Abstract
Brainstem catecholamine (CA) neurones have wide projections and an arousal-state-dependent activity pattern. They are thought to modulate the processing of sensory information and also participate in the control of breathing. Mice with lethal genetic defects that include CA neurones have abnormal respiratory control at birth. Also the A6 region (locus coeruleus), which contains CA neurones sensitive to CO2in vitro, is one of many putative central chemoreceptor sites. We studied the role of CA neurones in the control of breathing during sleep and wakefulness by specifically lesioning them with antidopamine β-hydroxylase–saporin (DBH-SAP) injected via the 4th ventricle. After 3 weeks there was a 73–84% loss of A5, A6 and A7 tyrosine hydroxylase (TH) immunoreactive (ir) neurones along with 56–60% loss of C1 and C2 phenyl ethanolamine-N-methyltransferase (PNMT)-ir neurones. Over the 3 weeks, breathing frequency decreased significantly during air and 3 or 7% CO2 breathing in both wakefulness and non-REM (NREM) sleep. The rats spent significantly less time awake and more time in NREM sleep. REM sleep time was unaffected. The ventilatory response to 7% CO2 was reduced significantly in wakefulness at 7, 14 and 21 days (−28%) and in NREM sleep at 14 and 21 days (−26%). Breathing variability increased in REM sleep but not in wakefulness or NREM sleep. We conclude that CA neurones (1) promote wakefulness, (2) participate in central respiratory chemoreception, (3) stimulate breathing frequency, and (4) minimize breathing variability in REM sleep.
Catecholamine (CA) neurones located in the brainstem project widely to forebrain, hindbrain and spinal cord (Hökfelt et al. 1984; Berridge & Waterhouse, 2003). Their natural activity pattern is state dependent; they fire substantially in wakefulness, and less so in NREM and REM sleep (Jacobs, 1986; Berridge & Waterhouse, 2003). They modulate the processing of sensory information in wakefulness (Foote et al. 1980; Aston-Jones et al. 2000; Berridge & Waterhouse, 2003) and participate in sleep cycle regulation, promoting wakefulness (Hobson et al. 1975; Saper et al. 2001; Ouyang et al. 2004). They are also involved in the control of breathing (Erickson & Milhorn, 1984; Guyenet et al. 1993; Oyamada et al. 1998; Dawid-Milner et al. 2001; Hilaire et al. 2004; Viemari et al. 2004) and cardiovascular function (Morilak et al. 1987; Levine et al. 1990; Curtis et al. 1993; Murase et al. 1993; Schreihofer & Guyenet, 2000; Dampney & Horiuchi, 2003; Swoap et al. 2004). Our interest lies in the role of CA neurones in the control of breathing, especially in central chemoreception, during both sleep and wakefulness.
CA neurones are labelled after retrograde tracing of viral vectors injected into phrenic motoneurones suggesting their involvement in respiratory control (Dobbins & Feldman, 1994). Evidence from Rnx mutant mice (Shirasawa et al. 2000), neonatal rat brainstem studies (Hilaire et al. 2004), and adult anaesthetized rats (Jodkowski et al. 1997; Dawid-Milner et al. 2001) indicate that CA A5 neurones can affect breathing frequency. In respect to the A6 region fetal Phox2a mutant mice, which die within 24 h of birth and have absent A6 CA neurones (Morin et al. 1997), breathe more slowly and with more variability than heterozygous controls (Viemari et al. 2004). Pharmacological and lesion studies showed that A6 CA neurones stimulate breathing frequency and are essential for the development of a normal respiratory rhythm (Hilaire et al. 2004).
Central chemoreceptors are thought by some to reside at or near the ventral surface of the medulla (see Loeschcke, 1982; Nattie, 1999; Feldman et al. 2003) and recent data support involvement of neurones in the retrotrapezoid nucleus (Nattie & Li, 2002) that are glutamatergic (Mulkey et al. 2004) and in the medullary raphe that are serotonergic (Nattie et al. 2004; Richerson, 2004) as chemoreceptor cells. We adhere to the view that central chemoreception is present at widespread sites within the hindbrain and cerebellum (Coates et al. 1993; Nattie, 2001; Nattie & Li, 2002; Feldman et al. 2003; Nattie et al. 2004) including regions rich in CA neurones. CO2 stimulation increases c-fos expression in CA neurones of the A5 and A6 regions (Haxhiu et al. 1996) and increases the firing rate of A6 cells in reduced preparations (Pineda & Aghajanian, 1997; Oyamada et al. 1998; Ballantyne & Scheid, 2000; Filosa et al. 2002) and in anaesthetized rats in vivo (Elam et al. 1981). Focal acidification of the A6 region increases ventilatory output (phrenic nerve activity) in anaesthetized cats and rats (Coates et al. 1993).
We hypothesize that CA neurones (1) produce an excitatory drive that affects breathing frequency and its variability predominantly in wakefulness when these neurones are most active and (2) participate in central chemoreception. To test these hypotheses, we have specifically lesioned brainstem CA neurones by injection of DBH-SAP into the 4th ventricle of rats and measured ventilation during sleep and wakefulness over 21 days. IgG-SAP is used as a control of similar size, which is not taken up into the cells.
Methods
General
The rats were housed in a room with a light, rest period from midnight to noon and a dark, active period from noon to midnight. Food and water were available ad libitum. All the experiments were performed between 8 a.m. and 3 p.m. All procedures were within the guidelines of the National Institutes of Health for animal use and care and were approved by the Dartmouth College Institutional Animal Use and Care Committee. A total of 33 male Sprague-Dawley rats (250–350 g) were used of which 16 completed the entire 4-week protocol. There were two main experimental groups: (1) lesion with DBH-SAP injection (n = 7); control with IgG-SAP injection (n = 6). We also made 24 h sleep measurements in additional lesioned rats (N = 3).
