Abstract
The anterior cingulate cortex (ACC) is critically involved in processing the affective component of pain sensation. Visceral hypersensitivity is a characteristic of irritable bowel syndrome. Electrophysiological activity of the ACC with regard to visceral sensitization has not been characterized. Single ACC neuronal activities in response to colorectal distension (CRD) were recorded in control, sham-treated rats and viscerally hypersensitive (EA) rats (induced by chicken egg albumin injection, i.p). The ACC neurones of controls failed to respond to 10 or 30 mmHg CRD; only 22% were activated by 50 mmHg CRD. Among the latter, 16.4% exhibited an excitatory response to CRD and were labelled ‘CRD-excited’ neurones. In contrast, CRD (10, 30 and 50 mmHg) markedly increased ACC neuronal responses of EA rats (10%, 28% and 47%, respectively). CRD produced greater pressure-dependent increases in ACC spike firing rates in EA rats compared with controls. Splanchnicectomy combined with pelvic nerve section abolished ACC responses to CRD in EA rats. Spontaneous activity in CRD-excited ACC neurones was significantly higher in EA rats than in controls. CRD-excited ACC neurones in control and EA rats (7 of 16 (42%) and 8 of 20 (40%), respectively) were activated by transcutaneous electrical and thermal stimuli. However, ACC neuronal activity evoked by noxious cutaneous stimuli did not change significantly in EA rats. This study identifies CRD-responsive neurones in the ACC and establishes for the first time that persistence of a heightened visceral afferent nociceptive input to the ACC induces ACC sensitization, characterized by increased spontaneous activity of CRD-excited neurones, decreased CRD pressure threshold, and increased response magnitude. Enhanced ACC nociceptive transmission in viscerally hypersensitive rats is restricted to visceral afferent input.
Visceral hypersensitivity is a key factor in the pathophysiology of gastrointestinal functional disorders. Noxious visceral stimuli such as colorectal distension (CRD) produce vigorous cardiovascular and visceromotor responses (Ness & Gebhart, 1988). CRD also produces avoidance behaviour in rats (Ness & Gebhart, 1988) and pain in humans (Lipkin & Sleisenger, 1957; Ness et al. 1990). Patients with irritable bowel syndrome (IBS) have lower pain thresholds during rectal distension, accompanied by the development of excessive reflex motor activity in the rectum (Mertz et al. 1995; Verne et al. 2001). Given the complexity of the afferent system, functional gastrointestinal disorders are likely to be heterogeneous. Abnormalities that up-regulate signal intensity anywhere in the afferent system can induce hypersensitivity and pain. It has been shown that peripheral sensitization results from an increase in the sensitivity and excitability of the afferent nerve itself and/or the dorsal horn of the spinal cord (Gebhart, 2000; Lin & Al-Chaer, 2003). The brain interprets and influences the perception of pain-sensation signals transmitted from the gut. The anterior cingulate cortex (ACC) is a major cortical component of the limbic loop system, and its functional relationship to emotional and motivational responses has been well described (Vogt & Robert, 1993). The ACC is involved in pain processing and encoding of negative affect in humans, which results in pain-related unpleasantness (Talbot et al. 1991; Tolle et al. 1999). Surgical lesions of this area do not remove the sensation of pain but they remove the associated emotional response and suffering (Davis et al. 1994; Hutchison et al. 1999). Experiments in animals demonstrate that the ACC receives nociceptive inputs (Sikes & Vogt, 1992; Traub et al. 1996). Research suggests that ACC neuronal activity in rodents is related to stimulus–reward learning (Orona & Gabriel, 1983; Bussey et al. 1997). The ACC also has direct neural connections to autonomic effector areas (dorsal motor nucleus of the vagus, amygdala and hypothalamus). Electrical stimulation of the ACC evokes transient changes in arterial pressure, gastric motility and gastric secretion (Hurley-Gius & Neafsey, 1986; Cechetto & Saper, 1990). Lesions in the area of the ACC decrease the incidence of gastric pathologies produced by restraint stress (Lewin & Whitty, 1960; Henke, 1982). Given the association of the ACC with pain, affect and gut motor function, its relevance to IBS is important.
The literature is conspicuously devoid of a definitive study that demonstrates the neural electrophysiological activity of the ACC during processing of visceral nociceptive stimulation. We hypothesize that persistence of a heightened tonic visceral afferent nociceptive input to the ACC enhances the neuronal response of the ACC to noxious visceral stimulation in parallel with ACC neuronal plasticity. The current study was designed to characterize the electrophysiological properties of ACC neurones that are correlated with the induction of visceral hypersensitivity. Single ACC neuronal discharges were examined in control, sham-treated rats and in a viscerally hypersensitive rat model to determine ACC hypersensitivity to CRD. To determine whether a population of rostral ACC neurones is capable of discriminative coding for sensitivity, specifically visceral hypersensitivity, the responses evoked by noxious cutaneous stimuli were examined in CRD-excited ACC neurones in sham-treated and viscerally hypersensitive rats.
Methods
Materials
Unless otherwise noted all chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). Dibucaine (Nupercainal), a local anaesthetic ointment, was purchased from Rugby Laboratories (Norcross, GA, USA). Experiments were performed on adult male Sprague-Dawley rats (275–300 g) that were housed four per plastic cage. Animals were maintained on a 12 h light–12 h dark cycle (lights on at 07.00 h) and given access to food and water ad libitum. Experimental procedures were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Viscerally hypersensitive rat model – colonic anaphylaxis
The rats were sensitized to chicken egg albumin with an injection (i.p.) of 1 ml normal saline containing egg albumin (10 μg) as the antigen and aluminium hydroxide (10 mg) as the adjuvant. Beginning on day 3, colorectal anaphylaxis was induced. Each rat was placed in plastic tunnel. A silastic enema tube (i.d., 0.63 mm) was inserted into the anal canal to the rectum, a distance of 9–10 cm, and secured to the tail. Antigen solution was perfused through this tube at 50 μl min−1 for 30 min (egg albumin, 10 mg ml−1 in 40 mm d-glucose, made isotonic with NaCl). A polyethylene tube (i.d., 1.67 mm) attached to a balloon (length, 4.0 cm) lightly coated with a surgical lubricant was placed in the colon and secured to the base of the tail. Colorectal distension (30 mmHg for 30 s with a 3-min interval, and repeated five times) was performed 30 min after egg albumin instillation. This entire procedure was performed once a day on three consecutive days. ACC electrophysiological recordings were performed 5–7 days after the entire procedure. Successful sensitization was verified by the detection of aluminium hydroxide deposits in the abdominal cavity during laparotomy (Jiang et al. 2000). Rats without these deposits were excluded from further experiments. Previous studies have shown that intestinal anaphylaxis alters intestinal motility (Scott et al. 1998), enhances the activity of the intestinal mesenteric nerve (Nozdrachev et al. 1999), and triggers neuronal activations in the nucleus of the solitary tract (NTS) (Scott et al. 2000). Histamine and other mast-cell mediators may be responsible for these changes (Kreis et al. 2000). Rats injected with 1 ml normal saline (i.p.) served as the sham-treated controls. Three days after the saline injection, these rats were subjected to an intracolonic infusion of saline and CRD (30 mmHg, maintained for 5 min), performed on three consecutive days, without egg albumin challenge. Recording studies were conducted 5–7 days after the third day using the same protocol as used with the sensitized rats.
