Abstract
The pathways involved in Ca2+ signalling in the uterus remain incompletely understood, impairing our ability to prevent preterm and difficult labours. In this review we focus on two elements in the pathway of Ca2+ signalling that have recently emerged as playing important roles: membrane lipid rafts and the sarcoplasmic reticulum. We examine the evidence for lipid rafts in the uterus and discuss their functional role. We suggest that the increases in cytosolic [Ca2+] and contractility that occur with raft disruption are due, at least in part, to effects on large conductance Ca2+-activated K+ (BK) channels that are localized to rafts. The role of the SR in contributing to subsarcolemmal cytosolic microdomains in uterus is evaluated, along with its interactions with ion channels on the plasma membrane. Thus, signalling microdomains play an important, but incompletely understood, role in the uterus, and integrating them into other Ca2+ signalling pathways is a challenge for further research. We suggest that the role of the SR changes in pregnancy, from promoting quiescence via BK channels or SR Ca2+ uptake, to promoting Ca2+ entry and contractility at term, and relate data on lipid rafts to clinical outcome in obese pregnant women.
Introduction
Understanding the control of uterine contractility remains a key goal of many researchers. The motivation arises from both the richly complex nature of the problem and the need to prevent the enormous toll of preterm labours on neonatal mortality and morbidity. It is recognized that intracellular Ca2+ changes and the signals arising from these changes, are of paramount importance to contractility and its control (Wray et al. 2003). Much has been learnt in the last decade concerning Ca2+ signals in the myometrium and how these relate to uterine contractility. Two treatments for threatened preterm labour are based on affecting the change of intracellular Ca2+, the Ca2+ channel antagonists (e.g. nifedipine) and magnesium chloride. The global Ca2+ signal recorded from uterine cells arises from the opening of voltage-gated L-type Ca2+ channels. In this review we examine two regulators of this Ca2+ rise, identified by recent work as possible important physiological mechanisms by which uterine activity is influenced: lipid raft microdomains, and the internal Ca2+ store, the sarcoplasmic reticulum (SR). As we will show, interactions between these two structures also occur, further adding to their role in modulating signalling in uterine cells. Answers to some of the questions raised by these latest findings may provide new loci for therapeutic interventions for both preterm labours and dysfunctional labours, i.e. those characterized by small infrequent contractions, necessitating delivery by caesarean section.
Membrane microdomains and lipid rafts
The plasma membrane of mammalian cells has long been known to be composed of a variety of lipids and proteins and contain ion channels, pumps and exchangers. Studies of many different cell types have shown that these components of the membrane are not evenly distributed, and the notion of membrane microdomains has been developed. The best characterized such microdomains are those known as lipid rafts, domains enriched in cholesterol and sphingolipid. The decreased fluidity as a consequence of the high cholesterol, gives rise to these regions becoming ‘rafts’, floating in the membrane (Simons & Ikonen, 2000; Laude & Prior, 2004). While there may be speculation about the exact physical nature of rafts (Laude & Prior, 2004), it is accepted that they have associated with them (or excluded from them) a range of signalling components, including ion channels, receptors and enzymes, and hence the interest in them as modulators of contractility (Simons & Toomre, 2000; O'Connell et al. 2004). Caveolae, invaginations of the surface membrane, may be regarded as a type of raft, where the structure has been stabilized by the protein caveolin (Quest et al. 2004). If we are to translate these exciting emerging concepts into medical benefit, the challenge is to elucidate the physiological importance of these microdomains and increase understanding of the underlying mechanisms. As described below, it may be anticipated that both membrane and cytosolic microdomains are playing an important role in uterine signalling.