The rats were anaesthetized with ketamine (100 mg kg−1i.m.) and xylazine (15 mg kg−1i.p.). Hindlimb withdrawal and corneal reflexes were tested to monitor the depth of anaesthesia. Responses resulted in administration of supplemental anaesthesia in the form of a quarter of the initial dose. The skull and a portion of the abdomen were shaved and the skin cleansed with a povidone–iodine solution and 70% alcohol. The head was placed into a Kopf stereotaxic holder and two EEG electrodes were screwed into the right side of the skull. One was over frontal cortex (2 mm anterior to bregma and 2 mm lateral to midline), another over parietal cortex (3.5 mm posterior to bregma, 2 mm lateral) and an earth lead placed between the two 3 mm lateral to midline. For the EMG, a pair of wire electrodes was threaded through the nuchal muscles. A sterile telemetry temperature probe (TA-F20, Data Sciences, St Paul, MN, USA) was placed in the abdominal cavity. After recovery and baseline data collection, each rat was re-anaesthetized and received a microinjection of either DBH-SAP or IgG-SAP into the fourth ventricle. A small incision was made on the top of the skull, and a ∼2 mm diameter hole was drilled 2.3–3.0 mm caudal from lambda in the midline. The microinjection was made by using a 5 μl Hamilton syringe with a 28 gauge needle inserted 7.1–7.4 mm below the dorsal surface of the skull. Each microinjection lasted at least 5 min and the needle remained in position for another 5 min before removal. The incision was sutured.
Protocol
After at least 7 days recovery from the EEG/EMG placement, baseline ventilation V̇E, tidal volume (VT), frequency (f), and oxygen consumption were measured at least twice in each rat while breathing room air, 3 and 7% CO2 in air during sleep and wakefulness. Each rat then received one injection (2.2 μl, 5 μg) of (a) IgG-SAP or (b) DBH-SAP (Advanced Targeting Systems, San Diego, CA, USA). Ventilatory measurements were made on days 7–9, 14–16 and 21–23 after the injection. For this protocol after the rat was acclimatised to the plethysmograph chamber data were obtained over 30–45 min of breathing air and each of the CO2 mixtures. This allowed ample time for awake and NREM periods but REM periods were not consistently observed. In order to obtain breathing data in REM, sleep cycling and breathing in air were separately measured in five control and four lesioned rats during 4 h prior to and ∼10 days after the injection. We also analysed the variability of breathing in wakefulness, NREM and REM sleep using 400 breaths in each state. In a separate set of rats (N = 3) sleep cycling was recorded over 24 h prior to and ∼21 days after the injection.
Data analysis
We determined sleep and wakefulness using EEG and EMG electrode signals, the fast Fourier transform (FFT) of the EEG signal analysed in 3.6 s epochs at delta (0.3–5 Hz), theta (6–9 Hz) and sigma (10–17 Hz) frequency bands, and behavioural observations. We used criteria as previously described (Nattie & Li, 2002; Nattie et al. 2004). In our case, we measured breathing only during quiet wakefulness. In active wakefulness the activity of the rat in the plethysmograph interferes with reliable measurement of breathing. For 4 h and 24 h sleep cycling data SleepSign sleep analysis software (Kissei Comtec America, Inc.) was used. We analysed the amount of time the rat spent in NREM and REM sleep and in wakefulness during each experiment. Ventilation was measured using a flow-through whole-body plethysmograph as previously described (Nattie & Li, 2002; Nattie et al. 2004). The volume of the plethysmograph was 7.6 l with a 3.5 l top to protect the head pedestal. The plethysmograph was connected by a high resistance leak to a similarly sized reference chamber. The inflow gas for the plethysmograph chamber was humidified and controlled by a flow meter at a minimum of 1.4 l min−1 to prevent rebreathing of exhaled gas. The outflow was matched to the inflow via a flow meter connected to a vacuum system. Approximately 100 ml min−1 of outflow gas served O2 and CO2 analysers (Applied Electrochemistry). We measured chamber pressure by transducer and calibrated the plethysmograph with multiple 0.3 ml injections. We measured chamber temperature by thermometer and rat body temperature by telemetry continuously.
For calculation of ventilatory data, we used the DataPac 2000 system to determine the pressure deflections and the respiratory cycle time for each of 100–300 breaths at defined sleep and wake periods breathing air, 3% and 7% CO2. Sighs, sniffing and recording artifacts were edited from analysis. These data were exported to Sigmaplot 6.0 (SPSS, Inc., Chicago, IL, USA) with f, VT and V̇E per 100 g of body weight calculated for each breath using plethysmograph and body temperatures for that time period. We obtained two to four measurement periods for NREM sleep and wakefulness as a baseline prior to 3% or 7% CO2 exposure and one or two periods during the 30–40 min of CO2 exposure. In our two experimental groups, we compared V̇E, VT and f breathing air, 3% or 7% CO2 in NREM sleep and wakefulness on days 7–9, 14–16 and 21–23 to the baseline data for that treatment in each rat. We defined the ‘CO2 response’ as the Δ V̇E breathing 7% (or 3%) CO2 minus that in air breathing. Oxygen consumption was calculated using the Fick principle and the difference in O2 content between gas entering and leaving the plethysmograph and the flow rate and normalized to ml (g body weight)−1 h−1. We monitored CO2 content of the outflow gas continuously to ensure that no build-up of CO2 took place within the chamber.
For breathing variability, we applied the Poincaré analysis in which the duration of each breath is plotted versus the duration of the next breath. We calculated the width of the variation perpendicular to (SD1) and along the line of identify (SD2) and calculated the area of the ellipse that describes the distribution of the points (Brennan et al. 2001).
Anatomy
Three weeks after injection following all physiological experiments the rats were anaesthetized with ketamine and xylazine then transcardially perfused with 200 ml saline followed by 300–500 ml of chilled 4% paraformaldehyde (4% in 0.1 m phosphate buffer, pH 7.4). The brain was removed and post-fixed overnight in 4% paraformaldehyde at 4°C, then cryoprotected for 48 h in 30% sucrose. The brains were sectioned at 30 μm thickness on a Leica cryostat for immunohistochemical staining of tyrosine hydroxylase (TH) or phenylethanolamine-N-methytransferase (PNMT). For the individual reactions (TH, PNMT) we used the diaminobenzidine (DAB) reaction for visualization. All immunohistochemical procedures were performed by using free-floating sections at room temperature. We used 0.1 m phosphate buffer (PB) for TH and PNMT quenched by 3% H2O2 and then blocked with 5% normal goat serum (NGS). The sections were incubated with either a rabbit polyclonal antibody against PNMT (1: 10 000, Chemicon), or a mouse monoclonal antibody against TH (1: 10 000, Sigma) for 48 h at 4°C followed by a biotinylated goat anti-rabbit or anti-mouse IgG overnight at 4°C (1: 500, Vector Laboratories). An avidin–biotin–horseradish peroxidase procedure with DAB was used to visualize PNMT and TH staining. These sections were used for all cell counts. All the sections were mounted and dehydrated with graded alcohol (25–100% EtOH), cleared with xylene and cover-slipped.