Electrophysiological recording of ACC neurones
The ACC is one of the three divisions of the prefrontal cortex in the rat, the other two being the agranular insular and orbitofrontal areas. Definitions of this region vary; for example, Zilles & Wree (1998) defined the ACC as comprising cortical subregions Cg1, Cg2 and Cg3, while Paxinos & Watson (1998) referred to Cg3 as the prelimbic cortex. In the current study, the ACC is defined as the cingulate cortex, area 2 (Cg2) and prelimbic cortex together with the overlying cingulate cortex, area 1 (Cg1) (Paxinos & Watson, 1998). Rats were anaesthetized with intraperitoneal injection of a mixture of α-chloralose (80 mg kg−1) and urethane (800 mg kg−1). The anaesthetic was periodically supplemented every 3.5 h with one-quarter of the initial dose given intravenously. Adequate depth of anaesthesia was established in a separate series of experiments without immobilization, by frequent observation of the absence of heart rate change and withdrawal reflexes after pinching the skin or subcutaneous electrical stimulation. Body temperature was maintained at 36.5 ± 5°C using a heating blanket. The electrocardiogram was monitored continuously. Each rat was placed in a stereotaxic frame, suspended from spine clamps at the thoracic and lumbar levels to reduce respiratory movement artifact. A craniotomy was performed. An opening was made 1.0–5.0 mm anterior to the bregma and 0.1–2.0 mm lateral to the midline to record neurones in the cingulate cortex. All surgical cut margins were covered with Nupercainal. Heart rate and respiratory rate were monitored throughout the experiment to ensure a consistent anaesthetic response. Signs of arousal such as increased heart rate and respiratory rate and coordinated movements were absent, but ACC neuronal activity remained robust. Glass microelectrodes with tip diameters of 0.08 μm and impedance of 20–40 MΩ were filled with neurobiotin (4%; Vector Laboratories, Burlingame, CA, USA) in 1.0 m KCl-Tris buffer (pH 7.6) and lowered into the rostral ACC using a micromanipulator (coordinates: 1.7–3.7 mm anterior to bregma, 0.3–1.0 mm lateral to midline, 1.5–3.5 mm ventral to brain surface). After penetrating the surface of the cortex and avoiding blood vessels near the midline, the recording electrode was advanced until the spontaneous activity of a single unit could be accurately discriminated from the background neuronal noise. The recording had uniform spike amplitude and could be maximized and separated from neighbouring neurones. Noise levels were typically 40–60 μV. Only well-isolated neurones that showed a signal-to-noise ratio of at least 4 : 1 were analysed.
The signals were amplified by a high-input impedance preamplifier (A-M Systems, Carlsborg, WA, USA), displayed, and stored on a PC with a 166-MHz pentium processor using Axoscope software (Axon Instruments, Union City, CA, USA). Data were recorded digitally (Datawave Systems, Thornton, CO, USA).
Labelling and histological identification of recording sites – juxtacellular injection
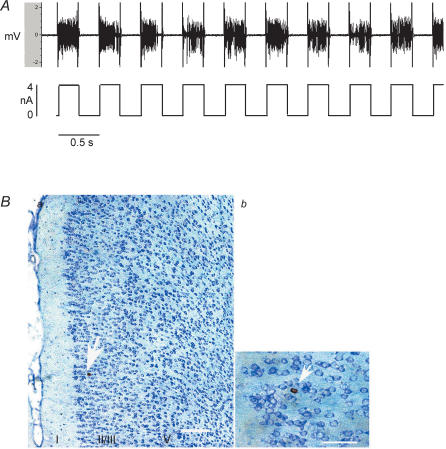
On completion of the experiment, recorded neurones were labelled by injecting neurobiotin using the technique of ‘juxtacellular’ iontophoresis (Pinault, 1996). Continuous electrophysiological control ensured that the neurones remained alive. The micropipette was positioned as close to the cell as possible. Using the bridge circuit of the recording amplifier, the marker was applied in pulses (250 ms on and 250 ms off). The intensity of the DC current was gradually increased from 2 to 8 nA (anode in the pipette). After a delay of several seconds, the electrical behaviour of first the background noise and then the recorded neuronal spike firing changed suddenly and significantly, confirming that the microelectrode tip was in the juxtacellular position (see Fig. 2B). A large amplitude extracellular action potential was consistent with a close approach of the aperture of the micropipette to the cell membrane. Staining is probably due to local and reversible disruption of the cell membrane under the influence of the current (i.e. electroporation). The presence of spike firing during the 250-ms on-period of the current pulse indicated that the neurone was filled efficiently by the marker (see Fig. 2A). These pulses produced bursts of action potentials that were continuously monitored, ensuring that the recorded cell was labelled and allowing the current intensity to be adjusted to the lowest level required to maintain a sufficient firing level and to prevent cellular damage (Schreihofer & Guyenet, 1997). If the discharge was absent during this period, stained cells were not observed. Both the amplitude and discharge frequency of the action potentials varied spontaneously in a reversible manner during the injection period. Therefore, it was necessary either to increase the current intensity temporarily or to move the microelectrode a few micrometers up or down. In this study, the juxtacellular current was delivered over 5–8 min. The animals were allowed to survive for 6–8 h after the juxtacellular injection of neurobiotin, and then were deeply anaesthetized with pentobarbital sodium and perfused transcardially with 50 ml PBS (pH 7.4) followed by 4% paraformaldehyde in 0.1 m PBS. The brain was removed and placed in cold (4°C) fixative for 4 days and then transferred to cold phosphate-buffered 30% sucrose for 48 h. Serial coronal sections (50 μm) were cut with a cryostat along the path of electrode penetration and mounted on gelatin-coated slides. Sections were incubated with peroxidase-conjugated avidin–biotin complex (1 : 100; ABC: Vector Laboratories). We used 3,3′-diaminobenzidinetetra-HCl, nickel-ammonium sulphate and 0.001% hydrogen peroxide in Tris-buffered saline (0.05 m, pH 7.4) to produce the distinctive black ABC reaction product. Thionine was used as a counterstain.
Figure 2. Juxtacellular labelling of an identified anterior cingulate cortex (ACC) neurone with neurobiotin.
A, Juxtacellular iontophoresis with positive current pulses (250 ms on/250 ms off, lower tracing shows current) of 2–4 nA produced bursts of action potentials that were continuously monitored and served as a measure of the magnitude of membrane disruption that resulted in cell labelling with neurobiotin. B, microphotograph of neurones in the ACC labelled with neurobiotin a, thionine-stained coronal section shows the laminar distribution of distension-excited neurones in the ACC. b, higher magnification of the neurobiotin-labelled pyramidal ACC neurone located in layer II/III in a (arrow). Scale bars: a, 250 μm; b, 50 μm.
Experimental design–ACC neuronal activity in response to colorectal distension in control and viscerally hypersensitive rats
ACC neuronal spontaneous discharge was monitored for 2 min to confirm the stability of the basal firing frequency. The basal firing rate was assessed over 30 s to quantify the resting discharge in both control and viscerally hypersensitive (EA) rats. Every neurone isolated on the basis of spontaneous activity was studied to determine its response to CRD. Graded pressure distension was produced by rapidly injecting saline into the balloon over 1 s and maintaining the distension for 30 s. Pressure within the balloon was monitored by connecting the catheter via a three-way stopcock to a pressure transducer (World Precision Instruments, Sarasota, FL, USA). Neurones responding to 50 mmHg CRD were tested twice to make sure the responses were consistent and repeatable. A neurone was deemed responsive to CRD if its spike firing rate increased or decreased by at least 10% from its predistension baseline level. All neurones activated by 50 mmHg CRD were tested with 10 and 30 mmHg CRD (each for 30 s with a 5-min interval) to reproduce a stimulus–response curve. Efforts were made to obtain at least two trials at each level of randomly applied pressure distension. Neuronal discharge rates were measured for 30 s before, 30 s during, and 120 s after CRD, and evaluated on a time histogram (5-s bin width). Consistent monitoring of each neurone was ensured by careful study of the firing pattern, the amplitude and the waveform of each spike. To examine the systemic effects that may have influenced ACC responses evoked by CRD, blood pressure was continuously monitored throughout the ACC recording experiments in five control rats and five EA rats. Consistent with observations of the orbital cortex (Follett & Dirks, 1994) and somatosensory cortex in anaesthetized rats (Follett & Dirks, 1994), ACC neuronal responses to 10 and 30 mmHg CRD in our anaesthetized rat studies were not associated with changes in blood pressure. However, 50 mmHg CRD induced a decrease in blood pressure of ≤ 5 mmHg in one control rat and one EA rat, whereas 80 mmHg CRD caused a significant decrease of mean blood pressure (by ∼8–15 mmHg) in all 10 rats.