Uterine caveolae
Caveolae are a particular feature of the membranes of uterine smooth muscle. The uterus expresses all three isoforms of caveolin (Taggart et al. 2000) and caveolae may increase in number towards the end of pregnancy (Turi et al. 2001; but also see Ciray et al. 1995). There is evidence that caveolae numbers are under hormonal control. Thus, oestrogen down-regulates the number of caveolae and the level of caveolin in rat uterine smooth muscle (Turi et al. 2001). In addition the oestrogen receptor may well be localized in caveolae, as their disruption with methyl β-cyclodextrin (MCD; a chelator of cholesterol) or caveolin-1 down-regulation has been found to activate oestrogen receptor α expression, suggesting that when it is localized to caveolae it is inhibited. Impairment of this inhibitory mechanism has been linked to 17β-oestradiol-stimulated mammary tumorigenesis (Zhang et al. 2005). Another powerful uterotonic hormone, oxytocin also appears to act via lipid rafts; the activity of its receptor is reduced if rafts are disrupted (Klein et al. 1995). A marked dependence of its binding function on cholesterol content suggests that the receptor exists in it high-affinity state only when it is in caveolae. It seems pertinent then to ask, what is the functional relevance of lipid rafts in the uterus?
Lipid rafts and the uterus
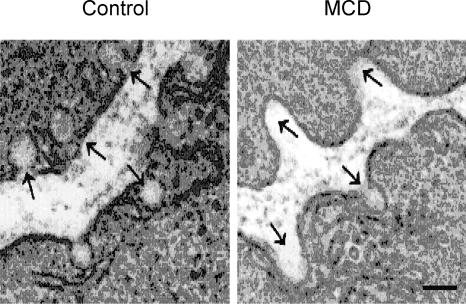
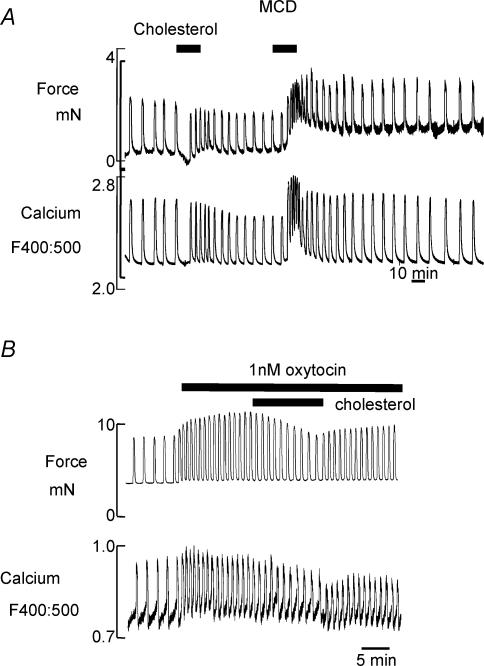
Figure 1 shows an electron micrograph of a uterine cell, and the arrows indicate caveolae before and after treatment with MCD (Smith et al. 2005). As can be seen, cholesterol extraction, which can be verified biochemically, disrupts caveolae. We have used MCD to explore the functional consequences of this raft disruption to uterine Ca2+ signalling and contractility (Kendrick et al. 2004; Smith et al. 2005). As Fig. 2 shows, modulation of cholesterol content of the myometrial plasma membrane has a profound effect on both Ca2+ and force. The spontaneous contractions and associated Ca2+ transients of longitudinal muscle, from rat and human myometrium, were greatly enhanced by cholesterol extraction and raft disruption (Fig. 2A). The same result was found if cholesterol was reduced using the bacterial enzyme, cholesterol oxidase (Smith et al. 2005). Interestingly if cholesterol is increased in the membrane, force and Ca2+ are markedly reduced, or even abolished. This was the case even in preparations stimulated with oxytocin, to increase the contractile drive on the tissue (Fig. 2B). Application of low density lipoproteins (LDLs), which will also increase cholesterol, was also detrimental to myometrial force and Ca2+ signalling (Smith et al. 2005). Recent studies on both pregnant and non-pregnant human myometrium have shown the same findings; elevation of cholesterol is deleterious for uterine Ca2+ signalling and reduces the force of contraction; the clinical implications of these findings are discussed later.