Cell counting methods
Brainstem sections were cut at 30 μm thickness in a cryostat with two of every four successive sections stained for TH or PNMT. To evaluate the effects of our DBH-SAP injections on brainstem CA neurones we counted the number staining for TH in the A5, A6 and A9 regions and the number staining for PNMT at the C3 and C1 regions. We counted all somatic cell profiles in each area of interest. A5, A6, A7, A9 and C1 regions were counted on one side only. The C3 region is in the midline. Each section counted for any marker was 30 μm in thickness and separated from the next counted section by 90 μm. For the A5 region we applied the approach of Byrum et al. (1984) as shown in their Fig. 1. TH-positive cells were counted in a small rectangle in the ventrolateral pons and medulla over the entire length of this cell group, a rostral-caudal range of just over 2 mm. A6 cells were counted over the entire length of this cell group in the dorso-lateral pons along the lateral margin of the fourth ventricle and extending caudally for approximately 1.5 mm (Hökfelt et al. 1984). A7 TH-ir neurones were counted in six to nine sections rostrally starting at the first evidence of TH-ir cells ventral to the most rostral aspect of A6. A9 TH-positive cells were counted in four successive sections beginning at about P = 5.8 (Hökfelt et al. 1984). The C1 PNMT-ir cells were counted in the ventrolateral medulla for their entire rostral–caudal extent, which is approximately 2.7 mm (Hökfelt et al. 1984). The C3 PNMT-ir cells were counted in the centre of the dorsal medulla for their entire rostral–caudal extent, a distance of approximately 1.5 mm (Hökfelt et al. 1984).
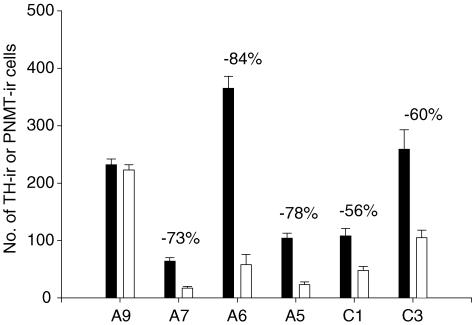
Figure 1. DBH-SAP injections (open bars; n = 6) decrease the number of TH-ir neurones in A7, A6 and A5 regions and the number of PNMT-ir neurones in C1 and C3 regions as compared to IgG-SAP injections (filled bars; N = 5).
Mean ± s.e.m. values are shown. The number of immunoreactive cell profiles was significantly lower in treated compared to control rats in A6 (P < 0.001; t test), A5 (P < 0.001), A7 (P < 0.001), C3 (P < 0.003) and C1 (P < 0.005).
Statistics
Counts of somatic cell profiles of TH-ir and PNMT-ir neurones were compared at each CA neuronal location between the IgG-SAP and DBH groups by t test. For analysis of ventilatory data we used a two-way repeated measures ANOVA with V̇E, VT, and f as the repeated measures variable and state (awake, NREM sleep), treatment (IgG-SAP, DBH-SAP) and CO2 (room air, 3% CO2, 7% CO2) as categorical variables. To evaluate the ‘CO2 response’ we calculated the Δ V̇E (ΔVT and Δf) comparing values in 3 or 7% CO2 to the corresponding air breathing values. These were analysed with the same two-way repeated measures ANOVA approach. For analysis of breathing variability we applied a two-way repeated measures ANOVA for each parameter of the Poincaré analysis (SD1, SD2, area) with baseline and 10 days as repeated measures and treatment (IgG-SAP, DBH-SAP) and state (awake, NREM, REM) as categorical variables. For analysis of sleep–wake time within the 4 h measurement periods we applied a two-way repeated measures ANOVA with baseline and 10 days as the repeated measure and treatment (IgG-SAP, DBH-SAP) and state (awake, NREM, REM) as categorical variables. In each case, post hoc comparisons of within-group effects were made using Dunnett's test and the t statistic. For analysis of treatment effects on sleep–wake time in the 24 h group, we applied a two-way repeated measures ANOVA with baseline and 21 days as repeated measures and treatment (IgG-SAP, DBH-SAP) and time (each 2 h period over the 24 h) as categorical factors.
Results
Cell counts
Figure 1 shows the mean ± s.e.m. of the total number of counted immunoreactive cell profiles for each region in IgG-SAP injected controls (N = 5) and DBH-SAP lesioned rats (N = 6). For each region of interest the number of sections counted and the rostral-to-caudal length were not different between the groups.
In the A6 region, we counted cell profiles over 1248 ± 61 μm in the control group and 1480 ± 67 μm in the lesion group. DBH-SAP decreased the number of TH-ir cell profiles by 84% on average from 365 ± 21 to 58 ± 18 (P < 0.001). In the A5 region, we counted cell profiles over 2232 ± 111 μm in the control group and 2180 ± 147 μm in the lesion group. DBH-SAP injections decreased the number of TH-ir cell profiles by 78% on average from 104 ± 9 to 23 ± 5 (P < 0.001). In the A7 region, we counted cell profiles in six to nine sections per rat. DBH-SAP decreased the number of TH-ir cell profiles from 64 ± 6 to 17 ± 3, a 73% decrease (P < 0.01). In the more rostral A9 region, we counted cell profiles in four consecutive sections. DBH-SAP injections had no significant effect on the number of TH-ir cell profiles with 232 ± 10 and 223 ± 9 being the counts.
In the C3 region, we counted cell profiles over 1440 ± 114 μm in the control group and 1640 ± 106 μm in the lesion group. DBH-SAP injections decreased the number of PNMT-ir cell profiles by 56% on average from 108 ± 13 to 48 ± 7 (P < 0.003). In the C1 region, we counted cell profiles over 2722 ± 222 μm in the control group and 2640 ± 161 μm in the lesion group. DBH-SAP injections decreased the number of PNMT-ir cell profiles by 60% on average from 259 ± 34 to 105 ± 13 (P < 0.005).