Experimental design–afferent neuronal pathways responsible for mediating ACC responses evoked by CRD
Sensory information from the distal colon and rectum travels to the CNS through two distinct anatomic pathways: the lumbar splanchnic nerves, which terminate in the thoracolumbar spinal cord, and the paired pelvic nerves, which terminate in the lumbosacral spinal cord. To determine the contribution of the splanchnic and pelvic nerves in the mediation of the ACC neuronal response to CRD, splanchnicectomy combined with pelvic nerve section was performed in viscerally hypersensitive (EA) rats 1 h before the electrophysiological recording.
Splanchnicectomy combined with pelvic nerve section
The mesentery, intestine, spleen and pancreas were placed on the animal's right flank and covered with sterile saline-soaked gauze. The lobes of the liver were pushed upwards. The coeliac plexus was identified on the aorta between its junction with the coeliac and superior mesenteric artery. The greater, lesser and least splanchnic nerves leave the trunk at the level of T12, T13, L1 and L2 and project to the coeliac–superior mesenteric ganglion complex. All splanchnic nerves were sectioned between the coeliac plexus and the suprarenal ganglion. Care was taken to avoid damaging the adrenal nerves and coeliac branches of the vagus nerves.
In the rat, 2–5 lumbar splanchnic nerves that leave the L2–L5 paravertebral ganglion are variable in size and in some cases are absent. The rostral lumbar splanchnic nerves join the intermesenteric nerves that connect the coeliac–superior mesenteric ganglion complex with the inferior mesenteric ganglion. Splanchnicectomy should eliminate these pathways. Only the lumbar splanchnic nerve from L5 directly joins the inferior mesenteric ganglion (Baron et al. 1988). The inferior mesenteric ganglion is small and lies on the surface of the inferior mesenteric artery close to the origin of the two common iliacs. In our pilot studies, we observed that splanchnicectomy combined with pelvic nerve section abolished the ACC neuronal responses to CRD, so in the current study we did not section the L5 lumbar splanchnic nerve.
The bifurcation of the vena cava into the common iliac vein was used as a landmark to locate the pelvic nerve. The pelvic nerve was located where it crosses the internal iliac vein, and the vein disappears into the dorsal muscle of the rat. A 4-mm section of pelvic nerve was performed. The procedure was repeated on the opposite side.
Experimental design–ACC neuronal response to noxious cutaneous stimuli
Transcutaneous electrical stimulation
In a separate group of rats, CRD-excited neurones were subjected to noxious cutaneous stimuli. Transcutaneous electrical stimulation (TCES) was applied through a pair of stainless steel stimulating electrodes inserted subcutaneously into the hindpaw. Previous studies have shown that nociceptive-specific ACC neurones have large, usually bilateral, receptive fields, sometimes covering the whole body surface (Sikes & Vogt, 1992; Yamamura et al. 1996). The hindpaw was chosen to determine whether distinct subgroups of ACC neurones were sensitive specifically to visceral but not somatic stimulation. Electrical square-wave stimuli (duration, 2 ms) were delivered with increasing intensity (2–10 mA). The appearance of one or several peaks of activation was monitored by direct examination of the oscilloscope screen. The effect of repeated application of an electrical stimulus (50 trials, 10.0 Hz) was analysed by building a peristimulus histogram. Previous studies have shown that stimuli over 5 mA reliably evoke pain in the conscious rat without causing tissue damage. Villanueva et al. (1989) observed that neurones in the reticular formation code for the number of Aδ and C fibres activated with TCES and that the maximal response to C-fibre stimulation occurs after 12-mA pulses.
Thermal stimulation
TCES-activated neurones were tested using noxious thermal stimulation, which consisted of immersing the metatarsus of the contralateral hindpaw in a hot water bath. For nociceptive neurones, graded temperatures (40°C–52°C) were applied over 30 s with a pause of 3 min between successive stimuli. A response was considered positive when the magnitude of response was greater than 50% of the ongoing activity for the neurones with ongoing activity > 1 Hz. In fact, positive responses were generally greatly above these criteria and negative responses were often clearly null. Because higher temperatures could inflict tissue damage, only two series were conducted in a single animal.
Data analysis and statistics
Single neuronal responses were examined using Datapac 2000 (RUN Technologies, Mission Viejo, CA, USA). The prestimulus discharge frequency was assessed for 30 s to quantify the resting discharge. The discharge frequency during CRD or cutaneous stimulation was also measured for 30 s. The mean and standard deviation of ACC neuronal firing during the 30-s control period was compared with the activity after CRD or cutaneous stimulation. Statistical comparisons of the percentages of ACC neurones activated by CRD in varies experimental groups of rats were made using the Pearson χ2 test. Data involving spontaneous firings in various experimental groups were evaluated using Dunnett's T3 multiple comparisons method following one-way analysis of variance (ANOVA). Statistical comparisons of the CRD-pressure responses in various groups were made using the one-way repeated measures ANOVA followed by multiple comparison adjusted by Bonferroni's test. Results were expressed as means ± s.e.m. P < 0.05 was considered statistically significant.
Results
ACC neuronal activity in control rats
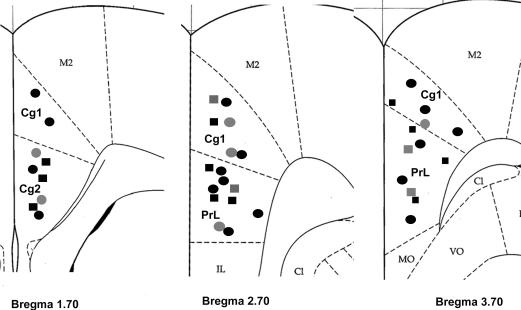
A total of 19 control rats were examined. Only one neurone per rat was labelled with neurobiotin. Neurobiotin labelling failed in four of 19 neurones. In two rats, the juxtacellular configurations were lost during the injection and could not be recovered with a momentary increase in current amplitude. These rats were excluded from the study. Histology studies revealed an absence of stained cells in two rats, presumably because neurobiotin was deposited extracellularly and then taken up or washed out of the tissue. Recording electrodes were successfully placed in the ACC of 15 rats (Fig. 1). From these 15 rats, 67 neurones were recorded from (Table 1). Ease of access to the recording site, the stability of surface landmarks on the skull, and ease of stereotaxic electrode placement contributed to this success. Histological localization of the CRD-responsive neurones showed that most were located in the cingulate cortex, area 1 (Cg1) and the prelimbic cortex. The greatest number of cells were located in layer II/III; where 13 of 15 of the cells responded to CRD (Fig. 2). One cell was located in layer I and one cell in layer V.
Figure 1. Location of neurones recorded in the anterior cingulate cortex (ACC).
Coronal sections from the caudal to rostral regions of the ACC (i.e. bregma 1.70, 2.70 and 3.70 mm) show the electrophysiological recording sites (adapted from the atlas of the rat brain by Paxinos & Watson (1998)). Squares and circles indicate ACC neurones in the control and EA rats, respectively. Black symbols represent CRD-excited neurones and grey symbols represent CRD-inhibited neurones. C1, claustrum; Cg1, cingulate cortex, area 1; Cg2, cingulate cortex, area 2; IL, intralimbic cortex; M2, secondary motor cortex; MO, medial orbital cortex; PrL, prelimbic cortex; VO, ventral orbital cortex.