Figure 1. Uterine caveolae and cholesterol.
Electron micrograph of rat myometrium, showing caveolae in control (untreated) uterus, and the disruptive effect of cholesterol extraction with methyl β-cyclodextrin (MCD). Scale bar 0.2 μm. From Smith et al. (2005).
Figure 2. Cholesterol manipulation and uterine function.
A, simultaneous recording of force and Ca2+ (indo-1 ratio) in a strip of myometrium taken from human myometrium. Methyl β-cyclodextrin (MCD, 15 mm) was used to extract cholesterol, and cholesterol was replenished using 0.5 mm cholesterol (Zhang & Wray, unpublished data). B, the effect of increasing cholesterol in the presence of oxytocin (1 nm) in myometrium form pregnant rat. Taken from Smith et al. (2005)
Lipid rafts and function in other smooth muscles
The effects of lipid raft disruption appear to be smooth muscle and agonist specific. Thus in ureteric smooth muscle, MCD treatment selectively reduced Ca2+ signalling and phasic contractions (Babiychuck et al. 2002). Tonic contraction to 40 mm K+ solution or carbachol were not affected by MCD, but the phasic components of these stimulants were abolished. This selective effect of cholesterol depletion is in agreement with an earlier study in blood vessels that showed MCD reducing efficacy of some but not all agonists (Dreja et al. 2002). Elevated cholesterol is considered to underly much vascular dysfunction, but a direct effect via rafts remains to be confirmed. High cholesterol is also thought to impair gall bladder contractility (Chen et al. 1999) and be a key factor in the pathogenesis of cholesterol gallstones, but the effects of raft disruption do not appear to have been examined. Further functional studies in other smooth muscles are required before any general conclusions can be drawn.
As mentioned above, rafts have been suggested to serve as platforms for signal transduction (Bastiaanse et al. 1997; O'Connell et al. 2004) and so the question arises, which specific targets can explain the functional effects on the uterus?
The effects on uterine Ca2+ signalling were found in the presence and absence of oxytocin, and in pregnant and non-pregnant animals and women. This suggests then that the mechanism is one fundamental to Ca2+ signalling (Smith et al. 2005).
BK channels
The enhancement of cytosolic uterine Ca2+ signalling by MCD suggests that lipid rafts contain elements of signalling pathways that reduce force; thus when rafts are disrupted these inhibitory signals are reduced. Such a mechanism would be analogous to the inhibitory control of eNOS and oestrogen receptors (Goligorsky et al. 2002), and would be explicable if K+ channels were the target. Although there is evidence in other tissues for both KATP (Sampson et al. 2004) and KV (Martens et al. 2004) channels being localized to lipid rafts, in myometrium the large conductance, Ca2+-sensitive K+ (BK) channel may be more important.
There is growing evidence from biochemical and molecular biology studies that the BK channel is located in caveolae (Babiychuck et al. 2002; Brainard et al. 2005). The opening of BK channels produces membrane hyperpolarization and relaxation of the uterus, as opening of voltage-gated Ca2+ channels is reduced (Anwer et al. 1993; Wray et al. 2003). Furthermore in freshly dispersed myocytes from rat myometrium, we have recently found that MCD reduces outward current due to BK channels, consistent with BK channels being located in rafts (Shmygol & Wray, 2005).
Recent data have suggested that coupling between myometrial β2-adrenoreceptors and BK channels facilitates uterine relaxation (Chanrachakul et al. 2004). If localization of BK channels to rafts is necessary for their proper functioning, then this may be an additional mechanism to regulate uterine contractility. It has already been shown that BK expression changes with gestation in many species (Khan et al. 1993; Eghbali et al. 2003) and may also be regulated by clustering at the surface membrane (Eghbali et al. 2003); transient movement into and out of rafts would add another element to their control.