Oxygen consumption and body temperature
Oxygen consumption and body temperature did not differ between the IgG-SAP control and DBH-SAP lesion groups at any of the four measurement days nor did they differ with time. In controls mean (± s.e.m.) oxygen consumption at baseline was 1.18 ± 0.02 ml h (g body wt)−1 and at 21 days was 1.15 ± 0.04 ml h (g body wt)−1 while in the lesion group it was 1.21 ± 0.11 and 1.14 ± 0.01 ml h (g body wt)−1, respectively. Body temperature in controls at baseline was 38.0 ± 0.08°C and at 21 days was 37.8 ± 0.12°C while in the lesion group it was 38.1 ± 0.11 and 37.9 ± 0.09°C, respectively.
Ventilation
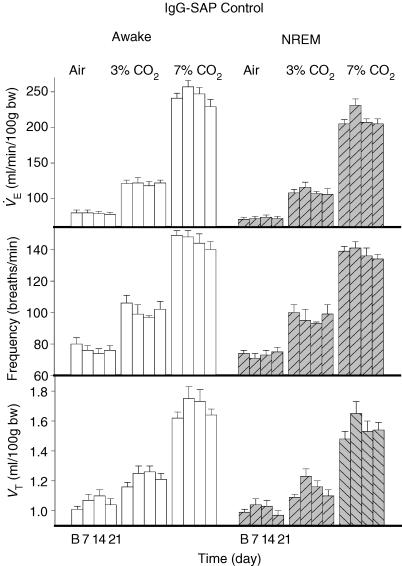
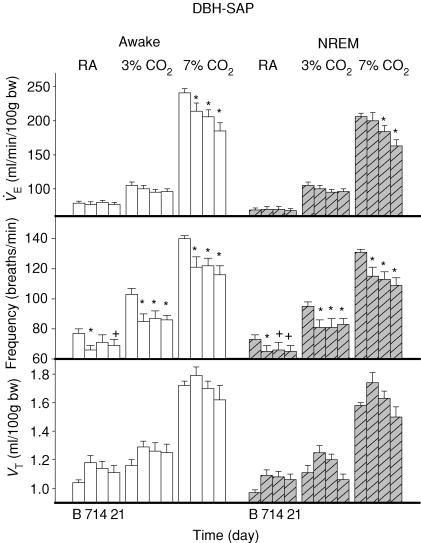
V̇E (Figs 2 and 3) decreased significantly over the time course of the experiment (P < 0.001) with significant interactions with treatment (P < 0.001), CO2 (P < 0.001) and with treatment and CO2 together (P < 0.002). There was no interaction of the V̇E effect with state but there was a significant effect of state as a categorical variable (P < 0.001). Thus V̇E was significantly less in NREM sleep overall without any variation in this sleep effect over the post-baseline time course of the experiment. In 7% CO2 V̇E was significantly decreased at 7, 14 and 21 days in wakefulness (P < 0.01) and at 14 (P < 0.05) and 21 days (P < 0.01) in NREM sleep. Post hoc comparison showed no treatment effect in 3% CO2 or in air breathing.
Figure 2. IgG-SAP effects.
IgG-SAP injections have no effect on ventilation V̇E, tidal volume (VT), and frequency (f) while breathing air, 3% CO2 or 7% CO2 in wakefulness (open bars) or NREM sleep (hatched bars) over 7, 14 and 21 days after baseline (B).
Figure 3. DBH-SAP effects.
DBH-SAP injections: (1) had no effect on ventilation V̇E (top row) in wakefulness (open bars) or NREM sleep (hatched bars) in air breathing (left panels) or 3% CO2 (middle panels) but significantly decreased V̇E (*P < 0.01, +P < 0.05; post hoc Dunnett's test after two-way repeated measures ANOVA; see text) in 7% CO2 (right panels) at 7, 14 and 21 days in wakefulness and at 14 and 21 days in NREM sleep; (2) decreased breathing frequency (f) (middle row) at all times in 3 and 7% CO2 and at 7 and 14 days breathing room air awake and at 7, 14, and 21 days in NREM sleep; and (3) had no significant effect on tidal volume (VT; bottom row) in any condition.
Breathing frequency (f) (Figs 2 and 3) decreased significantly over the time course of the experiment (P < 0.01) with significant interactions with treatment (P < 0.001) and CO2 (P < 0.001) There was no interaction of the f effect with state but there was a significant effect of state as a categorical variable (P < 0.001). Thus f was significantly less in NREM sleep overall without any variation in this sleep effect over the post-baseline time course of the experiment. The absence of a significant interaction between the effect on f, treatment and CO2 indicates that the effect on f is present at all levels of CO2 (air, 3% CO2 and 7% CO2). Given the a priori hypothesis that treatment would decrease f at all levels of CO2, we made post hoc comparisons. Frequency was significantly decreased in 7% CO2 at 7, 14 and 21 days in wakefulness and NREM sleep (P < 0.01), in 3% CO2 at 7, 14 and 21 days in wakefulness and NREM sleep (P < 0.01) and in air breathing during wakefulness at 7 (P < 0.01) and 21 (P < 0.05) but not 14 days and in NREM sleep at 7 (P < 0.01), 14 and 21 (P < 0.05) days.
Tidal volume (VT) (Figs 2 and 3) increased over the post-baseline time course of the experiment (P < 0.001) but there was no significant interaction with treatment, state or CO2. There was a significant effect of state as a categorical variable. Thus VT was significantly less in NREM sleep overall without any variation in this sleep effect over the post-baseline time course of the experiment.