Table 1.
ACC neuronal responses to colorectal distension (CRD)
| Sham-treated controls | EA | EA sham nerve sections | EA nerve sections | |
|---|---|---|---|---|
| Number of rats | 15 | 22 | 11 | 6 |
| Number of neurons recorded | 67 | 41 | 22 | 36 |
| Spontaneous firings (spikes s−1) | 0.86 ± 0.03 | 1.66 ± 0.07** | 1.66 ± 0.03** | 1.68 ± 0.06** |
| CRD non-responsive (%) | 77.6 | 46.3 | 50 | 100 |
| CRD excited (%) | ||||
| 10, 30, 50 mmHg | 0 | 9.75* | 9 | 0 |
| 30 mmHg | 0 | 29.3* | 36.3* | 0 |
| 50 mmHg | 16.4 | 41.5* | 45.5* | 0 |
| CRD inhibited (%) | 6 | 12 | 4.5 | 0 |
P < 0.05 versus sham-treated controls and EA nerve section. Pearson χ2 test.
P < 0.05 versus sham-treated controls. Dunnett's T3 multiple comparisons method following one-way analysis of variance (ANOVA), F statistic is 236.536 with the degrees of freedom of 3 and 50.
All of the recordings had uniform spike amplitude and could be minimized and separated from the recordings of the neighbouring neurones. Spontaneous activity was sufficiently stable to permit recording for 45–60 min. Basal ACC neuronal activity was assessed over 30 s. In the control rats, spontaneous activity was characterized as 0.86 ± 0.03 spikes s−1. From the 15 rats, 67 neurones were reported. Among the 67 neurones, 52 (77.6%) showed no response to CRD and were referred to as CRD-non-responsive; histological identification of these neurones was not performed. Fifteen of 67 neurones responded to CRD; only 50 mmHg, not 10 or 30 mmHg, evoked any effect. Eleven of 67 neurones (16.4%) exhibited an excitatory response characterized by increased spike firings from baseline (0.86 ± 0.5 spikes s−1) to 1.4 ± 0.3 spikes s−1 and thus were referred to as CRD-excited neurones (Fig. 3). Four neurones (6% of total 67 neurones) were inhibited by 50 mmHg CRD (CRD-inhibited neurones); their spontaneous activity was reduced from 0.87 ± 0.2 to 0.31 ± 0.05 spikes s−1. No laminar cortical organization was observed in the ACC with respect to CRD-excited or CRD-inhibited neurones. CRD-inhibited neurones did not appear to cluster nor did they seem to be isolated from the excitatory neurones. None of the neurones showed a combination of excitatory and inhibitory responses at 10, 30 or 50 mmHg CRD.
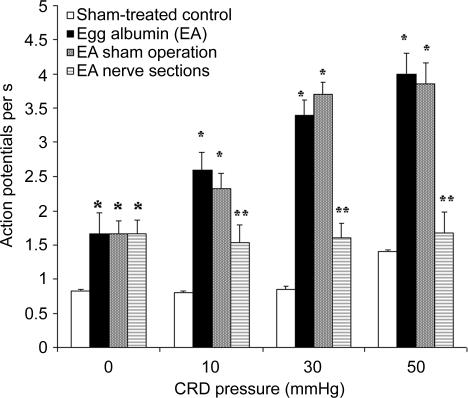
Figure 3. Spike firing rates of colorectal distension (CRD)-excited neurones of the anterior cingulate cortex (ACC) in response to graded pressure distension in sham-treated, viscerally hypersensitive (EA) rats and in EA rats after acute nerve sectioning or sham operation.
Results collected from 11 and 17 CRD-excited neurones from sham-treated controls and viscerally hypersensitive (EA) rats, respectively. CRD pressure ≤ 50 mmHg had no effect on ACC neuronal firing in control rats. In EA rats, the basal firing rate (CRD pressure, 0 mmHg) was higher and neural firings in response to both low- and high-pressure distension were markedly increased. Another set of results was collected from 36 neurones in six EA rats after acute splanchnicectomy combined with pelvic nerve section. All 36 neurones failed to respond to CRD. Nerve section had no effect on the enhanced spontaneous basal firings. In 11 sham-operated, EA rats, 10 of 20 neurones increased spike firings in response to 50 mmHg CRD, eight neurones increased spike firings in response to 30 and 50 mmHg CRD, and two neurones responded to all CRD pressures (10, 30 and 50 mmHg). P≤ 0.05 versus sham-treated control (Dunnett's T3 test following one-way ANOVA). P≤ 0.05 versus sham-treated control rats. **P≤ 0.05 versus EA sham operation. (One-way repeated measures ANOVA followed by Bonferroni's test).
ACC neuronal responses to graded CRD in sensitized rats
A total of 27 EA rats were studied. During the injection of neurobiotin, the juxtacellular configurations were lost in three rats. These rats were excluded from further study. Histology studies revealed an absence of stained cells in one rat, and in another, the stained neurone was outside the ACC region. The data from these two animals were not presented. Recording electrodes were successfully placed in the ACC of 22 EA rats (Fig. 1). From these 22 EA rats, 41 neurones were characterized and recorded from (Table 1). Three types of neurones were classified according to their CRD response: 19 of 41 (46.3%) were CRD-non-responsive and 22 of 41 (53.7%) were activated by CRD. Of the latter 22 activated neurones, 17 of 41 neurones (41.5%) were CRD-excited neurones; of which, 4 of 41 (9.8%) were excited by 10, 30 and 50 mmHg CRD, 12 of 41 (29.3%) were excited by 30 and 50 mmHg CRD, and 17 of 41 (41.5%) were only excited by 50 mmHg CRD. In EA rats, the average spontaneous activity recorded in the CRD-excitatory neurones was significantly higher than that in the control rats (1.66 ± 0.7 spikes s−1 in the control rats; versus, 0.83 ± 0.07 spikes s−1 in the EA rats; Fig. 3). ACC spike firings in response to 10, 30 and 50 mmHg CRD increased from baseline (1.66 ± 0.15 spikes s−1) to 2.6 ± 0.5, 3.4 ± 0.6 and 4.0 ± 0.5 spikes s−1, respectively. Mean neuronal firing frequencies are shown in Fig. 3. Original action potential recordings are presented in Fig. 4. These observations suggest that viscerally hypersensitive rats have a reduced CRD pressure threshold and an increased ACC neuronal excitability. Five of the 22 neurones (22.7%) were CRD-inhibited neurones; ACC neuronal discharge was reduced from baseline (1.66 ± 0.02 spikes s−1) to 0.39 ± 0.03 spikes s−1 at a distension pressure of 50 mmHg. Original action potential recordings of a CRD-inhibited neurone are presented in Fig. 5. Histological localization of the CRD-responsive neurones in EA rats is shown in Fig. 1. Twenty neurones were located in layer II/III, and two cells were located in layer V. The morphological properties of CRD-responsive neurones were not characterized.
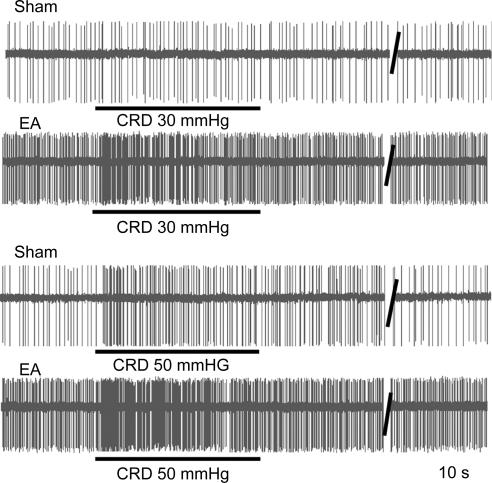
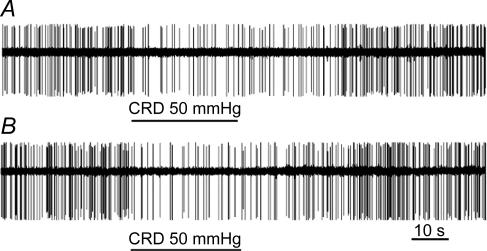
Figure 4. Recordings of colorectal distension (CRD)-excited anterior cingulate cortex (ACC) neurones in response to CRD (30 and 50 mmHg) in sham-treated and viscerally hypersensitive (EA) rats.