From microdomains to microsignals
One of the main regulators of BK channels is intracellular Ca2+, and in particular Ca2+ release from the SR has been implicated in activating these channels, as well as Ca2+-activated Cl− channels. We have recently shown that the SR plays a pivotal in controlling the refractory period in ureteric smooth muscle (Burdyga & Wray, 2005). Thus Ca2+ release from the SR may be anticipated to have a marked influence on excitability and hence contractility of the uterus (Wray et al. 2003) and this will now be discussed. As we will also describe, microdomains in the cytoplasm, under the microdomains in the plasma membrane, are also thought to play a substantial role in regulating contraction and Ca2+ signalling.
Sarcoplasmic reticulum structure
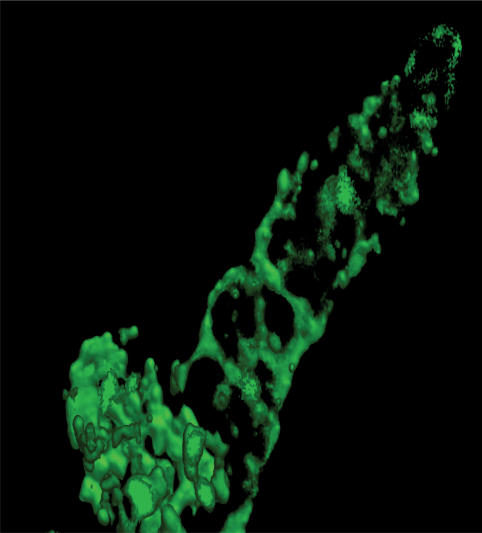
Modern imagining techniques have allowed an appreciation of the beautiful reticular pattern of the SR of the uterine myocytes (Fig. 3): an interconnecting membrane system of tubules and cisternae found throughout the cytoplasm. In uterus it makes up around 6% of the total smooth muscle cell volume (Shmygol & Wray, 2004) and increases in volume during pregnancy.
Figure 3. Uterine SR.
An xyz image of the sarcoplasmic reticulum (SR) from a rat uterine myocyte, obtained on a Perkin-Elmer Ultraview confocal system, and outlined with bodipy–ryanodine (Shmygol & Wray, unpublished work).
The SR is able to take up Ca2+ against the electrochemical gradient due to ATP-dependant Ca2+ pumps (SERCA) in the SR membrane. In the uterus there is evidence of physiological regulation of pump activity; expression of the dominant isoform (SERCA2b) is up-regulated during pregnancy (Khan et al. 1993; Tribe et al. 2000a).
In smooth muscle cells, Ca2+ is released from the SR by IP3- and Ca2+ (ryanodine)-dependant channels. In myometrium, all three isoforms of the IP3 channel (Morgan et al. 1996) and the ryanodine channel (RyR) (Martin et al. 1999) have been found. Golovina & Blaustein (1997) first proposed that although the smooth muscle SR appeared continuous, its Ca2+ stores were organized into spatially distinct units that could have specific physiology. There is indeed evidence for this occurrence in myometrium and it is discussed in more detail in Shmygol & Wray (2004).
SR compartments
In pregnant rat uterine myocytes, fluorescent labelling of ryanodine and thapsigargin (which binds to SERCA) is non-homogeneous and is clearly concentrated around the nucleus (deep SR) and close to the cell membrane (superficial SR; Shmygol & Wray, 2004). It is not yet known if these regions differ in their expression of either IP3 or RyR release channels or SERCA, or if they are the functionally distinct store identified by some workers in other smooth muscles (e.g. Flynn et al. 2001; Wray et al. 2005). Van Breemen et al. (1986) first suggested from work on vascular tissue that the superficial SR had a specific function, that it buffered Ca2+ by taking up a fraction of the Ca2+ that enters the cell through the plasmalemma, and that the deep SR supplies Ca2+ for the contractile machinery. Studies on rat (Shmigol et al. 1999) and human (Young et al. 2001; Young & Zhang, 2004) myocytes lend support to this functional separation between deep cytosolic and subplasmalemmal calcium concentrations in the myometrium. The close apposition between superficial SR and parts of the plasma membrane has led to the suggestion that it is a special signalling domain (Blaustein et al. 2002) and will result not only in different [Ca2+] from bulk cytosol, but also different [Na+], due to preferential expression of the α2 subunit of the Na+ pump. Although little explored in the uterus to date, we have preliminary evidence of gestational changes in Na+ pump in myometrium (Floyd et al. 2003).