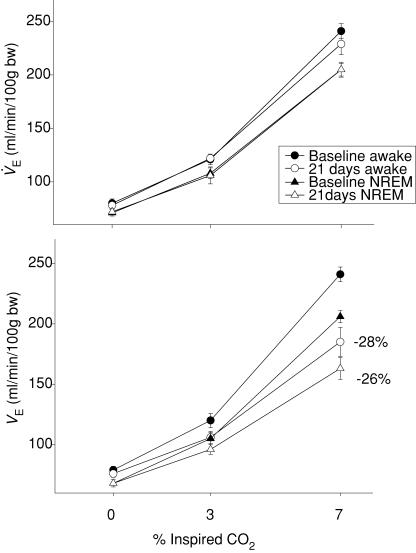
To emphasize the effect of DBH-SAP lesions on the CO2 response, we show CO2 response curves of V̇E for the 21-day data in wakefulness and NREM sleep in control and lesion groups (Fig. 4). The ‘CO2 response’ ΔV̇E) decreased over the post-baseline time course of the experiment (P < 0.001) and there was a significant interaction with treatment (P < 0.001), CO2 (P < 0.02), and with treatment and CO2 together (P < 0.05). There was no interaction of the Δ V̇E repeated measures effect with state but there was a significant effect of state as a categorical variable indicating that Δ V̇E was less in NREM sleep. Post hoc analysis showed that in 7% CO2, Δ V̇E was significantly decreased in wakefulness at 7 (P < 0.05) and at 14 and 21 days (P < 0.01) and in NREM sleep at 21 days (P < 0.01). At 21 days the ‘CO2 response’ breathing 7% CO2 was decreased by 28% in wakefulness and by 26% in NREM sleep. In 3% CO2 the ‘CO2 response’ was decreased by 33% in wakefulness and NREM sleep. These effects did not reach statistical significance so while we conclude that there is no treatment effect at the lower stimulus level we can warn that this conclusion is tentative. Analysis of Δf showed a significant effect over the post-baseline time course of the experiment (P < 0.001) but there was no significant interaction with state, CO2 or treatment (P= 0.06). Analysis of ΔVT showed no significant effect.
Figure 4. CO2 response before and after IgG-SAP and anti-DBH-SAP.
IgG-SAP injections had no effect on the V̇E CO2 response (top panel) in wakefulness or NREM sleep examined at 21 days after injection. DBH-SAP injections decreased the V̇E CO2 response in wakefulness or NREM sleep examined 21 days after injection. •, baseline data for wakefulness; ▴, NREM sleep; ○ and ▵, data obtained at 21 days. The percentage values indicate the percentage decrease compared for 7% CO2 at 21 days.
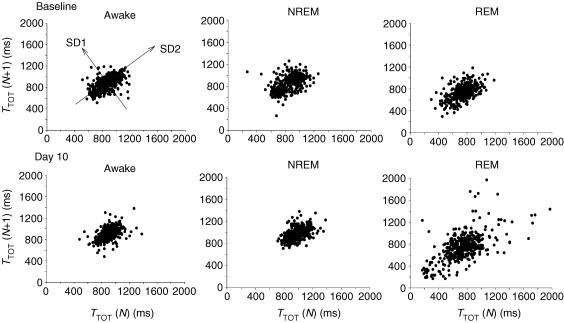
The variability of breath duration
Figure 5 shows for a single animal the Poincaré plots of the duration of a breath, TTOT, versus the duration of the subsequent breath, TTOT (N+ 1), for 400 breaths in each state (awake, NREM sleep and REM sleep) at baseline and at day 10 after injection. After DBH-SAP injections, the variability of breath duration increased in REM sleep but not in wakefulness or NREM sleep. To quantify the distribution of points we used the parameters SD1 and SD2 as shown on the upper left panel and the area of the ellipse that fits the distribution of points (not shown) (Brennan et al. 2001). In this animal SD1, SD2 and area did not differ between baseline and day 10 in the awake and NREM states. In REM sleep, SD1, SD2 and ellipse area were significantly greater at day 10 by 23, 32 and 131%, respectively. Average data for five lesion and four control rats are shown in Table 1. For each parameter SD1, SD2 and ellipse area, there was no significant lesion effect in wakefulness or in NREM sleep. In REM sleep all parameters showed a significant increase after the lesions. For SD1 there was a significant interaction of state, treatment, and SD1 (P < 0.01). SD1 at day 10 was greater than baseline in the lesion group (P < 0.05) and greater than day 10 in controls (P < 0.05). For SD2 the interaction term was not significant but a post hoc comparison based on the a priori hypothesis showed day 10 greater than baseline in the lesion group (P < 0.01) and greater than day 10 in the control group (P < 0.01). For the area, the interactive term was significant (P < 0.01) and post hoc analysis showed day 10 greater than baseline in the lesion group (P < 0.01) and greater than day 10 in the controls (P < 0.01). We repeated the lesions in four additional rats and extended the variability analysis out for 3 weeks and found no further disruption of variability. In REM sleep, there were also significantly more breaths longer than 1.5 s, a long breath duration for the rat. The number of breaths out of 400 that were longer than 1.5 s was 6.4 ± 0.7 and 3.0 ± 0.6 at baseline and day 10 in the control group and 2.4 ± 0.7 and 11.3 ± 2.3 in the lesion group (day 10 in lesion group significantly different from baseline and from day 10 in control group; P < 0.001 for interaction term; P < 0.05 post hoc).
Figure 5. Poincaré plots.
Poincaré plots graph for one rat the duration of a breath, TTOT, versus the duration of the subsequent breath, TTOT (N+ 1), for 400 breaths in each state (awake, NREM sleep, REM sleep) at baseline (top row) and at day 10 (bottom row) after injection. The variability of breath duration increases in REM sleep (right panels) but not in wakefulness (left panels) or NREM sleep (middle panels) after DBH-SAP lesions. SD1 and SD2 shown on the upper left panel and the area of the ellipse that fits the distribution of points (not shown) are parameters that quantify the point spread (Brennan et al. 2001). In this animal SD1, SD2 and area do not differ significantly between states in the baseline condition nor do they vary between baseline and day 10 in wakefulness and NREM sleep. In REM sleep, SD1, SD2 and area are significantly greater at day 10 by 23, 32 and 131% greater, respectively, as compared to awake or NREM at day 10 or to REM at baseline. See Table 1 for average data.
Table 1.