In sham-treated rats, there was a slight increase in response to 50 mmHg CRD, whereas both low- and high-pressure distension evoked increased responses in the rats sensitized with chicken egg albumin.
Figure 5. Recordings of colorectal distension (CRD)-inhibited neurones of the anterior cingulate cortex (ACC).
A, response to CRD in sham-treated rats; B, response to CRD in viscerally hypersensitive rats.
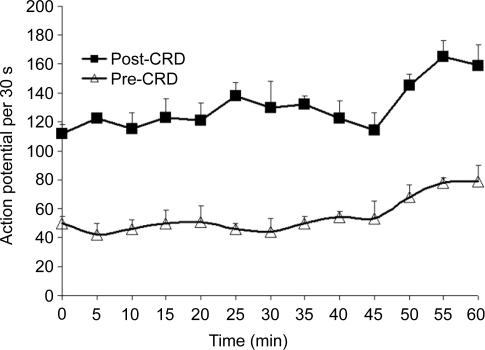
To evaluate the variability in response to repeated CRD trials, six neurones from a separate group of four EA rats were subjected to 13 trials of 50 mmHg CRD repeated at 5-min intervals (Fig. 6). The neural response to distension remained consistently excitatory. However, spontaneous activity increased dramatically after the 10th repetition (45 min), more than doubling, which suggests that sensitization of the ACC was achieved at the 10th repetition of CRD.
Figure 6. Responses of a single anterior cingulate cortex (ACC) neurone to 13 trials of 50 mmHg colorectal distension (CRD) repeated at 5-min intervals.
Neuronal responses to colorectal distension remained consistently excitatory. Spontaneous activity more than doubled after the 10th trial at 45 min, which suggests that sensitization occurred.
The response latency between the start of stimulation and the onset of the increase in spike firings was estimated to be 2.0 ± 0.19 s. Spike firings recorded from the ACC were obviously polysynaptic in nature, probably involving afferent nerves in the spinothalamic and thalamocortical tracts.
Completed stimulus–response tests of 16 of 17 CRD-excitatory neurones from EA rats confirmed the reliability of the ACC responses. Neurones that could not be held long enough to determine stimulus–response curves were classified according to their response to the standard CRD pressure of 50 mmHg.
In a separate group of seven EA rats, splanchnicectomy plus pelvic nerve section was performed 1 h prior to electrophysiological recording. Six neurones in each rat were tested in response to 30 and 50 mmHg CRD. Histological studies showed that recording electrodes were successfully placed in the ACC of six of seven EA rats following nerve sectioning. A total of 36 neurones were reported (Table 1). Sham operations were performed in another 11 EA rats that served as controls. Neurobiotin-labelling was successful in all 11 sham-operated EA rats. The basal firings of EA rats with intact nerves and EA rats following sham nerve section were 1.66 ± 0.07 and 1.66 ± 0.03 spikes s−1, respectively. Acute nerve sectioning did not change the increased spontaneous ACC neuronal firings in EA rats, which averaged 1.68 ± 0.06 spikes s−1 (Fig. 3 and Table 1). None of the neurones showed excitatory or inhibitory responses, which suggests that nerve sectioning completely eliminated the ACC neuronal responses evoked by CRD (Fig. 3). In the rats following sham nerve sectioning, 22 neurones were characterized (Table 1); 11 of 22 (50%) were CRD-non-responsive and 11 of 22 were activated by CRD. Of these 11 neurones, 10 neurones (45.5%) showed increased spike firings in response to 50 mmHg CRD. Eight neurones (36.4%) responded to 30 and 50 mmHg CRD and two neurones (9.1%) responded to all CRD pressures (10, 30 and 50 mmHg). One neurone was inhibited by CRD (Table 1). These observations suggest that peripheral afferent inputs are transmitted through the pelvic and splanchnic nerves to the ACC, evoking ACC neuronal responses to CRD.
Responses of CRD-excited ACC neurones to transcutaneous electrical stimulation
In eight control rats, 16 CRD-excited ACC neurones were identified. Transcutaneous electrical stimulation (TCES) of the contralateral hindpaw evoked a response in seven of 16 (43.8%) CRD-excited ACC neurones. In 11 EA rats, 20 CRD-excited ACC neurones were identified. TCES of the hindpaw evoked a response in eight of 20 (40%) CRD-excited ACC neurones. These neurones responded to repeated high intensity (6 mA) electrical stimulation with a strong and lasting increase in firing. ACC neuronal responses after subjecting the contralateral hindpaw to repetitive TCES at different intensities are shown in Figs 7 and 8. There was no significant difference in the average depths of the neurones with respect to the presence or absence of cutaneous responses to visceral stimulation (CRD).
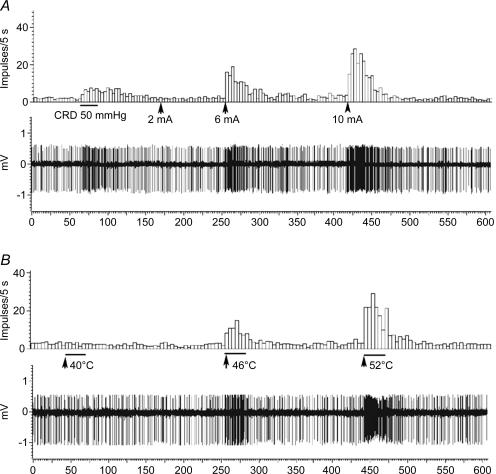
Figure 7. The responses of one anterior cingulate cortex (ACC) neurone to colorectal distension (CRD) and transcutaneous electrical stimulation (A) and thermal stimulation (B) of the hindpaw of a sham-treated control rat.
CRD (50 mmHg) caused a slight increase in ACC firings. Electrical stimulation (square wave, 2 ms duration, 2–10 mA, 10.0 Hz, 50 trials) and thermal stimulation with moderate to noxious temperatures (40°C–52°C) caused monotonic increases in spike firings (5 s per bin).
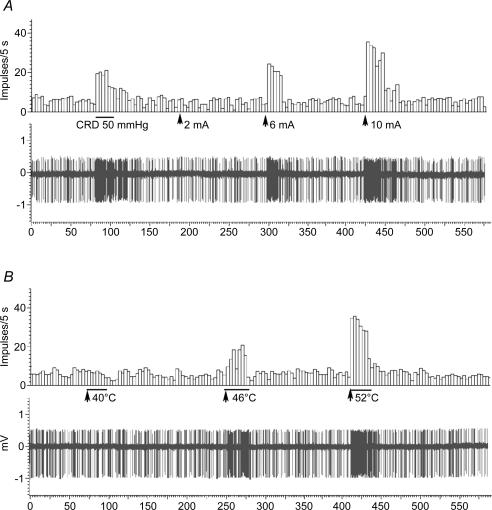
Figure 8. Anterior cingulate cortex (ACC) neuronal responses evoked by colorectal distension (CRD), transcutaneous electrical stimulation (A) and thermal stimulation (B) in a viscerally hypersensitive rat.
