty are what causes the clustering of the action potentials in the detrusor, and what exactly is the role of the interstitial cells both in the detrusor and urethra. A better understanding of this would help drug companies develop therapeutic treatments which could help a huge number of people currently suffering from bladder overactivity.
References
- Abrams P. The overactive bladder and its treatments: consensus conference. BJU Int. 2000;85(Suppl 2) [Google Scholar]
- Andersson KE, Garcia-Pascual A, Forman A, Tottrup A. Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand. 1991;141:133–134. doi: 10.1111/j.1748-1716.1991.tb09056.x. [DOI] [PubMed] [Google Scholar]
- Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. discussion 68–73. [DOI] [PubMed] [Google Scholar]
- Brading AF, Teramoto T, Nakayama S, Bramich N, Inoue R, Fujii K, Mostwin J. The relationship between the electrophysiological properties of lower urinary tract smooth muscles and their function in vivo. In: Bolton TB, Tonita T, editors. Smooth Muscle Excitation. London: Academic Press; 1996. pp. 403–415. [Google Scholar]
- Bradley JE, Anderson UA, Woolsey SM, Thornbury KD, McHale NG, Hollywood MA. Characterization of T-type calcium current and its contribution to electrical activity in rabbit urethra. Am J Physiol Cell Physiol. 2004;286:C1078–C1088. doi: 10.1152/ajpcell.00463.2003. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Brading AF. Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol. 1996;492:185–198. doi: 10.1113/jphysiol.1996.sp021300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater M, Davies JR, Brading AF. Regional variations in the neural control of the female pig urethra. Br J Urol. 1995;76:730–740. doi: 10.1111/j.1464-410x.1995.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Bridgewater M, MacNeil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol. 1993;150:223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KY, Wu C, Sui GP, Fry CH. Role of the T-type Ca2+ current on the contractile performance of guinea pig detrusor smooth muscle. Neurourol Urodyn. 2003;22:77–82. doi: 10.1002/nau.10081. [DOI] [PubMed] [Google Scholar]
- Coolsaet BL. Bladder compliance and detrusor activity during the collection phase. Neurourol Urodyn. 1985;4:263–273. [Google Scholar]
- Coolsaet BL, Van Duyl WA, Van Os-Bossagh P, De Bakker HV. New concepts in relation to urge and detrusor activity. Neurourol Urodyn. 1993;12:463–471. doi: 10.1002/nau.1930120504. [DOI] [PubMed] [Google Scholar]
- Crowe R, Burnstock G. A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra? J Urol. 1989;141:414–422. doi: 10.1016/s0022-5347(17)40785-3. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Cowan W, Daniel VP. Structural bases for neural and myogenic control of human detrusor muscle. Can J Physiol Pharmacol. 1983;61:1247–1273. doi: 10.1139/y83-183. [DOI] [PubMed] [Google Scholar]
- Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–1390. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- Drake MJ, Harvey IJ, Gillespie JI. Autonomous activity in the isolated guinea pig bladder. Exp Physiol. 2003;88:19–30. doi: 10.1113/eph8802473. [DOI] [PubMed] [Google Scholar]
- Fabiyi AC, Gopalakrishnan M, Lynch JJ, 3rd, Brioni JD, Coghlan MJ, Brune ME. In vivo evaluation of the potency and bladder-vascular selectivity of the ATP-sensitive potassium channel openers (–)-cromakalim, ZD6169 and WAY 133537 in rats. BJU Int. 2003;91:284–290. doi: 10.1046/j.1464-410x.2003.03069.x. [DOI] [PubMed] [Google Scholar]
- Foster CD, Speakman MJ, Fujii K, Brading AF. The effects of cromakalim on the detrusor muscle of human and pig urinary bladder. Br J Urol. 1989;63:284–294. doi: 10.1111/j.1464-410x.1989.tb05191.x. [DOI] [PubMed] [Google Scholar]
- Fry CH, Brading AF, Hussain M, Lewis SA, Takeda M, Tuttle JB, Uvelius B, Wood DN, Drake MJ. Cell biology. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publication Ltd; 2005. pp. 313–362. [Google Scholar]
- Fry CH, Hussain M, McCarthy C, Ikeda Y, Sui GP, Wu C. Recent advances in detrusor muscle function. Scand J Urol Nephrol Suppl. 2004:20–25. doi: 10.1080/03008880410015138. [DOI] [PubMed] [Google Scholar]
- Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990;99:779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol. 1995;24:159–187. doi: 10.1007/BF01181533. [DOI] [PubMed] [Google Scholar]
- Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int. 2004;93:478–483. doi: 10.1111/j.1464-410x.2003.04667.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Shieh CC. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin Ther Targets. 2004;8:437–458. doi: 10.1517/14728222.8.5.437. [DOI] [PubMed] [Google Scholar]
- Greenland JE, Dass N, Brading AF. Intrinsic urethral closure mechanisms in the female pig. Scand J Urol Nephrol Suppl. 1996:75–80. [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003a;140:146–158. doi: 10.1038/sj.bjp.0705319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003b;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004a;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004b;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA, Sergeant GP, McHale NG, Thornbury KD. Activation of Ca2+-activated Cl− current by depolarizing steps in rabbit urethral interstitial cells. Am J Physiol Cell Physiol. 2003a;285:C327–C333. doi: 10.1152/ajpcell.00413.2002. [DOI] [PubMed] [Google Scholar]
- Hollywood MA, Woolsey S, Walsh IK, Keane PF, McHale NG, Thornbury KD. T- and L-type Ca2+ currents in freshly dispersed smooth muscle cells from the human proximal urethra. J Physiol. 2003b;550:753–764. doi: 10.1113/jphysiol.2003.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovou JW, Hill SJ, Birmingham AT. Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J Urol. 1990;144:775–779. doi: 10.1016/s0022-5347(17)39590-3. [DOI] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig) Pflugers Arch. 1985a;405:340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflugers Arch. 1985b;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- Mattiasson A, Andersson K-E, Andersson P-O, Larsson B, Sjögren C, Uvelius B. Nerve-mediated functions in the circular and longitudinal muscle layers of the proximal female rabbit urethra. J Urol. 1990;143:155–160. doi: 10.1016/s0022-5347(17)39901-9. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Brading AF. Inactivation of the voltage-dependent Ca2+ channel current in smooth muscle cells isolated from the guinea-pig detrusor. J Physiol. 1993a;471:107–127. doi: 10.1113/jphysiol.1993.sp019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Brading AF. Evidence for multiple open states of the Ca2+ channels in smooth muscle cells isolated from the guinea-pig detrusor. J Physiol. 1993b;471:87–105. doi: 10.1113/jphysiol.1993.sp019892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Brading AF. Possible contribution of long open state to noninactivating Ca2+ current in detrusor cells. Am J Physiol. 1995;269:C48–C54. doi: 10.1152/ajpcell.1995.269.1.C48. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Brading AF, Tomita T. Studies of longitudinal tissue impedance in various smooth muscles. Prog Clin Biol Res. 1990;327:375–378. [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley GNA. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Fry CH. Inward calcium currents in cultured and freshly isolated detrusor muscle cells: evidence of a T-type calcium current. J Urol. 2001;165:621–626. doi: 10.1097/00005392-200102000-00084. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Fry CH. A description of Ca2+ channels in human detrusor smooth muscle. BJU Int. 2003;92:476–482. doi: 10.1046/j.1464-410x.2003.04356.x. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H. Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol. 2004;172:748–752. doi: 10.1097/01.ju.0000130419.32165.6b. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Brading AF. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br J Pharmacol. 1996;118:635–642. doi: 10.1111/j.1476-5381.1996.tb15448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Spread of excitation in smooth muscle. In: Seralakis N, Wood JD, editors. Frontiers in Smooth Muscle Research. New York: Wiley-Liss; 1990. pp. 361–373. [PubMed] [Google Scholar]
- Turner WH, Brading AF. Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther. 1997;75:77–110. doi: 10.1016/s0163-7258(97)00038-7. [DOI] [PubMed] [Google Scholar]