The variability of breath duration (mean ± S.E.M.) in wakefulness, NREM and REM sleep at baseline conditions (B) and at 10 days (10) after DBH-SAP (n = 4) or IgG-SAP (n = 5) injections
| Awake | NREM | REM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG-SAP | DBH-SAP | IgG-SAP | DBH-SAP | IgG-SAP | DBH-SAP | |||||||
| B | 10 | B | 10 | B | 10 | B | 10 | B | 10 | B | 10 | |
| SD1 | 81 | 77 | 76 | 73 | 71 | 77 | 78 | 70 | 93 | 87 | 85 | 108*# |
| (6) | (10) | (4) | (4) | (9) | (7) | (8) | (8) | (5) | (2) | (3) | (8) | |
| SD2 | 175 | 142 | 150 | 127 | 110 | 128 | 139 | 144 | 170 | 152 | 166 | 223*# |
| (25) | (17) | (13) | (9) | (16) | (20) | (16) | (30) | (5) | (9) | (13) | (31) | |
| Area | 43.9 | 35.7 | 35.8 | 29.0 | 25.6 | 31.1 | 34.9 | 32.2 | 50.1 | 41.4 | 44.0 | 75.0*# |
| (× 103) | (5.6) | (8.2) | (3.9) | (2.7) | (5.9) | (5.8) | (7.3) | (7.5) | (3.7) | (2.4) | (2.5) | (12.2) |
SD1, SD2 (both in ms) and area (defined in Fig. 5) were determined from 400 breaths in each case.
Significantly different from control value at day 10;
significantly different DBH-SAP value at baseline (B); P < 0.05.
Sleep
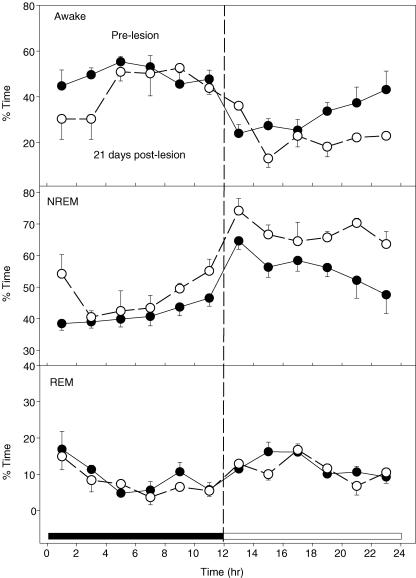
We analysed the effects of the DBH-SAP lesions on sleep–wake state during 4 h data collections periods obtained at baseline and 10 days after injection while breathing air. The lesioned rats spent less time awake and more time in NREM sleep. At 10 days the time awake for the lesion group (N = 9) was 30 ± 2% (S.E.M.) versus 37.1 ± 2.7% for baseline (P < 0.05) while the time in NREM sleep was 66 ± 2%versus 58 ± 2.2% for baseline (P < 0.05). In the control group the time awake was 38 ± 2.3% at baseline and 41 ± 3% at 10 days while the NREM sleep time was 54 ± 1.4% at baseline and 56 ± 1.7% at 10 days. In three rats we obtained 24 h sleep–wake data at baseline and at 21 days after injection of DBH-SAP (Fig. 6). At 21 days compared to baseline these rats were awake less (P < 0.04) and in NREM sleep more (P < 0.001). REM time was unaffected. This effect on sleep–wake time was small. Over the 24 h average time awake went from 37 ± 3% at baseline to 32 ± 3% at day 21; NREM sleep went from 51 ± 2% at baseline to 58 ± 2% at day 21.
Figure 6. Percentage time in wakefulness, NREM and REM sleep.
DBH-SAP injections decrease the percentage of time spent in wakefulness (top panel) and increase the percentage of time spent in NREM sleep (middle panel) over 24 h measured 21 days after the injections. There was no effect on the percentage time spent in REM sleep (bottom panel). •, baseline pre-lesion data; ○, data 21 days after injection. Mean ± s.e.m. values are shown for each 2-h period. The active, dark 12 h is at left; the quiet, light 12 h at the right. Data are from 3 rats. (P < 0.04 for wakefulness; P < 0.001 for NREM sleep).
Discussion
CA-cell-specific lesions
Our goal was to produce a widely distributed and significant loss of CA neurones by injection of this toxin (DBH-SAP) into the ventricular system. This is in essence similar to a knock-out mouse for CA neurones but produced in the adult thus avoiding the complications of any CA neuronal effects on development or unpredictable effects of gene deletion. We substantially reduced the numbers of brainstem CA neurones while avoiding non-specific tissue damage by injection into the 4th ventricle of a conjugate of the ribosomal toxin saporin with a monoclonal antibody to the CA-specific enzyme dopamine β-hydroxylase (DBH). Direct injection or dialysis into brain tissue by insertion of a needle or probe produces tissue damage visible at low power; this was not the case in the present experiments. The antibody binds to DBH in exposed vesicular membranes, the complex is endocytosed and saporin is released to kill the cell (Wrenn et al. 1996; Madden et al. 1999). Our lesion effects are similar to those of Wrenn et al. (1996) who injected the DBH-SAP into the lateral ventricle. The questions are: do CA neurones participate in chemoreception and/or in the determination of the breathing frequency and its variability in a state-dependent manner?
Brainstem CA neurones and breathing
Based on prior published observations from anaesthetized rats (Guyenet et al. 1993; Jodkowski et al. 1997; Oyamada et al. 1998; Dawid-Milner et al. 2001) and various mutant mice (Shirasawa et al. 2000; Hilaire et al. 2004; Viemari et al. 2004) we expected an effect on breathing frequency. Hilaire et al. (2004) concluded that A6 noradrenergic neurones provide a tonic excitatory stimulus that maintains breathing frequency while A5 neurones probably slow breathing frequency. Our results show that substantial lesions of brainstem CA neurones slow breathing frequency during air and CO2 breathing in adult rats and that this effect is present in both wakefulness and in NREM sleep. We conclude that brainstem CA neurones provide a tonic excitatory drive that maintains a normal frequency in both states. The size of the lesion effect on frequency in air breathing was small being ∼6% but in 3% and 7% CO2 the decrease in frequency was ∼16–17%. V̇E was unchanged in air breathing as was oxygen consumption so the effect of the lesions on frequency probably did not disturb alveolar ventilation or arterial PCO2.
Part of our hypothesis was that any effect of our lesions on breathing frequency would be greater in wakefulness when these neurones are most active. This was not the case. Our lesions reduced f equally in quiet wakefulness and NREM sleep.