CRD (50 mmHg) caused a marked increase in neuronal firings of the anterior cingualte cortex of the viscerally hypersensitive rat (see Fig. 3). However, neuronal spike firings evoked by electrical and thermal stimulation did not changed compared with spike firings of the sham-treated rat shown in Fig. 7 (see Fig. 9).
Responses of CRD-excited ACC neurones to graded thermal stimulation
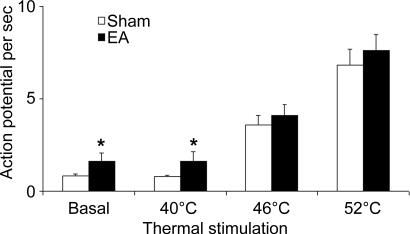
To evaluate the excitability of ACC neurones evoked by natural heat stimulation, all of the CRD-excited neurones that were activated by TCES were examined for their response to water at different temperatures. Immersion of the contralateral hindpaw in water at 40°C produced no measurable electrophysiological effect. All seven TCES-excited neurones from the control rats, and seven of the eight TCES-excited neurones from EA rats were activated by the application of thermal stimuli within the noxious range, 46–52°C. The magnitude of the response was directly related to the temperature applied to the hindpaw. In control rats, ACC firing increased from baseline (0.85 ± 0.06 spikes s−1) to 3.2 ± 0.4 spikes s−1 at 46°C and to 6.4 ± 0.5 spikes s−1 at 52°C. In EA rats, ACC firing increased from baseline (1.67 ± 0.25 spikes s−1) to 4.4 ± 0.45 spikes s−1 at 46°C and to 7.8 ± 0.85 spikes s−1 at 52°C. The original recordings presented in Figs 7 and 8 show that ACC neuronal responses monotonically increased their discharges in response to the application of thermal stimuli within the noxious range. Although basal firing rates were significantly higher in EA rats compared with control rats, proportional increases in the ACC firing rates in response to thermal stimuli were not significantly different in EA compared with control rats (Fig. 9). Histological studies showed that all neurones were successfully labelled, and most were located in Cg1 and prelimbic cortex (data not shown).
Figure 9. Discharge of anterior cingulate cortex (ACC) neurones in response to thermal stimulation.
Data from CRD-excited neurones in sham-treated and EA rats (seven neurones per group) that were activated by transcutaneous electrical stimulation. Application of water at 40°C to the hindpaw produced no measurable electrophysiological effect. ACC neurones in sham-treated and EA rats increased their discharges in response to the application of thermal stimulation within the noxious range (46°C–52°C). Note that basal firing rates were significantly higher in EA rats compared with sham-treated rats. However, proportional increases in ACC firing rates in response to thermal stimulion were not significantly different between sham-treated and EA rats. *P < 0.05 compared with sham-treated rats.
Discussion
Hypersensitivity to visceral distending stimuli has been shown in patients with IBS. Cumulative evidence from brain imaging studies suggests alterations in sensory CNS signal processing by the CNS in IBS. Functional magnetic resonance imaging (fMRI) studies are beginning to address the possible neural mechanisms of hyperalgesia in patients with IBS. All fMRI studies to date report that compared with healthy controls, patients with IBS often exhibit altered activation of regions involved in pain processing, such as the ACC, thalamus, insula and prefrontal cortex, in response to experimental and anticipated rectal pain (Silverman et al. 1997; Mertz et al. 2000; Mayer et al. 2000; Kern & Shaker, 2003). fMRI is blood oxygenation level-dependent (BOLD) imaging, measuring neuronal activity indirectly via its assumed haemodynamic correlate. BOLD fMRI reflects changes in cerebral blood volume, cerebral blood flow and oxygen consumption.
Inconsistencies exist in BOLD fMRI studies (Bonaz et al. 2000; Naliboff et al. 2000; Sidhu et al. 2004); some researchers report an increase in ACC responses, while others report a decrease or absence of ACC responses. Such variation emphasizes the need to learn how to interpret the BOLD fMRI signal in terms of the neuronal synaptic activity and action potentials in the brain (Arthurs & Boniface, 2002). There are compelling reasons to focus on the action potential; for example, the action potential is the only way neurones communicate over distance. The long-range projection signals from these principle neurones are identified mainly in single-cell recordings.
In this study, we recorded single ACC (Cg1, Cg2 and prelimbic cortex) neuronal discharges in response to CRD in sham-treated rats and in a well-characterized rat model of visceral hypersensitivity induced by intraperitoneal injection of chicken egg albumin followed by repeated colonic challenge with egg albumin and CRD to evoke colonic anaphylaxis. Previous studies have shown that intestinal anaphylaxis enhances the activity of the intestinal mesenteric nerve (Nozdrachev et al. 1999) and triggers neuronal activations in the nucleus of the solitary tract (NTS) (Scott et al. 2000). Histamine and other mast-cell mediators may be responsible for these changes (Kreis et al. 2000). We showed in the control rat that ACC neurones were not activated by non-noxious CRD (10 and 30 mmHg). Only 16% of ACC neurones showed a mild increase in firing rates in response to 50 mmHg CRD, which is comparable to the pressure at which human subjects undergoing CRD demonstrate changes in visceromotor function and report painful sensations (Ness et al. 1990). These observations suggest that ACC visceral nociceptive neurones are not easily excitable under normal conditions. In the viscerally hypersensitive EA rats, however, 41.5% of ACC neurones were identified as CRD-excited neurones. Graded CRD produced a marked, pressure-dependent increase in ACC spike firing rates in visceral hypersensitive rats. Further, the basal firing rates of CRD-excited ACC neurones in EA rats were markedly higher compared with those of the control rats. This report provides, for the first time, direct electrophysiological evidence of the sensitization of the ACC in viscerally hypersensitive rats.
The responses of ACC neurones to CRD are qualitatively similar to the responses of the spinal dorsal horn (Ness & Gebhart, 1987, 1998), the somatosensory cerebral (SI) cortex neurones (Follett & Dirks, 1994), and the ventral posterolateral nucleus of the thalamus (Al-Chaer et al. 1996). The major differences are in the relative proportions of neurones excited and inhibited by CRD at the various levels of the neural axis. In the spinal dorsal horn, 79% of neurones that responded to CRD were excited and 21% were inhibited (Ness & Gebhart, 1987, 1998). In the SI cortical neurones, 33% were excited and 52% were inhibited by CRD (Follett & Dirks, 1994). In contrast, a small group of ventral posterolateral neurones (5%) were inhibited by CRD (Al-Chaer et al. 1996). In the current study, 6% and 12% of ACC neurones were inhibited by noxious CRD in control and viscerally hypersensitive rats, respectively.
Ness & Gebhart (1998) showed that thoracolumbar and lumbosacral spinal segments receive afferent input from the colon via distinct pathways; the splanchnic and pelvic nerves. Brierley et al. (2004) showed that the splanchnic and pelvic pathways contain distinct populations of mechanosensitive afferents. In the current study, we showed that the combination of splanchnicectomy and pelvic nerve section abolished ACC responses to CRD in EA rats. Our observations suggest that afferent neuronal pathways transmitting colorectal input to the ACC are independent of the vagus nerve. However, acute nerve section failed to prevent the enhancement of ACC spontaneous firings in the sensitized rats, suggesting that the subspinal peripheral ongoing activity is not required to maintain a higher level of ACC spontaneous activity in visceral hypersensitive rats, a phenomenon that probably originates centrally rather than peripherally.