SR and local cytosolic Ca2+ releases
Van Breemen's model required that for SR Ca2+ to be maintained at steady state, accumulated Ca2+ is released into the subplasmalemmal space (referred to as ‘vectorial Ca2+ release’) and extruded through the cell membrane. There is currently a great deal of interest in how smooth muscle activity might be regulated by interaction of vectorial Ca2+ release and specific membrane microdomains. These studies have been greatly helped by confocal microscopy allowing the direct visualization of Ca2+ release from the SR, Ca2+ sparks from RyRs and Ca2+ puffs from IP3Rs.
Spontaneous transient outward currents (STOCs) are associated with Ca2+ sparks and BK channels activation in many smooth muscles (Burdyga & Wray, 2005). STOCs have been recorded in some uterine myocytes but no reports of Ca2+ spark activity appear to have been made, perhaps because Ca2+ release channels are not sufficiently clustered. It is however, clear that the SR has an important role in controlling myometrial excitability and contraction.
SR and uterine contraction
In the mouse, rat and human myometrium pharmacological inhibition of the SR Ca2+ pump, e.g. by cyclopiazonic acid (CPA), causes depletion of Ca2+ from the SR and increases cytosolic [Ca2+] and contraction (Taggart & Wray, 1998; Tribe et al. 2000b; Noble & Wray, 2002; Kupittayanant et al. 2002; Matthew et al. 2004). In rats, these studies have also provided evidence of physiological regulation of the myometrial SR; 60th developmental regulation (inhibition of the SR caused greater stimulation of neonatal uterus compared with adult uterus; Noble & Wray, 2002) and during pregnancy when SR Ca2+ depletion caused increased stimulation in late compared with early pregnancy (Taggart & Wray, 1998). These data are supported by Tribe et al. (2000b) who found in human myometrium that SR inhibition caused greater stimulation in labouring compared with non-labouring uterus. In order to understand how the SR might limit contraction in vivo we must question how SR Ca2+ depletion stimulates contraction.
SR and ion channels
Depletion of SR Ca2+ was shown to depolarize and contract vascular smooth muscle in a manner similar to agents which inhibited BK channels (Nelson et al. 1995). However, in uterus Taggart & Wray (1998) found that prior inhibition of BK channels with iberiotoxin did not inhibit further potentiation with CPA, and in cultured human uterine myocytes, Young & Zhang (2004) have recently shown that depletion of SR Ca2+ with CPA increased BK channel activity.
An alternative explanation is that SR Ca2+ depletion activates store-operated calcium entry (SOCE). SOCE is well described in non-excitable cells and increasingly in smooth muscle. SOCE has been demonstrated in primary cultured (Tribe et al. 2000a) and immortalized pregnant human myometrial cells stimulated with oxytocin (Monga et al. 1999), although caution must be used when applying these data on phenotypically altered cells to intact preparations of uterus. In rat myometrium, we have recently shown that a large component of the CPA-induced rise in basal Ca2+ is due to SOCE (Noble et al. 2005). The putative SOCE channels are thought to be members of the Trp C family and interestingly Dalrymple et al. (2004) have recently reported an up-regulation of specific Trp C mRNA and protein during human labour.
We know that oxytocin and prostaglandins are important myometrial agonists during labour and that they induce IP3 receptor activation and SR Ca2+ release. We might therefore speculate that as pregnancy progresses SERCA is up-regulated, and SR Ca2+ uptake is the dominant process until labour, when agonist release of SR Ca2+ allows SOCE and increased uterine excitability.