Chemoreception
There is evidence indicating that brainstem CA neurones participate in central chemoreception. For A5 neurones, CO2 stimulation increases c-fos expression (Haxhiu et al. 1996) and CA release (Rentero et al. 1997). For A6 neurones CO2 stimulation increases c-fos expression (Haxhiu et al. 1996) and A6 neurones are chemosensitive in reduced preparations (Pineda & Aghajanian, 1997; Oyamada et al. 1998; Ballantyne & Scheid, 2000; Filosa et al. 2002). In anaesthetized rats, systemic hypercapnia increases A6 neurone firing even after carotid body denervation (Elam et al. 1981) and focal acidification of the A6 region increases ventilatory output (phrenic nerve activity) indicating a direct role in chemoreception (Coates et al. 1993).
Putative chemoreceptor neurones at or near the ventral medullary surface have been identified as glutamatergic (Mulkey et al. 2004), serotonergic (Richerson, 2004), and other (Okada et al. 2002; Ribas-Salgueiro et al. 2005). We contend that central chemoreception is present at many hindbrain locations and involves many different neuronal phenotypes (see Nattie, 2001; Nattie & Li, 2002; Feldman et al. 2003; Nattie et al. 2004). Our lesion data support the hypothesis that CA neurones participate in central chemoreception. Lesions of brainstem CA neurones reduced the ventilatory response to systemic hypercapnia in wakefulness and in NREM sleep. That our large lesions produced a 26–28% decrease in the CO2 response indicates that other central chemoreceptor sites and the carotid body also contribute. Our findings were significant only with the larger stimulus of 7% CO2 breathing and not with 3% CO2 exposure. We can firmly conclude that at high CO2 stimulus levels brainstem CA neurones are important in central chemoreception. We note that at 3% CO2 exposure there was a similar per cent decrease in the ΔV̇E value comparing CO2 with room air baseline. While this did not reach statistical significance we suggest caution in rejecting the idea that CA neurones also participate in chemoreception at lower stimulus intensities. Studies with more animals in each group may uncover an effect at the lower stimulus level. We also expected a greater effect of our lesion on the CO2 response in wakefulness than in NREM sleep given the higher firing rate of CA neurones in wakefulness. This was not observed suggesting that CA neurones are important in chemoreception in both wakefulness and NREM sleep even at the lower firing rate of NREM sleep. It may be that the CO2 stimulus increases the firing rate of CA neurones by a similar amount in both states.
Note that we cannot conclude from these data that CA neurones are themselves the chemoreceptors, although they may well be CO2/pH sensitive as suggested by studies of A6 neurones in slices (Pineda & Aghajanian, 1997; Oyamada et al. 1998; Ballantyne & Scheid, 2000; Filosa et al. 2002) and by the observation that focal acidification at A6 increases respiratory output (Coates et al. 1993). CA neurones could also modulate inputs from other neurones and from the carotid body, which are chemosensitive.
Variability of breathing
Heart rate variability reflects non-linear control system output and the balance of sympathetic and parasympathetic activity in heart rate control (Kamen et al. 1996; Brennan et al. 2001). The normal variability of breath duration is affected by many factors and in general reflects some optimal control system function that lies between an absence of any variability at one extreme and a clearly periodic breathing pattern at the other (Bruce & Daubenspeck, 1995; Khoo, 2000; BuSha & Stella, 2002). That is, some level of variability is indicative of a normal, healthy control system with many feed-back loops and many sources of excitatory and inhibitory inputs.
We reasoned that CA neuronal activity, which is state dependent, would alter the variability of breath duration in a state-dependent manner, and that our lesions would affect variability less in sleep. This hypothesis is based on the assumption that CA neurones provide input to the respiratory control system that contributes to the normal variability of breath duration. CA neurone lesions had no effect on variability in wakefulness or NREM sleep indicating that in these states other inputs maintain normal variability. However, in REM sleep, the state normally associated with the lowest firing rate of CA neurones, the lesions increased variability even at this low firing rate. We suggest that in REM sleep breathing variability is directly influenced by brainstem CA neurones. For example, it may be that in REM sleep, breathing rate and rhythm are determined by the pre-Bötzinger complex (McKay et al. 2005), which is responsive to CA inputs, whereas in wakefulness and NREM sleep, rate and rhythm are determined by more complex circuits, which are affected by non-CA inputs. Alternatively, central chemoreceptor afferent inputs during REM could provide a needed drive for normal variability and these could be reduced in our lesioned rats.
CA neurones and sleep
CA neurones have long been thought to be important in sleep regulation (Hobson et al. 1975). DBH−/− knock-out mice with a total absence of CA neurones (Thomas et al. 1998) are awake less and in NREM sleep more (Ouyang et al. 2004). Our results support this. Substantial and specific depletion of CA neurones results in less wakefulness and more sleep. CA neurones promote wakefulness but the small quantitative effect of these substantial lesions suggests that they play an accessory role in sleep regulation; they are one of three groups of neurones that promote wakefulness (Saper et al. 2001).
Significance – CA neurones, CCHS and SIDS
Central congenital hypoventilation syndrome (CCHS) is an autosomal dominant disorder with a high likelihood of a mutation in Phox2b, a strong determinant of the noradrenergic system (Weese-Mayer et al. 2003, 2004). CCHS patients hypoventilate in sleep, requiring ventilatory support at night, and have reduced or absent responses to CO2 and hypoxia. Mice heterozygous for Phox2b have sleep-disordered breathing and have been suggested as a partial animal model for CCHS (Durand et al. 2005). Insofar as specific lesions of brainstem CA neurones decrease breathing frequency and chemosensitivity and in REM sleep increase the variability of breathing with a higher incidence of prolonged breath durations, one important defect in CCHS may be attributed to the loss of CA neurones.
The Sudden Infant Death Syndrome (SIDS) has been linked to CA neurone abnormalities (Obonai et al. 1998). Genes related to the embryological development of the autonomic nervous system including the CA neurones are abnormal in 15.2% of SIDS cases (Weese-Mayer et al. 2004). SIDS may arise from genetic, or other, alterations in the function of brainstem serotonergic (Kinney et al. 2003) and/or CA neurones, both of which are important in the development and maintenance of normal autonomic function and breathing.
What do brainstem catecholaminergic neurones do?