From the dorsal horn, neural signals ascend via the spinothalamic and spinoreticular pathways and the dorsal column to the brainstem and thalamus. The lateral system (i.e. the spinothalamic tract) is thought to encode pain location, classification and intensity, whereas the medial system (i.e. the spinoreticular tract) encodes pain suffering and emotional reaction. The spinoreticular tract sends sensory information to the medial and intralaminar nuclei of the thalamus; the ascending pain message spreads bilaterally to the prefrontal cortex, including the ACC. Anatomical and electrophysiological studies suggest that the sources of nociceptive inputs to the ACC may be mediated by dorsal medial thalamic (MT) nuclei (Yamamura et al. 1996; Hsu & Shyu, 1997). However, there is an increasing body of experimental (Al-Chaer et al. 1996, 1998; Houghton et al. 2001) and clinical (Nauta et al. 1997) evidence that shows the importance of the dorsal column pathway in the processing and transmission of visceral nociception. Neurones in the dorsal column nuclei activate neurones in the contralateral ventral posterolateral nucleus of the thalamus through the medial lemniscus (Saab et al. 2004). Currently, it is unclear whether the ACC neurones are activated by the dorsal column–ventral posterolateral pathway. Further studies are required to clarify the pathways from the subdivisions of nuclei of the thalamus to the ACC that are responsible for mediating ACC sensitization in the viscerally hypersensitive rat.
It has been demonstrated that peripheral sensitization occurs, caused by increased sensitivity and excitability of the afferent nerves and/or the dorsal horn of the spinal cord (Zhuo et al. 2002; Lin & Al-Chaer, 2003). In our rat model, colorectal anaphylaxis resulted in sensitization of high-threshold receptors and brought into play previously unresponsive silent nociceptors (Nozdrachev et al. 1999; Kreis et al. 2000). Once peripheral sensitization occurred, these nociceptors began to respond to the innocuous stimuli. We showed that viscerally hypersensitive rats have enhanced ACC spontaneous activity, decreased CRD pressure threshold to stimulate ACC neurones, and increased ACC response magnitude. The ACC forms a large region around the rostrum of the corpus callosum. It is involved in emotional and attentive responses to internal and external stimulation (Rainville et al. 1997). Recent neuroimaging and electrophysiological studies in humans have shown that pain activates several limbic sites, including the ACC (Hutchison et al. 1999). Experiments in animals have demonstrated that the ACC receives nociceptive inputs (Traub et al. 1996; Sikes & Vogt, 1992). Chronic pain is reduced in patients with ACC lesions (Davis et al. 1994). We propose that the level of activation in the ACC evoked by noxious CRD is a determinant in emotional and behavioural reactions to pain.
fMRI scanning has shown spatially distinct ACC activation during visceral and cutaneous noxious stimulation (Verne et al. 2001). Visceral stimulation activates a more anterior part of the ACC compared with cutaneous stimulation (Silverman et al. 1997; Lotze et al. 2001). Because our pilot studies revealed that most caudal ACC neurones (i.e. bregma −1.4 to +1.5 mm) did not respond to CRD (data not shown), we studied rostral ACC neurones. To determine whether distinct subgroups of rostral ACC neurones were sensitized specifically by visceral (CRD) but not somatic (cutaneous) stimulation, we examined the response of CRD-excited ACC neurones to transcutaneous electrical stimulation (TCES) of the hindpaw. Two groups of neurones in the rostral ACC were identified. One group of CRD-exited ACC neurones (∼60%) was activated exclusively by CRD stimulation. These neurones failed to respond to TCES, which suggests involvement of a population of rostral ACC neurones in visceral nociception and a possible discriminative aspect of visceral nociception. The other group of rostral ACC neurones (∼40%) responded to both CRD and TCES. The interaction between visceral and cutaneous nociception has been recognized in previous studies (Zhuo & Gebhart, 1992; Accarino et al. 1995). Limb nociceptive stimulation and CRD have been widely used to investigate the interaction between visceral and cutaneous nociception. Noxious somatic afferent input from the hindlimb has been shown to facilitate visceral hyperalgesia (CRD) in the rat (Miranda et al. 2004). The effect can be blocked by ionotropic glutamate receptor antagonists. Further, sensitization occurs at the lower spinal level and is independent of supraspinal influence (Peles et al. 2004). In humans, an fMRI study revealed that both rectal distension and cutaneous heat stimulation (right hand immersed in a heated circulating water bath) evoked an increase in neural activity within the same brain structures of patients with IBS (Verne et al. 2001). Further, rectal administration of lidocaine reversed the visceral hyperalgesia (CRD) and secondary cutaneous hyperalgesia in patients with IBS (right foot immersed in a heated water bath) (Verne et al. 2003). However, neuronal activities of a single cell in response to visceral or somatic stimulation could not be differentiated in these studies. In this study, to quantitatively identify the neuronal excitability in response to visceral and natural noxious cutaneous stimulation, we examined the ACC neuronal activity evoked by 50 mmHg CRD and graded thermal stimulation in sham-treated, control rats and viscerally hypersensitive rats. We showed that compared with ACC neurones in control rats, a group of ACC neurones in viscerally hypersensitive rats exhibited enhanced activities evoked by CRD. However, neuronal responses evoked by cutaneous noxious heat stimulation did not change significantly. Our results demonstrate, for the first time, that a population of rostral ACC neurones is capable of discriminative coding for hypersensitivity, specifically, visceral hypersensitivity. In this study, neither cutaneous receptive fields of CRD-non-responsive neurones nor CRD-inhibitory neurones were identified.
The midbrain periaqueductal grey (PAG) contains distinct neural substrates that initiate passive or active emotional coping strategies (Bandler et al. 2000). The segregation of visceral and somatic input to the ACC may parallel that observed in the PAG. Keay et al. (1993, 1994) have shown in animal studies that the ACC is interconnected with the PAG in columnar fashion, and that noxious stimulation of the skin increases c-fos labelling in the lateral PAG in the rat, whereas stimulation of the viscera increases c-fos labelling in the ventrolateral PAG. Correspondingly, behavioural reactions in rats evoked by microstimulation in the lateral and dorsolateral PAG are similar to those produced by cutaneous pain, whereas behavioural reactions evoked by microstimulation in the ventrolateral PAG are similar to those produced by visceral pain (Keay & Bandler, 1993; Keay et al. 1994). Consistent with previous functional and neuroanatomic evidence, our observations support the concept that the ACC has functionally distinct subregions.
The ACC acts as an integrating centre that has wide inputs and outputs (Hurley et al. 1991). Electrical stimulation of the ACC has been shown to elicit a variety of visceral or autonomic responses in a number of species (Cechetto & Saper, 1990). Hurley-Gius & Neafsey (1986) reported that low intensity electrical stimulation of the ACC inhibited ongoing gastric motility in anaesthetized rats. Henke (1982) showed that bilateral ACC lesion prevented gastric ulcers caused by restraint stress in the rat. Hence, the enhanced ACC activity induced by CRD in EA rats can cause gastrointestinal dysfunction. The altered activity of the gut may further increase the excitation of the nociceptors. The present study showed enhanced ACC spontaneous activity, a decrease in the CRD pressure threshold, and an increase in the response magnitude in EA rats. Further, a population of rostral ACC neurones is capable of hypersensitive-discriminative coding, specifically for visceral hypersensitivity; an observation that may provide a functional basis to understand visceral pain in human IBS.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-51717 (Y.L.) and P30-DK 34933 (C.O.). The authors wish to thank Ms Lingling Zhang, Center for Statistical Consultation and Research, University of Michigan, for providing expertise in the statistical analysis.