Obesity, channels and labour – translational research
It is interesting to speculate that the elevation of cholesterol, which occurs in obese women, could contribute to poor uterine contractility in vivo. Obesity is associated with an increase of both cholesterol and LDLs, and there is a further elevation of both in pregnancy (Gostynski et al. 2004). There is an increased risk of caesarean section associated with pregnancy in obese women (Crane et al. 1997). The underlying reason for the increased risk of surgery is not known, and it is tempting to speculate that the dyslipidaemia in these women is causing poor contractility, leading to dysfunctional labour, and hence the need for surgical intervention. This is supported by our preliminary data (Kendrick et al. 2004), showing poor contractility with increasing body mass index, this, in turn, being the reason that a caesarean section is needed, rather than obstruction or fetal distress. During the onset of labour we conclude that changes in lipid rafts and caveolae may have a role, perhaps by their clustering or the sorting of regulatory components of cell signalling, into or out of them. We can also speculate that changes in channel expression and SR function needed for successful parturition may not occur adequately in those women suffering dysfunctional labour, but there are at present no data on this.
Summary
As our understanding of signalling becomes ever more complex, and as we learn more about local signals, domains and environments, it is important that the functional relevance, under physiological conditions, of putative pathways be tested. In this brief review we have attempted to relate some of the exciting findings from membrane biology and signalling biochemistry to the control of myometrial, and indicate future therapeutic potential.
Acknowledgments
We are grateful to the MRC for supporting this work and J.Z. to ORS for a scholarship. We also thank our colleagues Drs T. Burdyga, A. Shmygol, S. Quenby and E. Babiychuk for many helpful discussions.
References
- Anwer K, Oberti C, Perez J, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol. 1993;265:C967–C985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- Babiychuck EB, Draeger A, Burdyga TV, Wray S. Extraction of cholesterol abolishes phasic contraction of rat and guinea-pig ureter. J Physiol. 2002;543.P:82P. [Google Scholar]
- Bastiaanse EM, Hold KM, Van der Laarse A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovascular Res. 1997;33:272–283. doi: 10.1016/s0008-6363(96)00193-9. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA, Song H, Choate J, Lencesova L, Robinson SW, Wier WG. Organization of Ca2+ stores in vascular smooth muscle: functional implications. Novartis Found Symp. 2002;246:125–137. [PubMed] [Google Scholar]
- Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289:C49–C57. doi: 10.1152/ajpcell.00399.2004. [DOI] [PubMed] [Google Scholar]
- Burdyga T, Wray S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature. 2005;436:559–562. doi: 10.1038/nature03834. [DOI] [PubMed] [Google Scholar]
- Chanrachakul B, Pipkin FB, Khan RN. Contribution of coupling between human myometrial beta2-adrenoreceptor and the BKCa channel to uterine quiescence. Am J Physiol Cell Physiol. 2004;287:C1747–C1752. doi: 10.1152/ajpcell.00236.2004. [DOI] [PubMed] [Google Scholar]
- Chen Q, Amaral J, Biancani P, Behar J. Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology. 1999;116:678–685. doi: 10.1016/s0016-5085(99)70190-3. [DOI] [PubMed] [Google Scholar]
- Ciray HN, Guner H, Hakansson H, Tekelioglu M, Roomans GM, Ulmsten U. Morphometric analysis of gap junctions in nonpregnant and term pregnant human myometrium. Acta Obstet Gynecol Scand. 1995;74:497–504. doi: 10.3109/00016349509024378. [DOI] [PubMed] [Google Scholar]