CA neurones are of importance in normal blood pressure regulation in awake mice (Swoap et al. 2004) and during physiological stress in anaesthetized rats (Schreihofer & Guyenet, 2000). In unanaesthetized cats (see Levine et al. 1990) A6 CA neurones were activated by stresses like noise, restraint, hypogylcaemia, increased temperature and as part of the ‘defense reaction’ precipitated by exposure to a threatening situation. More recent studies have linked the firing of A6 CA neurones to the presence, in wakefulness, of new sensory stimuli (Aston-Jones et al. 2000; Berridge & Waterhouse, 2003). These studies suggest that LC neurones modulate behaviours based on novel sensory input – that the LC governs attentiveness.
It is of interest that A6 CA neurones respond both to physiological stimuli that arise within internal milieu and to sensory stimuli from the external environment. Their efferent projections modulate behaviour and homeostatic physiology. Our results indicate their involvement as modulators of breathing frequency in wakefulness and in NREM sleep, and breathing variability in REM sleep and as one of many central chemoreceptor sites. We suggest that brainstem CA neurones may link the control of breathing, and perhaps blood pressure, to demands imposed by both internal stresses and external environmental changes.
Acknowledgments
This work was supported by a grant from the National Heart, Lung and Blood Institute (HL 28066). We acknowledge the technical assistance of Qiang Wei Fu.
References
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Ballantyne D, Scheid P. Mammalian brainstem chemosensitive neurons: linking them to respiration in vitro. J Physiol. 2000;525:567–577. doi: 10.1111/j.1469-7793.2000.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability. IEEE Trans Biomed Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- Bruce EN, Daubenspeck JA. Mechanisms and analysis of ventilatory instability. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 2nd. New York: Marcel Dekker; 1995. pp. 285–314. [Google Scholar]
- BuSha BF, Stella MH. State and chemical drive modulate respiratory variability. J Appl Physiol. 2002;93:685–696. doi: 10.1152/japplphysiol.00951.2001. [DOI] [PubMed] [Google Scholar]
- Byrum CE, Stornetta R, Guyenet PG. Electrophysiological properties of spinally-projecting A5 noradrenergic neurons. Brain Res. 1984;303:15–29. doi: 10.1016/0006-8993(84)90206-3. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Drolet G, Valentino RJ. Hemodynamic stress activates locus coeruleus neurons of unanesthetized rats. Brain Res Bull. 1993;31:737–744. doi: 10.1016/0361-9230(93)90150-a. [DOI] [PubMed] [Google Scholar]
- Dampney RAL, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Dawid-Milner M, Lara J, Gonzales-Baron S, Spyer K. Respiratory effects of stimulation of cell bodies of the A5 region in anesthetized rats. Pflug Arch. 2001;441:434–443. doi: 10.1007/s004240000450. [DOI] [PubMed] [Google Scholar]
- Dobbins EC, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rats. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Durand E, Dauger S, Pattyn A, Gaultier C, Goridis C, Gallego J. Sleep-disordered breathing in newborn mice heterozygous for the transcription factor Phox 2b. Amer J Respir Crit Care Medical. 2005;172:238–243. doi: 10.1164/rccm.200411-1528OC. [DOI] [PubMed] [Google Scholar]
- Elam M, Yao T, Thorén P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotonergic neurons of the rat brainstem. J Comp Neurol. 1984;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Feldman JR, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol. 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol Regul Integrative Comp Physiol. 1993;264:R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol. 1996;105:23–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generation by the pontine A5 and A6 groups in rodents. Resp Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M. Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, Vol. 2, Classical Transmitters in the CNS, Part I. Elsevier; 1984. pp. 277–379. [Google Scholar]
- Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol. 1997;82:377–381. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- Kamen PW, Krum H, Tonkin AM. Poincaré plot of heart rate variability allows quantitative display of parasympathetic nervous activity in humans. Clin Sci. 1996;91:201–208. doi: 10.1042/cs0910201. [DOI] [PubMed] [Google Scholar]
- Khoo MCK. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, et al. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- Levine ES, Litto WJ, Jacobs BL. Activity of cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res. 1990;531:189–195. doi: 10.1016/0006-8993(90)90773-5. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JF. Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholamine neurons of the ventrolateral medulla in rats using anti-DβH-saporin. Am J Physiol Regulatory Integrative Comp Physiol. 1999;277:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Fornal CA, Jacobs BL. Effects of physiological manipulations on locus coeruleus neuronal activity in freely moving cats. II. Cardiovascular challenge. Brain Res. 1987;422:24–31. doi: 10.1016/0006-8993(87)90536-1. [DOI] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;18:411–423. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Murase S, Takayama M, Nosaka S. Chemical stimulation of the nucleus locus coeruleus: cardiovascular responses and baroreflex modification. Neurosci Lett. 1993;153:1–4. doi: 10.1016/0304-3940(93)90062-p. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonai T, Yashuhara M, Nakamura T, Takashima S. Catecholamine neurons alteration in the brainstem of sudden infant death syndrome victims. Pediatrics. 1998;101:285–288. doi: 10.1542/peds.101.2.285. [DOI] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Mückenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem–spinal cord of the neonatal rat. J Physiol. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neurosci. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Rentero N, Bruandet N, Pequignot JM, Quintin L. Catechol changes in the rat rostral ventrolateral medulla following changes in systemic CO2. Am J Physiol Regul Integrative Comp Physiol. 1997;273:R947–R955. doi: 10.1152/ajpregu.1997.273.3.R947. [DOI] [PubMed] [Google Scholar]
- Ribas-Salgueiro JL, Gaytán SP, Ribas J, Pásaro R. Characterization of efferent projections of chemosensitive neurons in the caudal parapyramidal area of the rat brain. Brain Res Bull. 2005;66:235–248. doi: 10.1016/j.brainresbull.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DBH-saporin. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R729–R742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Arata A, Onimaru H, Roth K, Brown G, Horning S, Arata S, Okumora K, Sasazuki T, Kornmeyer S. Rnx deficiency results in congenital central hypoventilation. Nat Genet. 2000;24:287–290. doi: 10.1038/73516. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh−/− mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Bévengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravitz EM, Zhou L, Maher BS, Silvestri JM, Curran ME, Marazita ML. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A. 2003;123:267–278. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Curran ME, Silvestri JM, Marazita ML. Sudden infant death syndrome: case × control frequency differences at genes pertinent to early autonomic nervous system embryologic development. Pediatr Res. 2004;56:391–955. doi: 10.1203/01.PDR.0000136285.91048.4A. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Res. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]