References
- Accarino A, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in the irritable bowel syndrome. Gastroenterology. 1995;108:636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Feng Y, Willis WD. A role for the dorsal column in nociceptive visceral input into the thalamus of primates. J Neurophysiol. 1998;79:3143–3150. doi: 10.1152/jn.1998.79.6.3143. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol. 1996;76:2661–2674. doi: 10.1152/jn.1996.76.4.2661. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci. 2002;25:27–31. doi: 10.1016/s0166-2236(00)01995-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Baron R, Janig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical originization of lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- Bonaz BL, Papillon E, Baciu M, Segebarth C, Bost R, Le Bas J-F, Fournet J. Central processing of rectal pain in IBS patients: an fMRI study. Gastroenterology. 2000;118:A615. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RCW, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Role of the cerebral cortex in autonomic function. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Function. New York: Oxford University Press; 1990. pp. 208–223. [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, Dostrovsky JO. Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain. 1994;59:189–199. doi: 10.1016/0304-3959(94)90071-X. [DOI] [PubMed] [Google Scholar]
- Follett KA, Dirks B. Characterization of responses of primary somatosensory cerebral cortex neurons to noxious visceral stimulation in the rat. Brain Res. 1994;656:27–32. doi: 10.1016/0006-8993(94)91362-5. [DOI] [PubMed] [Google Scholar]
- Follett KA, Dirks B. Responses of neurons in ventrolateral orbital cortex to noxious visceral stimulation in the rat. Brain Res. 1995;669:157–162. doi: 10.1016/0006-8993(94)01200-2. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Henke PG. The telencephalic limbic system and gastric pathology. Neurosci Biobehav Rev. 1982;6:381–390. doi: 10.1016/0149-7634(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Houghton AK, Wang CC, Westlund KN. Do nociceptive signals from the pancreas travel in the dorsal column? Pain. 2001;89:207–220. doi: 10.1016/s0304-3959(00)00364-x. [DOI] [PubMed] [Google Scholar]
- Hsu MM, Shyu BC. Electrophysiological study of the connection between medial thalamus and anterior cingulate cortex in the rat. Neuroreport. 1997;8:2701–2707. doi: 10.1097/00001756-199708180-00013. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Hurley-Gius KM, Neafsey EJ. The medial frontal cortex and gastric motility: microstimulation results and their possible significance for the overall pattern of organization of rat frontal and parietal cortex. Brain Res. 1986;365:241–248. doi: 10.1016/0006-8993(86)91635-5. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kreis ME, Eastwood C, Kirkup AJ, Humphrey PP, Grundy D. 5-HT(3) and histamine H(1) receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 2000;119:1267–1275. doi: 10.1053/gast.2000.19461. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci Lett. 1993;154:23–26. doi: 10.1016/0304-3940(93)90162-e. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Owler B, Depaulis A, Bandler R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994;61:727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Kern M, Shaker R. Further characterization of human brain processing of viscero-sensation: the role of gender and a word of caution. Gastroenterology. 2003;124:1975–1977. doi: 10.1016/s0016-5085(03)00554-7. [DOI] [PubMed] [Google Scholar]
- Kreis ME, Muller M, Zittel TT, Glatzle J, Grundy D. Mediators of neuronal activation in the rat brainstem following intestinal anaphylaxis. Neurosci Lett. 2000;289:45–48. doi: 10.1016/s0304-3940(00)01265-9. [DOI] [PubMed] [Google Scholar]
- Lewin W, Whitty CW. Effects of anterior cingulate stimulation in conscious human subjects. J Neurophysiol. 1960;23:445–447. doi: 10.1152/jn.1960.23.4.445. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Lipkin M, Sleisenger MH. Studies of visceral pain: measurements of stimulus intensity and duration associated with the onset of pain in esophagus, ileum and colon. J Clin Invest. 1957;37:28–34. doi: 10.1172/JCI103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Wietek B, Birbaumer N, Ehrhardt J, Grodd W, Enck P. Cerebral activation during anal and rectal stimulation. Neuroimage. 2001;14:1027–1034. doi: 10.1006/nimg.2001.0901. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Derbyshire S, Naliboff BD. Cerebral activation in irritable bowel syndrome. Gastroenterology. 2000;119:1418–1419. doi: 10.1053/gast.2000.20116. [DOI] [PubMed] [Google Scholar]
- Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distension. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern MA, Chang L, Mayer EA. Evidence for decreased activation of central fear circuits by expected aversive visceral stimuli in IBS patients. Gastroenterology. 2000;118:A137. [Google Scholar]
- Nauta HJ, Hewitt E, Westlund KN, Willis WD. Surgical interruption of a midline dorsal column visceral pain pathway. Case report and review of the literature. J Neurosurg. 1997;86:538–542. doi: 10.3171/jns.1997.86.3.0538. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13–L2 spinal cord of the rat. J Neurophysiol. 1998;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Metcalf AM, Gebhart GF. A psychophysical study in humans using phasic colonic distention as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- Nozdrachev AD, Akoev GN, Filippova LV, Sherman NO, Lioudyno MI, Makarov N. Changes in afferent impulse activity of small intestine mesenteric nerves in response to antigen challenge. Neuroscience. 1999;94:1339–1342. doi: 10.1016/s0306-4522(99)00377-2. [DOI] [PubMed] [Google Scholar]
- Orona E, Gabriel M. Multiple-unit activity of the prefrontal cortex and mediodorsal thalamic nucleus during acquisition of discriminative avoidance behavior in rabbits. Brain Res. 1983;263:295–312. doi: 10.1016/0006-8993(83)90322-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. 4. San Diego, California, USA: Academic Press; 1998. [Google Scholar]
- Peles S, Miranda A, Shaker R, Sengupta JN. Acute nociceptive somatic stimulus sensitizes neurones in the spinal cord to colonic distension in the rat. J Physiol. 2004;560:291–302. doi: 10.1113/jphysiol.2004.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Saab CY, Park YC, Al-Chaer ED. Thalamic modulation of visceral nociceptive processing in adult rats with neonatal colon irritation. Brain Res. 2004;1008:186–192. doi: 10.1016/j.brainres.2004.01.083. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labelling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Scott RB, Tan DT, Miampamba M, Sharkey KA. Anaphylaxis-induced alterations in intestinal motility: role of extrinsic neural pathways. Am J Physiol. 1998;275:G812–G821. doi: 10.1152/ajpgi.1998.275.4.G812. [DOI] [PubMed] [Google Scholar]
- Scott RB, Tan DT, Sharkey KA. Effect of splanchnectomy on jejunal motility and fos expression in brain stem after intestinal anaphylaxis in rat. Am J Physiol Gastrointest Liver Physiol. 2000;279:G990–G997. doi: 10.1152/ajpgi.2000.279.5.G990. [DOI] [PubMed] [Google Scholar]
- Sidhu H, Kern M, Shaker R. Absence of increasing cortical fMRI activity volume in response to increasing visceral stimulation in IBS patients. Am J Physiol Gastrointest Liver Physiol. 2004;287:G425–G435. doi: 10.1152/ajpgi.00490.2003. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Ziglgansberger W, Willoch F, Schwaiger M, Conrad B, Bertenstain P. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol. 1999;45:40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett. 1996;215:165–168. doi: 10.1016/0304-3940(96)12978-5. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Villanueval L, Bing Z, Bouhassira D, LeBars D. Encoding of electrical, thermal and mechanical noxious stimuli by subnucleus reticularis dorsalis neurons in the rat medulla. J Neurophysiol. 1989;61:391–402. doi: 10.1152/jn.1989.61.2.391. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Robert WS. Anterior cingulate cortex and the medial pain system. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus, a Comprehensive Handbook. Boston, Basel, Berlin: Birkhauser; 1993. pp. 313–344. [Google Scholar]
- Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R. Morphological and electrophysiological properties of ACCx nociceptive neurons in rats. Brain Res. 1996;735:83–92. doi: 10.1016/0006-8993(96)00561-6. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Inhibition of a cutaneous nociceptive reflex by a noxious visceral stimulus is mediated by spinal cholinergic and descending serotonergic systems in the rat. Brain Res. 1992;585:7–18. doi: 10.1016/0006-8993(92)91185-h. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Sengupta J, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophsysiol. 2002;87:2225–2236. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The Rat Nervous System. Vol. 1. Sydney: Academic Press; 1998. pp. 375–415. [Google Scholar]