- Crane SS, Wojtowycz MA, Dye TD, Aubry RH, Artal R. Association between pre-pregnancy obesity and the risk of cesarean delivery. Obstet Gynecol. 1997;89:213–216. doi: 10.1016/S0029-7844(96)00449-8. [DOI] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Poston L, Tribe RM. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor and interleukin-1 beta. J Clin Endocrinol Metabolism. 2004;89:1291–1300. doi: 10.1210/jc.2003-031428. [DOI] [PubMed] [Google Scholar]
- Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol. 2002;22:1272. doi: 10.1161/01.atv.0000023438.32585.a1. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Toro L, Stefani E. Diminished surface clustering and increased perinuclear accumulation of large conductance Ca2+-activated K+ channel in mouse myometrium with pregnancy. J Biol Chem. 2003;278:45311–45317. doi: 10.1074/jbc.M306564200. [DOI] [PubMed] [Google Scholar]
- Floyd R, Mobasheri A, Martin-Vasallo P, Wray S. Na,K-ATPase isoforms in pregnant and nonpregnant rat uterus. Ann N Y Acad Sci. 2003;986:614–616. doi: 10.1111/j.1749-6632.2003.tb07263.x. [DOI] [PubMed] [Google Scholar]
- Flynn ERM, Bradleyt KN, Muir TC, McCarron JG. Functionally separate intracellular Ca2+ stores in smooth muscle. J Biol Cemistry. 2001;276:36411–36418. doi: 10.1074/jbc.M104308200. [DOI] [PubMed] [Google Scholar]
- Goligorsky MS, Li H, Brodsky S, Chen J. Relationaships between caveolae and eNOS. everything in proximity and the proximity of everything. Am J Physiol Renal Physiol. 2002;283:F1–F10. doi: 10.1152/ajprenal.00377.2001. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Gostynski M, Gutzwiller F, Kuulasmaa K, Doring A, Ferrario M, Grafnetter D, Pajak A. Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes Relat Metab Disord. 2004;28:1082–1090. doi: 10.1038/sj.ijo.0802714. [DOI] [PubMed] [Google Scholar]
- Kendrick AJ, Zhang J, Tattersall M, Bricker L, Quenby S, Wray S. Calcium signalling, caveolae and human myometrial contractility. J Physiol. 2004;560P:C50. [Google Scholar]
- Khan R, Smith SK, Morrison JJ, Ashford MLJ. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proc Royal Soc Lond B Biol Sci. 1993;251:9–15. doi: 10.1098/rspb.1993.0002. [DOI] [PubMed] [Google Scholar]
- Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34:13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- Kupittayanant S, Luckas MJM, Wray S. Effects of inhibitng the sarcoplasmic reticulum on spontaneous and oxytocin-induced contractions of human myometrium. Br J Obstet Gynaecol. 2002;109:289–296. doi: 10.1111/j.1471-0528.2002.01110.x. [DOI] [PubMed] [Google Scholar]
- Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membrane Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JR, O'Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Martin C, Hyvelin JM, Chapman KE, Marthan R, Ashley RH, Savineau JP. Pregnant rat myometrial cells show heterogeneous ryanodine- and caffeine-sensitive calcium stores. Am J Physiol. 1999;46:C243–C252. doi: 10.1152/ajpcell.1999.277.2.C243. [DOI] [PubMed] [Google Scholar]
- Matthew AJG, Kupittayanant S, Burdyga TV, Wray S. Characterization of contractile activity and intracellular Ca2+ signalling in mouse myometrium. J Soc Gynecol Investig. 2004;11:207–212. doi: 10.1016/j.jsgi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Monga M, Campbell DF, Sanborn BM. Oxytocin stimulated capacitative calcium entry in human myometrial cells. Am J Obstet Gynecol. 1999;181:424–429. doi: 10.1016/s0002-9378(99)70573-9. [DOI] [PubMed] [Google Scholar]
- Morgan JM, De Smedt H, Gillespie JI. Identification of three isoforms of the InsP3 receptor in human myometrial smooth muscle. Pflugers Arch. 1996;431:697–705. doi: 10.1007/BF02253832. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Noble K, Tengah A, Wray S. The role of the sarcoplasmic reticulum (SR) increases with gestation in pregnant rat myometrium: is store-operated calcium entry (SOCE) involved. J Physiol. 2005;568P:PC47. [Google Scholar]
- Noble K, Wray S. The role of the sarcoplasmic reticulum in neonatal uterine smooth muscle: enhanced role compared to adult rat. J Physiol. 2002;545:557–566. doi: 10.1113/jphysiol.2002.031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell KM, Martens JR, Tamkun MM. Localization of ion channels to lipid Raft domains within the cardiovascular system. Trends Cardiovasc Med. 2004;14:37–42. doi: 10.1016/j.tcm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signalling and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circulation Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- Shmigol AV, Eisner DA, Wray S. The role of the sarcoplasmic reticulum as a calcium sink in uterine smooth muscle cells. J Physiol. 1999;520:153–163. doi: 10.1111/j.1469-7793.1999.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmygol A, Wray S. Functional architecture of the SR calcium store in uterine smooth muscle. Cell Calcium. 2004;35:501–508. doi: 10.1016/j.ceca.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Shmygol A, Wray S. Effect of cholesterol extraction on [Ca2+]i transients and K+ currents in rat uterine myocytes. J Physiol. 2005;000.P:565P. [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nature Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smith RD, Babiychuk EB, Noble K, Draeger A, Wray S. Increased cholesterol decreases uterine activity: functional effects of cholesterol in pregnant rat myometrium. Am J Physiol Cell Physiol. 2005;288:C982–C988. doi: 10.1152/ajpcell.00120.2004. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Leavis P, Feron O, Morgan KG. Inhibition of PKCalpha and rhoA translocation in differentiated smooth muscle by a caveolin scaffolding domain peptide. Exp Cell Res. 2000;258:72–81. doi: 10.1006/excr.2000.4891. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J Physiol. 1998;511:133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribe RM, Moriarty P, Poston L. Calcium homeostatic pathways change with gestation in human myometrium. Biol Reprod. 2000a;63:748–755. doi: 10.1095/biolreprod63.3.748. [DOI] [PubMed] [Google Scholar]
- Tribe RM, Moriarty P, Poston L. Calcium homeostatic pathways change with gestation in human myometrium. Biol Reprod. 2000b;63:748–755. doi: 10.1095/biolreprod63.3.748. [DOI] [PubMed] [Google Scholar]
- Turi A, Kiss AL, Mullner N. Estrogen downregulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int. 2001;25:785–794. doi: 10.1006/cbir.2001.0769. [DOI] [PubMed] [Google Scholar]
- van Breemen C, Lukeman S, Leijten P, Yamamoto H, Loutzenhiser R. The role of superficial SR in modulating force development induced by Ca entry into arterial smooth muscle. J Cardiovasc Pharmacol. 1986;8:S111–S116. doi: 10.1097/00005344-198600088-00023. [DOI] [PubMed] [Google Scholar]
- Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wray S, Jones K, Kupittayanant S, Matthew AJG, Monir-Bishty E, Noble K, Pierce SJ, Quenby S, Shmygol AV. Calcium signalling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Young RC, Schumann R, Zhang P. Intracellular calcium gradients in cultured human uterine smooth muscle: a functionally important subplasmalemmal space. Cell Calcium. 2001;29:183–189. doi: 10.1054/ceca.2000.0182. [DOI] [PubMed] [Google Scholar]
- Young RC, Zhang P. Functional separation of deep cytoplasmic calcium from subplasmalemmal space calcium in cultured human uterine smooth muscle cells. Cell Calcium. 2004;36:11–17. doi: 10.1016/j.ceca.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shen P, Coleman M, Zou W, Loggie BW, Smith LM, Wang Z. Caveolin-1 down-regulation activates estrogen receptor alpha expresion and leads to 17beta-estradiol-stimulated mammary tumorigenesis. Anticancer Res. 2005;25:369–375. [PubMed] [Google Scholar]